Abstract
To reveal the changes on the stock of the litter layer and its nutrient storage capacity during Moso bamboo expansion in subtropical coniferous and broad-leaved forests, permanent plots were set up in the transitional zone in Wuxie National Park, Zhuji, Zhejiang, China. The plots contained conifer and broad-leaved forests (CFs), transition forests (TFs), and Moso bamboo forests (MFs), which represented three stages of the expansion of Moso bamboo to the surrounding forests. Litter samples were collected and analyzed by un-decomposed, semi-decomposed, and decomposed layers. The stock of the litter layer, the content and storage of the main nutrient elements, and their release rate were measured. It was revealed that the stock of the litter layer and each decomposition layer decreased as the bamboo expands. However, the litter decomposition rate exhibited a positive correlation with the expansion of Moso bamboo, which might be due to the change in the physical properties of the litter. Meanwhile, there were no significant differences in the un-decomposed and semi-decomposed layers of the litter contents of C, N, and P between the three forests, but the contents of C, N, and P in the decomposed layer gradually decreased with the expansion of Moso bamboo. There were no remarkable differences in the N content, C/N, C/P, and lignin/N values of the un-decomposed layer of the three forests, indicating that the litter quality was not the principal reason affecting the decomposition rate. The total nutrient storage in the litter layer decreased significantly with the bamboo expansion, and the release rate of nutrient elements increased, which was adverse to the accumulation and storage of the nutrients. The material cycle of the original forest ecosystem is likely to deteriorate gradually with the bamboo expansion.
1. Introduction
Plant litter is an important part of forest ecosystems [1] and is the key nutrient pool of the forest ecosystem [2]. Meanwhile, the litter layer formed on the ground surface has a good water retention and heat insulation, which can effectively reduce soil erosion, buffer the impact of extreme weather such as extreme temperature and extreme precipitation, and maintain the stability of the soil environment [3]. In addition, the litter layer may have a broad threshold of litter accumulation in different types of communities, which has the potential to either enhance or reduce the productivity and variety of plants [4].
The litter layer often contains un-decomposed, semi-decomposed, and decomposed litter, which contains leaf, flower, fruit, stem, and botanical remains [5]. The composition, quantity, and dynamics of litter layers varies greatly due to differences in plant community types, composition, environmental conditions, and human disturbance activities [6,7]. The growth and decline of the litter layer composition and reserves reflect the differences and the dynamic characteristics of plant communities. However, current research mainly focuses on the yield and decomposition of the litter [2,8], studies about the stock and nutrients of the litter and the correlation between the litter and related factors are still rarely reported [9,10].
CFs and MFs have a widespread distribution in the subtropical area of China, and the two forest types often grow adjacent to each other. CFs are in the middle process of the development of a coniferous plant community to an evergreen broad-leaved plant community [11,12]. It has the advantages of high productivity, rich biodiversity, and complex and diverse ecosystems. Moso bamboo occupies about 73 percent of the bamboo forest area in China [13] and shows an increasing trend in recent years [14]. As a typical clonal plant with a strong expansion ability, Moso bamboo could continuously extend into the adjacent forests to form TFs or even pure bamboo forests [15]. Neighboring forest communities are greatly threatened by the presence of Moso bamboo, so the potential invasiveness of Moso bamboo in subtropical regions has been considered in recent studies [16,17,18].
The litter quantity and quality may change, and the composition and structure of the forest community are simplified [19], along with the change of the site microhabitat during the expansion of MFs [20]. The study of the litter layer is very important to understand the changing stock characteristics of the litter layer in the forest during bamboo expansion, mastering the changes of nutrients and energy in the community, understanding the mechanism of community seedling regeneration, and deepening the understanding of the Moso bamboo expansion mechanism. However, due to the lack of research on the storage and nutrient characteristics of the litter layer in different degrees of bamboo expansion [21], issues such as the litter storage and composition, and how the nutrient content of the litter layer evolves are still unclear.
Therefore, in this study we chose a representative location where Moso bamboo has been encroaching upon the CFs in China. We applied the “Space for Time” method [22] to study the dynamic characteristics of the litter stock and main nutrient elements during different stages of bamboo expansion. Revealing the changes in the stock and nutrient properties of the litter layer during Moso bamboo expansion can provide a basis for studying the matter cycling and energy conversion laws of the forest ecosystem, which is of great significance for strengthening the management and maintaining soil fertility of the mixed forest ecosystem after Moso bamboo expansion.
2. Materials and Methods
2.1. Research Site
This research was conducted at Wuxie National Forest Park (120°2′40″ E; 29°44′15″ N), Zhuji city, Zhejiang Province, China. This area falls within the north-eastern extension of the Longmen Mountains range, and it is characterized by a subtropical humid monsoon climate. The region experiences four distinct seasons, with an average annual temperature of 16.3 degrees and an average annual rainfall of 1374 mm [23]. The dominant soil type is Ferralsols, and bedrock is mainly volcanic rocks and granite. The combination of CF trees and bamboos results in the formation of a mixed forest between the two forest types. The main species in the CFs include Liquidambar formosana, Schima superba, and Pinus massoniana, etc.
2.2. Plot Setting
In May 2020, we chose three transects in the relatively gentle location in the mountains that covered a CF, a TF, and an MF. The plot’s elevation ranges from 210 to 230 m and consists of CFs, representing a forest stage unaffected by Moso bamboo expansion. TFs represent the forest stage with a moderate Moso bamboo expansion, with a bamboo to wood ratio of approximately 4:1. The MFs represent the forest stage heavily invaded by Moso bamboo, where the bamboos dominate the tree layers [16].
