Abstract
The Loess Plateau region of China suffers from severe soil erosion, and the selection of effective slope-protection vegetation is essential to prevent soil and water loss. This study focused on individual plants of common species in the Loess Plateau, such as Caragana korshinski Kom., Hippophae rhamnoides Linn., Pinus tabuliformis Carr., Robinia Pseudoacacia Linn., Populus tomentosa Carr., Prunus armeniaca Lam. The root spatial distribution, geometric morphology, and fractal characteristics of these plants were measured using the whole-root-excavation method, and the vertical pull-out force of their root systems was quantified using the in situ whole-plant root-pulling method. The results showed that H. rhamnoides dominates in the vertical spatial distribution of its root system through a larger number of inclined roots. C. korshinskii, P. tomentosa, R. pseudoacacia, and P. armeniaca dominate in the horizontal spatial distribution of their root systems through a greater number of horizontal roots. P. tabuliformis, on the other hand, achieves a relatively balanced distribution in both horizontal and vertical spaces through its well-developed taproot and numerous lateral roots. In terms of the geometric morphology and fractal characteristics of their root systems, H. rhamnoides and C. korshinskii exhibit a larger number of fine roots and complex branching, resulting in a higher total-root length, total-root surface area, and root fractal dimension. The soil-stabilizing ability of H. rhamnoides, C. korshinskii, and R. pseudoacacia was stronger, mainly influenced by their total-root length, total-root surface area, and inclined root quantity, and these species can be prioritized as typical vegetation for soil and water conservation in the construction of Loess Plateau vegetation. From the perspective of slope stabilization and soil conservation alone, we strongly recommend planting shrub vegetation in the Chinese Loess Plateau.
1. Introduction
The Loess Plateau is an area of widespread hills and valleys which is one of the most eroded regions in the world [1,2]. Its ecological environment is fragile, and its natural conditions are harsh, making it prone to geological disasters such as landslides and collapses [3]. The occurrence of these disasters is caused by the combined effect of natural and human factors [4]. Natural factors include climate-heavy rain that directly leads to soil erosion. The soil geology, sparse vegetation, and strong winds of the Loess Plateau cause severe weathering erosion of the surface bedrock of the mountains, and the loose structure of the Loess, in ridges and valleys, is prone to collapse under the action of rainwater erosion, resulting in frequent landslides and mudslides. The terrain’s numerous gullies in the Loess hilly area, as well as its large slope gradients and lengths, often result in frequent occurrence of disasters such as soil erosion and mountain landslides. Surface vegetation can effectively reduce soil erosion, protect the soil surface from raindrop impact, and disperse surface runoff [5,6]. However, the vegetation cover in the Loess Plateau is low, which reduces the soil’s ability to resist erosion. Human factors include unreasonable production of activities that accelerate soil erosion, such as the cultivation of wasteland and deforestation, which destroy the protective function of vegetation and lead to problems such as soil erosion and landslides [7]. Therefore, ecological restoration projects that focus on vegetation restoration are an effective means of targeted control of soil erosion and geological hazards [8].
Since the 1950s, a series of measures have been implemented in China to control soil erosion and landslides in order to protect the ecological environment [9]. For common shallow landslides, traditional engineering measures are generally used to prevent and reduce the occurrence of slope instability, soil displacement, and other hazards [10]. However, engineering methods have obvious limitations in terms of protection cycles and stability performance, making it difficult to achieve long-term and stable protection effects [11]. In addition, the engineering methods used tend to generate a large amount of debris, leading to secondary disasters and posing potential threats to the formation of mountain soil layers that are difficult to prevent [12,13]. The network structure formed by the development of plant roots not only spreads horizontally in the soil, but also anchors the main and lateral roots vertically in the soil, increasing the erosion resistance and cohesion of the soil, which helps to improve the stability of slope soils and reduce the probability of landslide disasters [14,15]. Vegetation-protection measures not only play an important role in suppressing large-scale soil movement and improving slope stability, but also play an important role in preventing soil erosion [16]. They also have a stable and long-term effect on the healthy and sustainable development of the local ecosystem, which is of great importance to the local economy [6,17].
Vegetative ecological restoration is a comprehensive topic that integrates multiple disciplines, such as plant and soil sciences [18]. For instance, vegetation can suppress the instability of slope soils to varying degrees, and plant root systems can increase the shear strength of the slope and improve its stability [19]. The mechanical effect of its slope-stabilizing function is mainly manifested in the mechanical interaction between the stem and massive root systems [20]. The plant increases the shear strength and mechanical performance of the root–soil composite through mechanical interactions, thereby strengthening the soil [21]. Lateral roots can strengthen the cohesion between plant roots and soil. Under the condition that the internal friction angle of the soil is kept constant, the mechanical restraining force of the roots can increase the tensile strength of the soil [22]. The main root and its longitudinally growing lateral roots, which reach the deep soil layer, increase the shear strength of the soil by anchoring and strengthening the principle of resisting slope sliding [23,24]. The root system can be divided into deep thick roots and shallow fine roots. The deep thick roots of plants have a certain strength and stiffness and play a role similar to anchoring in the slope, while the intertwined shallow fine roots in shallow slopes can be considered a three-dimensional pre-stressed reinforcement material [25,26].
