Responses of Soil Phosphorus Cycling-Related Microbial Genes to Thinning Intensity in Cunninghamia lanceolata Plantations
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Site
2.2. Experimental Design and Sample Collection
2.3. Physical and Chemical Measurements
2.4. Sequential Fractionation of Phosphorus
2.5. Genomic DNA Extraction and Metagenomic Sequencing Data Analysis
2.6. Gene Prediction and Taxonomic and Functional Annotations
2.7. Statistical Analysis
3. Results
3.1. Impacts of Thinning on Soil Properties and Phosphorus Fractions
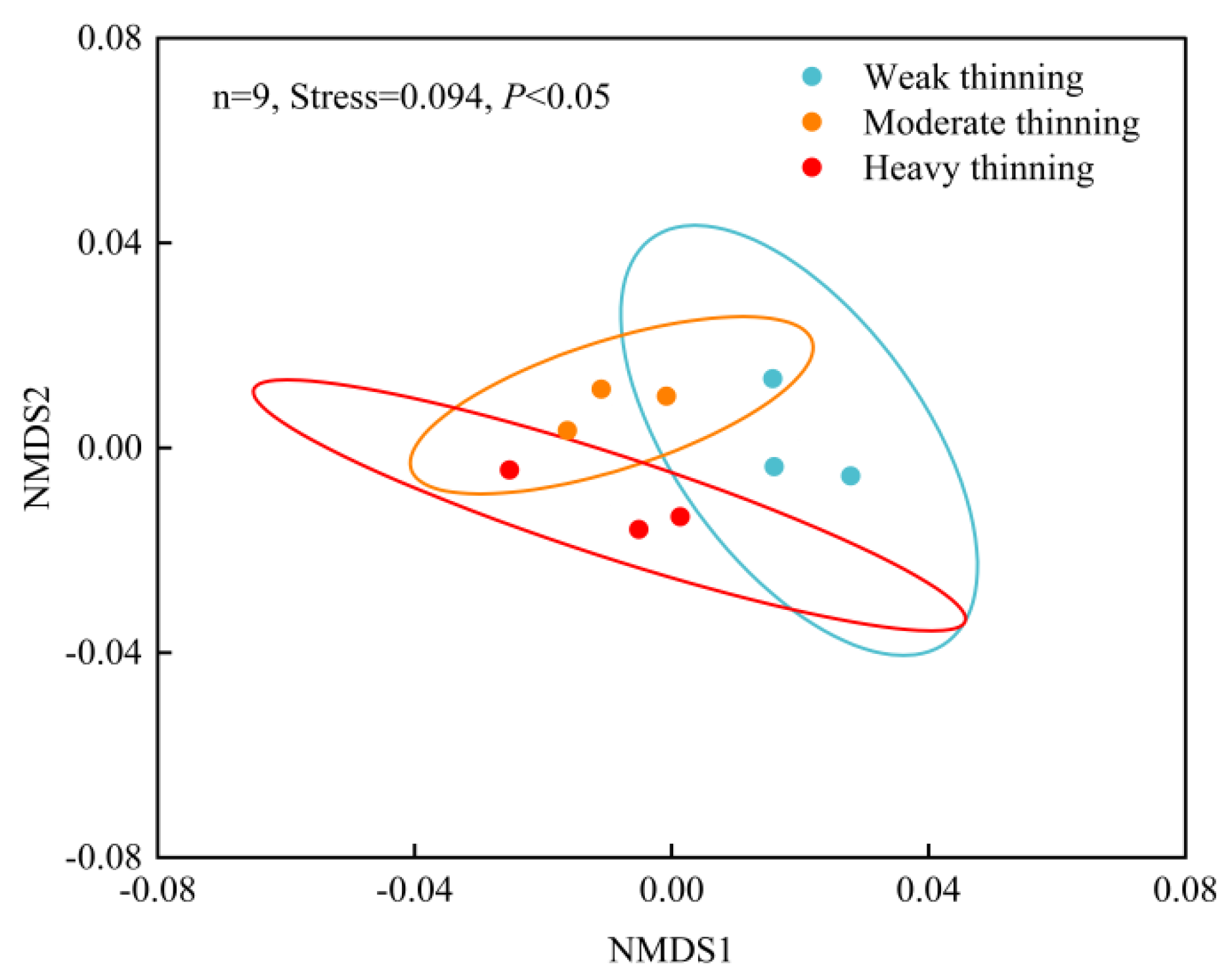
3.2. Composition of Soil Phosphorus Cycling-Related Genes
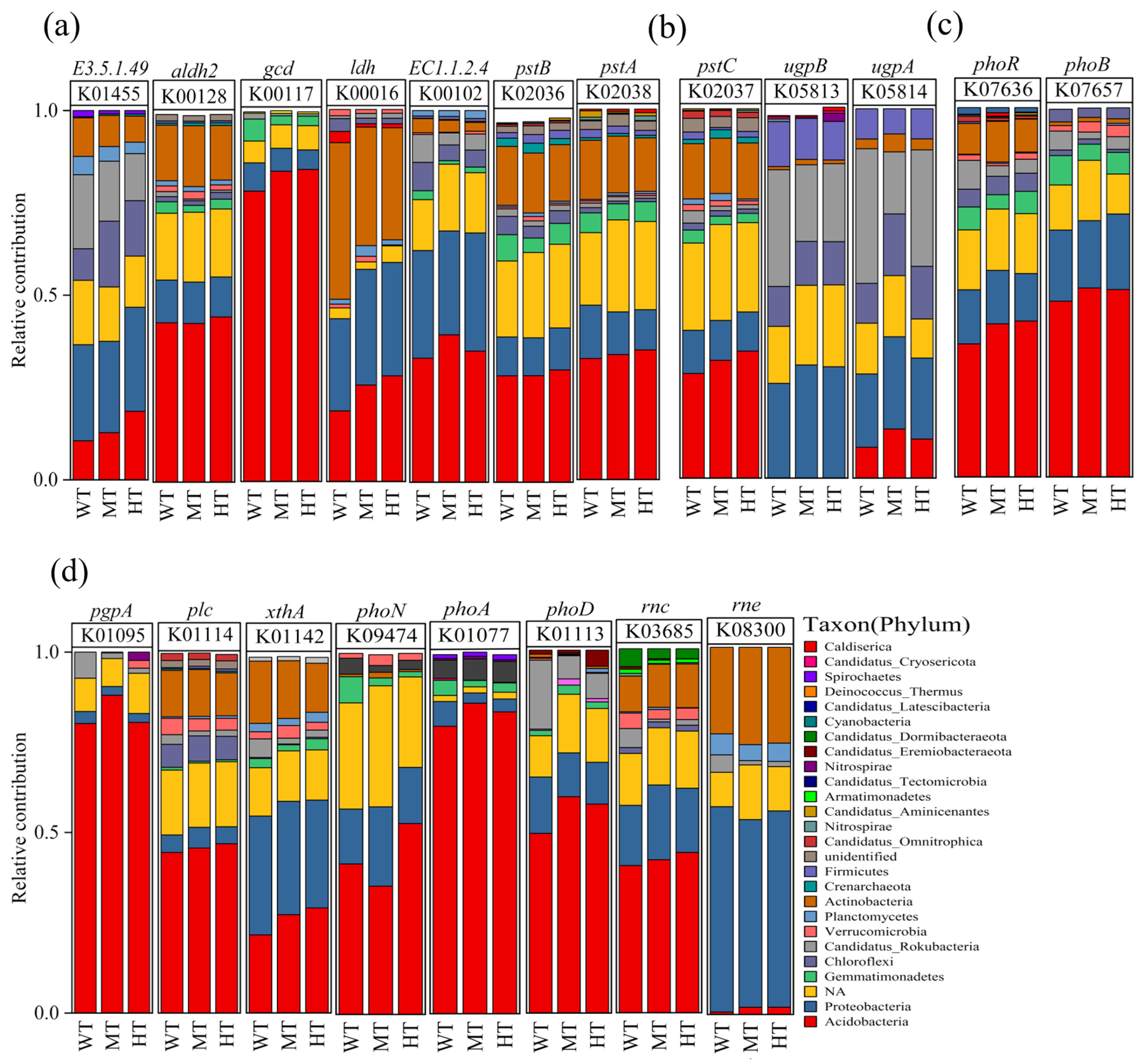
3.3. Microbial Composition and Contribution to the Genes Included in Phosphorus Cycling
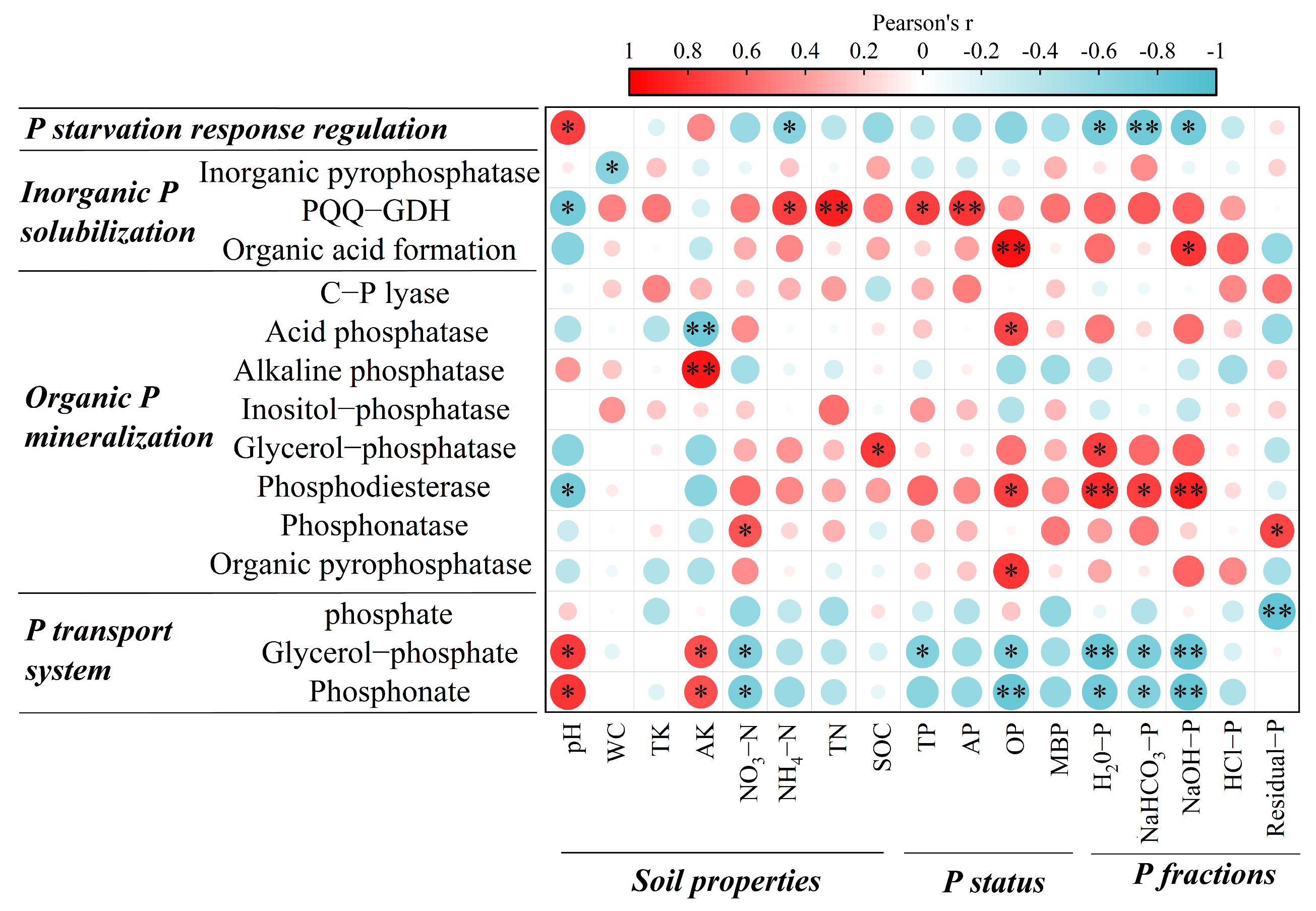
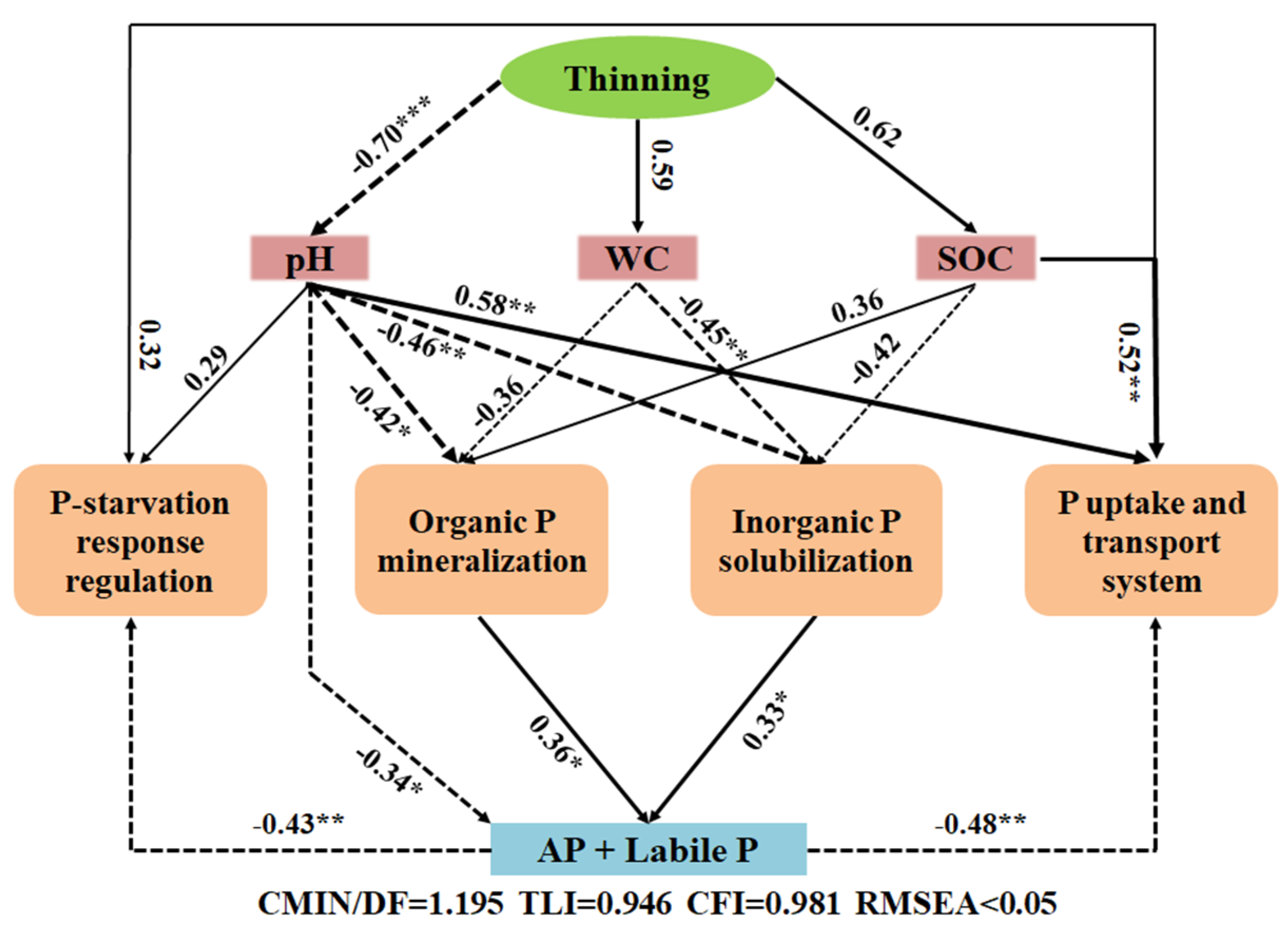
3.4. Correlations among Phosphorus Status, Soil Properties, and Functional Genes
4. Discussion
4.1. Effects of Different Thinning Intensities on Soil Phosphorus Fractions and Availabilities
4.2. Shifts in the Functional Genes of Soil Phosphorus Cycling after Different Thinning Treatments
4.3. Microbes Involved in Phosphorus Cycling under Different Thinning Conditions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- George, T.S.; Giles, C.D.; Menezes-Blackburn, D.; Condron, L.M.; Gama-Rodrigues, A.C.; Jaisi, D.; Lang, F. Organic Phosphorus in the Terrestrial Environment: A Perspective on the State of the Art and Future Priorities. Plant Soil 2017, 427, 191–208. [Google Scholar] [CrossRef]
- Hu, B.; Yang, B.; Pang, X.Y.; Bao, W.; Tian, G.L. Responses of Soil Phosphorus Fractions to Gap Size in a Reforested Spruce Forest. Geoderma 2016, 279, 61–69. [Google Scholar] [CrossRef]
- Cui, H.; Ou, Y.; Wang, L.X.; Wu, H.T.; Yan, B.X.; Li, Y.X. Distribution and Release of Phosphorus Fractions Associated with Soil Aggregate Structure in Restored Wetlands. Chemosphere 2019, 223, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, G.H.; Luo, X.Z.; Hou, E.Q.; Zheng, M.H.; Zhang, L.L.; He, X.J.; Shen, W.J.; Wen, D.Z. Mycorrhizal Fungi and Phosphatase Involvement in Rhizosphere Phosphorus Transformations Improves Plant Nutrition During Subtropical Forest Succession. Soil Biol. Biochem. 2021, 153, 108099. [Google Scholar] [CrossRef]
