Hydraulic Traits and Non-Structural Carbon Responses to Drought Stress in Reaumuria soongorica (Pall.) Maxim. and Salsola passerina Bunge
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Experimental Design
2.3. Water Relations Measurement
2.3.1. Leaf Water Potential
2.3.2. Hydraulic Conductivity
2.3.3. Vulnerability Curves and Hydraulic Safety Margin
2.3.4. Sapwood Specific Conductivity, Leaf Specific Conductivity, and Huber Value
2.3.5. Leaf and Stem Water Content
2.4. Photosynthesis and Gas Exchange Measurement
2.5. Non-Structural Carbohydrate Assay
2.6. Statistics
3. Results
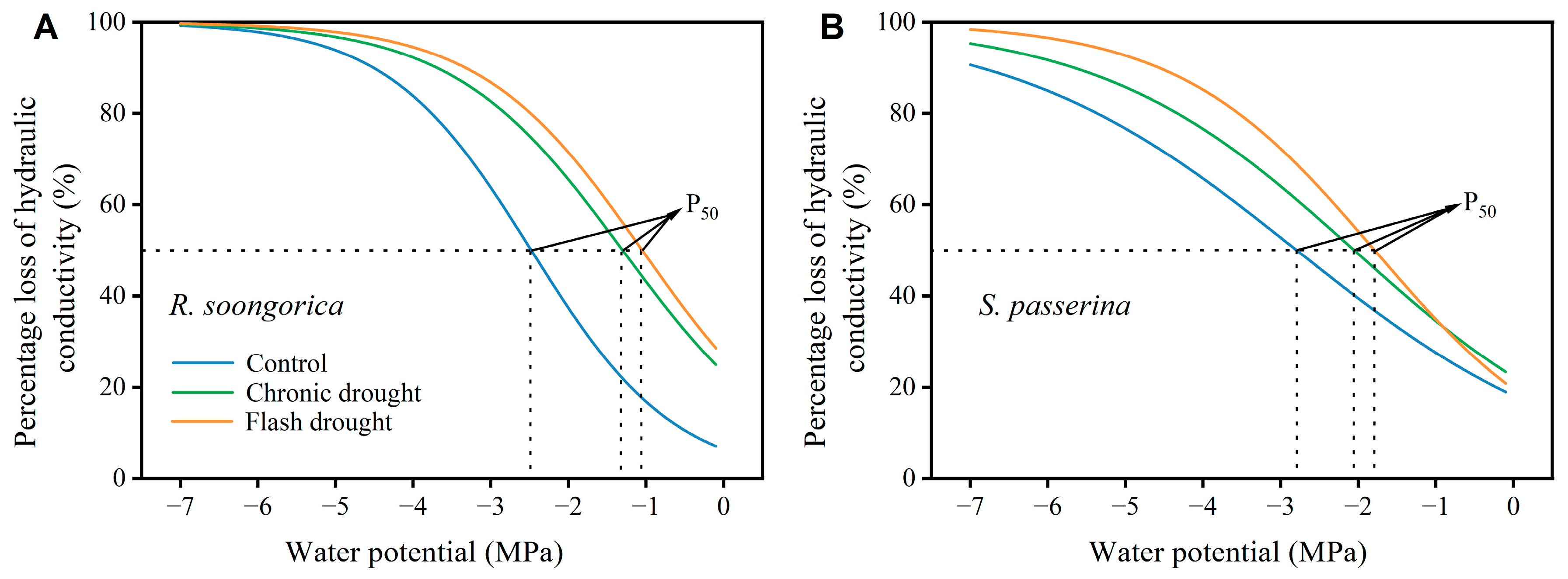
3.1. Plant Water Relation
3.2. Non-Structural Carbohydrate
3.3. Leaf Gas Exchange and Photosynthesis
3.4. Drought Response Strategies
3.5. Links among Hydraulic Traits, Gas Exchange, and Non-Structural Carbohydrate
4. Discussion
4.1. Response of Plant Traits to Drought
4.2. Drought Response Strategies
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, Y.J.; Choat, B.; Sterck, F.; Maenpuen, P.; Katabuchi, M.; Zhang, S.B.; Tomlinson, K.W.; Oliveira, R.S.; Zhang, Y.J.; Shen, J.X. Hydraulic prediction of drought-induced plant dieback and top-kill depends on leaf habit and growth form. Ecol. Lett. 2021, 24, 2350–2363. [Google Scholar] [CrossRef] [PubMed]
- Reichstein, M.; Bahn, M.; Ciais, P.; Frank, D.; Mahecha, M.D.; Seneviratne, S.I.; Zscheischler, J.; Beer, C.; Buchmann, N.; Frank, D.C. Climate extremes and the carbon cycle. Nature 2013, 500, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Anderegg, W.R.; Anderegg, L.D.; Kerr, K.L.; Trugman, A.T. Widespread drought-induced tree mortality at dry range edges indicates that climate stress exceeds species’ compensating mechanisms. Glob. Chang. Biol. 2019, 25, 3793–3802. [Google Scholar] [CrossRef] [PubMed]
- Brodribb, T.J.; Powers, J.; Cochard, H.; Choat, B. Hanging by a thread? Forests and drought. Science 2020, 368, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Tyree, M.T.; Sperry, J.S. Do woody plants operate near the point of catastrophic xylem dysfunction caused by dynamic water stress? Answers from a model. Plant Physiol. 1988, 88, 574–580. [Google Scholar] [CrossRef] [PubMed]
- Duan, H.; Li, Y.; Xu, Y.; Zhou, S.; Liu, J.; Tissue, D.T.; Liu, J. Contrasting drought sensitivity and post-drought resilience among three co-occurring tree species in subtropical China. Agric. For. Meteorol. 2019, 272, 55–68. [Google Scholar] [CrossRef]
- Adams, H.D.; Zeppel, M.J.; Anderegg, W.R.; Hartmann, H.; Landhäusser, S.M.; Tissue, D.T.; Huxman, T.E.; Hudson, P.J.; Franz, T.E.; Allen, C.D. A multi-species synthesis of physiological mechanisms in drought-induced tree mortality. Nat. Ecol. Evol. 2017, 1, 1285–1291. [Google Scholar] [CrossRef]
