Insights into the Root Sprouts of Toona fargesii in a Natural Forest: From the Morphology, Physiology, and Transcriptome Levels
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Endogenous Hormones Measurements
2.3. Soluble Sugar, Starch, Non-Structural Carbohydrate Measurements
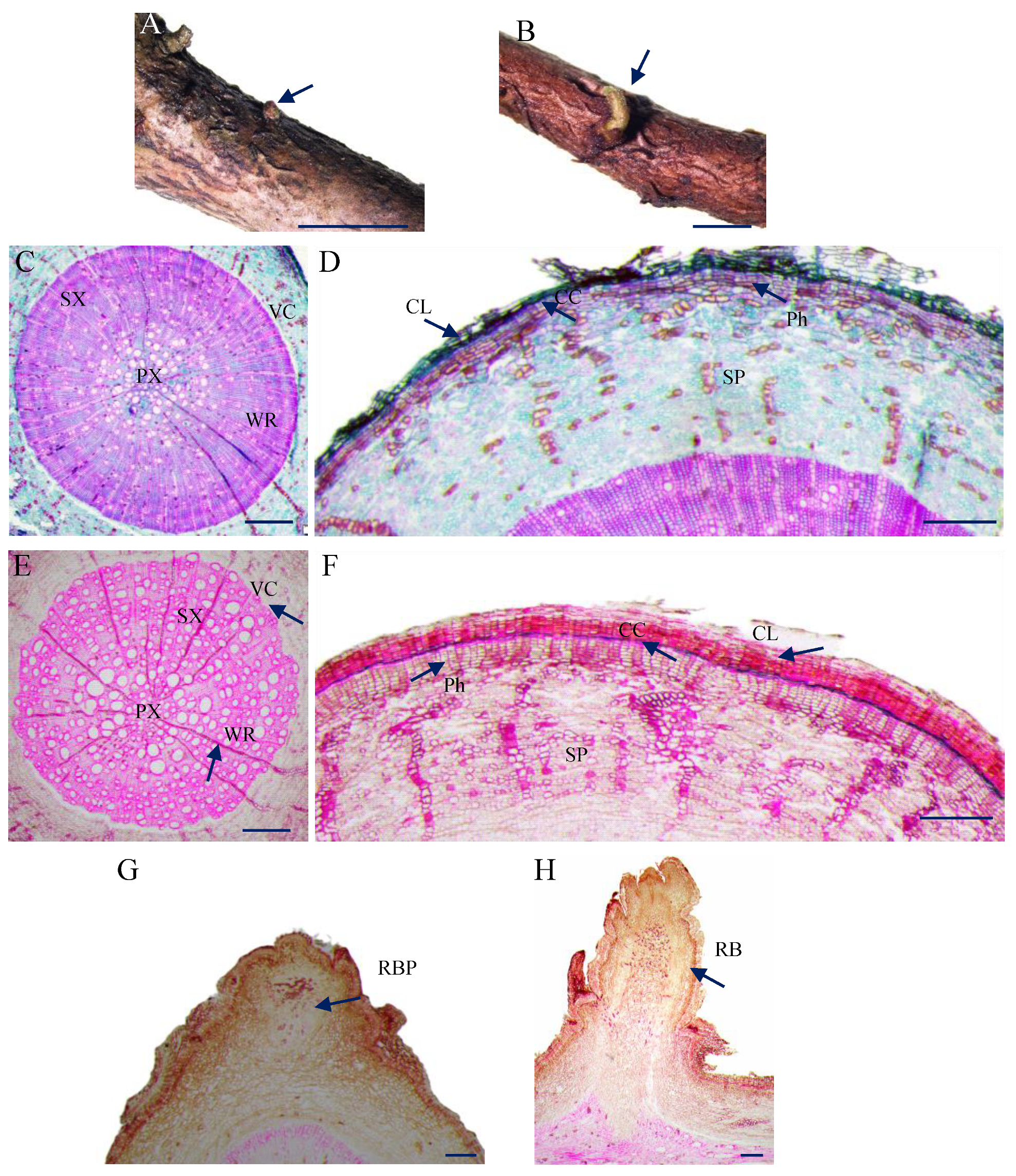
2.4. Histological Observation
2.5. Illumina and PacBio SMRT Sequencing
2.6. The Annotation, Enrichment, and Analysis of DEGs
2.7. The Validation of Candidate Genes by qPCR
2.8. Statistical Analysis
3. Results
3.1. Morphology and Anatomical Observation
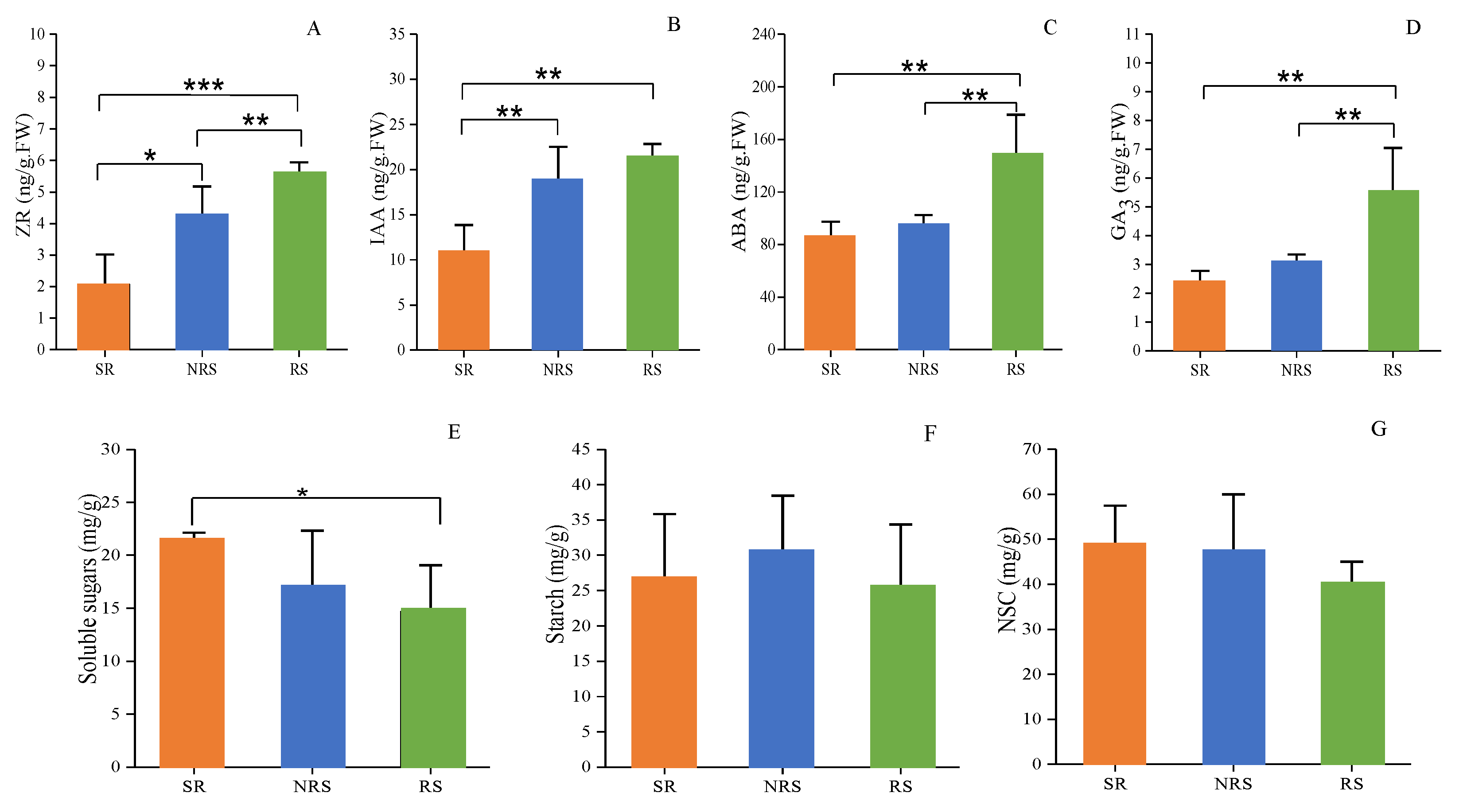
3.2. Phytohormone Levels in RS, NRS and SR
3.3. Non-Structural Carbohydrate Levels in RS, NRS and SR
3.4. Transcriptome Assembly and Annotation
3.5. Analysis of Differentially Expressed Genes (DEGs)
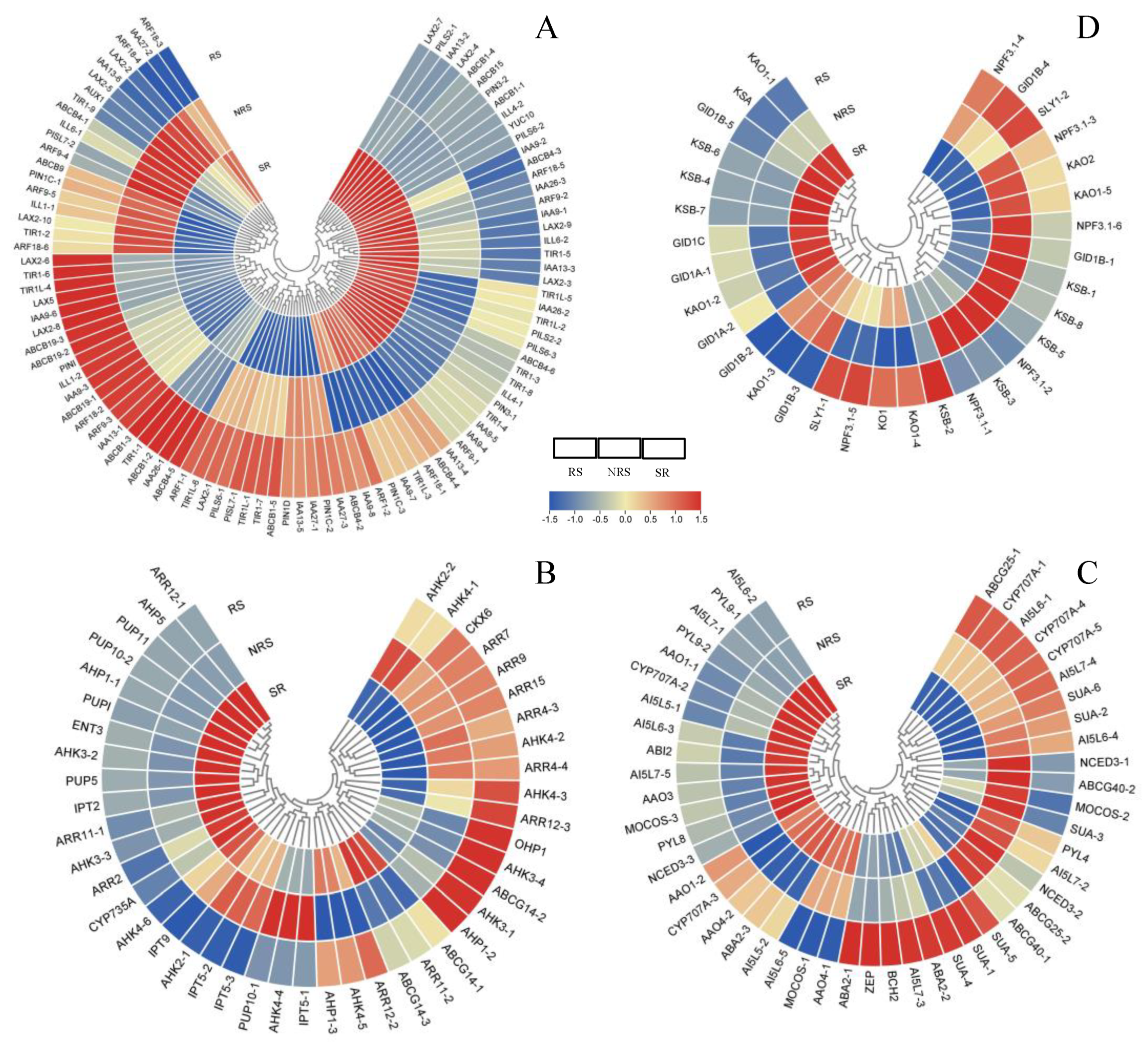
3.6. Hormone Pathways in Response to Root Sprouts
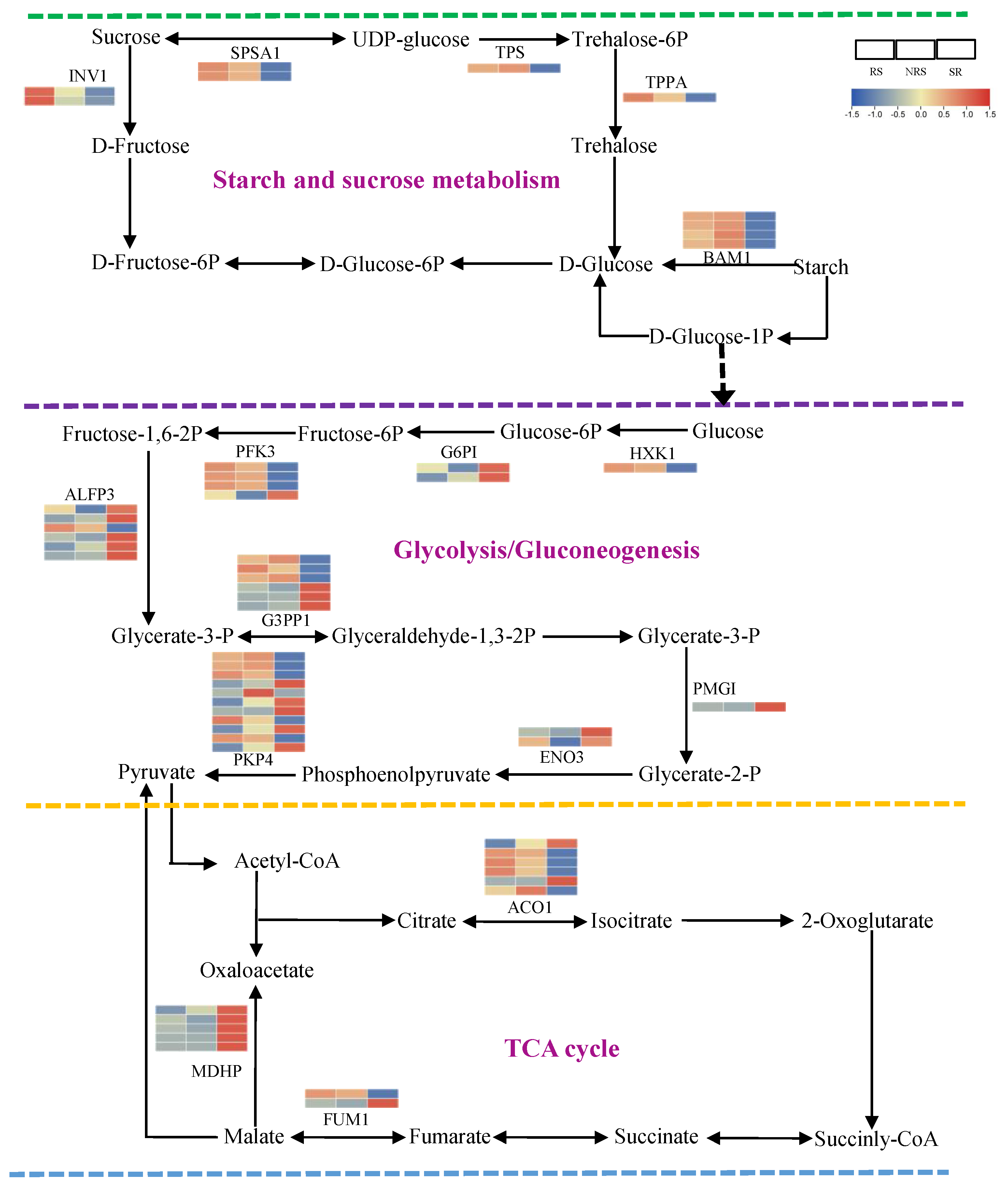
3.7. Nonstructural Carbohydrate Metabolism in Response to Root Sprouts
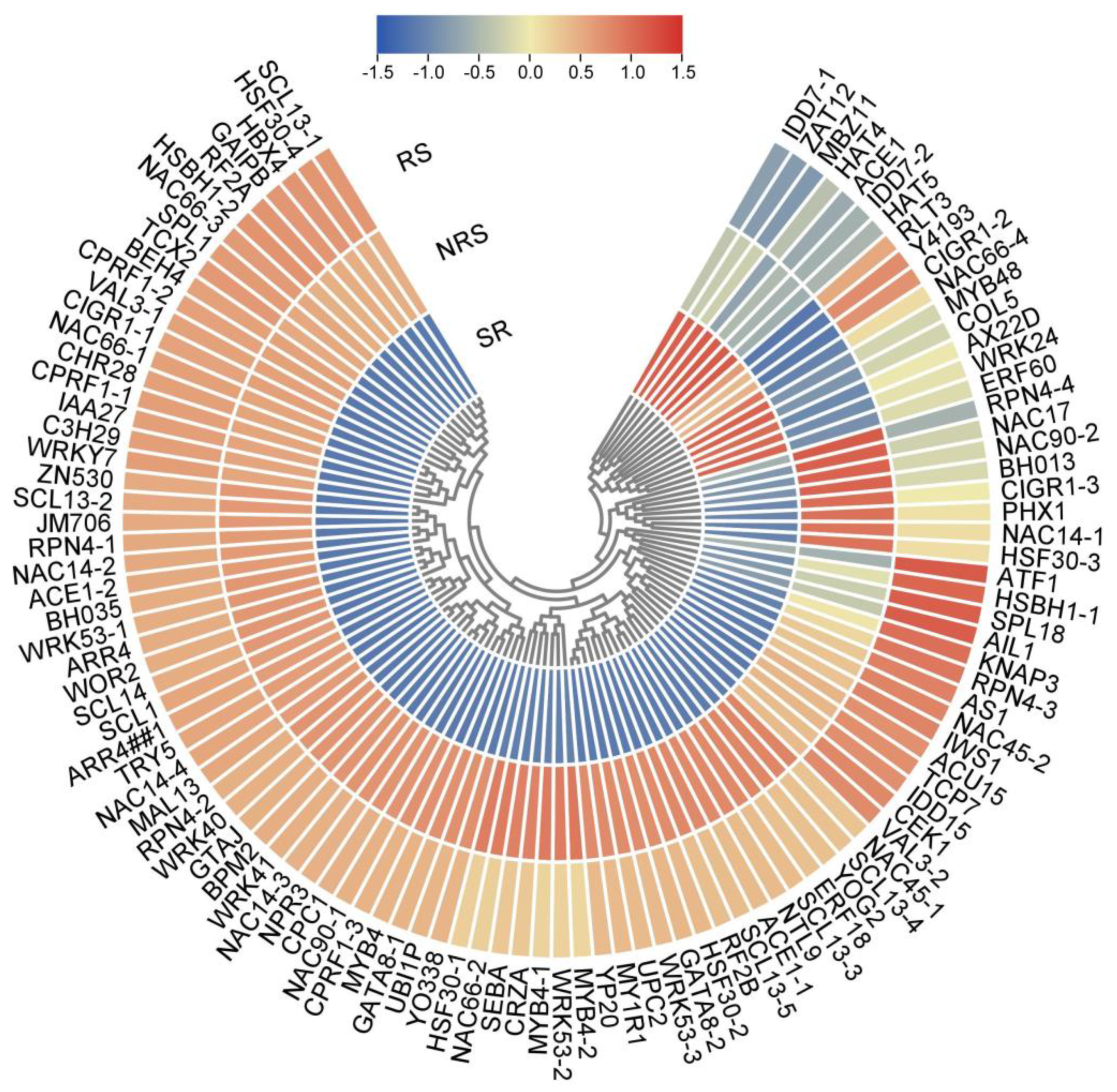
3.8. Transcription Factors of DEGs Involved in Root Sprouts
3.9. Validation by qRT-PCR
4. Discussion
4.1. The Phenomenon and Characteristics of Root Sprouting
4.2. The Regulation Underlying Root Sprouting in T. fargesii
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Olsthoorn, A.; Keltjens, W.; Van Baren, B.; Hopman, M. Influence of ammonium on fine root development and rhizosphere pH of Douglas-fir seedlings in sand. Plant Soil 1991, 133, 75–81. [Google Scholar] [CrossRef]
- Schier, G.A. Seasonal variation in sucker production from excised roots of Populus tremuloides and the role of endogenous auxin. Can. J. For. Res. 1973, 3, 459–461. [Google Scholar] [CrossRef]
- Bosela, M.J.; Ewers, F.W. The mode of origin of root buds and root sprouts in the clonal tree Sassafras albidum (Lauraceae). Am. J. Bot. 1997, 84, 1466–1481. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Shao, X.; Xue, P.; Tian, Y.; Xiao, Z.; Wu, Y. Root sprouting ability and growth dynamics of the rootsuckers of Emmenopterys henryi, a rare and endangered plant endemic to China. For. Ecol. Manag. 2017, 389, 35–45. [Google Scholar] [CrossRef]
- Kataoka, E.Y.; Alves, D.M.; Koch, I.; Souto, L.S. Are there buds in the roots of Aspidosperma spp. (Apocynaceae)? A comparative morphoanatomical study of underground organs. Flora 2019, 256, 92–99. [Google Scholar] [CrossRef]