- Rodrigues, M.; Pavinato, P.S.; Withers, P.J.; Teles, A.P.; Herrera, W.F. Legacy Phosphorus and No Tillage Agriculture in Tropical Oxisols of the Brazilian Savanna. Sci. Total Environ. 2016, 542, 1050–1061. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Y.; Yang, Y.; Zhang, C.; Niu, S.L.; Yang, H.; Yu, G.R.; Wang, H.M.; Blagodatskaya, E.; Kuzyakov, Y.; Tian, D.S.; et al. Contrasting Responses of Phosphatase Kinetic Parameters to Nitrogen and Phosphorus Additions in Forest Soils. Funct. Ecol. 2018, 32, 106–116. [Google Scholar] [CrossRef]
- Fang, X.M.; Zhang, X.L.; Zong, Y.Y.; Zhang, Y.; Wan, S.Z.; Bu, W.S.; Chen, F.S. Soil Phosphorus Functional Fractions and Tree Tissue Nutrient Concentrations Influenced by Stand Density in Subtropical Chinese Fir Plantation Forests. PLoS ONE 2017, 12, e0186905. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.F.; Lu, X.K.; Mo, J.M. Phosphorus Limitation on Photosynthesis of Two Dominant Understory Species in a Lowland Tropical Forest. J. Plant Ecol. 2014, 7, 526–534. [Google Scholar] [CrossRef]
- Chen, F.S.; Niklas, K.J.; Liu, Y.; Fang, X.M.; Wan, S.Z.; Wang, H.M. Nitrogen and Phosphorus Additions Alter Nutrient Dynamics but Not Resorption Efficiencies of Chinese Fir Leaves and Twigs Differing in Age. Tree Physiol. 2015, 35, 1106–1117. [Google Scholar] [CrossRef]
- Bergkemper, F.; Schöler, A.; Engel, M.; Lang, F.; Krüger, J.; Schloter, M.; Schulz, S. Phosphorus Depletion in Forest Soils Shapes Bacterial Communities Towards Phosphorus Recycling Systems. Environ. Microbiol. 2016, 8, 2767. [Google Scholar]
- Dai, Z.M.; Liu, G.F.; Chen, H.H.; Chen, C.R.; Wang, J.K.; Ai, S.Y.; Wei, D.; Li, D.M.; Ma, B.; Brookes, P.C. Long-Term Nutrient Inputs Shift Soil Microbial Functional Profiles of Phosphorus Cycling in Diverse Agroecosystems. ISME J. 2020, 14, 757–770. [Google Scholar] [CrossRef]
- Rawat, P.; Das, S.; Shankhdhar, D.; Shankhdhar, S.C. Phosphate-Solubilizing Microorganisms: Mechanism and Their Role in Phosphate Solubilization and Uptake. J. Soil Sci. Plant Nutr. 2021, 21, 49–68. [Google Scholar] [CrossRef]