- Sala, A.; Piper, F.; Hoch, G. Physiological mechanisms of drought-induced tree mortality are far from being resolved. New Phytol. 2010, 186, 274–281. [Google Scholar] [CrossRef]
- Brodribb, T.; Holbrook, N.; Edwards, E.; Gutiérrez, M. Relations between stomatal closure, leaf turgor and xylem vulnerability in eight tropical dry forest trees. Plant Cell Environ. 2003, 26, 443–450. [Google Scholar] [CrossRef]
- Blackman, C.J.; Creek, D.; Maier, C.; Aspinwall, M.J.; Drake, J.E.; Pfautsch, S.; O’grady, A.; Delzon, S.; Medlyn, B.E.; Tissue, D.T. Drought response strategies and hydraulic traits contribute to mechanistic understanding of plant dry-down to hydraulic failure. Tree Physiol. 2019, 39, 910–924. [Google Scholar] [CrossRef]
- Hochberg, U.; Windt, C.W.; Ponomarenko, A.; Zhang, Y.-J.; Gersony, J.; Rockwell, F.E.; Holbrook, N.M. Stomatal closure, basal leaf embolism, and shedding protect the hydraulic integrity of grape stems. Plant Physiol. 2017, 174, 764–775. [Google Scholar] [CrossRef]
- Salleo, S.; Lo Gullo, M.; Trifilo, P.; Nardini, A. New evidence for a role of vessel-associated cells and phloem in the rapid xylem refilling of cavitated stems of Laurus nobilis L. Plant Cell Environ. 2004, 27, 1065–1076. [Google Scholar] [CrossRef]
- Secchi, F.; Pagliarani, C.; Cavalletto, S.; Petruzzellis, F.; Tonel, G.; Savi, T.; Tromba, G.; Obertino, M.M.; Lovisolo, C.; Nardini, A. Chemical inhibition of xylem cellular activity impedes the removal of drought-induced embolisms in poplar stems–new insights from micro-CT analysis. New Phytol. 2021, 229, 820–830. [Google Scholar] [CrossRef] [PubMed]
- Tomasella, M.; Nardini, A.; Hesse, B.D.; Machlet, A.; Matyssek, R.; Häberle, K.-H. Close to the edge: Effects of repeated severe drought on stem hydraulics and non-structural carbohydrates in European beech saplings. Tree Physiol. 2019, 39, 717–728. [Google Scholar] [CrossRef] [PubMed]
- Salleo, S.; Trifilò, P.; Esposito, S.; Nardini, A.; Gullo, M.A.L. Starch-to-sugar conversion in wood parenchyma of field-growing Laurus nobilis plants: A component of the signal pathway for embolism repair? Funct. Plant Biol. 2009, 36, 815–825. [Google Scholar] [CrossRef] [PubMed]
- Zwieniecki, M.A.; Holbrook, N.M. Confronting Maxwell’s demon: Biophysics of xylem embolism repair. Trends Plant Sci. 2009, 14, 530–534. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Guo, W.; Yang, L.; Zou, Z.; Zhang, X.; Addo-Danso, S.D.; Zhou, L.; Li, S. Effects of drought stress on non-structural carbohydrates in different organs of Cunninghamia lanceolata. Plants 2023, 12, 2477. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Su, B.-L.; Han, Y.-G.; Wu, X.-H.; Zhou, W.-M.; Wang, Q.-W.; Zhou, L.; Yu, D.-P. Response of photosynthetic characteristics and non-structural carbohydrate accumulation of Betula ermanii seedlings to drought stress. J. Appl. Ecol. 2021, 32, 513–520. [Google Scholar]