Currently, the use of vegetation to stabilize slopes and control surface soil erosion is widely accepted in many countries and regions [27,28]. In China, the history of using plants to stabilize riverbank slopes dates back to the Ming Dynasty. Research on the use of plants to control soil erosion and stabilize slopes began to deepen in the 1930s. Early studies found that cutting down trees reduced slope stability and increased the frequency of mountain collapses [29,30]. In recent years, with the frequent occurrence of geological disasters such as soil erosion, landslides, and mudflows, research on the mechanisms of slope erosion and instability has received more attention [31]. Many scholars have conducted research on various aspects of the slope-stabilization effect of different types of plants [32]. By studying the spatial distribution heterogeneity of the root systems of Robinia Pseudoacacia and Platycladus orientalis in the Loess Plateau, the influence of plant root systems on the Loess Plateau terrace was revealed.
Studies have shown that plant root systems can inhibit surface soil erosion and shallow landslides on slopes [33,34]. As one of the main factors influencing the ability of vegetation to stabilize soil, the configuration of plant root systems refers to the complex combination of different components of the root system in space, which is often characterized by geometric and fractal features and topological structure [35,36]. Precisely because the configuration of the plant root system plays an important role in root-pull resistance, obtaining and quantifying the configuration features of the plant root system has become the basis for studying the mechanical properties of plant-root-system soil stabilization [33,34,37]. However, due to constraints such as field experimental equipment and high operational costs, current research on root-system mechanical properties mainly focuses on the tensile and shear resistance of individual roots, and there are relatively few experiments on the whole-root system for pulling and excavation [38]. This not only affects the quantification and accuracy of the plant-root-system configuration, but also limits the in-depth study of the soil-stabilization mechanism of vegetation’s root systems [39].
Although ecological construction work such as soil conservation using tree species has been carried out to protect soil resources and ensure food production, the basic theoretical research on the root function of water- and soil-conserving tree species is still relatively weak, which seriously affects the full play of the vegetation function efficiency. Therefore, this study focuses on six main woody plant species commonly used for water and soil conservation in the Loess Plateau, including Caragana korshinski Kom., Hippophae rhamnoides Linn., Pinus tabuliformis Carr., Robinia Pseudoacacia Linn., Populus tomentosa Carr., and Prunus armeniaca Lam. The aims of this study are (1) to quantify the differences of root-configuration characteristics of different soil- and water-conserving plants and (2) to evaluate the soil-retaining ability of the whole-root system of different plants, and determine the relationship between the soil-retaining ability of the whole-root system and the root configuration. The research results can provide a scientific basis for the selection of tree species in water- and soil-conserving vegetation construction in the Loess Plateau.
2. Materials and Methods
2.1. Study Area
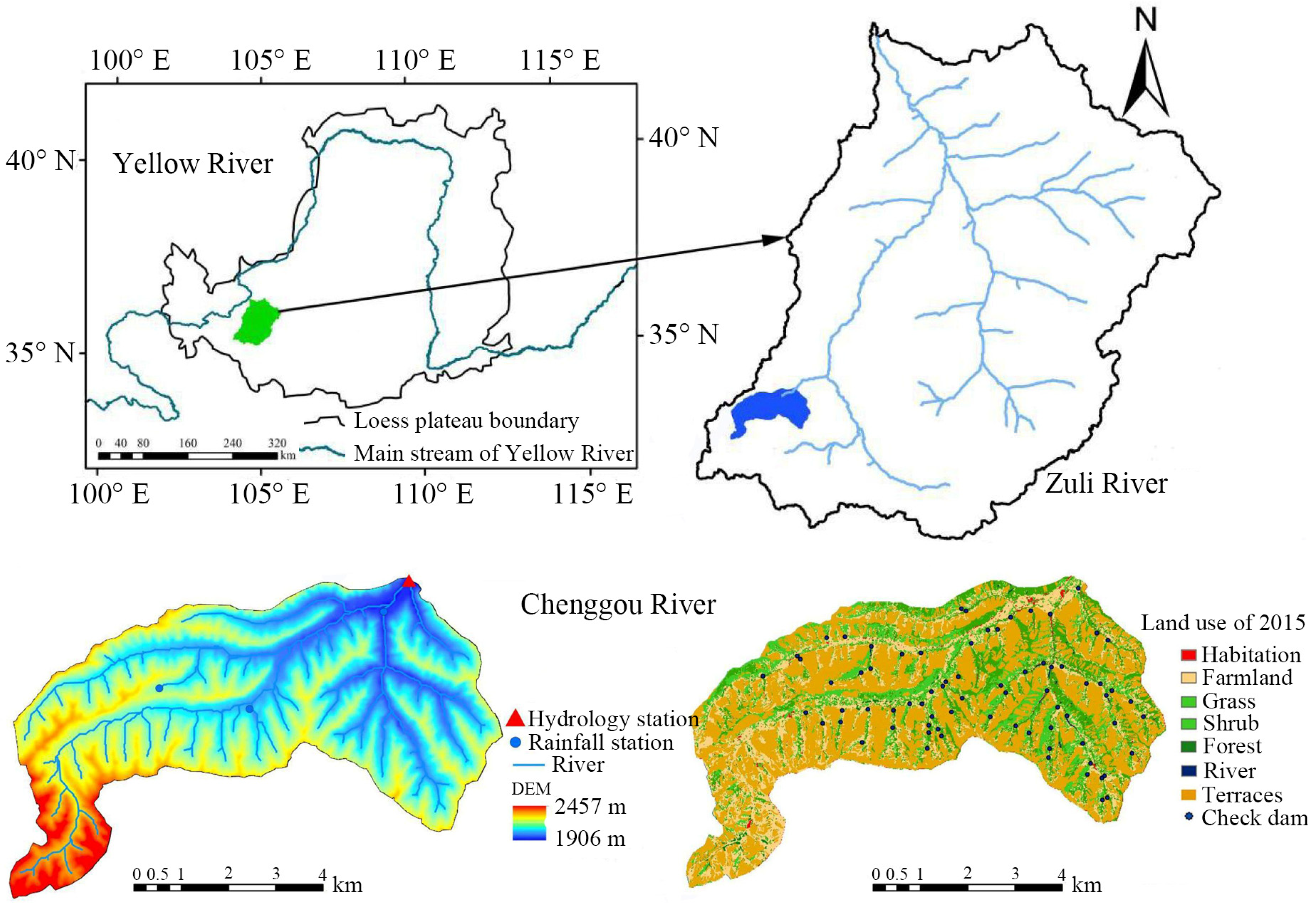
Fieldwork was performed from 3 August to 26 September 2022 in the Chengguohe River basin, a secondary tributary of the Zuli River in the Yellow River basin, with a total area of 161.37 km2. It is located between 104°14′15″–104°28′31″ E and 35°41′7″–35°35′10″ N. The elevation ranges from 1957 to 2273 m. The climate is a temperate semi-arid climate, with an average annual temperature of 6.3 °C. The average annual rainfall is 380 mm (1980–2020), with uneven temporal and spatial distribution, and most of the rainfall occurs during July to September, accounting for 67% of annual precipitation, most of which is in the form of heavy rain. The annual evaporation is up to 1500 mm. According to the land-use situation in 