- Pradhan, A.; Pahari, A.; Mohapatra, S.; Mishra, B.B. Phosphate-Solubilizing Microorganisms in Sustainable Agriculture: Genetic Mechanism and Application. In Advances in Soil Microbiology: Recent Trends and Future Prospects: Volume 2: Soil-Microbe-Plant Interaction; Adhya, T.K., Mishra, B.B., Annapurna, K., Verma, D.K., Kumar, U., Eds.; Springer Singapore: Singapore, 2017; pp. 81–97. [Google Scholar]
- Yao, Q.M.; Li, Z.; Song, Y.; Wright, S.J.; Guo, X.; Tringe, S.G.; Tfaily, M.M.; Pasa-Tolic, L.; Hazen, T.C.; Turner, B.L.; et al. Community Proteogenomics Reveals the Systemic Impact of Phosphorus Availability on Microbial Functions in Tropical Soil. Nat. Ecol. Evol. 2018, 2, 499–509. [Google Scholar] [CrossRef]
- Lu, J.L.; Jia, P.; Feng, S.W.; Wang, Y.T.; Zheng, J.; Ou, S.N.; Wu, Z.H.; Liao, B.; Shu, W.S.; Liang, J.L.; et al. Remarkable Effects of Microbial Factors on Soil Phosphorus Bioavailability: A Country-Scale Study. Glob. Chang. Biol. 2022, 28, 4459–4471. [Google Scholar] [CrossRef]
- Wang, C.Q.; Xue, L.; Jiao, R.Z. Soil Phosphorus Fractions, Phosphatase Activity, and the Abundance of Phoc and Phod Genes Vary with Planting Density in Subtropical Chinese Fir Plantations. Soil Tillage Res. 2021, 209, 104946. [Google Scholar] [CrossRef]
- Richardson, A.E.; Simpson, R.J. Soil Microorganisms Mediating Phosphorus Availability Update on Microbial Phosphorus. Plant Physiol. 2011, 156, 989–996. [Google Scholar] [CrossRef] [PubMed]
- Grafe, M.; Goers, M.; von Tucher, S.; Baum, C.; Zimmer, D.; Leinweber, P.; Vestergaard, G. Bacterial Potentials for Uptake, Solubilization and Mineralization of Extracellular Phosphorus in Agricultural Soils Are Highly Stable under Different Fertilization Regimes. Environ. Microbiol. Rep. 2018, 10, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, Y.J.; Wanner Barry, L. Global Regulation by the Seven-Component Pi Signaling System. Curr. Opin. Microbiol. 2010, 13, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.J.; Gu, H.D.; Liu, J.J.; Wei, D.; Zhu, P.; Cui, X.A.; Zhou, B.K.; Chen, X.L.; Jin, J.; Liu, X.B.; et al. Metagenomic Strategies Uncover the Soil Bioavailable Phosphorus Improved by Organic Fertilization in Mollisols. Agric. Ecosyst. Environ. 2023, 349, 108462. [Google Scholar] [CrossRef]