- Zhang, T.; Cao, Y.; Chen, Y.; Liu, G. Non-structural carbohydrate dynamics in Robinia pseudoacacia saplings under three levels of continuous drought stress. Trees 2015, 29, 1837–1849. [Google Scholar] [CrossRef]
- Li, M.-Y.; Fang, L.-D.; Duan, C.-Y.; Cao, Y.; Yin, H.; Ning, Q.-R.; Hao, G.-Y. Greater risk of hydraulic failure due to increased drought threatens pine plantations in Horqin Sandy Land of northern China. For. Ecol. Manag. 2020, 461, 117980. [Google Scholar] [CrossRef]
- Zhang, S.-B.; Zhang, J.-L.; Cao, K.-F. Divergent hydraulic safety strategies in three co-occurring Anacardiaceae tree species in a Chinese savanna. Front. Plant Sci. 2017, 7, 2075. [Google Scholar] [CrossRef]
- Brodribb, T.J.; McAdam, S.A.M.; Jordan, G.J.; Martins, S.C.V. Conifer species adapt to low-rainfall climates by following one of two divergent pathways. Proc. Natl. Acad. Sci. USA 2014, 111, 14489–14493. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, R.S.; Eller, C.B.; Barros, F.d.V.; Hirota, M.; Brum, M.; Bittencourt, P. Linking plant hydraulics and the fast–slow continuum to understand resilience to drought in tropical ecosystems. New Phytol. 2021, 230, 904–923. [Google Scholar] [CrossRef]
- Wyse, S.V.; Macinnis-Ng, C.M.; Burns, B.R.; Clearwater, M.J.; Schwendenmann, L. Species assemblage patterns around a dominant emergent tree are associated with drought resistance. Tree Physiol. 2013, 33, 1269–1283. [Google Scholar] [CrossRef]
- González-Rebeles, G.; Terrazas, T.; Méndez-Alonzo, R.; Paz, H.; Brodribb, T.J.; Tinoco-Ojanguren, C. Leaf water relations reflect canopy phenology rather than leaf life span in Sonoran Desert trees. Tree Physiol. 2021, 41, 1627–1640. [Google Scholar] [CrossRef]
- Johnson, D.M.; Domec, J.C.; Carter Berry, Z.; Schwantes, A.M.; McCulloh, K.A.; Woodruff, D.R.; Wayne Polley, H.; Wortemann, R.; Swenson, J.J.; Scott Mackay, D. Co-occurring woody species have diverse hydraulic strategies and mortality rates during an extreme drought. Plant Cell Environ. 2018, 41, 576–588. [Google Scholar] [CrossRef] [PubMed]
- Chaves, M.M.; Maroco, J.P.; Pereira, J.S. Understanding plant responses to drought—From genes to the whole plant. Funct. Plant Biol. 2003, 30, 239–264. [Google Scholar] [CrossRef] [PubMed]
- Vargas, G.G.; Brodribb, T.J.; Dupuy, J.M.; González-M, R.; Hulshof, C.M.; Medvigy, D.; Allerton, T.A.; Pizano, C.; Salgado-Negret, B.; Schwartz, N.B. Beyond leaf habit: Generalities in plant function across 97 tropical dry forest tree species. New Phytol. 2021, 232, 148–161. [Google Scholar] [CrossRef]
