Physiological Responses and Ecological Benefits of Water Uptake by Populus euphratica Leaves in Arid Areas
Abstract
:1. Introduction
2. Materials and Methods
2.1. Overview of the Study Area
2.2. Sample Plot Design
2.3. Test Methods
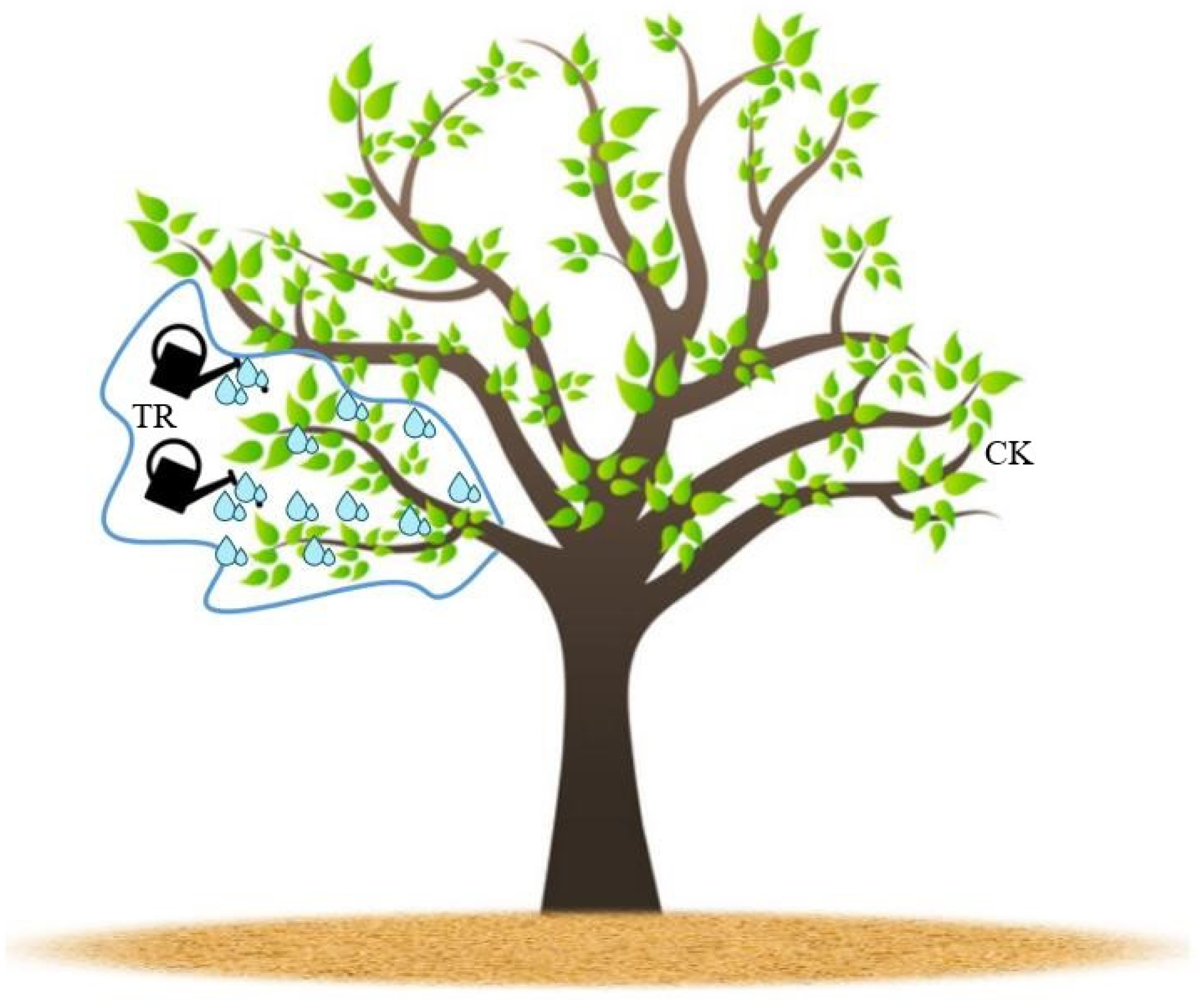
2.3.1. In Situ Field Control Test
2.3.2. Measurement of Leaf Water Potential at Dawn and Noon
2.3.3. Measurement of Photosynthetic Parameters
2.3.4. Measurement of Osmoregulatory and Antioxidant Enzymes
2.3.5. Water Droplet Experiment
2.3.6. Determination of Water Absorption Parameters
2.4. Data Processing
3. Results
3.1. Response of Populus euphratica Leaves to Changes in Condensation Water
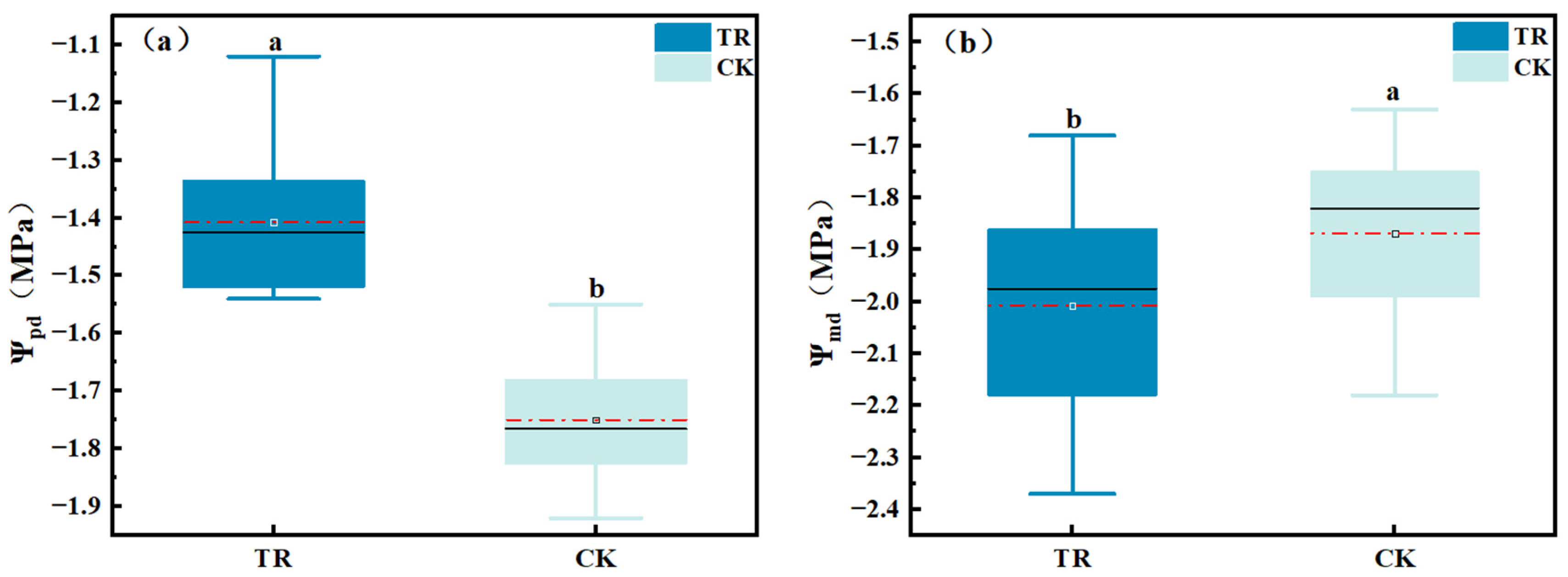
3.1.1. Variability in Leaf Water Potential under Condensation Water Treatment
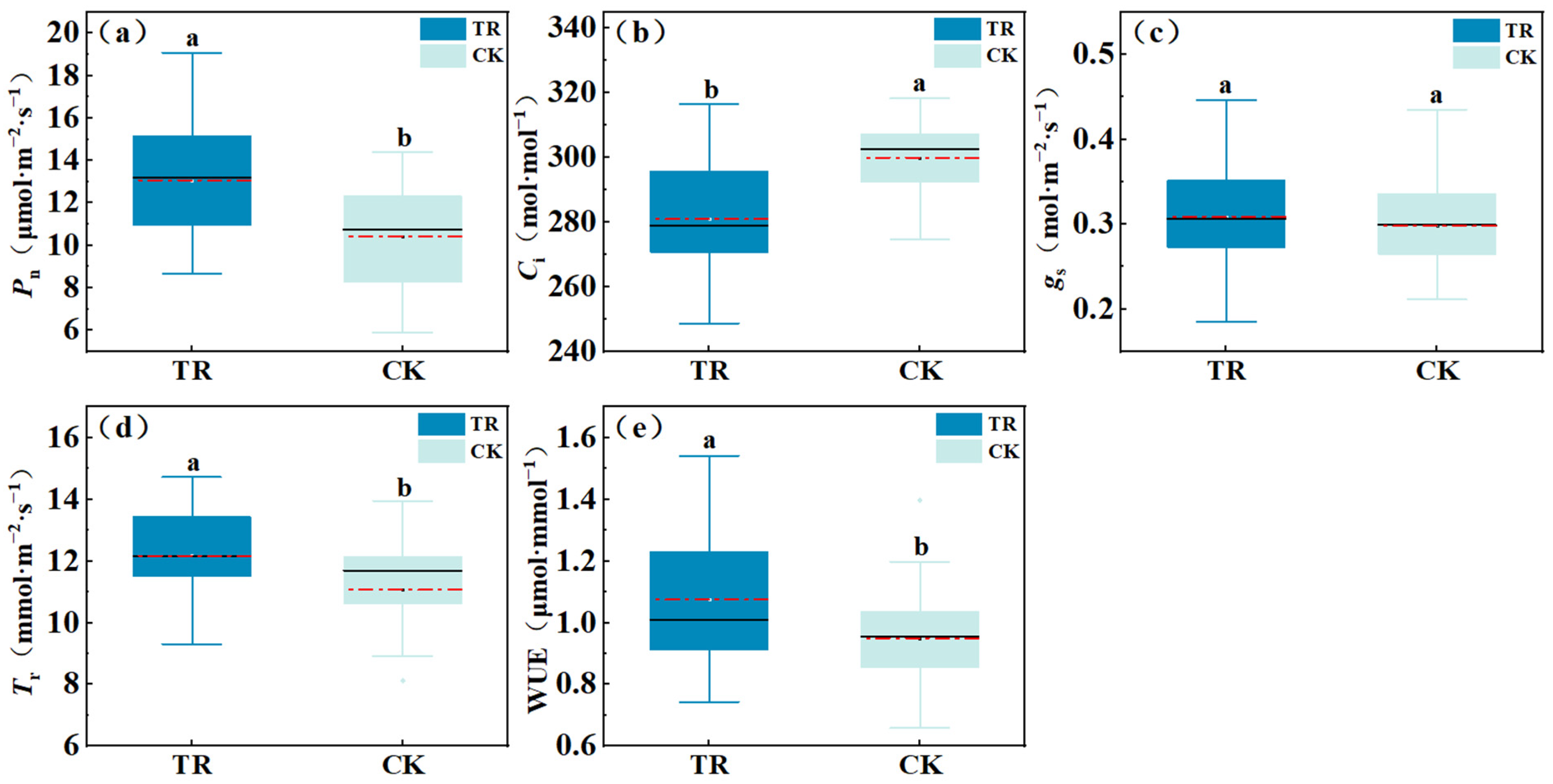
3.1.2. Differences in Photosynthetic Parameters under Condensation Water Treatment
3.1.3. Differences in Osmoregulation and Antioxidant Enzymes under Condensation Water Treatment
3.2. Leaf Wettability
Variability Analysis of Contact Angle with Different Water Droplet Volume Sizes
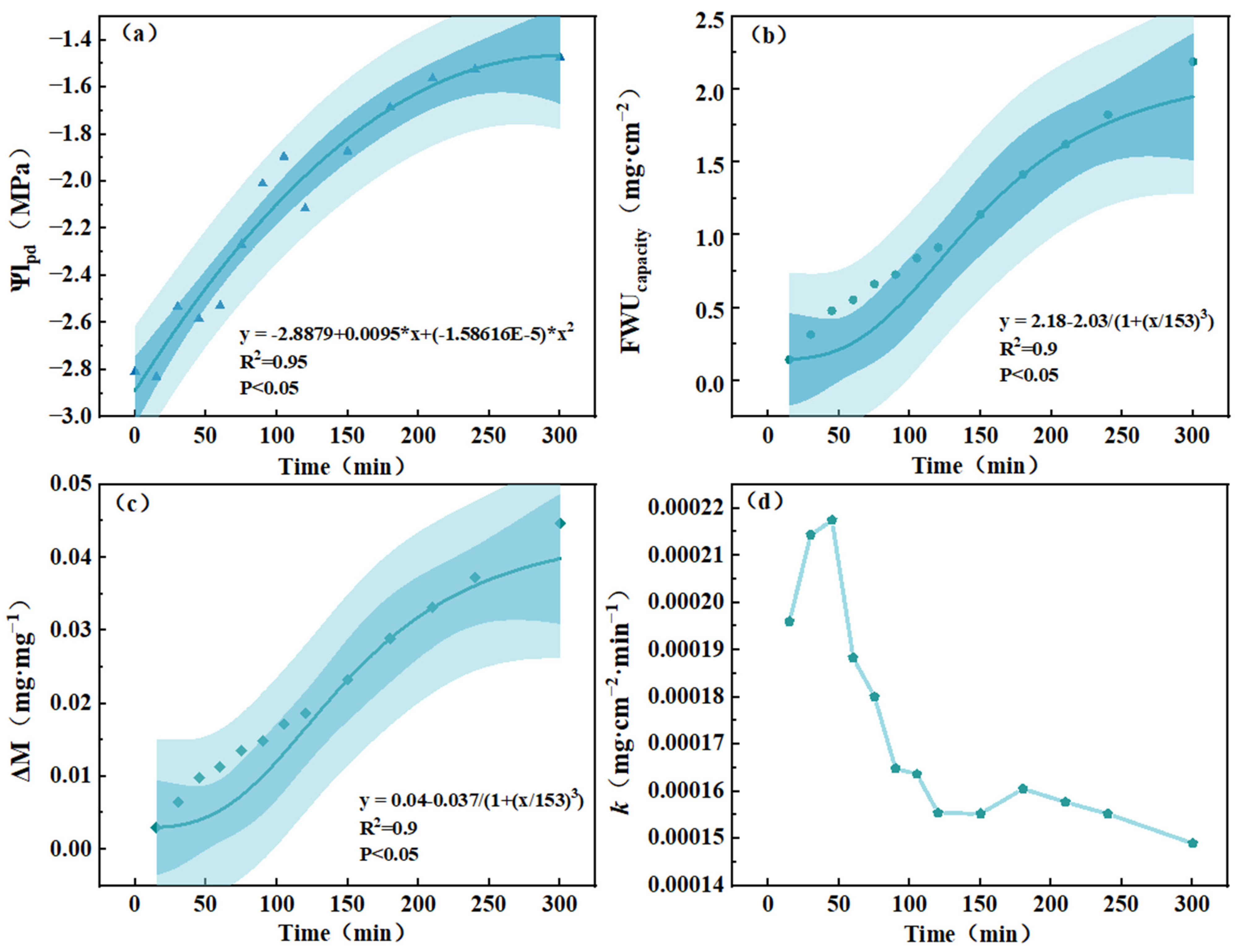
3.3. The Change Rule of Water Absorption Characteristics of Populus euphratica Leaves over Time
3.4. Relationship among Water Uptake, Leaf Structure, and Hydrodynamic Parameters of Populus euphratica Leaves
4. Discussion
4.1. Physiological Water Pattern of P. euphratica Leaves under Condensate Treatment
4.2. Patterns of Leaf Wettability and Water Uptake Characteristics
4.3. Contribution of FWU to Ecosystem Evapotranspiration
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jiang, T.; Zhen, W.G.; Yu, X.X.; Jia, G.D.; Zi Erdie, B.H. Leaf water absorption of Platycladus orientalis under drought stress in Beijing. Acta Ecol. Sin. 2022, 42, 1429–1440. [Google Scholar]