- Chen, J.Q.; Zhao, G.Y.; Wei, Y.H.; Dong, Y.H.; Hou, L.Y.; Jiao, R.Z. Isolation and Screening of Multifunctional Phosphate Solubilizing Bacteria and Its Growth-Promoting Effect on Chinese Fir Seedlings. Sci. Rep. 2021, 11, 9081. [Google Scholar] [CrossRef]
- Wang, S.L.; Zhang, W.D.; Sanchez, F. Relating Net Primary Productivity to Soil Organic Matter Decomposition Rates in Pure and Mixed Chinese Fir Plantations. Plant Soil 2010, 334, 501–510. [Google Scholar] [CrossRef]
- Ye, Y.; Sun, X.; Zhao, J.; Chen, X.; Wang, M.; Li, J.; Guan, Q. Thinning Alters the Network Patterns and Keystone Taxa of Rhizosphere Soil Microbial Communities in Chinese Fir Plantation. Appl. Soil Ecol. 2023, 189, 104956. [Google Scholar] [CrossRef]
- Wic Baena, C.; Andrés-Abellán, M.; Lucas-Borja, M.E.; Martínez-García, E.; García-Morote, F.A.; Rubio, E.; López-Serrano, F.R. Thinning and Recovery Effects on Soil Properties in Two Sites of a Mediterranean Forest, in Cuenca Mountain (South-Eastern of Spain). For. Ecol. Manag. 2013, 308, 223–230. [Google Scholar] [CrossRef]
- Mosca, E.; Montecchio, L.; Scattolin, L.; Garbaye, J. Enzymatic Activities of Three Ectomycorrhizal Types of Quercus robur L. in Relation to Tree Decline and Thinning. Soil Biol. Biochem. 2007, 39, 2897–2904. [Google Scholar] [CrossRef]
- Overby, S.T.; Hart, S.C. Short-Term Belowground Responses to Thinning and Burning Treatments in Southwestern Ponderosa Pine Forests of the USA. Forests 2016, 7, 45. [Google Scholar] [CrossRef]
- Zhou, T.; Wang, C.K.; Zhou, Z.H. Thinning Promotes the Nitrogen and Phosphorous Cycling in Forest Soils. Agric. For. Meteorol. 2021, 311, 108665. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, J.; Gong, Z.; Xu, W.L.; Mou, Z. Gcd Gene Diversity of Quinoprotein Glucose Dehydrogenase in the Sediment of Sancha Lake and Its Response to the Environment. Int. J. Environ. Res. Public Health 2018, 16, 1. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Hao, Y.B.; Yan, Z.Q.; Kang, E.; Wang, J.Z.; Zhang, K.R.; Li, Y.; Wu, H.D.; Kang, X.M. Long-Term Degradation from Marshes into Meadows Shifts Microbial Functional Diversity of Soil Phosphorus Cycling in an Alpine Wetland of the Tibetan Plateau. Land Degrad. Dev. 2022, 33, 628–637. [Google Scholar] [CrossRef]
