Mixed-Effects Height Prediction Model for Juniperus procera Trees from a Dry Afromontane Forest in Ethiopia
Abstract
:1. Introduction
2. Materials and methods
2.1. Study Area
2.2. Data Collection
2.3. Juniperus procera Hochst. ex. Endl.
2.4. Statistical Analysis
2.4.1. Base Model Selection
| No | Name | Mathematical Expression | References |
|---|---|---|---|
| M1 | Power | [62] | |
| M2 | Näslund | [63] | |
| M3 | Curtis | [18] | |
| M4 | Meyer | [64] | |
| M5 | Schumacher | [65] | |
| M6 | Michaelis–Menten | [66] | |
| M7 | Gomperz | [67] | |
| M8 | Logistic | [56] | |
| M9 | Chapman–Richards | [68,69] | |
| M10 | Weibull | [70] | |
| M11 | Lundqvist Korf | [62] | |
| M12 | Ratkowsky | [71] | |
| M13 | Hossfeld IV | [62] | |
| M14 | Johnson–Schumacher | [62] |
2.4.2. Nonlinear Mixed-Effects Modeling
2.4.3. Generalized Mixed-Effects Models
2.4.4. Calibration and Random Effect Estimation
3. Results
3.1. Base Model Selection
3.2. Nonlinear Mixed-Effects Model
3.3. Generalized Mixed Effects Model
3.4. Calibration Response
4. Discussion
4.1. Base Model Selection
4.2. Nonlinear Mixed Effect Model
4.3. The Use of Additional Stand Variables in Height Prediction
4.4. Random Effect Estimation and Model Calibration
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Asrat, Z.; Eid, T.; Gobakken, T.; Negash, M. Aboveground tree biomass prediction options for the Dry Afromontane forests in south-central Ethiopia. For. Ecol. Manag. 2020, 473, 118335. [Google Scholar] [CrossRef]
- Guerra-Hernández, J.; Arellano-Pérez, S.; González-Ferreiro, E.; Pascual, A.; Altelarrea, V.S.; Ruiz-González, A.D.; Álvarez-González, J.G.J.F.E. Developing a site index model for P. Pinaster stands in NW Spain by combining bi-temporal ALS data and environmental data. For. Ecol. Manag. 2021, 481, 118690. [Google Scholar] [CrossRef]
- Ruiz-Peinado, R.; Pretzsch, H.; Löf, M.; Heym, M.; Bielak, K.; Aldea, J.; Barbeito, I.; Brazaitis, G.; Drössler, L.; Godvod, K. Mixing effects on Scots pine (Pinus sylvestris L.) and Norway spruce (Picea abies (L.) Karst.) productivity along a climatic gradient across Europe. For. Ecol. Manag. 2021, 482, 118834. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, X.; Guo, Z. Estimation of tree height and aboveground biomass of coniferous forests in North China using stereo ZY-3, multispectral Sentinel-2, and DEM data. Ecol. Indic. 2021, 126, 107645. [Google Scholar] [CrossRef]
- Xu, Y.; Li, C.; Sun, Z.; Jiang, L.; Fang, J. Tree height explains stand volume of closed-canopy stands: Evidence from forest inventory data of China. For. Ecol. Manag. 2019, 438, 51–56. [Google Scholar] [CrossRef]
- Holdaway, R.J.; McNeill, S.J.; Mason, N.W.; Carswell, F.E. Propagating uncertainty in plot-based estimates of forest carbon stock and carbon stock change. Ecosystems 2014, 17, 627–640. [Google Scholar] [CrossRef]
- Hunter, M.; Keller, M.; Victoria, D.; Morton, D. Tree height and tropical forest biomass estimation. Biogeosciences 2013, 10, 8385–8399. [Google Scholar] [CrossRef]
- Larjavaara, M.; Muller-Landau, H.C. Measuring tree height: A quantitative comparison of two common field methods in a moist tropical forest. Methods Ecol. Evol. 2013, 4, 793–801. [Google Scholar] [CrossRef]
- Mehtätalo, L.; de-Miguel, S.; Gregoire, T.G. Modeling height-diameter curves for prediction. Can. J. For. Res. 2015, 45, 826–837. [Google Scholar] [CrossRef]
- Imani, G.; Boyemba, F.; Lewis, S.; Nabahungu, N.L.; Calders, K.; Zapfack, L.; Riera, B.; Balegamire, C.; Cuni-Sanchez, A. Height-diameter allometry and above ground biomass in tropical montane forests: Insights from the Albertine Rift in Africa. PLoS ONE 2017, 12, e0179653. [Google Scholar] [CrossRef]
- Feldpausch, T.R.; Banin, L.; Phillips, O.L.; Baker, T.R.; Lewis, S.L.; Quesada, C.A.; Affum-Baffoe, K.; Arets, E.J.; Berry, N.J.; Bird, M. Height-diameter allometry of tropical forest trees. Biogeosciences 2011, 8, 1081–1106. [Google Scholar] [CrossRef]
- Calama, R.; Montero, G. Interregional nonlinear height diameter model with random coefficients for stone pine in Spain. Can. J. For. Res. 2004, 34, 150–163. [Google Scholar] [CrossRef]
- Banin, L.; Feldpausch, T.R.; Phillips, O.L.; Baker, T.R.; Lloyd, J.; Affum-Baffoe, K.; Arets, E.J.; Berry, N.J.; Bradford, M.; Brienen, R.J. What controls tropical forest architecture? Testing environmental, structural, and floristic drivers. Glob. Ecol. Biogeogr. 2012, 21, 1179–1190. [Google Scholar] [CrossRef]