- Tomaszkiewicz, M.; Abou Najm, M.R.; Beysens, D.; Alameddine, I.; El-Fadel, M. Dew as a Sustainable Non-Conventional Water Resource: A Critical Review. Environ. Rev. 2015, 23, 425–442. [Google Scholar] [CrossRef]
- Hill, A.J.; Dawson, T.E.; Shelef, O.; Rachmilevitch, S. The Role of Dew in Negev Desert Plants. Oecologia 2015, 178, 317–327. [Google Scholar] [CrossRef]
- Cen, Y.; Liu, M.Z. Effects of Dew on Eco-Physiological Traits and Leaf Structures of Leymus chinensis and Agropyron Cristatum Grown under Drought Stress. Chin. J. Plant Ecol. 2017, 41, 1199–1207. [Google Scholar] [CrossRef]
- Zhuang, Y.L.; Zhao, W.Z. Study on the Ecological Effects of Condensed Water on an Annual Plant in a Temperate Desert. Arid Zone Res. 2009, 26, 526–532. [Google Scholar] [CrossRef]
- Zhuang, Y.L.; Zhao, W.Z. Experimental Study of Effects of Artificial Dew on Bassia dasyphylla and Agriophyllum squarrosum. J. Desert Res. 2010, 30, 1068–1074. [Google Scholar]
- Zhuang, Y.L.; Ratcliffe, S. Relationship between Dew Presence and Bassia dasyphylla Plant Growth. J. Arid Land 2012, 4, 8. [Google Scholar] [CrossRef]
- Li, L.C.; Gui, Z.Y.; Qin, S.G.; Zhang, Y.Q. Foliar condensate absorption capacity of four typical plant species and their physiological responses to water in the Mu Us Sandy Land of northwestern China. J. Beijing For. Univ. 2021, 43, 72–80. [Google Scholar]
- Fang, J. A Review on Eco-Hydrological Effects of Condensation Water. Sci. Cold Arid Reg. 2013, 7, 275–281. [Google Scholar] [CrossRef]
- Berry, Z.C.; Emery, N.C.; Gotsch, S.G.; Goldsmith, G.R. Foliar Water Uptake: Processes, Pathways, and Integration into Plant Water Budgets. Plant Cell Environ. 2019, 42, 410–423. [Google Scholar] [CrossRef] [PubMed]
- Stone, E.C. Dew as an Ecological Factor: I. A Review of the Literature. Ecology 1957, 38, 407–413. [Google Scholar] [CrossRef]