- Poorter, L.; Kitajima, K.; Mercado, P.; CHUBIna, J.; Melgar, I.; Prins, H.H. Resprouting as a persistence strategy of tropical forest trees: Relations with carbohydrate storage and shade tolerance. Ecology 2010, 91, 2613–2627. [Google Scholar] [CrossRef] [PubMed]
- Nano, C.E.; Clarke, P.J. How do drought and fire influence the patterns of resprouting in Australian deserts? Plant Ecol. 2011, 212, 2095–2110. [Google Scholar] [CrossRef]
- Dietze, M.C.; Clark, J.S. Changing the gap dynamics paradigm: Vegetative regeneration control on forest response to disturbance. Ecol. Monogr. 2008, 78, 331–347. [Google Scholar] [CrossRef]
- Keeley, J.E.; Bond, W.J.; Bradstock, R.A.; Pausas, J.G.; Rundel, P.W. Fire in Mediterranean Ecosystems: Ecology, Evolution and Management; Cambridge University Press: Cambridge, UK, 2011. [Google Scholar]
- Lawes, M.J.; Adie, H.; Russell-Smith, J.; Murphy, B.; Midgley, J.J. How do small savanna trees avoid stem mortality by fire? The roles of stem diameter, height and bark thickness. Ecosphere 2011, 2, 1–13. [Google Scholar] [CrossRef]
- Midgley, J.; Cowling, R. Regeneration patterns in Cape subtropical transitional thicket; where are all the seedlings? S. Afr. J. Bot. 1993, 59, 496–499. [Google Scholar]
- Bellingham, P.J.; Sparrow, A.D. Multi-stemmed trees in montane rain forests: Their frequency and demography in relation to elevation, soil nutrients and disturbance. J. Ecol. 2009, 97, 472–483. [Google Scholar] [CrossRef]
- Klimešová, J.; Martínková, J. Intermediate growth forms as a model for the study of plant clonality functioning: An example with root sprouters. Evol. Ecol. 2004, 18, 669–681. [Google Scholar] [CrossRef]
- Klimešová, J.; Klimeš, L. Resprouting of herbs in disturbed habitats: Is it adequately described by Bellingham-Sparrow’s model? Oikos 2003, 103, 225–229. [Google Scholar] [CrossRef]
- Bartušková, A.; Malíková, L.; Klimešová, J. Checklist of root-sprouters in the Czech flora: Mapping the gaps in our knowledge. Folia Geobot. 2017, 52, 337–343. [Google Scholar] [CrossRef]
- Clarke, P.J.; Lawes, M.; Midgley, J.J.; Lamont, B.; Ojeda, F.; Burrows, G.; Enright, N.; Knox, K. Resprouting as a key functional trait: How buds, protection and resources drive persistence after fire. New Phytol. 2013, 197, 19–35. [Google Scholar] [CrossRef]