- Sileshi, G.W.; Nath, A.J.; Kuyah, S. Allometric scaling and allocation patterns: Implications for predicting productivity across plant communities. Front. For. Glob. Chang. 2023, 5, 1084480. [Google Scholar] [CrossRef]
- Raptis, D.I.; Kazana, V.; Kazaklis, A.; Stamatiou, C. Mixed-effects height–diameter models for black pine (Pinus nigra Arn.) forest management. Trees 2021, 35, 1167–1183. [Google Scholar] [CrossRef]
- Temesgen, H.; Zhang, C.; Zhao, X. Modelling tree height–diameter relationships in multi-species and multi-layered forests: A large observational study from Northeast China. For. Ecol. Manag. 2014, 316, 78–89. [Google Scholar] [CrossRef]
- Temesgen, H.; v Gadow, K. Generalized height–diameter models–an application for major tree species in complex stands of interior British Columbia. Eur. J. For. Res. 2004, 123, 45–51. [Google Scholar] [CrossRef]
- Curtis, R.O. Height-diameter and height-diameter-age equations for second-growth Douglas-fir. For. Sci. 1967, 13, 365–375. [Google Scholar]
- Dorado, F.C.; Diéguez-Aranda, U.; Anta, M.B.; Rodríguez, M.S.; von Gadow, K. A generalized height–diameter model including random components for radiata pine plantations in northwestern Spain. For. Ecol. Manag. 2006, 229, 202–213. [Google Scholar] [CrossRef]
- Ercanli, I. Nonlinear mixed effect models for predicting relationships between total height and diameter of Oriental beech trees in Kestel, Turkey. Rev. Chapingo Ser. Cienc. For. Del Ambiente 2015, 21, 185–202. [Google Scholar] [CrossRef]
- Pinheiro, J.C.; Bates, D.M. Theory and Computational Methods for Linear Mixed-Effects Models. In Mixed-Effects Models in S and S-PLUS; Springer: Berlin/Heidelberg, Germany, 2000; pp. 57–96. [Google Scholar]
- Ogana, F.N.; Ercanli, I. Modelling height-diameter relationships in complex tropical rain forest ecosystems using deep learning algorithm. J. For. Res. 2022, 33, 883–898. [Google Scholar] [CrossRef]
- Sharma, R.P.; Vacek, Z.; Vacek, S.; Kučera, M. Modelling individual tree height–diameter relationships for multi-layered and multi-species forests in central Europe. Trees 2019, 33, 103–119. [Google Scholar] [CrossRef]
- Ogana, F.N.; Holmström, E.; Sharma, R.P.; Langvall, O.; Nilsson, U. Optimizing height measurement for the long-term forest experiments in Sweden. For. Ecol. Manag. 2023, 532, 120843. [Google Scholar] [CrossRef]
- Gomez-Garcia, E.; Fonseca, T.F.; Crecente-Campo, F.; Almeida, L.R.; Dieguez-Aranda, U.; Huang, S.; Marques, C.P. Height-diameter models for maritime pine in Portugal: A comparison of basic, generalized and mixed-effects models. Iforest-Biogeosci. For. 2015, 9, 72. [Google Scholar] [CrossRef]
- Ciceu, A.; Garcia-Duro, J.; Seceleanu, I.; Badea, O. A generalized nonlinear mixed-effects height–diameter model for Norway spruce in mixed-uneven aged stands. For. Ecol. Manag. 2020, 477, 118507. [Google Scholar] [CrossRef]
- Lima, R.B.d.; Görgens, E.B.; Elias, F.; de Abreu, J.C.; Baia, A.L.; de Oliveira, C.P.; Silva da Silva, D.A.; Batista, A.P.B.; Lima, R.C.; Sotta, E.D. Height-diameter allometry for tropical forest in northern Amazonia. PLoS ONE 2021, 16, e0255197. [Google Scholar] [CrossRef] [PubMed]
- Kearsley, E.; Moonen, P.C.; Hufkens, K.; Doetterl, S.; Lisingo, J.; Boyemba Bosela, F.; Boeckx, P.; Beeckman, H.; Verbeeck, H. Model performance of tree height-diameter relationships in the central Congo Basin. Ann. For. Sci. 2017, 74, 1–13. [Google Scholar] [CrossRef]
- Mugasha, W.; Mauya, E.; Njana, A.; Karlsson, K.; Malimbwi, R.; Ernest, S. Height-diameter allometry for tree species in tanzania mainland. Int. J. For. Res. 2019, 2019, 4832849. [Google Scholar] [CrossRef]
- Ogana, F.N. A mixed-effects height-diameter model for Gmelina arborea Roxb stands in Southwest Nigeria. J. For. Res. 2022, 27, 1–7. [Google Scholar] [CrossRef]
- Panzou, G.J.L.; Bocko, Y.E.; Mavoungou, A.Y.; Loumeto, J.-J. Height–diameter allometry in African monodominant forest close to mixed forest. J. Trop. Ecol. 2021, 37, 98–107. [Google Scholar] [CrossRef]
- Sebrala, H.; Abich, A.; Negash, M.; Asrat, Z.; Lojka, B. Tree allometric equations for estimating biomass and volume of Ethiopian forests and establishing a database. Trees For. People 2022, 9, 100314. [Google Scholar] [CrossRef]