As shown in Figure 1, the length and width of each transect was 80 m and 30 m, respectively. A buffer zone of 5 m was designated around each horizontal transect. Within the remaining area, three 20 m × 20 m sampling plots were established, spaced 5 m apart [16] (Figure 1). The Quadrat method was used to investigate the plant community, and the specific survey methods can be found in reference [16]. The basic characteristics and main tree species’ composition among the three forests were surveyed (Table 1).
Figure 1.
Transects, sampling plots, and small quadrats in this study.

Table 1.
Survey of the three forests in the studied area [16]. The important value is equal to (relative abundance, relative frequency, relative significance)/3.
2.3. Stock of the Litter Layer
In March 2021, we randomly arranged six 1 m × 1 m sampling points in every 20 m × 20 m sampling plot [24]. Litter was collected from every small quadrat and then taken back to the lab. According to the litter layer stratification criteria [25], the litters were divided into un-decomposed, semi-decomposed, and decomposed layers. We classified the un-decomposed layer of litter to dead leaves and others (deadwood, barks, fruits, flowers, etc.), and then classified the dead leaves of the dominant species (broad leaves, needles, bamboo leaves, etc.). The samples were dried at 70 °C to a constant weight, weighed, and the dry matter weight per unit area of each component was calculated.
2.4. Chemical Analyses
The C, N, P, and lignin contents of the litter were analyzed after mixing the samples from different litter layers of each sampling plot. The K2Cr2O7 oxidation method was used to determine the total C content of the litter [26]. The total N content of the litter was measured using the micro-Kjeldahl method with a Kjeldahl nitrogen analyzer (Hanon K9860, Hanon Group, Jinan, China), after digestion with H2SO4 and H2O2. The total P and lignin content of the litter was measured using the colorimetric method [27].
2.5. Statistical Analysis
The nutrient stocks of the litter layer and each decomposition stage was calculated as follows: , where Dij was the stock of nutrient j in the decomposition layer i (kg/hm2), Wi was the litter stock in the decomposition layer i (kg/hm2), and Cij was the content of nutrient j in the decomposition layer i (g/kg). The release rate of the nutrients from the un-decomposed (or semi-decomposed) litter layer was calculated as follows: , where αi was the release rate of nutrients i in the un-decomposed (or semi-decomposed) layer, Ai was the difference between the nutrient stocks i in the un-decomposed (or semi-decomposed) layer and semi-decomposed (or decomposed) layer (kg/hm2), and Bi was the nutrient stock i in the un-decomposed (or semi-decomposed) layer (kg/hm2). The decomposed layer was converted into soil organic matter, so the nutrient release rate of the decomposed layer was not calculated in this study.
SPSS 26.0 software was used to process the data and a test of the significance of difference (p < 0.05) was performed using the one-way ANOVA and LSD methods. The graphs were plotted using Origin 2021 9.8.0 software. Data in the table represent the average ± standard deviations.
3. Results
3.1. Stock and Composition of the Litter Layer
The stock of the litter layer in the three forests were 4.17, 4.30, and 2.13 t/hm2, respectively, and the stock of the litter layer in MFs was less than that of CFs and TFs (p < 0.05; Figure 2). The stratification of the litter layers into different stands was obvious, all with un-decomposed, semi-decomposed, and decomposed layers. The stock of each litter layer of MFs was lower than that of CFs and TFs, and the difference between the un-decomposed layers was the highest (p < 0.05). In CFs and TFs, the percentage of stock in the un-decomposed layer to the litter layer was 43.45% and 48.70%, respectively. The stock of the un-decomposed layer was obviously higher than that of both the semi-decomposed and decomposed layers (p < 0.05). However, there were no obvious differences among the un-decomposed layer, semi-decomposed, and decomposed layer of MFs (p > 0.05), the percentage of stock in the un-decomposed layer to the litter layer was only 32.84%. The percentage of stock in the semi-decomposed layer and the decomposed layer to the litter layer were 31.18% and 35.99%, respectively, indicating that about 2/3 of the litter had been transformed into semi-decomposed and decomposed, and more than 35% had been transformed into humus.
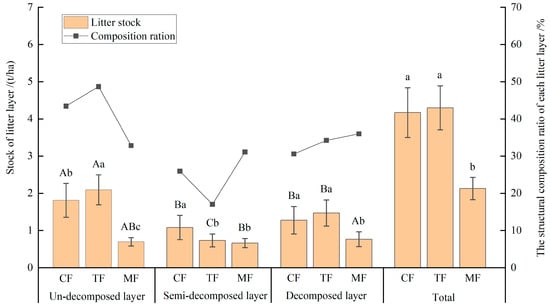
Figure 2.
Stock and composition of the litter layer in the studied forests (SFs, TFs, and MFs). Different capital letters indicate the differences between different decomposition layers of the same forest (p < 0.05), and different lowercase letters indicate significant differences between different forests (p < 0.05).
As Figure 3 shows, the proportion of leaf stock in the un-decomposed layer of the three forests exceeded 50% of the total litter stock, and MFs were the highest, reaching 89%. In general, as the expansion of the bamboo intensifies, the proportion of conifers increased at first and then decreased, the proportion of broad leaves continued to decrease, and the proportion of bamboo leaves increased significantly. The others such as deadwood, bark, fruits, and flowers increased slightly and then decreased significantly.