- De Moraes, M.G.; de Carvalho, M.A.M.; Franco, A.C.; Pollock, C.J.; Figueiredo-Ribeiro, R.d.C.L. Fire and drought: Soluble carbohydrate storage and survival mechanisms in herbaceous plants from the Cerrado. Bioscience 2016, 66, 107–117. [Google Scholar] [CrossRef]
- Pausas, J.G.; Lamont, B.B.; Paula, S.; Appezzato-da-Glória, B.; Fidelis, A. Unearthing belowground bud banks in fire-prone ecosystems. New Phytol. 2018, 217, 1435–1448. [Google Scholar] [CrossRef] [PubMed]
- Klimešová, J.; Herben, T.; Martínková, J. Disturbance is an important factor in the evolution and distribution of root-sprouting species. Evol. Ecol. 2017, 31, 387–399. [Google Scholar] [CrossRef]
- Hissae Hayashi, A.; Appezzato-da-Glória, B. Resprouting from roots in four Brazilian tree species. Rev. De Biol. Trop. 2009, 57, 789–800. [Google Scholar] [CrossRef] [PubMed]
- Cline, M.G. Apical dominance. Bot. Rev. 1991, 57, 318–358. [Google Scholar] [CrossRef]
- Wan, X.; Landhäusser, S.M.; Lieffers, V.J.; Zwiazek, J.J. Signals controlling root suckering and adventitious shoot formation in aspen (Populus tremuloides). Tree Physiol. 2006, 26, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Eliasson, L. Growth regulators in Populus tremula IV. Apical dominance and suckering in young plants. Physiol. Plant. 1971, 25, 263–267. [Google Scholar] [CrossRef]
- Schier, G.A. Apical Dominance in Multishoot Cultures from Aspen Roots; Oxford University Press: Oxford, UK, 1972. [Google Scholar]
- Van Staden, J.; Davey, J. The synthesis, transport and metabolism of endogenous cytokinins. Plant Cell Environ. 1979, 2, 93–106. [Google Scholar] [CrossRef]
- Jean, S.A.; Pinno, B.D.; Nielsen, S.E. Trembling aspen root suckering and stump sprouting response to above ground disturbance on a reclaimed boreal oil sands site in Alberta, Canada. New For. 2019, 50, 771–784. [Google Scholar] [CrossRef]
- Frey, B.R.; Lieffers, V.J.; Landhäusser, S.M.; Comeau, P.G.; Greenway, K.J. An analysis of sucker regeneration of trembling aspen. Can. J. For. Res. 2003, 33, 1169–1179. [Google Scholar] [CrossRef]
- Li, W.; Zhai, L.; Strauss, S.H.; Yer, H.; Merewitz, E.; Chen, J.; Wang, X.; Zhuang, W.; Fang, C.; Chen, Y. Transgenic reduction of cytokinin levels in roots inhibits root-sprouting in Populus. Plant Physiol. 2019, 180, 1788–1792. [Google Scholar] [CrossRef] [PubMed]