2015, terraced fields, forests, grasslands, shrubs, cultivated land, water surfaces, and residential areas account for 47.35%, 0.54%, 10.78%, 16.24%, 20.76%, 0.1%, and 0.73% of the total watershed area, respectively (Figure 1). The soils in the area are yellow loam and channel saline soils developed on loess parent materials. The bulk density of the soil in the experimental area of the Chengguohe River basin ranges from 1.0 to 1.30 g/cm, the soil volumetric water content ranges from 6.71% to 15.62%, and the total soil porosity ranges from 34.2% to 37.2%. Slope-protection vegetation in the basin includes the shrubs C. korshinskii, H. rhamnoides, and the trees P. tabuliformis, R. pseudoacacia, P. tomentosa, and P. armeniaca [40].

Figure 1.
Study area.
C. korshinskii is a plant of the Fabaceae family, a shrub, sometimes a small tree, 1–4 m tall. Old branches are golden yellow and glossy, while young branches are covered with soft white hairs. Its feather-like compound leaves have 6–8 pairs of leaflets and stipules persistent. Petioles drooping: leaflets are lanceolate or narrowly ovate, pointed or slightly blunt-tipped, with spiny tips, and gray-green in color [41].
H. rhamnoides is a deciduous shrub of the Rhamnaceae family. It grows 1–2.5 m tall, with many thorns, strong and upper or lateral buds. Young branches are brownish-green, while old branches are gray-black and rough; buds are large and golden-yellow or rust-colored. The flowers appear before the leaves, and the plant is dioecious [42].
P. tabuliformis is an evergreen coniferous tree in the Pinaceae family, growing up to 25 m tall with a trunk diameter of up to 1 m. The lower part of the bark is grayish brown and irregularly scaly. The larger branches are flat or upwardly sloping, while old trees have flat tops. The smaller branches are stout, with male cones, cylindrical, 1.2–1.8 cm in length, borne in a spike at the lower part of the new branch [43].
R. pseudoacacia has a shallow but developed root system, is wind-resistant, and is a tree species suitable for sand fixation and soil conservation [44].
P. tomentosa is a deciduous tree, about 30 m tall, with a trunk diameter of about 1m. The trunk is straight and the bark is grayish green to grayish white, with diamond-shaped pores and longitudinal fissures at the base of old trees. Young branches are hairy and later fall off [45].
P. armeniaca is a shrub or small tree, 2–5 m tall, with dark-gray bark. The small branches are hairless, sparsely and softly hairy when young, and grayish brown or light reddish brown. The leaf blade is ovate or nearly circular, 5–10 cm long and 4–7 cm wide, with a long, gradually pointed or tapering tip, a circular or nearly heart-shaped base, and fine, blunt serrations on the margin. The leaf surfaces are glabrous, with short, soft hairs on their veins [46].
2.2. Characteristics of Spatial Distribution of Root Systems
We fully considered site conditions, growth and development stages, and competition among individuals. Three similar and healthy individual trees were selected as samples for each tree species, and a total of 18 sample shrubs and trees were selected. Table 1 showed the basic characteristics of the sample shrubs and trees. The entire root system was excavated using a combination of trenching and root tracing methods to investigate its configuration [47]. Specifically, a 2 m × 0.6 m × 1.6 m trench was dug 2 m away from the plant, and the soil around the plant was cleared into the trench using a small shovel. The accumulated sand in the trench was continuously removed until the entire root system, with a diameter of >3 mm, was completely exposed (reconstruction of the root configuration with a diameter of <3 mm is difficult to achieve in the study), while trying to maintain the position of the roots in their natural space. The horizontal range, depth, and angle (θ) between the primary root and the ground were measured. The number of horizontal roots (0° ≤ θ < 30°), oblique roots (30° ≤ θ < 60°), and vertical roots (60° ≤ θ ≤ 90°) were counted, as well as the total number of primary roots. The ratio of root width to root depth was also calculated.

Table 1.
A brief overview of the sampling areas for six species of shrubs and trees.