- Christoffersen, B.O.; Gloor, M.; Fauset, S.; Fyllas, N.M.; Galbraith, D.R.; Baker, T.R.; Kruijt, B.; Rowland, L.; Fisher, R.A.; Binks, O.J. Linking hydraulic traits to tropical forest function in a size-structured and trait-driven model (TFS v. 1-Hydro). Geosci. Model. Dev. 2016, 9, 4227–4255. [Google Scholar] [CrossRef]
- Zhang, Z.; Shan, L.; Li, Y.; Wang, Y. Belowground interactions differ between sympatric desert shrubs under water stress. Ecol. Evol. 2020, 10, 1444–1453. [Google Scholar] [CrossRef]
- Su, P.; Yan, Q.; Xie, T.; Zhou, Z.; Gao, S. Associated growth of C3 and C4 desert plants helps the C3 species at the cost of the C4 species. Acta Physiol. Plant. 2012, 34, 2057–2068. [Google Scholar] [CrossRef]
- Urli, M.; Porté, A.J.; Cochard, H.; Guengant, Y.; Burlett, R.; Delzon, S. Xylem embolism threshold for catastrophic hydraulic failure in angiosperm trees. Tree Physiol. 2013, 33, 672–683. [Google Scholar] [CrossRef] [PubMed]
- Brodribb, T.; Feild, T. Stem hydraulic supply is linked to leaf photosynthetic capacity: Evidence from New Caledonian and Tasmanian rainforests. Plant Cell Environ. 2000, 23, 1381–1388. [Google Scholar] [CrossRef]
- Sperry, J.; Donnelly, J.; Tyree, M. A method for measuring hydraulic conductivity and embolism in xylem. Plant Cell Environ. 1988, 11, 35–40. [Google Scholar] [CrossRef]
- Chen, Y.J.; Maenpuen, P.; Zhang, Y.J.; Barai, K.; Katabuchi, M.; Gao, H.; Kaewkamol, S.; Tao, L.B.; Zhang, J.L. Quantifying vulnerability to embolism in tropical trees and lianas using five methods: Can discrepancies be explained by xylem structural traits? New Phytol. 2021, 229, 805–819. [Google Scholar] [CrossRef] [PubMed]
- Pammenter, N.V.; Van der Willigen, C. A mathematical and statistical analysis of the curves illustrating vulnerability of xylem to cavitation. Tree Physiol. 1998, 18, 589–593. [Google Scholar] [CrossRef] [PubMed]
- Choat, B.; Jansen, S.; Brodribb, T.J.; Cochard, H.; Delzon, S.; Bhaskar, R.; Bucci, S.J.; Feild, T.S.; Gleason, S.M.; Hacke, U.G. Global convergence in the vulnerability of forests to drought. Nature 2012, 491, 752–755. [Google Scholar] [CrossRef]
- Burgess, S.S.; Pittermann, J.; Dawson, T.E. Hydraulic efficiency and safety of branch xylem increases with height in Sequoia sempervirens (D. Don) crowns. Plant Cell Environ. 2006, 29, 229–239. [Google Scholar] [CrossRef]
- Osaki, M.; Shinano, T.; Tadano, T. Redistribution of carbon and nitrogen compounds from the shoot to the harvesting organs during maturation in field crops. Soil. Sci. Plant Nutr. 1991, 37, 117–128. [Google Scholar] [CrossRef]
- Lefcheck, J.S. piecewiseSEM: Piecewise structural equation modelling in r for ecology, evolution, and systematics. Methods Ecol. Evol. 2016, 7, 573–579. [Google Scholar] [CrossRef]