- Burkhardt, J. Hygroscopic Aerosols on Plant Surfaces: Nutrients or Desiccants? Geochim. Cosmochim. Acta 2009, 13, A177. [Google Scholar] [CrossRef]
- Feng, T.J.; Zhang, Z.Q.; Zhang, L.X. Review on the Influencing Factors and Functions of Condensated Water in Arid and Semi-Arid Ecosystems. Acta Ecol. Sin. 2022, 41, 456–468. [Google Scholar] [CrossRef]
- Darby, A.; Dragulji, D.; Glunk, A.; Gotsch, S.G. Habitat Moisture Is an Important Driver of Patterns of Sap Flow and Water Balance in Tropical Montane Cloud Forest Epiphytes. Oecologia 2016, 182, 357–371. [Google Scholar] [CrossRef]
- Gotsch, S.G.; Nadkarni, N.; Darby, A.; Glunk, A.; Dix, M.; Davidson, K.; Dawson, T.E. Life in the Treetops: Ecophysiological Strategies of Canopy Epiphytes in a Tropical Montane Cloud Forest. Ecol. Monogr. 2016, 85, 393–412. [Google Scholar] [CrossRef]
- Boanares, D.; Isaias, R.R.M.S.; De Sousa, H.C.; Kozovits, A.R. Strategies of Leaf Water Uptake Based on Anatomical Traits. Plant Biol. J. 2018, 20, 848–856. [Google Scholar] [CrossRef]
- Pan, L.Z.; Guo, W.; Wang, T.; Wang, Y.p.; Yang, S.J. Research progress on foliar water uptake. Plant Physiol. J. 2021, 57, 19–32. [Google Scholar] [CrossRef]
- Boanares, D.; Jovelina da-Silva, C.; Mary Dos Santos Isaias, R.; Costa França, M.G. Oxidative Metabolism in Plants from Brazilian Rupestrian Fields and Its Relation with Foliar Water Uptake in Dry and Rainy Seasons. Plant Physiol. Biochem. 2020, 146, 457–462. [Google Scholar] [CrossRef]
- Berry, Z.C.; Hughes, N.M.; Smith, W.K. Cloud Immersion: An Important Water Source for Spruce and Fir Saplings in the Southern Appalachian Mountains. Oecologia 2014, 174, 319–326. [Google Scholar] [CrossRef]
- Gotsch, S.G.; Asbjornsen, H.; Holwerda, F.; Goldsmith, G.R.; Weintraub, A.E.; Dawson, T.E. Foggy Days and Dry Nights Determine Crown-level Water Balance in a Seasonal Tropical Montane Cloud Forest. Plant Cell Environ. 2014, 37, 261–272. [Google Scholar] [CrossRef]