- Bell, T.L.; Ojeda, F. Underground starch storage in Erica species of the Cape Floristic Region–differences between seeders and resprouters. New Phytol. 1999, 144, 143–152. [Google Scholar] [CrossRef]
- Bell, D.T. Ecological response syndromes in the flora of southwestern Western Australia: Fire resprouters versus reseeders. Bot. Rev. 2001, 67, 417–440. [Google Scholar] [CrossRef]
- Knox, K.; Clarke, P. Nutrient availability induces contrasting allocation and starch formation in resprouting and obligate seeding shrubs. Funct. Ecol. 2005, 19, 690–698. [Google Scholar] [CrossRef]
- Kushwah, S.; Laxmi, A. The interaction between glucose and cytokinin signaling in controlling Arabidopsis thaliana seedling root growth and development. Plant Signal. Behav. 2017, 12, e1312241. [Google Scholar] [CrossRef]
- Sakr, S.; Wang, M.; Dédaldéchamp, F.; Perez-Garcia, M.-D.; Ogé, L.; Hamama, L.; Atanassova, R. The sugar-signaling hub: Overview of regulators and interaction with the hormonal and metabolic network. Int. J. Mol. Sci. 2018, 19, 2506. [Google Scholar] [CrossRef] [PubMed]
- Kotov, A.A.; Kotova, L.M.; Romanov, G.A. Signaling network regulating plant branching: Recent advances and new challenges. Plant Sci. 2021, 307, 110880. [Google Scholar] [CrossRef] [PubMed]
- Wiley, E.; King, C.M.; Landhäusser, S.M. Identifying the relevant carbohydrate storage pools available for remobilization in aspen roots. Tree Physiol. 2019, 39, 1109–1120. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, J.; Wang, Z.; Zhu, Q.; Wang, W. Hormonal changes in the grains of rice subjected to water stress during grain filling. Plant Physiol. 2001, 127, 315–323. [Google Scholar] [CrossRef]
- He, Z. Guidance to Experiment on Chemical Control in Crop Plants; Beijing Agricultural University Press: Beijing, China, 1993; pp. 60–68. [Google Scholar]
- Loewus, F.A. Improvement in anthrone method for determination of carbohydrates. Anal. Chem. 1952, 24, 219. [Google Scholar] [CrossRef]
- Salmela, L.; Rivals, E. LoRDEC: Accurate and efficient long read error correction. Bioinformatics 2014, 30, 3506–3514. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Niu, B.; Zhu, Z.; Wu, S.; Li, W. CD-HIT: Accelerated for clustering the next-generation sequencing data. Bioinformatics 2012, 28, 3150–3152. [Google Scholar] [CrossRef]