2.3. Geometric Morphological Parameters of Root Systems
Based on the diameter (d) of the roots (d is the average diameter of a certain root’s length. When measuring root diameter, we take measurements at three points along the upper, middle, and lower sections of a single root. And then, we calculate the average of these three measurements to determine the root diameter), the root systems of the sample trees were classified into four size classes: fine roots (d < 2 mm), small roots (2 mm ≤ d < 5 mm), medium roots (5 mm ≤ d < 10 mm), and coarse roots (d ≥ 10 mm). The diameter and length of roots larger than 2 mm were measured manually, and the surface area and volume of the roots were calculated based on the assumed cylindrical shape of the roots and the measured diameter and length. Finally, the surface area and volume of roots in each size class were calculated based on the number of roots in each class. The length, surface area, and volume of fine roots were obtained using a scanner (Epson Expression 12000XL, Nagano-ken, Japan) in grayscale mode and analyzed using software (Win-RHIZO Pro, LA2004, Vancouver, BC, Canada).
2.4. Root Fractal Feature Parameters Parameters
The fractal characteristic parameters of the root system were determined using the box-counting dimension method. First, based on the average diameter of the primary lateral roots, three lateral root segments with diameters close to the average diameter were counted as samples for each sample tree in the northeast, northwest, and south directions, respectively. These samples were photographed with a camera to obtain images of the same scale. Second, the digital images were imported into ArcGIS 10.5, and an appropriate threshold was selected to convert the root system image into a binary image. Finally, the binary image was divided into squares of side length r, and the number of squares intersecting the root system, nr, was counted. As the side length of the square, r, gradually decreases, the number of squares intersecting the root system, nr, gradually increases. The corresponding nr values at different levels of r were plotted with lgr as the abscissa and lgnr as the ordinate, and the linear equation was fitted as follows:
where nr is the number of square grids intercepted by the root system; r is the side length of the square; the negative slope of the regression line, FD, represents the fractal dimension; and the lgk intercept represents the fractal abundance.
lgnr = −FDlgr + lgk
2.5. The Whole-Root-System Vertical Pull-Out Force
Whole-plant pull-out tests were performed in situ using a universal mechanical testing machine (S9M, Shanghai, China). The pull-out force and root displacement were recorded every second using a data-acquisition instrument. The curve of pull-out force versus displacement was plotted, and the maximum peak value in the curve corresponded to the maximum vertical pull-out force of the whole-root system of the tested sample. A total of 48 individual plants were selected for pull-out tests, of which 26 were successfully tested, including 5 C. korshinskii individuals, 5 H. rhamnoides individuals, 4 P. tabuliformis individuals, 3 R. pseudoacacia individuals, 5 P. tomentosa individuals, and 4 P. armeniaca individuals.
2.6. Data Analysis
We conducted a one-way analysis of variance (ANOVA) to assess variations in root spatial distribution, length, surface area, volume, fractal characteristics, and average maximum uprooting force among different species. Building upon the ANOVA, we employed the Tukey’s HSD post hoc test to further analyze differences between groups. This post hoc test enables us to identify which species exhibit significant differences and provides information about the extent of these differences. We collected root samples from six different species and grouped these samples according to their respective species. To minimize random errors, we conducted randomization within each species group and incorporated appropriate repetitions to ensure the reliability of statistical results. Measurements were taken for parameters including root spatial distribution, length, surface area, volume, fractal characteristics, and average maximum uprooting force. These measurements ensured a comprehensive understanding of root structures. Pearson correlation analysis was employed to assess the relationship between root-structure parameters and maximum uprooting force. This aided us in comprehending the interrelations among these parameters. Additionally, a randomness test was conducted on single-sample-variable values to validate the randomness of the experimental sampling. The median was used as the dividing point value for data dichotomy, further enhancing the reliability and robustness of the data. All statistical analyses were conducted using SPSS 18.0 software, ensuring the scientific rigor and precision of our analytical processes.
3. Results
3.1. Spatial Distribution Characteristics of Root Systems of Different Tree Species
The average horizontal range of the root system of the main slope-protection tree species varied from 1.41 to 2.21 m. Among them, the horizontal range of the root system of P. tomentosa was the largest, significantly higher than that of H. rhamnoides, P. armeniaca, and P. tabuliformis. The average depth of the root system varied from 1.02 to 1.91 m, with the root depth of H. rhamnoides being significantly higher than that of other tree species. From the analysis of root width–depth ratio, the ratio of H. rhamnoides is 1.19, which was the smallest among all tree species, indicating its advantage in the vertical distribution of the root system. C. korshinskii, P. tomentosa, R. pseudoacacia, and P. armeniaca had relatively large root width–depth ratios, indicating their advantage in the horizontal distribution of the root system (Table 2).

Table 2.
Root spatial distribution characteristic parameters of different species of shrubs and trees (n = 3).
The total number of primary roots for different tree species ranged from 16.02 to 35.54 per plant. C. korshinskii had the largest total number of primary roots, significantly higher than that of P. tomentosa, R. pseudoacacia, P. armeniaca, and P. tabuliformis, while H. rhamnoides had the smallest total number of primary roots. Analyzing the relationship between the number of horizontal, oblique, and vertical primary roots, H. rhamnoides had a significantly higher number of oblique primary roots than horizontal and vertical primary roots, indicating that it mainly relied on oblique roots. In contrast, C. korshinskii, P. tomentosa, R. pseudoacacia, P. armeniaca, and P. tabuliformis had significantly more horizontal primary roots than their respective oblique and vertical primary roots, indicating that they mainly relied on horizontal roots (Table 2).