- Lu, D.J.; Li, C.Z.; Sokolwski, E.; Magen, H.; Chen, X.Q.; Wang, H.Y.; Zhou, J.M. Crop Yield and Soil Available Potassium Changes as Affected by Potassium Rate in Rice–Wheat Systems. Field Crops Res. 2017, 214, 38–44. [Google Scholar] [CrossRef]
- Börling, K.; Otabbong, E.; Barberis, E. Phosphorus Sorption in Relation to Soil Properties in Some Cultivated Swedish Soils. Nutr. Cycl. Agroecosystems 2001, 59, 39–46. [Google Scholar] [CrossRef]
- Guo, F.M.; Yost Russell, S. Partitioning Soil Phosphorus into Three Discrete Pools of Differing Availability. Soil Sci. 1998, 163, 822–833. [Google Scholar] [CrossRef]
- Liang, J.L.; Liu, J.; Jia, P.; Yang, T.T.; Zeng, Q.W.; Zhang, S.C.; Liao, B.; Shu, W.S.; Li, J.T. Novel Phosphate-Solubilizing Bacteria Enhance Soil Phosphorus Cycling Following Ecological Restoration of Land Degraded by Mining. ISME J. 2020, 14, 1600–1613. [Google Scholar] [CrossRef]
- Bowman, R.A.; Cole, C.V. Anexploratory method for fractionation of organic phosphorus from grassland soils. Soil Sci. 1978, 125, 95–101. [Google Scholar] [CrossRef]
- Chun, X.; Zhao, Y.S.; Xin, Y.; Li, J.X.; Liang, D.Z. Soil Phosphorus Forms and Phosphorus Solubilizing Bacteria Distribution after Restoration from Seriously Burning in Greater Khingan Mountain Areas, China. Chin. J. Appl. Ecol. 2020, 31, 388–398. (In Chinese) [Google Scholar]
- Johnson, A.H.; Frizano, J.; Vann, D.R. Biogeochemical Implications of Labile Phosphorus in Forest Soils Determined by the Hedley Fractionation Procedure. Oecologia 2003, 135, 487–499. [Google Scholar] [CrossRef] [PubMed]
- De Schrijver, A.; Vesterdal, L.; Hansen, K.; De Frenne, P.; Augusto, L.; Achat, D.L.; Staelens, J.; Baeten, L.; De Keersmaeker, L.; De Neve, S.; et al. Four Decades of Post-Agricultural Forest Development Have Caused Major Redistributions of Soil Phosphorus Fractions. Oecologia 2012, 169, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Q.; Song, M.H.; Wang, C.M.; Dou, X.M.; Wang, X.Q.; Li, X.Y. Mechanisms Underlying Soil Microbial Regulation of Available Phosphorus in a Temperate Forest Exposed to Long-Term Nitrogen Addition. Sci. Total Environ. 2023, 904, 166403. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.; Zaidi, A.; Ahemad, M.; Oves, M.; Wani, P.A. Plant Growth Promotion by Phosphate Solubilizing Fungi—Current Perspective. Arch. Agron. Soil Sci. 2010, 56, 73–98. [Google Scholar] [CrossRef]