- Pereyra, D.A.; Bucci, S.J.; Arias, N.S.; Ciano, N.; Cristiano, P.M.; Goldstein, G.; Scholz, F.G. Grazing Increases Evapotranspiration without the Cost of Lowering Soil Water Storages in Arid Ecosystems. Ecohydrology 2017, 10, e1850. [Google Scholar] [CrossRef]
- Meng, X.Y.; Meng, B.C.; Wang, Y.J.; Liu, Z.W.; Ji, X.N.; Yu, D.L. Influence of Climate Change and Human Activities on Water Resources inEbinur Lake in Recent 60 Years. J. China Hydrol. 2015, 35, 90–96. [Google Scholar]
- Qin, W.H.; Meng, W.Q. Desert Salt Lake—Abi Lake National Nature Reserve. World Life 2020, 2020, 16–27. [Google Scholar]
- Li, Y.; Zibibula, S.; Dong, Y.; Sheng, Y.C.; Tian, J. Analysis of Variation Rules and Abrupt Changes of Precipitation in Aibi Lake Oasis. Water Sav. Irrig. 2017, 10, 41–45. [Google Scholar]
- Zhang, X.N.; Li, Y.; He, X.M.; Lu, G.H. Effects of soil water and salinity on relationships between desert plantfunctional diversity and species diversity. Chin. J. Ecol. 2019, 38, 2354–2360. [Google Scholar] [CrossRef]
- Sun, Q.X.; Yang, X.D.; Li, B.R.; Kong, C.C.; Zhou, J.; Lu, G.H. Effects of hydraulic traits on the species abundance distribution pattern ofdesert plant communities. Arid Zone Res. 2023, 40, 412–424. [Google Scholar] [CrossRef]
- Rosado, B.H.P.; Holder, C.D. The Significance of Leaf Water Repellency in Ecohydrological Research: A Review. Ecohydrology 2013, 6, 150–161. [Google Scholar] [CrossRef]
- Holder, C.D. Leaf Water Repellency of Species in Guatemala and Colorado (USA) and Its Significance to Forest Hydrology Studies. J. Hydrol. 2007, 336, 147–154. [Google Scholar] [CrossRef]
- Shi, H.; Wang, H.X.; Li, Y.Y. Wettability on plant leaf surfaces and its ecological significance. Acta Ecol. Sin. 2011, 31, 4287–4298. [Google Scholar]
- Liang, X.; Su, D.; Yin, S.; Wang, Z. Leaf Water Absorption and Desorption Functions for Three Turfgrasses. J. Hydrol. 2009, 376, 243–248. [Google Scholar] [CrossRef]