3.2. Geometric Characteristics of Root System
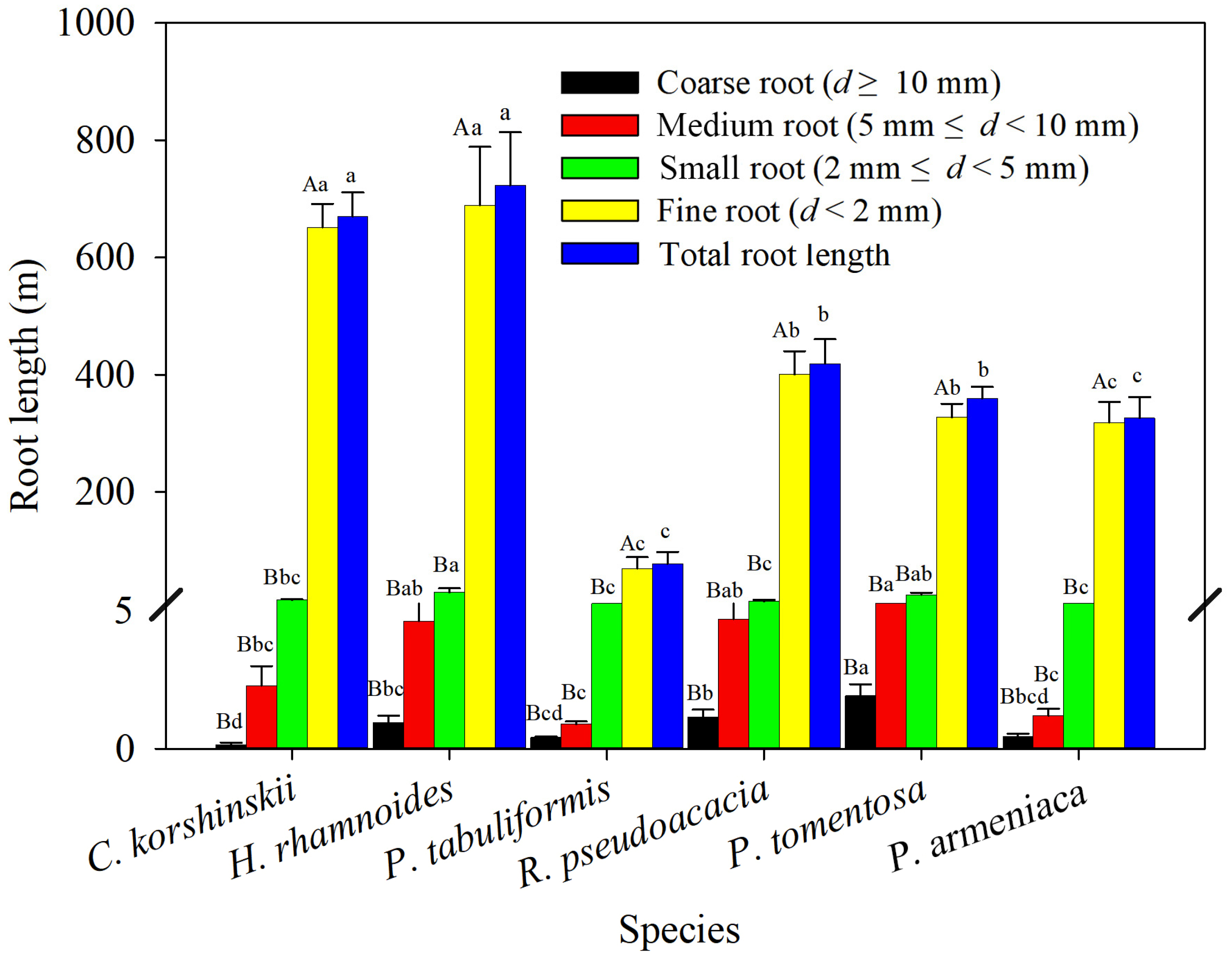
Indicators such as root length, root surface area, and root volume are commonly used to characterize the geometric properties of root systems. These indicators directly affect the size of the root–soil interface, thereby affecting the efficiency of water and nutrient uptake and the ability of roots to hold soil. As shown in Figure 2, the total-root length of H. rhamnoides and C. korshinskii is significantly larger than that of other tree species, while the total-root length of P. tomentosa, R. pseudoacacia, and P. armeniaca is significantly larger than that of P. tabuliformis. Analyzing the root length of coarse and medium roots, C. korshinskii has the longest root length, while P. tabuliformis has the shortest root length. P. tomentosa has the longest root length of coarse and medium roots, significantly higher than those of C. korshinskii, P. armeniaca, and P. tabuliformis. Analyzing the root length of small roots and fine roots, P. tabuliformis has the shortest root length, while H. rhamnoides has the longest root length of small roots and fine roots, significantly higher than those of R. pseudoacacia and P. armeniaca. The root length of the main tree species decreases with increasing root diameter, and the proportion of fine roots in the total-root length is 90.2%–97.7%. Analysis of variance showed that the length of fine roots is significantly greater than that of small, medium, and coarse roots, indicating that fine roots are the main contributors to total-root length.
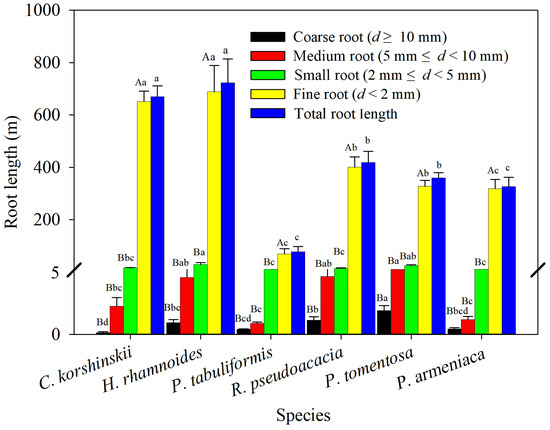
Figure 2.