- Bai, J.H.; Ye, X.; Jia, J.; Zhang, G.; Zhao, Q.; Cui, B.; Liu, X. Phosphorus sorption-desorption and effects of temperature, pH and salinity on phosphorus sorption in marsh soils from coastal wetlands with different flooding conditions. Chemosphere 2017, 188, 677–688. [Google Scholar] [CrossRef]
- Reed, S.C.; Townsend, A.R.; Taylor, P.G.; Cleveland, C.C. Phosphorus Cycling in Tropical Forests Growing on Highly Weathered Soils. In Phosphorus in Action. Soil Biology; Bünemann, E., Oberson, A., Frossard, E., Eds.; Springer: Berlin/Heidelberg, Germany, 2021; Volume 26. [Google Scholar]
- Li, M.; Cozzolino, V.; Mazzei, P.; Drosos, M.; Monda, H.; Hu, Z.; Piccolo, A. Effects of Microbial Bioeffectors and P Amendements on P Forms in a Maize Cropped Soil as Evaluated by 31p–Nmr Spectroscopy. Plant Soil 2018, 427, 87–104. [Google Scholar] [CrossRef]
- Alori, E.T.; Glick, B.R.; Babalola, O. Microbial Phosphorus Solubilization and Its Potential for Use in Sustainable Agriculture. Front. Microbiol. 2017, 8, 971. [Google Scholar] [CrossRef]
- Mori, T.; Wang, S.H.; Wang, C.; Chen, J.; Peng, C.; Zheng, M.H.; Huang, J.; Wang, F.M.; Liu, Z.F.; Mo, J.M.; et al. Effects of Long-Term Phosphorus Addition on Soil Ratios of Phosphomonoesterase to Phosphodiesterase in Three Tropical Forests. J. Plant Ecol. 2023, 16, rtac091. [Google Scholar] [CrossRef]
- Ragot, S.A.; Kertesz, M.A.; Bünemann, E.K. Phod Alkaline Phosphatase Gene Diversity in Soil. Appl. Environ. Microbiol. 2015, 81, 7281–7289. [Google Scholar] [CrossRef]
- Wu, Z.; Haack, S.E.; Lin, W.; Li, B.; Wu, L.; Fang, C.; Zhang, Z. Soil Microbial Community Structure and Metabolic Activity of Pinus Elliottii Plantations across Different Stand Ages in a Subtropical Area. PLoS ONE 2015, 10, e0135354. [Google Scholar] [CrossRef]