- Sisay, K.; Thurnher, C.; Belay, B.; Lindner, G.; Hasenauer, H. Volume and carbon estimates for the forest area of the amhara region in northwestern ethiopia. Forests 2017, 8, 122. [Google Scholar] [CrossRef]
- Asrat, Z.; Eid, T.; Gobakken, T.; Negash, M. Modelling and quantifying tree biometric properties of dry Afromontane forests of south-central Ethiopia. Trees 2020, 34, 1411–1426. [Google Scholar] [CrossRef]
- Tesfaye, M.A.; Gardi, O.; Bekele, T.; Blaser, J. Temporal variation of ecosystem carbon pools along altitudinal gradient and slope: The case of Chilimo dry afromontane natural forest, Central Highlands of Ethiopia. J. Ecol. Environ. Conserv. 2019, 43, 17. [Google Scholar] [CrossRef]
- Soromessa, T.; Kelbessa, E. Diversity and endemicity of Chilimo forest, central Ethiopia. Biosci. Discov. 2013, 4, 1–4. [Google Scholar]
- Tesfaye, M.A.; Bravo, F.; Ruiz-Peinado, R.; Pando, V.; Bravo-Oviedo, A. Impact of changes in land use, species and elevation on soil organic carbon and total nitrogen in Ethiopian Central Highlands. Geoderma 2016, 261, 70–79. [Google Scholar] [CrossRef]
- Mammo, S.; Kebin, Z.; Chimidi, A.; Ibrahim, H. Soil quality analysis for sustainability of forest ecosystem: The case of Chilimo-Gaji Forest, West Shewa Zone, Ethiopia. J. Environ. Earth Sci. 2019, 9, 1–9. [Google Scholar]
- Lemi, T.; Eshete, A.; Seid, G.; Mulugeta, S.; Egeta, D.; Teshome, M. Aboveground Biomass Models for Indigenous Tree Species in the Dry Afromontane Forest, Central Ethiopia. Int. J. For. Res. 2023, 2023, 4901521. [Google Scholar] [CrossRef]
- Duguma, L.A.; Hager, H.; Gruber, M. The community-state forest interaction in Menagesha Suba area, Ethiopia: The challenges and possible solutions. For. Trees Livelihoods 2009, 19, 111–128. [Google Scholar] [CrossRef]
- Friis, I.; Demissew, S.; Breugel, P.V. Atlas of the Potential Vegetation of Ethiopia; The Royal Danish Academy of Sciences and Letters: Copenhagen, Denmark, 2010. [Google Scholar]
- Mucheye, G.; Yemata, G. Species composition, structure and regeneration status of woody plant species in a dry Afromontane Forest, Northwestern Ethiopia. Cogent Food Agric. 2020, 6, 1823607. [Google Scholar] [CrossRef]
- Ali, F.; Khan, N.; Ali, K.; Khan, M.E.H.; Jones, D.A. Vegetation pattern and regeneration dynamics of the progressively declining Monotheca buxifolia forests in Pakistan: Implications for conservation. Sustainability 2022, 14, 6111. [Google Scholar] [CrossRef]
- Edwards, S.; Tadesse, M.; Hedberg, I. Flora of Ethiopia and Eritrea, Volume 2, Part 2: Canellaceae to Euphorbiaceae; The National Herbarium, Addis Ababa, Ethiopia, and Department of Systematic Botany: Uppsala, Switzerland, 1995. [Google Scholar]
- Edwards, S.; Tadesse, M.; Demissew, S.; Hedberg, I. Flora of Ethiopia and Eritrea, Volume 2, Part 1: Magnoliaceae to Flacourtiaceae; Addis Ababa, Ethiopia and Uppsala, Sweden; The National Herbarium, Addis Ababa University: Addis Ababa, Etiopia, 2000. [Google Scholar]
- Hedberg, I.; Hedberg, O.; Edwards, S. Flora of Ethiopia and Eritrea. Vol. 3, Pittosporaceae to Araliaceae; National Herbarium, Addis Ababa University: Addis Ababa, Etiopia, 1989. [Google Scholar]
- Hedberg, I.; Friis, I.; Edwards, S. Flora of Ethiopia and Eritrea. Asteraceae Volume 4 Part 2. Ethiopia; Department of Systematic Botany, Uppsala University: Uppsala, Sweden; National Herbarium, Addis Ababa University: Addis Ababa, Etiopia, 2004. [Google Scholar]
- Hedberg, I.; Edwards, S.; Nemomissa, S. Flora of Ethiopia and Eritrea. Vol. 4. Part 1, Apiaceae to Dipsacaceae; National Herbarium, Addis Ababa University: Addis Ababa, Etiopia, 2003. [Google Scholar]
- Pohjonen, V.; Pukkala, T. Juniperus procera Hocht. ex. Endl. in Ethiopian forestry. For. Ecol. Manag. 1992, 49, 75–85. [Google Scholar] [CrossRef]
- Negash, L.; Kagnew, B. Mechanisms for the successful biological restoration of the threatened African pencilcedar (Juniperus procera Hochst. ex. Endl., Cupressaceae) in a degraded landscape. For. Ecol. Manag. 2013, 310, 476–482. [Google Scholar] [CrossRef]
- Farjon, A. Juniperus procera. The IUCN Red List of Threatened Species 2013: E.T33217A2835242. Available online: https://www.iucnredlist.org/species/33217/2835242 (accessed on 3 April 2023).