- Latt, C.; Nair, P.; Kang, B. Reserve carbohydrate levels in the boles and structural roots of five multipurpose tree species in a seasonally dry tropical climate. For. Ecol. Manag. 2001, 146, 145–158. [Google Scholar] [CrossRef]
- Silva, E.N.; Ferreira-Silva, S.L.; Viégas, R.A.; Silveira, J.A.G. The role of organic and inorganic solutes in the osmotic adjustment of drought-stressed Jatropha curcas plants. Environ. Exp. Bot. 2010, 69, 279–285. [Google Scholar] [CrossRef]
- Handa, S.; Bressan, R.A.; Handa, A.K.; Carpita, N.C.; Hasegawa, P.M. Solutes contributing to osmotic adjustment in cultured plant cells adapted to water stress. Plant Physiol. 1983, 73, 834–843. [Google Scholar] [CrossRef] [PubMed]
- Birami, B.; Gattmann, M.; Heyer, A.G.; Grote, R.; Arneth, A.; Ruehr, N.K. Heat waves alter carbon allocation and increase mortality of Aleppo pine under dry conditions. Front. For. Glob. Chang. 2018, 1, 8. [Google Scholar] [CrossRef]
- Pangle, R.E.; Limousin, J.M.; Plaut, J.A.; Yepez, E.A.; Hudson, P.J.; Boutz, A.L.; Gehres, N.; Pockman, W.T.; McDowell, N.G. Prolonged experimental drought reduces plant hydraulic conductance and transpiration and increases mortality in a piñon–juniper woodland. Ecol. Evol. 2015, 5, 1618–1638. [Google Scholar] [CrossRef] [PubMed]
- Choat, B.; Brodribb, T.J.; Brodersen, C.R.; Duursma, R.A.; López, R.; Medlyn, B.E. Triggers of tree mortality under drought. Nature 2018, 558, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Duan, H.; Resco de Dios, V.; Wang, D.; Zhao, N.; Huang, G.; Liu, W.; Wu, J.; Zhou, S.; Choat, B.; Tissue, D.T. Testing the limits of plant drought stress and subsequent recovery in four provenances of a widely distributed subtropical tree species. Plant Cell Environ. 2022, 45, 1187–1203. [Google Scholar] [CrossRef]
- Sevanto, S.; Mcdowell, N.G.; Dickman, L.T.; Pangle, R.; Pockman, W.T. How do trees die? A test of the hydraulic failure and carbon starvation hypotheses. Plant Cell Environ. 2014, 37, 153–161. [Google Scholar] [CrossRef]
- Luo, D.; Wang, C.; Jin, Y.; Li, Z.; Wang, Z. Different hydraulic strategies under drought stress between Fraxinus mandshurica and Larix gmelinii seedlings. J. For. Res. 2022, 34, 99–111. [Google Scholar] [CrossRef]
- Anderegg, W.R.; Klein, T.; Bartlett, M.; Sack, L.; Pellegrini, A.F.; Choat, B.; Jansen, S. Meta-analysis reveals that hydraulic traits explain cross-species patterns of drought-induced tree mortality across the globe. Proc. Natl. Acad. Sci. USA 2016, 113, 5024–5029. [Google Scholar] [CrossRef]