- Zheng, Y.; Jiao, C.; Sun, H.; Rosli, H.G.; Pombo, M.A.; Zhang, P.; Banf, M.; Dai, X.; Martin, G.B.; Giovannoni, J.J. iTAK: A program for genome-wide prediction and classification of plant transcription factors, transcriptional regulators, and protein kinases. Mol. Plant 2016, 9, 1667–1670. [Google Scholar] [CrossRef]
- Sun, L.; Luo, H.; Bu, D.; Zhao, G.; Yu, K.; Zhang, C.; Liu, Y.; Chen, R.; Zhao, Y. Utilizing sequence intrinsic composition to classify protein-coding and long non-coding transcripts. Nucleic Acids Res. 2013, 41, e166. [Google Scholar] [CrossRef]
- Li, A.; Zhang, J.; Zhou, Z. PLEK: A tool for predicting long non-coding RNAs and messenger RNAs based on an improved k-mer scheme. BMC Bioinform. 2014, 15, 311. [Google Scholar] [CrossRef]
- Kang, Y.-J.; Yang, D.-C.; Kong, L.; Hou, M.; Meng, Y.-Q.; Wei, L.; Gao, G. CPC2: A fast and accurate coding potential calculator based on sequence intrinsic features. Nucleic Acids Res. 2017, 45, W12–W16. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.D.; Coggill, P.; Eberhardt, R.Y.; Eddy, S.R.; Mistry, J.; Mitchell, A.L.; Potter, S.C.; Punta, M.; Qureshi, M.; Sangrador-Vegas, A. The Pfam protein families database: Towards a more sustainable future. Nucleic Acids Res. 2016, 44, D279–D285. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Wang, M.; Zhang, T.; Tong, X.; Xiang, T. Mammalian CYP2E1 gene triggered changes of relative ions fluxes, CaM content and genes expression profiles in Petunia hybrida cells to enhance resistance to formaldehyde. Plant Cell Tissue Organ Cult. 2018, 135, 433–444. [Google Scholar] [CrossRef]
- Song, H.; Mao, W.; Duan, Z.; Que, Q.; Zhou, W.; Chen, X.; Li, P. Selection and validation of reference genes for measuring gene expression in Toona ciliata under different experimental conditions by quantitative real-time PCR analysis. BMC Plant Biol. 2020, 20, 450. [Google Scholar] [CrossRef] [PubMed]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Yan, Y.; Wei, M.; Li, Y.; Tao, H.; Wu, H.; Chen, Z.; Li, C.; Xu, J.-H. MiR529a controls plant height, tiller number, panicle architecture and grain size by regulating SPL target genes in rice (Oryza sativa L.). Plant Sci. 2021, 302, 110728. [Google Scholar] [CrossRef]