Root length of various species of shrubs and trees as measured within different root diameter classes. Different capital letters for the same planting indicate significant differences between different diameter classes of the same tree species, and different lowercase letters for the same diameter class indicate significant differences between different tree species of the same diameter class (p < 0.05) (n = 3).
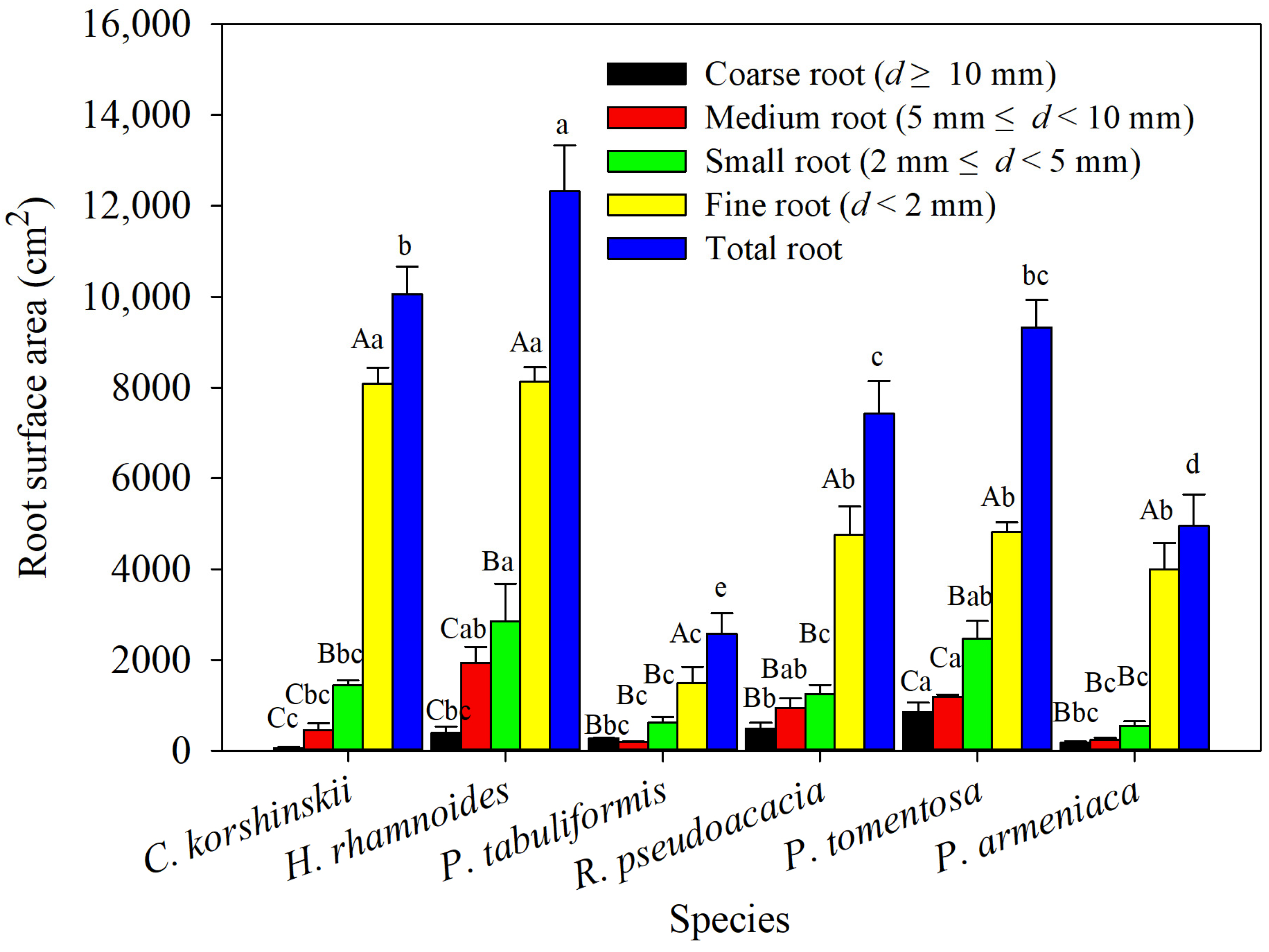
From Figure 3, it can be seen that H. rhamnoides has the largest total-root surface area, which is significantly higher than other tree species, while P. tabuliformis has the smallest total-root surface area, which is significantly lower than other tree species. Analysis of the differences in root surface area among different tree species in the same diameter class shows that P. tomentosa has the largest surface area for both coarse and medium roots, which is significantly higher than those of C. korshinskii, P. armeniaca, and P. tabuliformis, while H. rhamnoides has the largest surface area for small and fine roots, which is significantly higher than those of R. pseudoacacia, P. armeniaca, and P. tabuliformis. Except for the fact that the surface area of coarse roots in P. tabuliformis is larger than that of medium roots, the root surface area of the main tree species decreases with increasing root diameter. The proportion of the surface area of fine roots in the total-root surface area is 51.6%–80.8%, and the analysis of variance showed that the surface area of fine roots is significantly larger than that of small, medium, and coarse roots, indicating that the surface area of fine roots contributes the most to the total-root surface area. However, compared with root length, the proportion of fine-root surface area in the total-root surface area decreases, and the proportion of other diameter classes relatively increases.