- Shao, J.; Zhou, X.; Zhang, P.; Zhai, D.; Yuan, T.; Li, Z.; He, Y.; McDowell, N.G. Embolism resistance explains mortality and recovery of five subtropical evergreen broadleaf trees to persistent drought. Ecology 2023, 104, e3877. [Google Scholar] [CrossRef] [PubMed]
- Smith-Martin, C.M.; Muscarella, R.; Ankori-Karlinsky, R.; Delzon, S.; Farrar, S.L.; Salva-Sauri, M.; Thompson, J.; Zimmerman, J.K.; Uriarte, M. Hurricanes increase tropical forest vulnerability to drought. New Phytol. 2022, 235, 1005–1017. [Google Scholar] [CrossRef] [PubMed]
- Lens, F.; Sperry, J.S.; Christman, M.A.; Choat, B.; Rabaey, D.; Jansen, S. Testing hypotheses that link wood anatomy to cavitation resistance and hydraulic conductivity in the genus Acer. New Phytol. 2011, 190, 709–723. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Blackman, C.J.; Choat, B.; Rymer, P.D.; Medlyn, B.E.; Tissue, D.T. Drought tolerance traits do not vary across sites differing in water availability in Banksia serrata (Proteaceae). Funct. Plant Biol. 2019, 46, 624–633. [Google Scholar] [CrossRef] [PubMed]
- López, R.; Cano, F.J.; Choat, B.; Cochard, H.; Gil, L. Plasticity in vulnerability to cavitation of Pinus canariensis occurs only at the driest end of an aridity gradient. Front. Plant Sci. 2016, 7, 769. [Google Scholar] [CrossRef] [PubMed]
- Stojnić, S.; Suchocka, M.; Benito-Garzón, M.; Torres-Ruiz, J.; Cochard, H.; Bolte, A.; Cocozza, C.; Cvjetković, B.; De Luis, M.; Martinez-Vilalta, J. Variation in xylem vulnerability to embolism in European beech from geographically marginal populations. Tree Physiol. 2018, 38, 173–185. [Google Scholar] [CrossRef]
- Fu, P.-L.; Jiang, Y.-J.; Wang, A.-Y.; Brodribb, T.J.; Zhang, J.-L.; Zhu, S.-D.; Cao, K.-F. Stem hydraulic traits and leaf water-stress tolerance are co-ordinated with the leaf phenology of angiosperm trees in an Asian tropical dry karst forest. Ann. Bot. 2012, 110, 189–199. [Google Scholar] [CrossRef]
- Kondoh, S.; Yahata, H.; Nakashizuka, T.; Kondoh, M. Interspecific variation in vessel size, growth and drought tolerance of broad-leaved trees in semi-arid regions of Kenya. Tree Physiol. 2006, 26, 899–904. [Google Scholar] [CrossRef]
- Mota-Gutiérrez, D.; Arreola-González, G.; Aguilar-Romero, R.; Paz, H.; Cavender-Bares, J.; Oyama, K.; Gonzalez-Rodriguez, A.; Pineda-García, F. Seasonal variation in native hydraulic conductivity between two deciduous oak species. J. Plant Ecol. 2020, 13, 78–86. [Google Scholar] [CrossRef]
- Choat, B.; Ball, M.C.; Luly, J.G.; Holtum, J.A. Hydraulic architecture of deciduous and evergreen dry rainforest tree species from north-eastern Australia. Trees 2005, 19, 305–311. [Google Scholar] [CrossRef]