- Orwa, C.; Mutua, A.; Kindt, R.; Jamnadass, R.; Simons, A. Agroforestree Database: A Tree Reference and Selection Guide, Version 4.0; World Agroforestry Centre ICRAF: Nairobi, Kenia, 2009. [Google Scholar]
- Abrha, H.; Birhane, E.; Hagos, H.; Manaye, A. Predicting suitable habitats of endangered Juniperus procera tree under climate change in Northern Ethiopia. J. Sustain. For. 2018, 37, 842–853. [Google Scholar] [CrossRef]
- White, F. The vegetation of Africa, a descriptive memoir to accompany the UNESCO/AETFAT/UNSO vegetation map of Africa. UNESCO Nat. Resour. Res. 1983, 20, 356. [Google Scholar]
- Zeide, B. Analysis of growth equations. For. Sci. 1993, 39, 594–616. [Google Scholar] [CrossRef]
- Huang, S.; Titus, S.J.; Wiens, D.P. Comparison of nonlinear height–diameter functions for major Alberta tree species. Can. J. For. Res. 1992, 22, 1297–1304. [Google Scholar] [CrossRef]
- Mehtatalo, L.; Lappi, J. Biometry for Forestry and Environmental Data: With Examples in R; Chapman and Hall/CRC: Boca Raton, FL, USA, 2020. [Google Scholar]
- Corral-Rivas, S.; Álvarez-González, J.G.; Crecente-Campo, F.; Corral-Rivas, J.J. Local and generalized height-diameter models with random parameters for mixed, uneven-aged forests in Northwestern Durango, Mexico. For. Ecosyst. 2014, 1, 6. [Google Scholar] [CrossRef]
- Chenge, I.B. Height–diameter relationship of trees in Omo strict nature forest reserve, Nigeria. Trees For. People 2021, 3, 100051. [Google Scholar] [CrossRef]
- Ogana, F.N.; Corral-Rivas, S.; Gorgoso-Varela, J.J. Nonlinear mixed-effect height-diameter model for Pinus pinaster ait. and Pinus radiata d. Don. Cerne 2020, 26, 150–161. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Volume 1. [Google Scholar]
- Zeide, B.J.C.J.o.F.R. Accuracy of equations describing diameter growth. Can. J. For. Res. 1989, 19, 1283–1286. [Google Scholar] [CrossRef]
- Näslund, M. The thinning experiments of the Forest Research Institute in Scots pine stand. Medd. Från Statens Skogsförsöksanstalt 1937, 29, 1–169. [Google Scholar]
- Meyer, H.A. A mathematical expression for height curves. J. For. 1940, 38, 415–420. [Google Scholar]
- Schumacher, F.X. A new growth curve and its application to timber yield studies. J. For. 1939, 37, 819–820. [Google Scholar]
- Bates, D.M.; Watts, D.G. Relative curvature measures of nonlinearity. J. R. Stat. Soc. Ser. B 1980, 42, 1–16. [Google Scholar] [CrossRef]
- Medawar, P.B. The growth, growth energy, and ageing of the chicken’s heart. Proc. R. Soc. Lond. Ser. B-Biol. Sci. 1940, 129, 332–355. [Google Scholar]
- Richards, F.J. A flexible growth function for empirical use. J. Exp. Bot. 1959, 10, 290–301. [Google Scholar] [CrossRef]
- Chapman, D.G. Statistical problems in dynamics of exploited fisheries populations. In Proceedings of the 4th Berkeley Symposium on Mathematical Statistics and Probability, Santa Barbara, CA, USA, 20 June–30 July 1960; pp. 153–168. [Google Scholar]
- Yang, R.C.; Kozak, A.; Smith, J.H.G. The potential of Weibull-type functions as flexible growth curves. Can. J. For. Res. 1978, 8, 424–431. [Google Scholar] [CrossRef]
- Ratkowsky, D. Handbook of Nonlinear Regression Models; Marcel Dekker: New York, NY, USA, 1990. [Google Scholar]
- Mulamba, N.; Mock, J. Improvement of yield potential of the ETO blanco maize (Zea mays L.) population by breeding for plant traits [Mexico]. Egypt. J. Genet. Cytol. 1978, 7, 476–490. [Google Scholar]
- Özçelik, R.; Cao, Q.V.; Trincado, G.; Göçer, N. Predicting tree height from tree diameter and dominant height using mixed-effects and quantile regression models for two species in Turkey. For. Ecol. Manag. 2018, 419, 240–248. [Google Scholar] [CrossRef]
- Stegmann, G.; Jacobucci, R.; Harring, J.R.; Grimm, K.J. Nonlinear mixed-effects modeling programs in R. Struct. Equ. Model. 2018, 25, 160–165. [Google Scholar] [CrossRef]
- Cui, K.; Wu, X.; Zhang, C.; Zhao, X.; von Gadow, K. Estimating height-diameter relations for structure groups in the natural forests of Northeastern China. For. Ecol. Manag. 2022, 519, 120298. [Google Scholar] [CrossRef]
- Xie, L.; Widagdo, F.R.A.; Dong, L.; Li, F. Modeling height–diameter relationships for mixed-species plantations of Fraxinus mandshurica Rupr. and Larix olgensis Henry in Northeastern China. Forests 2020, 11, 610. [Google Scholar] [CrossRef]
- Zhang, X.; Fu, L.; Sharma, R.P.; He, X.; Zhang, H.; Feng, L.; Zhou, Z. A Nonlinear Mixed-Effects Height-Diameter Model with Interaction Effects of Stand Density and Site Index for Larix olgensis in Northeast China. Forests 2021, 12, 1460. [Google Scholar] [CrossRef]
- Pinheiro, J.; Bates, D.M. Nlme: Linear and Nonlinear Mixed Effects Models. R package Version 3.1-161. Available online: https://CRAN.R-project.org/package=nlme (accessed on 12 May 2022).