Figure 3.
The surface areas of roots of various species of shrubs and trees were analyzed using different root-diameter classes. Different capital letters for the same planting indicate significant differences between different diameter classes of the same tree species, and different lowercase letters for the same diameter class indicate significant differences between different tree species of the same diameter class (p < 0.05) (n = 3).
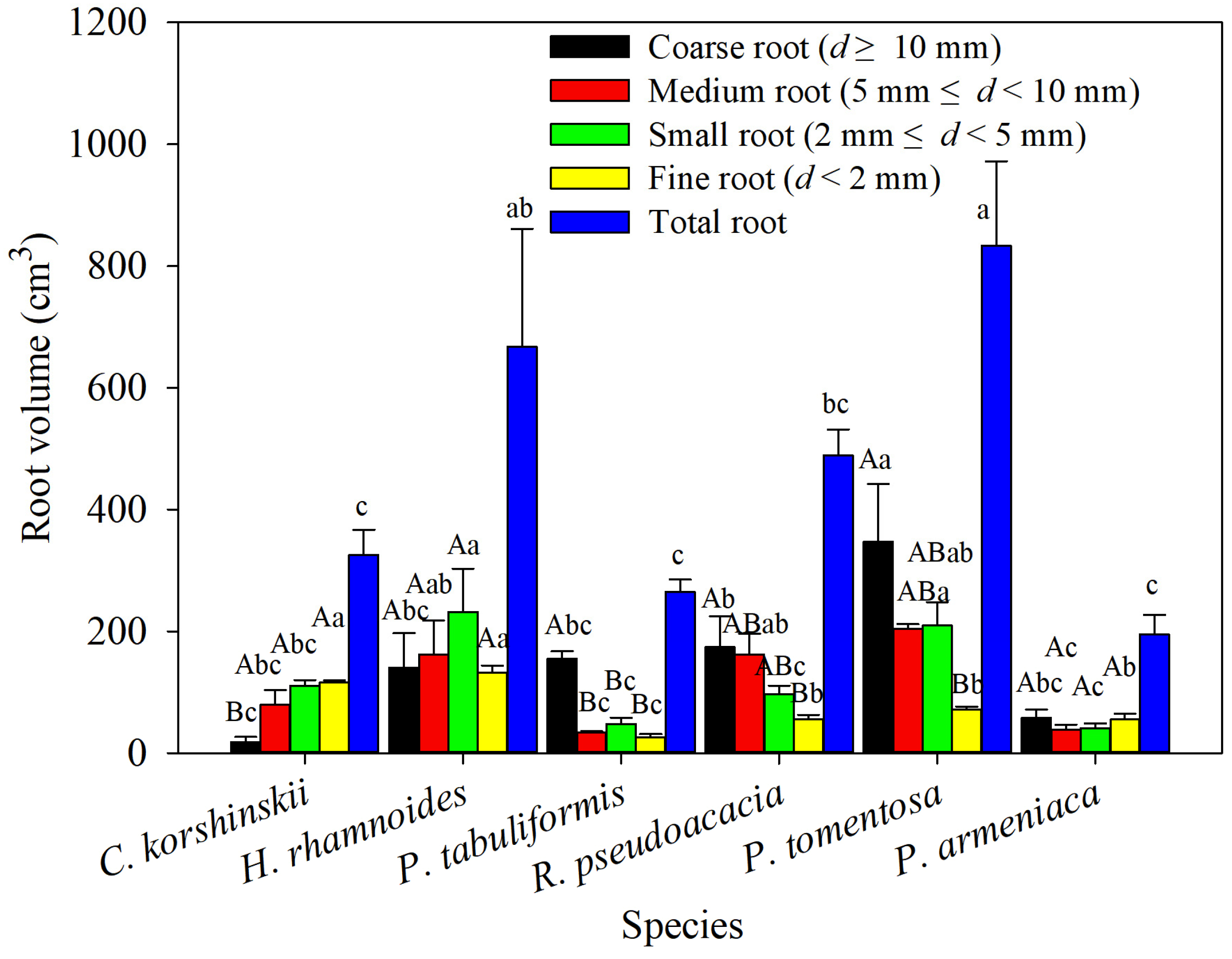
The total-root volume of the main tree species showed significant differences, with the highest total-root volume being found in P. tomentosa, significantly higher than that of C. korshinskii, R. pseudoacacia, P. armeniaca, and P. tabulaeformis. The lowest total-root volume was found in P. armeniaca, which was significantly lower than that of P. tomentosa and H. rhamnoides. Among different tree species in the same diameter class, the coarse and medium root volumes of P. tomentosa were significantly larger than those of C. korshinskii, P. armeniaca, and P. tabulaeformis, while the small- and fine-root volumes of H. rhamnoides were significantly larger than those of R. pseudoacacia, P. armeniaca, and P. tabulaeformis. Within the same tree species in different diameter classes, the coarse-root volume of R. pseudoacacia was significantly lower than other diameter classes, and the root volume increased with decreasing root diameter. The coarse-root volumes of P. tomentosa, R. pseudoacacia, and P. tabulaeformis were significantly higher than their fine-root volumes, and except for the small-root volumes of P. tomentosa and P. tabulaeformis, the root volumes of the three species decreased with decreasing root diameter. There was no significant difference in root volume between different diameter classes of H. rhamnoides and P. armeniaca (Figure 4).