- Qin, L. Formation Mechanism of Condensation Water and Its Ecological Effects of Halostachys caspica Community in Different Salinity Habitats. Ph.D. Thesis, Xinjiang University, Ürümqi, China, 2015. [Google Scholar]
- Zhang, J.; Zhang, Y.M.; Downing, A.; Cheng, J.H.; Zhou, X.B.; Zhang, B.C. The Influence of Biological Soil Crusts on Dew Deposition in Gurbantunggut Desert, Northwestern China. J. Hydrol. 2009, 379, 220–228. [Google Scholar] [CrossRef]
- Hao, X.M.; Li, C.; Guo, B.; Ma, J.X.; Ayup, M.; Chen, Z.S. Dew Formation and Its Long-Term Trend in a Desert Riparian Forest Ecosystem on the Eastern Edge of the Taklimakan Desert in China. J. Hydrol. 2012, 472–473, 90–98. [Google Scholar] [CrossRef]
- Zen, F.J.; Zhang, M.X.; Li, X.M. A review on the water physiological characteristics of Tamarix and its prospect. Chin. J. Appl. Ecol. 2002, 13, 611–614. [Google Scholar]
- Yang, X.J.; Du, J.H.; Zhang, C.J. Diurnal variations of water potential and its influencing factors in typical plantcommunities on coppice dunes of pingtan island. Acta Ecol. Sin. 2016, 36, 2614–2619. [Google Scholar]
- Fu, A.H.; Li, W.H.; Chen, Y.N. Factors Affecting Stem Water Potential of Tamarix ramosissima Ledeb. in the Extremely Arid Regions. J. Desert Res. 2012, 32, 730–736. [Google Scholar]
- Eller, C.B.; Lima, A.L.; Oliveira, R.S. Cloud Forest Trees with Higher Foliar Water Uptake Capacity and Anisohydric Behavior Are More Vulnerable to Drought and Climate Change. New Phytol. 2016, 211, 489–501. [Google Scholar] [CrossRef]
- Luo, D.D.; Wang, C.K. Plant Water-Regulation Strategies: Isohydric versus Anisohydric Behavior. J. Plant Ecol. 2017, 2017, 1020–1032. [Google Scholar] [CrossRef]