- Patrício, M.S.; Dias, C.R.; Nunes, L. Mixed-effects generalized height-diameter model: A tool for forestry management of young sweet chestnut stands. For. Ecol. Manag. 2022, 514, 120209. [Google Scholar] [CrossRef]
- Vonesh, E.; Chinchilli, V.M. Linear and Nonlinear Models for the Analysis of Repeated Measurements; CRC Press: Boca Raton, FL, USA, 1997. [Google Scholar]
- Camacho, E.A.R.; Rivas, S.C.; Hernández, J.A.L.; Durán, Á.A.C.; Carmona, J.X.; Nagel, J. Generalized height-diameter models with random effects for natural forests of central Mexico. CERNE 2022, 28, E-103033. [Google Scholar] [CrossRef]
- Johnson, K.A.; Goody, R.S. The original Michaelis constant: Translation of the 1913 Michaelis–Menten paper. Biochemistry 2011, 50, 8264–8269. [Google Scholar] [CrossRef]
- Fayolle, A.; Panzou, G.J.L.; Drouet, T.; Swaine, M.D.; Bauwens, S.; Vleminckx, J.; Biwole, A.; Lejeune, P.; Doucet, J.-L. Taller trees, denser stands and greater biomass in semi-deciduous than in evergreen lowland central African forests. For. Ecol. Manag. 2016, 374, 42–50. [Google Scholar] [CrossRef]
- Molto, Q.; Hérault, B.; Boreux, J.-J.; Daullet, M.; Rousteau, A.; Rossi, V. Predicting tree heights for biomass estimates in tropical forests–a test from French Guiana. Biogeosciences 2014, 11, 3121–3130. [Google Scholar] [CrossRef]
- Barbosa, R.I.; Ramirez-Narvaez, P.N.; Fearnside, P.M.; Villacorta, C.D.A.; Carvalho, L.C.d.S. Allometric models to estimate tree height in northern Amazonian ecotone forests. Acta Amaz. 2019, 49, 81–90. [Google Scholar] [CrossRef]
- Bronisz, K.; Mehtätalo, L. Mixed-effects generalized height–diameter model for young silver birch stands on post-agricultural lands. For. Ecol. Manag. 2020, 460, 117901. [Google Scholar] [CrossRef]
- Huang, S.; Wiens, D.P.; Yang, Y.; Meng, S.X.; Vanderschaaf, C.L. Assessing the impacts of species composition, top height and density on individual tree height prediction of quaking aspen in boreal mixedwoods. For. Ecol. Manag. 2009, 258, 1235–1247. [Google Scholar] [CrossRef]
- Sharma, M.; Yin Zhang, S. Height–diameter models using stand characteristics for Pinus banksiana and Picea mariana. Scand. J. For. Res. 2004, 19, 442–451. [Google Scholar] [CrossRef]
- Temesgen, H.; Hann, D.W.; Monleon, V.J. Regional height–diameter equations for major tree species of southwest Oregon. West. J. Appl. For. 2007, 22, 213–219. [Google Scholar] [CrossRef]
- Sullivan, M.J.; Lewis, S.L.; Hubau, W.; Qie, L.; Baker, T.R.; Banin, L.F.; Chave, J.; Cuni-Sanchez, A.; Feldpausch, T.R.; Lopez-Gonzalez, G. Field methods for sampling tree height for tropical forest biomass estimation. Methods Ecol. Evol. 2018, 9, 1179–1189. [Google Scholar] [CrossRef]