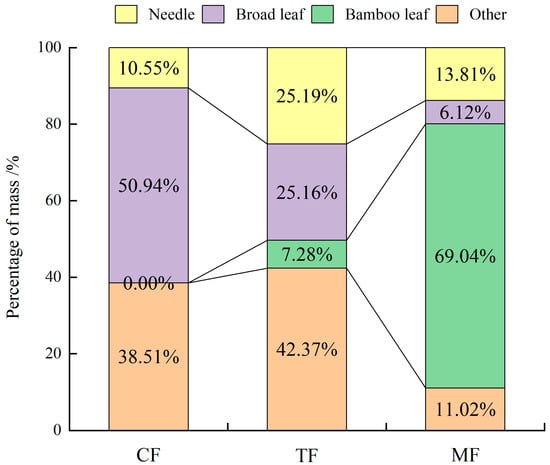
Figure 3.
Litter composition of un-decomposed layers in the studied forests.
3.2. C, N, P, and Lignin Contents in the Litter Layer
As the bamboo expands, the average content of C in the litter layer of the three forests presented a trend of rising and then decreasing significantly (p < 0.05). N and P content showed a downward trend overall, but not at a significant level (p > 0.05; Figure 4). The content of C, N, and P had no significant differences between the un-decomposed and semi-decomposed layers in the studied forests (p > 0.05); however, it was significantly reduced in the decomposed layer (p < 0.05). The average content of lignin in the litter layer significantly increased with the Moso bamboo expansion (p < 0.05), but the lignin content of the same decomposition layers between the three forests did not reach a significant level (p > 0.05).
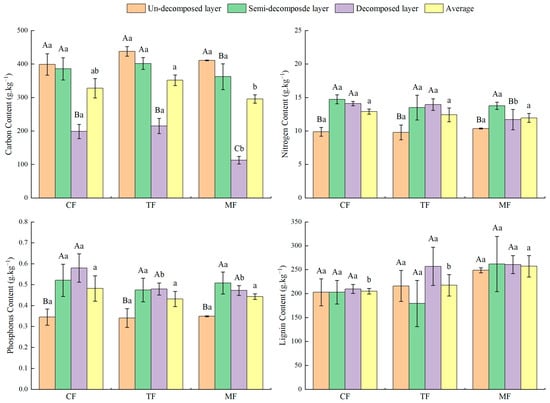
Figure 4.
Content of C, N, P, and lignin in the litter layer of the studied forests. Different capital letters indicate the differences between different decomposition layers of the same forest (p < 0.05), and different lowercase letters indicate significant differences between different forests (p< 0.05).
The content of C in the same forest decreased with the litter decomposition. Among them, the decomposed layer of CFs and TFs differs significantly from the un-decomposed and semi-decomposed layers (p < 0.05), but the difference between the un-decomposed and semi-decomposed layers was not significant (p > 0.05). The difference between the different decomposition layers of MFs was significant (p < 0.05).
The content of N in each decomposition layer of CFs and TFs increased with the litter decomposition. The un-decomposed layer of CFs and TFs differs significantly from the semi-decomposed and decomposed layer (p < 0.05), but the difference between the semi-decomposed and decomposed layer was not remarkable (p > 0.05). In MFs, the content of N in the semi-decomposed layer was the highest, and the un-decomposed layer was the lowest. The semi-decomposed layer of MFs differs significantly from the un-decomposed and decomposed layers (p < 0.05), but the difference between the un-decomposed and decomposed layers was not significant (p > 0.05).
The content of P in the same forest generally increased with the litter decomposition. The un-decomposed layer of the three forests differs significantly from the semi-decomposed layer and the decomposed layer (p < 0.05), but the difference between the semi-decomposed and decomposed layers was not significant (p > 0.05).
The content of lignin in the same stand generally increased with the litter decomposition, but the difference between different decomposition layers of the three forests was not significant (p > 0.05).
3.3. Stoichiometric Ratio of C, N, P, and Lignin in the Litter Layer
As Figure 5 shows, there were no obvious differences in the litter C/N and C/P ratios of un-decomposed and semi-decomposed layers in the three forests (p > 0.05), but the C/N ratio of the decomposed layer in MFs was significantly less than that of CFs and TFs, and the C/P ratio was significantly less than CFs (p < 0.05). In the same forest, the C/N and C/P ratios decreased with the litter decomposition, which was consistent with the trend of C content. It may be due to the high ratio of C/N and C/P in the litter of the un-decomposed layer and the enrichment phenomenon of N and P occurring during the decomposition of the litter or being slowly released, which results in the N and P content of the semi-decomposed and decomposed layers remaining at a high level. So, there were significant differences in the C/N and C/P ratios between the different decomposition layers (p < 0.05).
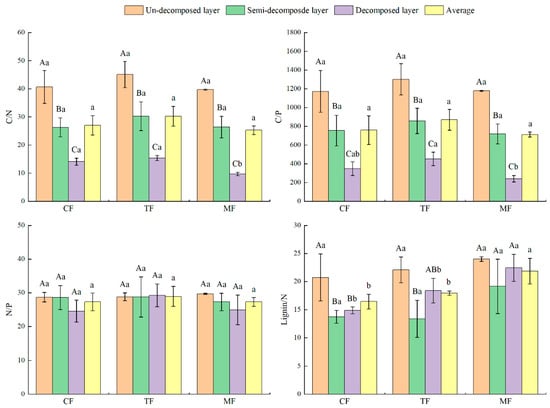
Figure 5.
C, N, P, and lignin stoichiometric ratios of the litter layer in the studied forests. Different capital letters indicate the differences between different decomposition layers of the same forest (p < 0.05), and different lowercase letters indicate significant differences between different forests (p < 0.05).