- Hayashi, A.; Penha, A.; Rodrigues, R.R.; Appezzato-da-Glória, B. Anatomical studies of shoot bud-forming roots of Brazilian tree species. Aust. J. Bot. 2001, 49, 745–751. [Google Scholar] [CrossRef]
- Kondhare, K.R.; Patil, A.B.; Giri, A.P. Auxin: An emerging regulator of tuber and storage root development. Plant Sci. 2021, 306, 110854. [Google Scholar] [CrossRef]
- Terasaka, K.; Blakeslee, J.J.; Titapiwatanakun, B.; Peer, W.A.; Bandyopadhyay, A.; Makam, S.N.; Lee, O.R.; Richards, E.L.; Murphy, A.S.; Sato, F. PGP4, an ATP binding cassette P-glycoprotein, catalyzes auxin transport in Arabidopsis thaliana roots. Plant Cell 2005, 17, 2922–2939. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.; Lee, S.H.; Cho, H.-T. P-Glycoprotein4 displays auxin efflux transporter–like action in Arabidopsis root hair cells and tobacco cells. Plant Cell 2007, 19, 3930–3943. [Google Scholar] [CrossRef]
- Geisler, M.; Aryal, B.; Di Donato, M.; Hao, P. A critical view on ABC transporters and their interacting partners in auxin transport. Plant Cell Physiol. 2017, 58, 1601–1614. [Google Scholar] [CrossRef]
- Blakeslee, J.J.; Bandyopadhyay, A.; Lee, O.R.; Mravec, J.; Titapiwatanakun, B.; Sauer, M.; Makam, S.N.; Cheng, Y.; Bouchard, R.; Adamec, J. Interactions among PIN-FORMED and P-glycoprotein auxin transporters in Arabidopsis. Plant Cell 2007, 19, 131–147. [Google Scholar] [CrossRef]
- Bailly, A.; Sovero, V.; Vincenzetti, V.; Santelia, D.; Bartnik, D.; Koenig, B.W.; Mancuso, S.; Martinoia, E.; Geisler, M. Modulation of P-glycoproteins by auxin transport inhibitors is mediated by interaction with immunophilins. J. Biol. Chem. 2008, 283, 21817–21826. [Google Scholar] [CrossRef]
- Yang, H.; Murphy, A.S. Functional expression and characterization of Arabidopsis ABCB, AUX 1 and PIN auxin transporters in Schizosaccharomyces pombe. Plant J. 2009, 59, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Krouk, G.; Lacombe, B.; Bielach, A.; Perrine-Walker, F.; Malinska, K.; Mounier, E.; Hoyerova, K.; Tillard, P.; Leon, S.; Ljung, K. Nitrate-regulated auxin transport by NRT1. 1 defines a mechanism for nutrient sensing in plants. Dev. Cell 2010, 18, 927–937. [Google Scholar] [CrossRef] [PubMed]