- Li, X.J. Diurnal variation in net photosynthetic rate and maininfluencing factors of Populus euphratica in extremely aridregion of the lower Heihe River. Master’s Thesis, Shanxi University, Taiyuan, China, 2021. [Google Scholar]
- Gao, L.; Yang, L.; Liu, R.X. Effects of soil moisture levels on photosynthesis, transpiration, and moisture useefficiency of female and male plants of Hippophae rhamnoides ssp. sinensis. Acta Ecol. Sin. 2009, 29, 6025–6034. [Google Scholar]
- Sobrado, M.A. Relation of Water Transport to Leaf Gas Exchange Properties in Three Mangrove Species. Trees 2000, 14, 258–262. [Google Scholar] [CrossRef]
- Wang, H.F.; Zhou, Y.Z.; Qin, L.; Gong, X.W.; Lu, G.H. Influence of Dew on Fluorescence Parameter and Water Use Efficiency ofHalostachys caspica in Different Salinity Habitats. Arid Zone Res. 2017, 34, 1124–1132. [Google Scholar] [CrossRef]
- Kidron, G.J. Analysis of Dew Precipitation in Three Habitats within a Small Arid Drainage Basin, Negev Highlands, Israel. Atmos. Res. 2000, 55, 257–270. [Google Scholar] [CrossRef]
- Vaadia, Y.; Waisel, Y. Water Absorption by the Aerial Organs of Plants. Physiol. Plant. 2010, 16, 44–51. [Google Scholar] [CrossRef]
- Wei, J.; Xu, C.; Li, K.X. Progress on superoxide dismutase and plant stress resistance. Plant Physiol. J. 2020, 56, 2571–2584. [Google Scholar] [CrossRef]
- Zhao, Y.L.; Deng, X.P. The Response Mechanism of Active Oxygen Species Removing System to Drought Stress. Acta Bot. Boreali-Occident. Sin. 2005, 25, 413–418. [Google Scholar]
- Li, J.M.; Deng, X.P. Photosynthetic System Characteristics of Transgenic Sweet Potato under Water Stress and Rewatering. J. Soil Water Conserv. 2007, 4, 193–196. [Google Scholar] [CrossRef]
- Munne-Bosch, S.; Alegre, L. Changes in Carotenoids, Tocopherols and Diterpenes during Drought and Recovery, and the Biological Significance of Chlorophyll Loss in Rosmarinus Officinalis Plants. Planta 2000, 210, 925–931. [Google Scholar] [CrossRef]