- Ramírez-Valiente, J.A.; Cavender-Bares, J. Evolutionary trade-offs between drought resistance mechanisms across a precipitation gradient in a seasonally dry tropical oak (Quercus oleoides). Tree Physiol. 2017, 37, 889–901. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Wang, L.; Wan, X. Relative contributions of hydraulic dysfunction and carbohydrate depletion during tree mortality caused by drought. AoB Plants 2018, 10, plx069. [Google Scholar] [CrossRef] [PubMed]
- Creek, D.; Blackman, C.J.; Brodribb, T.J.; Choat, B.; Tissue, D.T. Coordination between leaf, stem, and root hydraulics and gas exchange in three arid-zone angiosperms during severe drought and recovery. Plant Cell Environ. 2018, 41, 2869–2881. [Google Scholar] [CrossRef] [PubMed]






| Traits | Species | Water Treatment | Species × Water Treatment |
|---|---|---|---|
| KS | 8.50 * | 5.48 * | 0.87 |
| KL | 68.92 ** | 20.15 ** | 14.13 ** |
| Hv | 14.39 ** | 3.30 | 3.57 |
| P50 | 27.98 ** | 45.18 ** | 1.37 |
| ΨPD | 69.80 ** | 164.43 ** | 14.44 ** |
| ΨMD | 57.03 ** | 358.24 ** | 2.04 |
| HSM50 | 107.14 ** | 431.23 ** | 0.33 |
| LWC | 146.64 ** | 33.92 ** | 5.40 * |
| SWC | 122.15 ** | 47.95 ** | 10.42 ** |
| Traits | Species | Water Treatment | Species × Water Treatment |
|---|---|---|---|
| L-SSC | 48.34 ** | 61.34 ** | 0.32 |
| L-SC | 21.60 ** | 59.72 ** | 1.69 |
| L-NSC | 0.17 | 9.03 ** | 2.10 |
| S-SSC | 4.13 | 1060.56 ** | 125.35 ** |
| S-SC | 2.55 | 12.48 ** | 5.36 * |
| S-NSC | 5.66 * | 128.18 ** | 26.12 ** |
| Traits | Species | Water Treatment | Species × Water Treatment |
|---|---|---|---|
| gs | 28.10 ** | 93.38 ** | 0.26 |
| Pn | 63.74 ** | 1280.85 ** | 2.779 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Ma, J.; Xie, T.; Niu, F.; He, C.; Shi, Y.; Zhang, Z.; Zhang, J.; Shan, L. Hydraulic Traits and Non-Structural Carbon Responses to Drought Stress in Reaumuria soongorica (Pall.) Maxim. and Salsola passerina Bunge. Forests 2024, 15, 287. https://doi.org/10.3390/f15020287
Wang H, Ma J, Xie T, Niu F, He C, Shi Y, Zhang Z, Zhang J, Shan L. Hydraulic Traits and Non-Structural Carbon Responses to Drought Stress in Reaumuria soongorica (Pall.) Maxim. and Salsola passerina Bunge. Forests. 2024; 15(2):287. https://doi.org/10.3390/f15020287
Chicago/Turabian StyleWang, Hongyong, Jing Ma, Tingting Xie, Furong Niu, Cai He, Yating Shi, Zhengzhong Zhang, Jing Zhang, and Lishan Shan. 2024. "Hydraulic Traits and Non-Structural Carbon Responses to Drought Stress in Reaumuria soongorica (Pall.) Maxim. and Salsola passerina Bunge" Forests 15, no. 2: 287. https://doi.org/10.3390/f15020287
APA StyleWang, H., Ma, J., Xie, T., Niu, F., He, C., Shi, Y., Zhang, Z., Zhang, J., & Shan, L. (2024). Hydraulic Traits and Non-Structural Carbon Responses to Drought Stress in Reaumuria soongorica (Pall.) Maxim. and Salsola passerina Bunge. Forests, 15(2), 287. https://doi.org/10.3390/f15020287