- Bouguyon, E.; Brun, F.; Meynard, D.; Kubeš, M.; Pervent, M.; Leran, S.; Lacombe, B.; Krouk, G.; Guiderdoni, E.; Zažímalová, E. Multiple mechanisms of nitrate sensing by Arabidopsis nitrate transceptor NRT1. 1. Nat. Plants 2015, 1, 15015. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Mitchum, M.G.; Barnaby, N.; Ayele, B.T.; Ogawa, M.; Nam, E.; Lai, W.-C.; Hanada, A.; Alonso, J.M.; Ecker, J.R. Potential sites of bioactive gibberellin production during reproductive growth in Arabidopsis. Plant Cell 2008, 20, 320–336. [Google Scholar] [CrossRef] [PubMed]
- Silverstone, A.L.; Jung, H.-S.; Dill, A.; Kawaide, H.; Kamiya, Y.; Sun, T.-P. Repressing a repressor: Gibberellin-induced rapid reduction of the RGA protein in Arabidopsis. Plant Cell 2001, 13, 1555–1566. [Google Scholar] [CrossRef]
- Qin, F.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Achievements and challenges in understanding plant abiotic stress responses and tolerance. Plant Cell Physiol. 2011, 52, 1569–1582. [Google Scholar] [CrossRef]
- Zeevaart, J.A. Changes in the levels of abscisic acid and its metabolites in excised leaf blades of Xanthium strumarium during and after water stress. Plant Physiol. 1980, 66, 672–678. [Google Scholar] [CrossRef] [PubMed]
- Dietze, M.C.; Sala, A.; Carbone, M.S.; Czimczik, C.I.; Mantooth, J.A.; Richardson, A.D.; Vargas, R. Nonstructural carbon in woody plants. Annu. Rev. Plant Biol. 2014, 65, 667–687. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, E.S.; Jensen, G.S.; Maksaev, G.; Katims, A.; Sherp, A.M.; Haswell, E.S. Mechanosensitive channel MSL8 regulates osmotic forces during pollen hydration and germination. Science 2015, 350, 438–441. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.P.; Maksaev, G.; Jensen, G.S.; Murcha, M.W.; Wilson, M.E.; Fricker, M.; Hell, R.; Haswell, E.S.; Millar, A.H.; Sweetlove, L.J. MSL 1 is a mechanosensitive ion channel that dissipates mitochondrial membrane potential and maintains redox homeostasis in mitochondria during abiotic stress. Plant J. 2016, 88, 809–825. [Google Scholar] [CrossRef] [PubMed]
- Basu, D.; Haswell, E.S. The mechanosensitive ion channel MSL10 potentiates responses to cell swelling in Arabidopsis seedlings. Curr. Biol. 2020, 30, 2716–2728.e6. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Sun, G.; Zhai, X.; Xu, P.; Ma, L.; Deng, M.; Zhao, Z.; Yang, H.; Dong, Y.; Shang, Z. Genomic insights into the fast growth of paulownias and the formation of Paulownia witches’ broom. Mol. Plant 2021, 14, 1668–1682. [Google Scholar] [CrossRef]