- Wang, B.X.; Huang, J.C.; Wang, H. Relationship between proline accumulation and drought resistance in different plants under water stress conditions. Plant Physiol. J. 1989, 46–51. [Google Scholar]
- Li, D.Q.; Guo, Q.F.; Zhang, Y.Q.; Cheng, B. Studies on the physiological characteristics of drought resistance in winter wheat. Acta Agron. Sin. 1993, 19, 125–132. [Google Scholar]
- Yan, X.; Zhou, M.; Dong, X.; Zou, S.; Xiao, H.; Ma, X.F. Molecular Mechanisms of Foliar Water Uptake in a Desert Tree. AoB Plants 2015, 7, plv129. [Google Scholar] [CrossRef]
- Wang, X.; Xiao, H.; Cheng, Y.; Ren, J. Leaf Epidermal Water-Absorbing Scales and Their Absorption of Unsaturated Atmospheric Water in Reaumuria Soongorica, a Desert Plant from the Northwest Arid Region of China. J. Arid Environ. 2016, 128, 17–29. [Google Scholar] [CrossRef]
- Gong, X.W.; Lu, G.H.; He, X.M.; Sarkar, B.; Yang, X.-D. High Air Humidity Causes Atmospheric Water Absorption via Assimilating Branches in the Deep-Rooted Tree Haloxylon Ammodendron in an Arid Desert Region of Northwest China. Front. Plant Sci. 2019, 10, 573. [Google Scholar] [CrossRef]
- Wang, H.M.; Li, Z.K.; Yang, J.J. Effects of Leaf Hydrophilicity and Stomatal Regulation on Foliar Water Uptake Capacity of Desert Plants. Forests 2023, 14, 551. [Google Scholar] [CrossRef]
- Guzmán-Delgado, P.; Mason Earles, J.; Zwieniecki, M.A. Insight into the Physiological Role of Water Absorption via the Leaf Surface from a Rehydration Kinetics Perspective. Plant Cell Environ. 2018, 41, 1886–1894. [Google Scholar] [CrossRef]
- Berry, Z.C.; Smith, W.K. Ecophysiological Importance of Cloud Immersion in a Relic Spruce–Fir Forest at Elevational Limits, Southern Appalachian Mountains, USA. Oecologia 2013, 173, 637–648. [Google Scholar] [CrossRef]