With the intensification of bamboo expansion, the lignin/N ratio of the litter layer and different decomposition layers of the studied forests demonstrated an overall upward trend. Among them, the mean and decomposed lignin/N ratio of MFs were significantly higher than those of CFs and TFs (p < 0.05). In the same forest, the lignin/N ratio of the three forests decreased first and then increased with the decomposition of the litter. The un-decomposed layer of CFs was obviously higher than that of the semi-decomposed layer and the decomposed layer (p < 0.05). The un-decomposed layer of TFs was obviously higher than that of the semi-decomposed layer (p < 0.05). The difference between the different decomposition layers of other forests was not significant (p > 0.05).
3.4. Stock and Release Rate of C, N, and P of the Litter Layer
As shown in Table 2, the total nutrient element reserves in the litter layer decreased significantly with the Moso bamboo expansion. From CFs to MFs, it decreased by 806.50 kg/hm2. Overall, the stock of C in the litter layer and the same decomposition layer had a trend of rising at first and then falling in the studied forests. The stock of N and P in the litter layer and the same decomposition layer showed a downward trend. Except for the stock of C, N, and P between the semi-decomposed layers of MFs and TFs, the stock of nutrient elements in the litter layer and the same decomposition layer of MFs was less than CFs and TFs (p < 0.05). In addition, the percentage of total nutrient element reserves in the un-decomposed layer to the litter layer of MFs dropped to 46.01%, obviously lower than that of CFs (51.20%) and TFs (59.34%).

Table 2.
Nutrient stock and release rates of the litter layers in the studied forests/(kg/hm2).
In the same forest, the stock of different nutrient elements in the litter layer and each decomposition layer were C > N > P. With the decomposition of litter, carbon stocks overall showed a downward trend, and N and P stocks showed an upward trend. The total nutrient release rates of CFs, TFs, and MFs from the un-decomposed layer to the semi-decomposed layer were 41.42%, 67.51%, and 15.21%, respectively, while from the semi-decomposed layer to the decomposed layer were 37.30%, −10.87%, and 61.63%, respectively (Table 2). The total release rate of nutrients in the semi-decomposed layer was significantly less than that of the un-decomposed layer of CFs and TFs, while MFs significantly increased. The release rate of different nutrient elements from the un-decomposed layer to the semi-decomposed layer was −38.18%–67.87%, and from the semi-decomposed layer to the decomposed layer was −107.59%–63.99%, of which C was the highest, after N, and P was the lowest.
4. Discussion
4.1. Impacts of Moso Bamboo Expansion on the Stock of the Litter Layer
The stock of the litter layer mainly depends on the amount of litterfall and its decomposition rate [28], which is affected by biological factors, abiotic factors, and plant characteristics [29,30]. Under the same climatic conditions, the species composition, litter amount, and decomposition rate of different woodlands are different, so the stock of litter in different woodland litter layers and their decomposition layers is also different [7]. There were differences in the development degree, stand structure, and tree species composition of the three forests (Table 1), which affected the nature and decomposition of the forest litter layer and determined the stock and composition of the litter layer in different forests. The litter stocks of the three forests in the studied area showed TFs > CFs > MFs (Figure 2), which first increased and then decreased significantly with the expansion of bamboo. This may be closely related to the change of the stand structure in the three forests. Studies have shown that the litter stocks are significantly positively correlated with the average breast diameter and stand density of the forest [31]. In the middle stage of bamboo expansion, although the average breast diameter of the forest decreased, it was mainly small-breasted conifer and broad-leaved trees that died, with a retention of large-diameter trees, and an increase in stand density [16], so the stock of the litter layer increased. With the intensification of bamboo expansion, the original conifers and broad-leaved trees died in large numbers. The average breast diameter of the forest stands decreased, plant species decreased, woody plant diversity decreased, the proportion of large trees decreased, community hierarchy gradually became monolithic, and species metabolism slowed down, resulting in a decrease in the amount of litterfall, which was not conducive to the accumulation of litter on the surface.
Research shows that the stock of litter in the un-decomposed layer is not only related to the amount of litter falling in the community but also related to external environmental conditions and litter characteristics [32]. In this research, the percentage of litter stock in the un-decomposed layer of different forests was different, the litter stocks of the un-decomposed layer of the CFs and TFs were obviously higher than those of the semi-decomposed and decomposed layers (Figure 2, p < 0.05), which showed that the litter stock of the CFs and TFs were dominated by fresh litter. Our results are consistent with the study by Zhao et al. [33] on the litter stocks of native forest litter in the Maolan Karst. The decomposition of the surface litter layer mainly includes water leaching, photodegradation, natural fragmentation, soil animal feeding, and microbial decomposition [34]. The frequent precipitation and strong leaching in the study area led to the rapid loss and degradation of carbohydrates and water-soluble substances in the litter of the un-decomposed layer [35], while cellulose, lignin, and other substances are difficult to decompose, and accumulate in the semi-decomposed and decomposed layers, resulting in a decrease in the decomposition rate of litter, so that litter can accumulate [36]. However, there were no differences between the un-decomposed layer and the semi-decomposed and decomposed layers in the litter stock of MFs (Figure 2, p > 0.05). The results indicated that with the intensification of bamboo expansion, the ability of the un-decomposed litter to migrate to the semi-decomposed and decomposed layers and the litter decomposition rate increased. Among them, the proportion of defoliation content in the total litter of the MFs’ un-decomposed layer increased significantly (Figure 3), which may be one of the main causes for the increase in its decomposition rate, because the leaf cuticle is thinner and litter decomposes relatively quickly, so more litter is converted to soil organic matter [37].