- Zhou, J.; Ma, F.; Yao, Y.; Deng, M.; Chen, M.; Zhang, S.; Li, Y.; Yang, J.; Zhang, N.; Huang, J. Jujube witches’ broom phytoplasma effectors SJP1 and SJP2 induce lateral bud outgrowth by repressing the ZjBRC1-controlled auxin efflux channel. Plant Cell Environ. 2021, 44, 3257–3272. [Google Scholar] [CrossRef]
- Han, S.S.; Baek, S.J.; Lee, S.H.; Seo, S.T.; Seralathan, K.K. First Report of Aster Yellows Phytoplasma (16SrI-B) Associated with Witches’ Broom Disease of Melia azedarach var. japonica in Korea. J. Phytopathol. 2015, 163, 1055–1058. [Google Scholar] [CrossRef]
- Wang, L.; Ming, L.; Liao, K.; Xia, C.; Sun, S.; Chang, Y.; Wang, H.; Fu, D.; Xu, C.; Wang, Z. Bract suppression regulated by the miR156/529-SPLs-NL1-PLA1 module is required for the transition from vegetative to reproductive branching in rice. Mol. Plant 2021, 14, 1168–1184. [Google Scholar] [CrossRef]
- Schmidt, R.; Schippers, J.H. ROS-mediated redox signaling during cell differentiation in plants. Biochim. Biophys. Acta-Gen. Subj. 2015, 1850, 1497–1508. [Google Scholar] [CrossRef]
- Mittler, R. ROS Are Good. Trends Plant Sci. 2017, 22, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Zavattieri, M.A.; Frederico, A.M.; Lima, M.; Sabino, R.; Arnholdt-Schmitt, B. Induction of somatic embryogenesis as an example of stress-related plant reactions. Electron. J. Biotechnol. 2010, 13, 12–13. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, Q.; Ma, J.; Guo, C.; Zhong, Q.; Yu, W.; Jia, T.; Zhang, L. Insights into the Root Sprouts of Toona fargesii in a Natural Forest: From the Morphology, Physiology, and Transcriptome Levels. Forests 2024, 15, 335. https://doi.org/10.3390/f15020335
Cheng Q, Ma J, Guo C, Zhong Q, Yu W, Jia T, Zhang L. Insights into the Root Sprouts of Toona fargesii in a Natural Forest: From the Morphology, Physiology, and Transcriptome Levels. Forests. 2024; 15(2):335. https://doi.org/10.3390/f15020335
Chicago/Turabian StyleCheng, Qiangqiang, Jikai Ma, Chunce Guo, Qiuwei Zhong, Wanwen Yu, Ting Jia, and Lu Zhang. 2024. "Insights into the Root Sprouts of Toona fargesii in a Natural Forest: From the Morphology, Physiology, and Transcriptome Levels" Forests 15, no. 2: 335. https://doi.org/10.3390/f15020335