- Cassana, F.F.E.; Cleiton, B.; Oliveira, R.S.; Dillenburg, L.R. Effects of Soil Water Availability on Foliar Water Uptake of Araucaria angustifolia. Plant Soil 2016, 399, 147–157. [Google Scholar] [CrossRef]
- Cui, D.B. Charactertics of Root Suckers of Populus euphratica and Influencing Factors along the Aqikesu River bank in Ebinur Lake Nature Reserve. Master’s Thesis, Xinjiang Normal University, Ürümqi, China, 2012. [Google Scholar]
- Cavallaro, A.; Carbonell Silleta, L.; Pereyra, D.A.; Goldstein, G.; Scholz, F.G.; Bucci, S.J. Foliar Water Uptake in Arid Ecosystems: Seasonal Variability and Ecophysiological Consequences. Oecologia 2020, 193, 337–348. [Google Scholar] [CrossRef]




| Oxidative Metabolism | Abbreviation | Method |
|---|---|---|
| Malondialdehyde | MDA | enzyme marker |
| Superoxide dismutase | SOD | enzyme marker |
| Catalase | CAT | spectrophotometer |
| Soluble sugar | SS | enzyme marker |
| Proline | PRO | enzyme marker |
| Main Water Absorption Parameters | Unit | Formula |
|---|---|---|
| Foliar water uptake capacity (FWUcapacity) | mg·cm−2 | |
| Cumulative leaf water uptake (ΔM) | mg·mg−1 | |
| Leaf water absorption rate (k) | mg·cm−2·min−1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, J.; Li, Z.; Wang, H.; Lv, G.; Li, W.; Wang, H.; Wang, Y. Physiological Responses and Ecological Benefits of Water Uptake by Populus euphratica Leaves in Arid Areas. Forests 2024, 15, 430. https://doi.org/10.3390/f15030430
Tian J, Li Z, Wang H, Lv G, Li W, Wang H, Wang Y. Physiological Responses and Ecological Benefits of Water Uptake by Populus euphratica Leaves in Arid Areas. Forests. 2024; 15(3):430. https://doi.org/10.3390/f15030430
Chicago/Turabian StyleTian, Junhao, Zhoukang Li, Huimin Wang, Guanghui Lv, Wusong Li, Huifang Wang, and Yuchen Wang. 2024. "Physiological Responses and Ecological Benefits of Water Uptake by Populus euphratica Leaves in Arid Areas" Forests 15, no. 3: 430. https://doi.org/10.3390/f15030430