4.2. Impacts of Moso Bamboo Expansion on C, N, P, and Lignin Content and Their Stoichiometric Ratios in the Litter Layer
The nutrient content of litter depends on the uptake of soil nutrients by vegetation, which is associated with the plant characteristics and the content of soil nutrients [38]. In this study (Figure 4), the average N content of the litter layer (11.94–12.89 g/kg) was slightly higher than that of global woody plant litter N (10.9 g/kg), and the average P content (0.43–0.49 g/kg) was obviously lower than that of global woody plant litter P (0.85 g/kg) [39]. However, it was found that the average N and P contents of the litter layer of the three forests were not much different, and it is likely that the growth of vegetation in this area is limited by N and P elements, resulting in the convergence in the absorption of these limiting elements by vegetation [40].
Studies have shown that the change in the nutrient element content in litter is related to the release pattern of each element during litter decomposition [41]. In this research, the C content of the three forests decreased with decomposition (Figure 4), which may be associated with the consumption of litter organic matter via microbial activity [42]. Among the different decomposition layers, the C content of the litter was highest in the un-decomposed layer and gradually decreased with the intensification of decomposition, which was consistent with the conclusions of Li et al. [43] and Yu et al. [44]. The N and P contents of the three forests showed the mode of enrichment or first enrichment and then release (Figure 4). It is mainly due to the loss of soluble sugar, organic carbon, and other substances in the litter, which makes the mass loss of the litter obvious, while the release of N and P lags the mass loss [45], so results indicated that the C content gradually decreases and the N and P content increase relatively in our study. A strong enrichment mode is displayed, which might be related to the decomposition of litter from different forests in the study area being relatively strongly limited by N and P.
The decomposition of litter is largely affected by various factors like N content and C/N and lignin/N ratios [46]. Studies have indicated that the decomposition rate of litter is proportional to the initial N concentration [47,48], and the ratio of C/N, C/P, and lignin/N are significantly negatively correlated with the decomposition rate of the litter [49,50,51]. In this research, there were no significant differences in the N content and the ratio of C/N, C/P, and lignin/N of the un-decomposed layers of the three forests (Figure 5), indicating that initial nutrients in the litter were not the main reason affecting the decomposition rate of the litter in the three forests. The N/P ratio is often used to reflect which nutrients are limited by vegetation growth, and to a certain extent, it can also reflect the supply of nitrogen and phosphorus elements from the soil [52,53]. In this research, the average ratio of N/P in the litter of the three forests was 27.31–28.96, which was obviously higher than the N/P average of global woody vegetation (p < 0.05) [39]. In addition, Koerselman et al. [54] showed that litter decomposition was limited by P when N/P > 16, indicating that the plant growth and development in the study area was significantly limited by P [55].
4.3. Impacts of Moso Bamboo Expansion on Nutrient Element Reserves of the Litter Layer
The nutrient reserves of the litter layer are associated with the stock and nutrient content of the litter layer. In this study, with the bamboo expansion, the litter stocks decreased significantly (Figure 2) more than the decrease in each nutrient content (Figure 4). Therefore, the total reserves of litter nutrients decreasing with the bamboo expansion is consistent with the conclusions of Chen et al. [56], which showed that the total reserves of litter nutrients were mainly directly affected by the stocks of the litter layer [57]. However, the percentage of total nutrient element reserves in the un-decomposed layer to the total nutrient element reserves in the litter layer obviously decreased as the Moso bamboo expansion intensifies, which might be due to the high decomposition rate and nutrient release rate of litter in MFs (Table 2). The results indicated that with the bamboo expansion, the ability of nutrients to migrate to the humus layer was increased, which may be conducive to the return of nutrients. But it was unclear whether these elements will remain fixed in the humus, so more measurements should be taken in further studies to prove this conclusion.
During the bamboo expansion, the composition of above-ground plants continues to change, and the composition and quantity of the litter also changes. Therefore, long-term positioning studies in the future are needed to accurately reflect the effects of vegetation restoration on the stock of the litter layer and its C, N, and P stoichiometry. In addition, litter decomposition and nutrient release are affected by a combination of factors, like changes in soil microorganisms, animals, and understory microenvironments. Therefore, in the process of litter decomposition of different forests, the factors and their comprehensive driving mechanisms need to be further studied.
5. Conclusions
After the bamboo expansion, the stock of the litter layer and each decomposition layer decreased significantly, and the litter stock of MFs only accounted for about 50% of CFs or TFs. The contents of the main nutrient elements in the litter layer and each decomposition layer showed different characteristics with the bamboo expansion, among which the C content first increased and then decreased significantly, the N and P contents decreased overall, and the lignin content increased obviously. At the same time, the total reserves of litter nutrients decreased by 806.50 kg/hm2 from CFs to MFs, which was mainly directly affected by the reduced stocks of the litter layer. Although the ability of the un-decomposed litter to migrate to the semi-decomposed and decomposed layers and the litter decomposition rate increasing after the expansion, there were no obvious differences in the N content and the ratio of C/N, C/P, and lignin/N of the un-decomposed layers of the three forests, which means the change in the physical properties of the litter may be one of the main reasons affecting litter decomposition, independent of the substrate quality of litter. These results indicate that the nutrient migration to the humus layer capacity of the litter is increased after the bamboo expansion; however, due to the significant reduction in nutrients in the litter layer and each decomposition layer, was not conducive to the maintenance and accumulation of soil nutrients, and inhibited the formation of nutrient biological cycling and self-maintenance mechanisms in the ecosystem. The material cycle of the original forest ecosystem is likely to deteriorate gradually with the expansion of Moso bamboo.