- Zhi, R.; Deng, J.; Xu, Y.; Xu, M.; Zhang, S.; Han, X.; Yang, G.; Ren, C. Altered Microbial P Cycling Genes Drive P Availability in Soil after Afforestation. J. Environ. Manag. 2023, 328, 116998. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Barret, M.; Mooij, M.J.; Rice, O.; Morrissey, J.P.; Dobson, A.; Griffiths, B.; O’Gara, F. Long-Term Phosphorus Fertilisation Increased the Diversity of the Total Bacterial Community and the Phod Phosphorus Mineraliser Group in Pasture Soils. Biol. Fertil. Soils 2013, 49, 661–672. [Google Scholar] [CrossRef]
- Liu, J.; Cade-Menun, B.J.; Yang, J.; Hu, Y.; Liu, C.W.; Tremblay, J.; LaForge, K. Long-Term Land Use Affects Phosphorus Speciation and the Composition of Phosphorus Cycling Genes in Agricultural Soils. Front. Microbiol. 2018, 9, 1643. [Google Scholar] [CrossRef] [PubMed]
- Santos-Torres, M.; Romero-Perdomo, F.; Mendoza-Labrador, J.; Gutiérrez, A.Y.; Vargas, C.; Castro-Rincon, E.; Caro-Quintero, A.; Uribe-Velez, D.; Estrada-Bonilla, G.A. Genomic and Phenotypic Analysis of Rock Phosphate-Solubilizing Rhizobacteria. Rhizosphere 2021, 17, 100290. [Google Scholar] [CrossRef]
- Song, K.; Wang, Z.; Du, J.; Liu, L.; Zeng, L.; Ren, C. Wetland Degradation: Its Driving Forces and Environmental Impacts in the Sanjiang Plain, China. Environ. Manag. 2014, 54, 255–271. [Google Scholar] [CrossRef] [PubMed]
- Teng, Z.; Zhu, Y.; Li, M.; Whelan, M.J. Microbial Community Composition and Activity Controls Phosphorus Transformation in Rhizosphere Soils of the Yeyahu Wetland in Beijing, China. Sci. Total Environ. 2018, 628–629, 1266–1277. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; Bao, W.; Zhu, B.; Cheng, W. Responses of Soil Respiration and Its Temperature Sensitivity to Thinning in a Pine Plantation. Agric. For. Meteorol. 2013, 171–172, 57–64. [Google Scholar] [CrossRef]
- Ma, B.; Stirling, E.; Liu, Y.; Zhao, K.; Zhou, J.; Singh, B.K.; Tang, C.; Dahlgren, R.A.; Xu, J. Soil Biogeochemical Cycle Couplings Inferred from a Function-Taxon Network. Research 2021, 2021, 7102769. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.M.; Jia, X.X.; Zhang, G.L.; Shao, M.A. Soil Organic Phosphorus TransformationDuring Ecosystem Development: A Review. Plant Soil 2017, 417, 17–42. [Google Scholar] [CrossRef]