- Marshall, A.R.; Willcock, S.; Platts, P.J.; Lovett, J.C.; Balmford, A.; Burgess, N.D.; Latham, J.E.; Munishi, P.K.; Salter, R.; Shirima, D. Measuring and modelling above-ground carbon and tree allometry along a tropical elevation gradient. Biol. Conserv. 2012, 154, 20–33. [Google Scholar] [CrossRef]
- Tian, D.; Jiang, L.; Shahzad, M.K.; He, P.; Wang, J.; Yan, Y. Climate-sensitive tree height-diameter models for mixed forests in Northeastern China. Agric. For. Meteorol. 2022, 326, 109182. [Google Scholar] [CrossRef]
- van Breugel, M.; Hall, J.S.; Craven, D.J.; Gregoire, T.G.; Park, A.; Dent, D.H.; Wishnie, M.H.; Mariscal, E.; Deago, J.; Ibarra, D. Early growth and survival of 49 tropical tree species across sites differing in soil fertility and rainfall in Panama. For. Ecol. Manag. 2011, 261, 1580–1589. [Google Scholar] [CrossRef]
- Detto, M.; Muller-Landau, H.C.; Mascaro, J.; Asner, G.P. Hydrological networks and associated topographic variation as templates for the spatial organization of tropical forest vegetation. PLoS ONE 2013, 8, e76296. [Google Scholar] [CrossRef]
- Neophytou, C.; Weisser, A.-M.; Landwehr, D.; Šeho, M.; Kohnle, U.; Ensminger, I.; Wildhagen, H. Assessing the relationship between height growth and molecular genetic variation in Douglas-fir (Pseudotsuga menziesii) provenances. Eur. J. For. Res. 2016, 135, 465–481. [Google Scholar] [CrossRef]
- Sertse, D.; Gailing, O.; Eliades, N.G.; Finkeldey, R. Anthropogenic and natural causes influencing population genetic structure of Juniperus procera Hochst. ex Endl. in the Ethiopian highlands. Genet. Resour. Crop Evol. 2011, 58, 849–859. [Google Scholar] [CrossRef]
- Castaño-Santamaría, J.; Crecente-Campo, F.; Fernández-Martínez, J.L.; Barrio-Anta, M.; Obeso, J.R. Tree height prediction approaches for uneven-aged beech forests in northwestern Spain. For. Ecol. Manag. 2013, 307, 63–73. [Google Scholar] [CrossRef]
- Trincado, G.; VanderSchaaf, C.L.; Burkhart, H.E. Regional mixed-effects height–diameter models for loblolly pine (Pinus taeda L.) plantations. Eur. J. For. Res. 2007, 126, 253–262. [Google Scholar] [CrossRef]
- Van Laar, A.; Akça, A. Forest Mensuration; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2007; Volume 13. [Google Scholar]


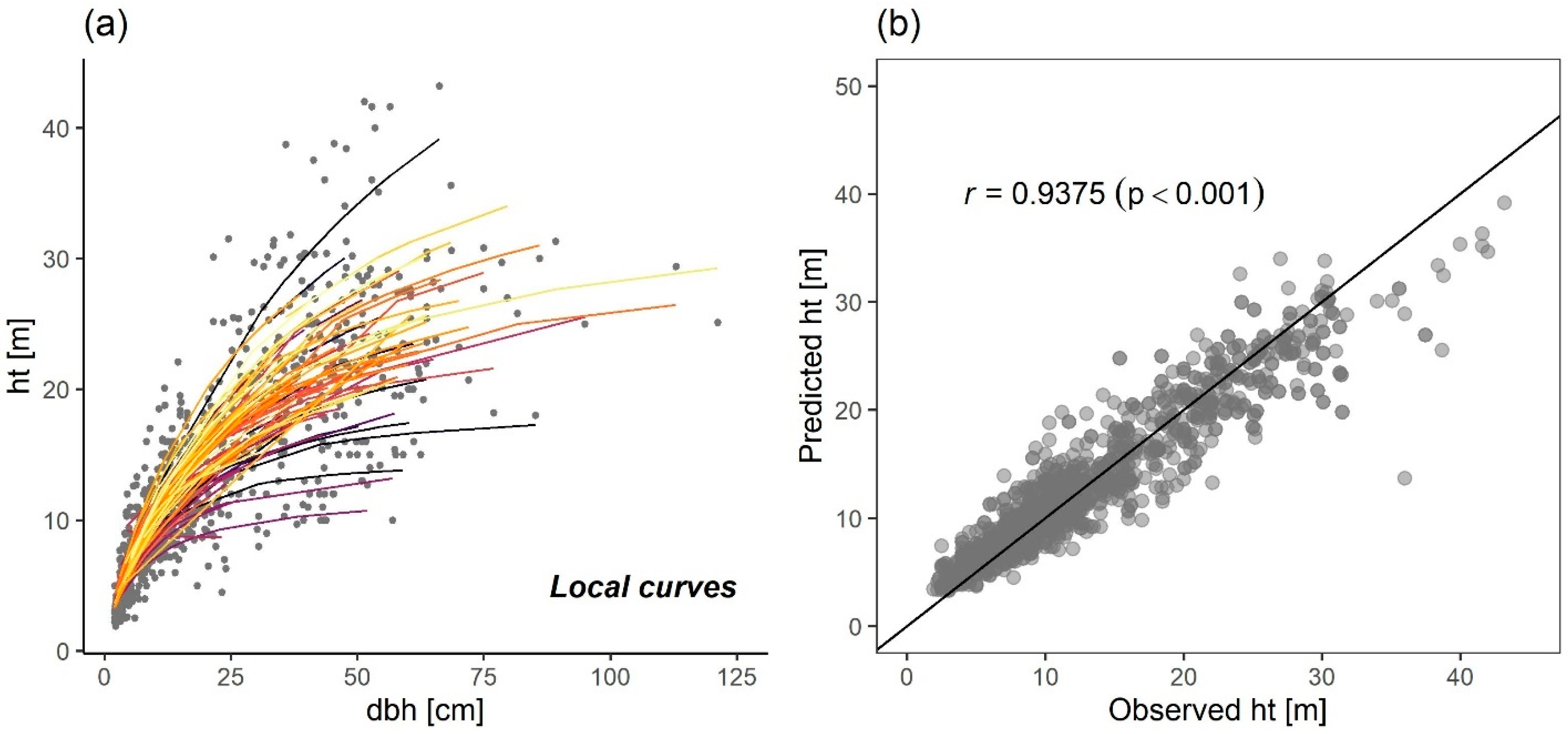

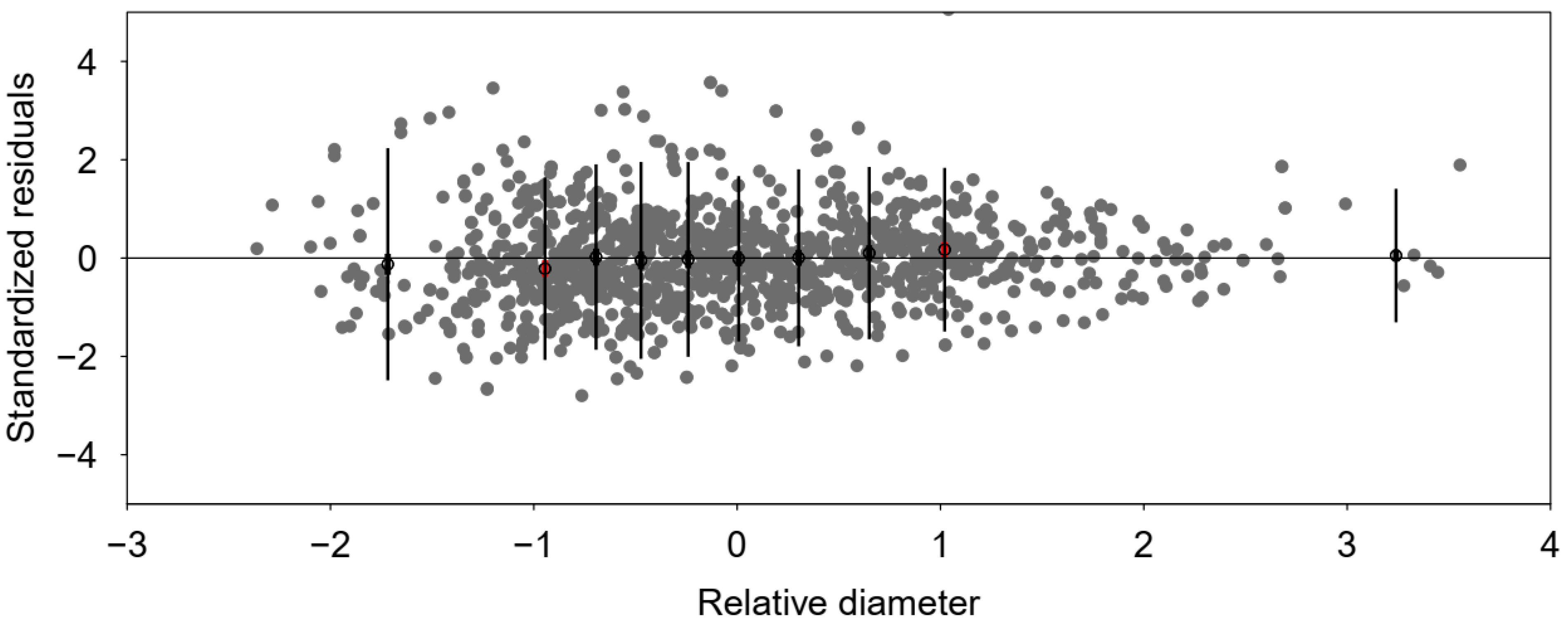

| Variables | Fitting Dataset—Chilimo Forest | Calibration Dataset—Menagesha Suba Forest | ||||||
|---|---|---|---|---|---|---|---|---|
| Mean | Min. | Max. | Std | Mean | Min. | Max. | Std | |
| dbh | 21.10 | 2.00 | 121.20 | 18.45 | 14.3 | 2.0 | 71.0 | 12.7 |
| ht | 13.55 | 2.00 | 43.22 | 7.76 | 9.9 | 1.6 | 28.0 | 6.2 |
| G | 11.27 | 0.01 | 87.60 | 15.89 | 2.0 | 0.01 | 16.9 | 4.4 |
| N | 484.48 | 25.00 | 2750 | 773.37 | 606.8 | 21.0 | 1149 | 365.8 |