Author Contributions
Conceptualization, X.C.; methodology, X.C.; validation, X.C.; formal analysis, X.C.; investigation, X.C.; resources, X.C.; data curation, X.C.; writing—original draft, X.C.; writing—review and editing, X.C. and Y.L.; visualization, X.C.; supervision, Y.L.; project administration, Y.L.; funding acquisition, Y.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Talent Project of Jiyang College of Zhejiang A&F University (NO. 05251700035; RQ2020B14), the National Natural Science Foundation of China (grant number: 32171786 and 31770680) and the “Leading Talents” R & D Program in Universities of Zhejiang, China (2022).
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors are thankful to Haochen Di, Ziqi Wang, and Yuxuan Hu for their help in field measurement.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Manzoni, S.; Jackson, R.B.; Trofymow, J.A.; Porporato, A. The global stoichiometry of litter nitrogen mineralization. Science 2008, 321, 684–686. [Google Scholar] [CrossRef]
- Giweta, M. Role of litter production and its decomposition, and factors affecting the processes in a tropical forest ecosystem: A review. J. Ecol. Environ. 2020, 44, 11. [Google Scholar] [CrossRef]
- Pan, K.; He, J.; Wu, N. Effect of forest litter on microenvironment conditions of forestland. Ying Yong Sheng Tai Xue Bao J. Appl. Ecol. 2004, 15, 153–158. [Google Scholar]
- Carson, W.P.; Peterson, C.J. The role of litter in an old-field community: Impact of litter quantity in different seasons on plant species richness and abundance. Oecologia 1990, 85, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Santos, S.L.d.; Valio, I.F. Litter accumulation and its effect on seedling recruitment in a Southeast Brazilian Tropical Forest. Braz. J. Bot. 2002, 25, 89–92. [Google Scholar] [CrossRef]
- Berg, B.; Matzner, E. Effect of N deposition on decomposition of plant litter and soil organic matter in forest systems. Environ. Rev. 1997, 5, 1–25. [Google Scholar] [CrossRef]
- Kavvadias, V.A.; Alifragis, D.; Tsiontsis, A.; Brofas, G.; Stamatelos, G. Litterfall, litter accumulation and litter decomposition rates in four forest ecosystems in northern Greece. For. Ecol. Manag. 2001, 144, 113–127. [Google Scholar] [CrossRef]
- Krishna, M.; Mohan, M. Litter decomposition in forest ecosystems: A review. Energy Ecol. Environ. 2017, 2, 236–249. [Google Scholar] [CrossRef]
- Martius, C.; Höfer, H.; Garcia, M.V.; Römbke, J.; Hanagarth, W. Litter fall, litter stocks and decomposition rates in rainforest and agroforestry sites in central Amazonia. Nutr. Cycl. Agroecosystems 2004, 68, 137–154. [Google Scholar] [CrossRef]
- Vaccaro, L.E.; Bedford, B.L.; Johnston, C.A. Litter accumulation promotes dominance of invasive species of cattails (Typha spp.) in Lake Ontario wetlands. Wetlands 2009, 29, 1036–1048. [Google Scholar] [CrossRef]
- Forrester, D.I. The spatial and temporal dynamics of species interactions in mixed-species forests: From pattern to process. For. Ecol. Manag. 2014, 312, 282–292. [Google Scholar] [CrossRef]
- Pretzsch, H.; Schütze, G. Transgressive overyielding in mixed compared with pure stands of Norway spruce and European beech in Central Europe: Evidence on stand level and explanation on individual tree level. Eur. J. For. Res. 2009, 128, 183–204. [Google Scholar] [CrossRef]
- Li, Y.; Feng, P. Bamboo resources in china based on the ninth national forest inventory data. World Bamboo Ratt 2019, 17, 45–48. [Google Scholar]
- Xu, Q.; Liang, C.; Chen, J.; Li, Y.; Qin, H.; Fuhrmann, J.J. Rapid bamboo invasion (expansion) and its effects on biodiversity and soil processes+. Glob. Ecol. Conserv. 2020, 21, e00787. [Google Scholar] [CrossRef]
- Bai, S.; Wang, Y.; Conant, R.T.; Zhou, G.; Xu, Y.; Wang, N.; Fang, F.; Chen, J. Can native clonal moso bamboo encroach on adjacent natural forest without human intervention? Sci. Rep. 2016, 6, 31504. [Google Scholar] [CrossRef]
- Chen, X.; Chen, X.; Huang, S.; Fang, D. Impacts of Moso bamboo (Phyllostachys pubescens) invasion on species diversity and aboveground biomass of secondary coniferous and. Physiol. Ecol. Trees Under Environ. Stress. 2023, 16648714, 112. [Google Scholar]
- Li, Y.; Li, Y.; Chang, S.X.; Xu, Q.; Guo, Z.; Gao, Q.; Qin, Z.; Yang, Y.; Chen, J.; Liang, X. Bamboo invasion of broadleaf forests altered soil fungal community closely linked to changes in soil organic C chemical composition and mineral N production. Plant Soil 2017, 418, 507–521. [Google Scholar] [CrossRef]