- Wang, H.; Teng, C.; Li, H.; Sun, X.; Jiang, C.; Lou, L.; Yue, C.; Zhang, Z. Microbial Community Shifts Trigger Loss of Orthophosphate in Wetland Soils Subjected to Experimental Warming. Plant Soil 2018, 424, 351–365. [Google Scholar] [CrossRef]

| Item | Weak Thinning | Moderate Thinning | Heavy Thinning |
|---|---|---|---|
| TP/mg kg−1 | 682.8 ± 39.1 b | 738.9 ± 41.5 ab | 816.4 ± 56.2 a |
| AP/mg kg−1 | 2.9 ± 0.12 b | 3.1 ± 0.2 ab | 3.5 ± 0.2 a |
| OP/mg kg−1 | 262.5 ± 19.1 b | 362.3 ± 24.9 a | 341.1 ± 24.5 a |
| MBP/mg kg−1 | 20.3 ± 4.4 b | 25.8 ± 5.4 ab | 33.2 ± 7.3 a |
| H2O-Pi/mg kg−1 | 3.3 ± 0.9 b | 4.8 ± 1.0 b | 9.3 ± 1.7 a |
| H2O-Po/mg kg−1 | 15.2 ± 2.7 a | 22.7 ± 3.7 a | 21.2 ± 3.7 a |
| NaHCO3-Pi/mg kg−1 | 15.4 ± 2.3 ab | 12.2 ± 3.0 b | 20.1 ± 3.6 a |
| NaHCO3-Po/mg kg−1 | 35.7 ± 5.9 a | 44.4 ± 5.9 a | 45.4 ± 5.3 a |
| NaOH-Pi/mg kg−1 | 55.8 ± 8.1 a | 76.2 ± 9.4 b | 88.1 ± 11.2 b |
| NaOH-Po/mg kg−1 | 100.3 ± 12.7 a | 154.4 ± 15.8 b | 138.5 ± 18.4 b |
| HCl-Pi/mg kg−1 | 37.9 ± 9.3 a | 31.7 ± 7.5 a | 41.0 ± 8.3 a |
| HCl-Po/mg kg−1 | 111.9 ± 13.2 b | 139.8 ± 14.9 a | 133.6 ± 10.1 ab |
| Residual-P/mg kg−1 | 290.9 ± 36.5 a | 246.4 ± 34.3 a | 303.5 ± 47.5 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, D.; Wang, J.; Chen, K.; Lan, W.; Ye, Y.; Ma, X.; Lin, K. Responses of Soil Phosphorus Cycling-Related Microbial Genes to Thinning Intensity in Cunninghamia lanceolata Plantations. Forests 2024, 15, 440. https://doi.org/10.3390/f15030440
Ma D, Wang J, Chen K, Lan W, Ye Y, Ma X, Lin K. Responses of Soil Phosphorus Cycling-Related Microbial Genes to Thinning Intensity in Cunninghamia lanceolata Plantations. Forests. 2024; 15(3):440. https://doi.org/10.3390/f15030440
Chicago/Turabian StyleMa, Dongxu, Jiaqi Wang, Kuaiming Chen, Weili Lan, Yiquan Ye, Xiangqing Ma, and Kaimin Lin. 2024. "Responses of Soil Phosphorus Cycling-Related Microbial Genes to Thinning Intensity in Cunninghamia lanceolata Plantations" Forests 15, no. 3: 440. https://doi.org/10.3390/f15030440
APA StyleMa, D., Wang, J., Chen, K., Lan, W., Ye, Y., Ma, X., & Lin, K. (2024). Responses of Soil Phosphorus Cycling-Related Microbial Genes to Thinning Intensity in Cunninghamia lanceolata Plantations. Forests, 15(3), 440. https://doi.org/10.3390/f15030440