| hd | 10.71 | 2.00 | 38.67 | 6.77 | 4.6 | 1.4 | 14 | 2.5 |
| Dd | 18.47 | 2.53 | 71.70 | 13.12 | 9.1 | 2.0 | 25.9 | 6.5 |
| Dq | 16.23 | 2.00 | 79.80 | 13.81 | 5.4 | 2.0 | 23.9 | 6.1 |
| Total sample | n = 1215 trees from 101 rectangular plots | n = 300 trees from 30 rectangular plots | ||||||
| No. | Model Forms | Random Effects |
|---|---|---|
| M1 | The random effect at β0 and β1 | |
| M2 | The random effect at β0 | |
| M3 | The random effect at β1 |
| Code | Sampling Alternatives |
|---|---|
| A1. | Utilizing measurements from selected trees with diameters near the quartiles (the 25th, 50th, and 75th percentiles) of the diameter distribution. |
| A2. | Utilizing measurements from selected trees with diameters close to the first and the second quartiles (25th and 50th percentiles) of the diameter distribution |
| A3. | Utilizing measurements from selected trees with diameters close to the first and the third quartiles (25th and 75th percentiles) of the diameter distribution |
| A4. | Utilizing measurements from selected trees with diameters close to the second and the third quartiles (50th and 75th percentiles) of the diameter distribution |
| A5. | Utilizing measurements from selected trees with diameters close to the second quartile (50th percentile) of the diameter distribution |
| A6. | Utilizing measurements from selected trees with diameters close to the quadratic mean diameter (Dq), the smallest diameter (dmin), and the largest diameter trees (dmax) in each sample plot |
| A7. | Utilizing measurements from the smallest diameter trees (dmin) in each sample plot |
| A8. | Utilizing measurements from 1–10 randomly selected trees in each sample plot |
| A9. | Utilizing measurements from 1–10 systematically selected largest diameter trees in each sample plot |
| Models | Parameters | Fit Statistics | Rank | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| β0 | β1 | β2 | Bias | Rank | RMSE | Rank | AIC | Rank | ∑ | Final | |
| M1 | 1.802 * | 0.659 * | 0.00 | 1 | 4.39 | 12 | 6398.13 | 11 | 24 | 8 | |
| M2 | 1.346 * | 0.195 * | 0.35 | 12 | 4.32 | 11 | 6389.49 | 9 | 32 | 11 | |
| M3 | 20.891 * | 8.041 * | 0.85 | 13 | 4.77 | 13 | 6531.36 | 13 | 39 | 13 | |
| M4 | 24.905 * | 0.043 * | 0.10 | 7 | 4.17 | 5 | 6322.19 | 6 | 18 | 6 | |
| M5 | 19.99 * | 7.102 * | 0.97 | 14 | 4.91 | 14 | 6586.78 | 14 | 42 | 14 | |
| M6 | 36.437 * | 32.113 * | 0.09 | 6 | 4.16 | 1 | 6314.99 | 2 | 9 | 1 | |
| M7 | 22.083 * | 2.415 * | 0.089 * | 0.16 | 10 | 4.24 | 9 | 6396.07 | 10 | 29 | 10 |
| M8 | 21.152 * | 6.891 * | 0.141 * | 0.23 | 11 | 4.31 | 10 | 6462.61 | 12 | 33 | 12 |
| M9 | 26.816 * | 0.034 * | 0.914 * | 0.08 | 3 | 4.16 | 2 | 6318.97 | 5 | 10 | 3 |
| M10 | 27.406 * | 0.044 * | 0.929 * | 0.08 | 3 | 4.16 | 3 | 6318.42 | 4 | 10 | 4 |