- Wu, C.; Mo, Q.; Wang, H.; Zhang, Z.; Huang, G.; Ye, Q.; Zou, Q.; Kong, F.; Liu, Y.; Geoff Wang, G. Moso bamboo (Phyllostachys edulis (Carriere) J. Houzeau) invasion affects soil phosphorus dynamics in adjacent coniferous forests in subtropical China. Ann. For. Sci. 2018, 75, 24. [Google Scholar] [CrossRef]
- Lima, R.A.; Rother, D.C.; Muler, A.E.; Lepsch, I.F.; Rodrigues, R.R. Bamboo overabundance alters forest structure and dynamics in the Atlantic Forest hotspot. Biol. Conserv. 2012, 147, 32–39. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, X.; Tao, J. Effects of different bamboo densities on understory species diversity and trees regeneration in an Abies faxoniana forest, Southwest China. Sci. Res. Essays 2012, 7, 660–668. [Google Scholar]
- Liu, X.; Fan, S.; Liu, G.; Peng, C. Changing characteristics of main structural indexes of community during the expansion of moso bamboo forests. Chin. J. Ecol. 2016, 35, 3165. [Google Scholar]
- Pickett, S.T. Space-for-time substitution as an alternative to long-term studies. In Long-Term Studies in Ecology: Approaches and Alternatives; Springer: New York, NY, USA, 1989; pp. 110–135. [Google Scholar]
- Chen, X.; Chen, H. Comparing environmental impacts of Chinese Torreya plantations and regular forests using remote sensing. Environ. Dev. Sustain. 2021, 23, 133–150. [Google Scholar] [CrossRef]
- Wu, H.; Xu, H. A review of sampling and modeling techniques for forest biomass inventory. Agric. Rural Stud. 2023, 1, 2. [Google Scholar] [CrossRef]
- Zheng, L.; Lu, L. Standing crop and nutrient characteristics of forest floor litter in China. J. Northwest For. Univ. 2012, 27, 63–69. [Google Scholar]
- Lu, R. Soil and Agro-Chemical Analytical Methods; China Agricultural Science and Technology Press: Beijing, China, 1999; pp. 146–195. [Google Scholar]
- Hatfield, R.; Grabber, J.; Ralph, J.; Brei, K. Using the acetyl bromide assay to determine lignin concentrations in herbaceous plants: Some cautionary notes. J. Agric. Food Chem. 1999, 47, 628–632. [Google Scholar] [CrossRef] [PubMed]
- Martins, W.B.R.; do Vale, R.L.; Ferreira, G.C.; de Andrade, V.M.S.; Dionísio, L.F.S.; Rodrigues, R.P.; de Assis Oliveira, F.; de Souza, G.M.P. Litterfall, litter stock and water holding capacity in post-mining forest restoration ecosystems, Eastern Amazon. Rev. Bras. De Ciências Agrárias 2018, 13, 1–9. [Google Scholar] [CrossRef]
- Pang, Y.; Tian, J.; Zhao, X.; Chao, Z.; Wang, Y.; Zhang, X.; Wang, D. The linkages of plant, litter and soil C: N: P stoichiometry and nutrient stock in different secondary mixed forest types in the Qinling Mountains, China. PeerJ 2020, 8, e9274. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yang, Q.; Qiao, Y.; Zhai, D.; Jiang, L.; Liang, G.; Sun, X.; Wei, N.; Wang, X.; Xia, J. Relative contributions of biotic and abiotic factors to the spatial variation of litter stock in a mature subtropical forest. J. Plant Ecol. 2019, 12, 769–780. [Google Scholar] [CrossRef]
- Hao, Q.; Yang, B.; Zhou, Y. Quantitative characteristics and impact factors of litter accumulation for Casuarina equisetifolia. J. For. Environ. 2020, 40, 356–362. [Google Scholar] [CrossRef]
- Leroy, C.J.; Marks, J.C. Litter quality, stream characteristics and litter diversity influence decomposition rates and macroinvertebrates. Freshw. Biol. 2006, 51, 605–617. [Google Scholar] [CrossRef]
- Zhao, C.; Long, J.; Li, J.; Liao, H.-K.; Liu, L.-F.; Zhang, M.-J. Litter stock and nutrient characteristics of decomposing litter layers in Maolan Karst primary forest in different slope directions. Chin. J. Ecol. 2018, 37, 296. [Google Scholar]
- Li, Q.; Zhou, D.; Chen, X. The accumulation, decomposition and ecological effects of above-ground litter in terrestrial ecosystem. Acta Ecol. Sin. 2014, 34, 3807–3819. [Google Scholar]
- Wang, F.; Yan, K.; Nam, K.; Zhu, G.; Peng, X.; Zhao, Z. The Wuxie debris flows triggered by a record-breaking rainstorm on 10 June 2021 in Zhuji City, Zhejiang Province, China. Landslides 2022, 19, 1913–1934. [Google Scholar] [CrossRef]
- Guo, J.; Yang, Y.; Chen, G.; Lin, P.; Xie, J. A review on litter decomposition in forest ecosystem. Sci. Silvae Sin. 2006, 42, 93. [Google Scholar]
- Cotrufo, M.F.; Wallenstein, M.D.; Boot, C.M.; Denef, K.; Paul, E. The M icrobial E fficiency-M atrix S tabilization (MEMS) framework integrates plant litter decomposition with soil organic matter stabilization: Do labile plant inputs form stable soil organic matter? Glob. Chang. Biol. 2013, 19, 988–995. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.; Zeng, L.; Xiao, W.; Huang, Z.; Geng, X.; Tan, B. Effect of litter substrate quality and soil nutrients on forest litter decomposition: A review. Acta Ecol. Sin. 2013, 33, 102–108. [Google Scholar] [CrossRef]
- Kang, H.; Xin, Z.; Berg, B.; Burgess, P.J.; Liu, Q.; Liu, Z.; Li, Z.; Liu, C. Global pattern of leaf litter nitrogen and phosphorus in woody plants. Ann. For. Sci. 2010, 67, 811. [Google Scholar] [CrossRef]
- Chang, Y.; Cao, J.; Li, J.; Pan, C.; Chen, Q.; Ma, L. Chemical properties of litter layers in coniferous forests of western Qinling Mountains. Chin. J. Ecol. 2009, 28, 1308. [Google Scholar]
- Akinyele, A.O.; Donald-Amaeshi, U. Leaf litter decomposition and nutrient release of three selected agroforestry tree species. Agrofor. Syst. 2021, 95, 559–570. [Google Scholar] [CrossRef]
- Moorhead, D.; Lashermes, G.; Recous, S.; Bertrand, I. Interacting microbe and litter quality controls on litter decomposition: A modeling analysis. PLoS ONE 2014, 9, e108769. [Google Scholar] [CrossRef]
- Li, X.; Han, S.; Hu, Y.; Zhao, Y. Decomposition of litter organic matter and its relations to C, N and P release in secondary conifer and broadleaf mixed forest in Changbai Mountains. Ying Yong Sheng Tai Xue Bao J. Appl. Ecol. 2008, 19, 245–251. [Google Scholar]
- Yu, L.; Fang, X.; Xiang, W.; Shi, J.; Liu, Z.; Li, L. Stoichiometry of Carbon, Nitrogen, and Phosphorus in Litter and Soil of Four Types of Subtropical Stand. Sci. Silvae Sin 2016, 52, 10–21. [Google Scholar]
- Han, W.; Fang, J.; Guo, D.; Zhang, Y. Leaf nitrogen and phosphorus stoichiometry across 753 terrestrial plant species in China. New Phytol. 2005, 168, 377–385. [Google Scholar] [CrossRef]
- Gessner, M.O.; Swan, C.M.; Dang, C.K.; McKie, B.G.; Bardgett, R.D.; Wall, D.H.; Hättenschwiler, S. Diversity meets decomposition. Trends Ecol. Evol. 2010, 25, 372–380. [Google Scholar] [CrossRef]
- Liu, P.; Huang, J.; Sun, O.J.; Han, X. Litter decomposition and nutrient release as affected by soil nitrogen availability and litter quality in a semiarid grassland ecosystem. Oecologia 2010, 162, 771–780. [Google Scholar] [CrossRef]
- Zhang, W.; Chao, L.; Yang, Q.; Wang, Q.; Fang, Y.; Wang, S. Litter quality mediated nitrogen effect on plant litter decomposition regardless of soil fauna presence. Ecology 2016, 97, 2834–2843. [Google Scholar] [CrossRef]
- García-Palacios, P.; McKie, B.G.; Handa, I.T.; Frainer, A.; Hättenschwiler, S. The importance of litter traits and decomposers for litter decomposition: A comparison of aquatic and terrestrial ecosystems within and across biomes. Funct. Ecol. 2016, 30, 819–829. [Google Scholar] [CrossRef]
- Liu, G.; Sun, J.; Tian, K.; Xiao, D.; Yuan, X. Long-term responses of leaf litter decomposition to temperature, litter quality and litter mixing in plateau wetlands. Freshw. Biol. 2017, 62, 178–190. [Google Scholar] [CrossRef]
- Qu, H.; Pan, C.; Zhao, X.; Lian, J.; Wang, S.; Wang, X.; Ma, X.; Liu, L. Initial lignin content as an indicator for predicting leaf litter decomposition and the mixed effects of two perennial gramineous plants in a desert steppe: A 5-year long-term study. Land Degrad. Dev. 2019, 30, 1645–1654. [Google Scholar] [CrossRef]
- Li, L.J.; Zeng, D.H.; Yu, Z.Y.; Fan, Z.P.; Mao, R.; Peri, P.L. Foliar N/P ratio and nutrient limitation to vegetation growth on Keerqin sandy grassland of North-east China. Grass Forage Sci. 2011, 66, 237–242. [Google Scholar] [CrossRef]
- von Oheimb, G.; Power, S.A.; Falk, K.; Friedrich, U.; Mohamed, A.; Krug, A.; Boschatzke, N.; Härdtle, W. N:P ratio and the nature of nutrient limitation in Calluna-dominated heathlands. Ecosystems 2010, 13, 317–327. [Google Scholar] [CrossRef]
- Koerselman, W.; Meuleman, A.F. The vegetation N: P ratio: A new tool to detect the nature of nutrient limitation. J. Appl. Ecol. 1996, 33, 1441–1450. [Google Scholar] [CrossRef]
- Güsewell, S.; Verhoeven, J.T. Litter N:P ratios indicate whether N or P limits the decomposability of graminoid leaf litter. Plant Soil 2006, 287, 131–143. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, S.; Li, L.; Gu, X.; Liu, Z.; Wang, L.; Fang, X. Stock and nutrient characteristics of litter layer at different vegetation restoration stages in subtropical region, China. Acta Ecol. Sin. 2020, 40, 4073–4086. [Google Scholar]
- Ma, W.; Zhao, Y.; Zhang, Q.; Arshad, A.; Shi, Q.; Yan, E. C: N: P stoichiometry in forest floor litter of evergreen broad-leaved forests at different successional stages in Tiantong, Zhejiang, eastern China. Chin. J. Plant Ecol. 2014, 38, 833. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).