| M11 | 98.175 * | 4.849 * | −0.302 * | 0.08 | 5 | 4.18 | 8 | 6310.83 | 1 | 14 | 5 |
| M12 | 31.394 * | 20.392 * | 5.819 * | 0.12 | 9 | 4.18 | 7 | 6335.11 | 8 | 24 | 9 |
| M13 | 36.768 * | 0.031 * | −0.995 * | 0.08 | 2 | 4.17 | 4 | 6316.97 | 3 | 9 | 2 |
| M14 | 33.337 * | 20.893 * | 7.296 * | 0.11 | 8 | 4.17 | 6 | 6329.15 | 7 | 21 | 7 |
| Components | Mixed-Effects Models | ||
|---|---|---|---|
| M1 | M2 | M3 | |
| Fixed parameters | |||
| β0 | 31.6506 (1.4959) | 32.3400 (1.0469) | 34.6591 (0.9960) |
| β1 | 23.8515 (1.6557) | 25.0679 (1.2465) | 28.8907 (1.6301) |
| Random variance components | |||
| std () | 12.1889 | 5.4512 | |
| std () | 11.9283 | 7.7571 | |
| cor (, ) | 0.9380 | ||
| σ2 | 0.8463 | 0.9313 | 0.8122 |
| δ | 0.4145 | 0.4018 | 0.4704 |
| Model performance | |||
| RMSE (m) | 2.6924 | 2.9144 | 3.2499 |
| Bias (m) | 0.0432 | 0.0112 | −0.0566 |
| AIC | 5858.75 | 6051.30 | 6316.07 |
| Components | M4 |
|---|---|
| Fixed parameters | |
| β0 | 28.4695 (1.3859) |
| β1 | 13.4697 (2.1315) |
| β2 | −1.3338 (0.1496) |
| β3 | 0.1139 (0.0383) |
| Random variance components | |
| std () | 11.3006 |
| std () | 11.1352 |
| cor (, ) | 0.9510 |
| σ2 | 0.8019 |
| δ | 0.4327 |
| Model performance | |
| RMSE (m) | 2.6921 |
| Bias (m) | 0.0724 |
| AIC | 5860.53 |
| No | Subsample | N | Model RMSE | |
|---|---|---|---|---|
| Local | Generalized | |||
| A0 | The best local model | 1215 | 2.6924 | 2.6921 |
| A1 | Quartiles (1,2,3) | 3 | 2.2320 | 2.4311 |
| A2 | Quartiles (1,2) | 2 | 2.5344 | 2.9109 |
| A3 | Quartiles (1,3) | 2 | 2.2873 | 2.5345 |
| A4 | Quartiles (2,3) | 2 | 2.3376 | 2.6929 |
| A5 | Quartiles (2) | 1 | 2.6680 | 3.1421 |
| A6 | Dq, dmin, dmax | 3 | 2.1354 | 2.4517 |
| A7 | dmin | 1 | 2.9954 | 3.2069 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teshome, M.; Braz, E.M.; Torres, C.M.M.E.; Raptis, D.I.; de Mattos, P.P.; Temesgen, H.; Rubio-Camacho, E.A.; Sileshi, G.W. Mixed-Effects Height Prediction Model for Juniperus procera Trees from a Dry Afromontane Forest in Ethiopia. Forests 2024, 15, 443. https://doi.org/10.3390/f15030443
Teshome M, Braz EM, Torres CMME, Raptis DI, de Mattos PP, Temesgen H, Rubio-Camacho EA, Sileshi GW. Mixed-Effects Height Prediction Model for Juniperus procera Trees from a Dry Afromontane Forest in Ethiopia. Forests. 2024; 15(3):443. https://doi.org/10.3390/f15030443
Chicago/Turabian StyleTeshome, Mindaye, Evaldo Muñoz Braz, Carlos Moreira Miquelino Eleto Torres, Dimitrios Ioannis Raptis, Patricia Povoa de Mattos, Hailemariam Temesgen, Ernesto Alonso Rubio-Camacho, and Gudeta Woldesemayat Sileshi. 2024. "Mixed-Effects Height Prediction Model for Juniperus procera Trees from a Dry Afromontane Forest in Ethiopia" Forests 15, no. 3: 443. https://doi.org/10.3390/f15030443