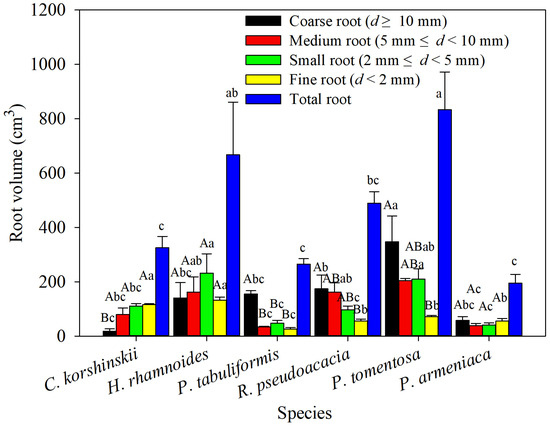
Figure 4.
The root volume of various species of shrubs and trees was analyzed across different root-diameter classes. Different capital letters for the same planting indicate significant differences between different diameter classes of the same tree species, and different lowercase letters for the same diameter class indicate significant differences between different tree species of the same diameter class (p < 0.05) (n = 3).
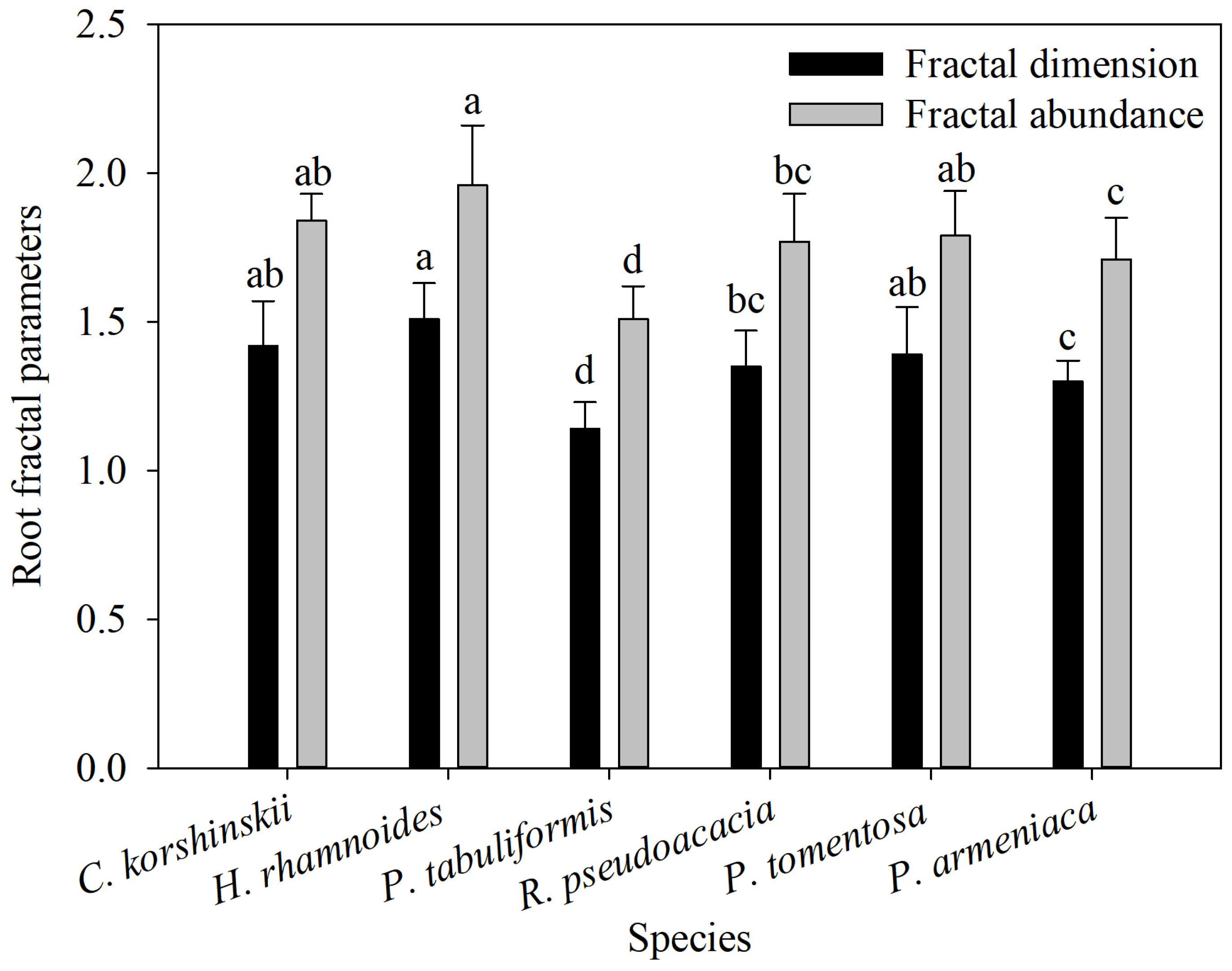
3.3. Root Characteristic Fractal Parameters
The fractal dimension of the root system represents the branching ability of the root system, while the fractal abundance reflects the spatial expansion volume of the root system. The fractal dimensions of the root systems of the main tree species range from 1.14 to 1.51. The fractal dimensions and fractal abundance of the root systems of H. rhamnoides, C. korshinskii, and P. tomentosa are significantly higher than those of P. armeniaca and P. tabulaeformis. The fractal dimensions and fractal abundance of the root system of R. pseudoacacia are significantly lower than those of H. rhamnoides, but significantly higher than those of P. tabulaeformis (Figure 5).

Figure 5.
Root fractal characteristic parameters of different species of shrubs and trees. Different letters meant significant difference among different tree species at 0.05 level (n = 9).
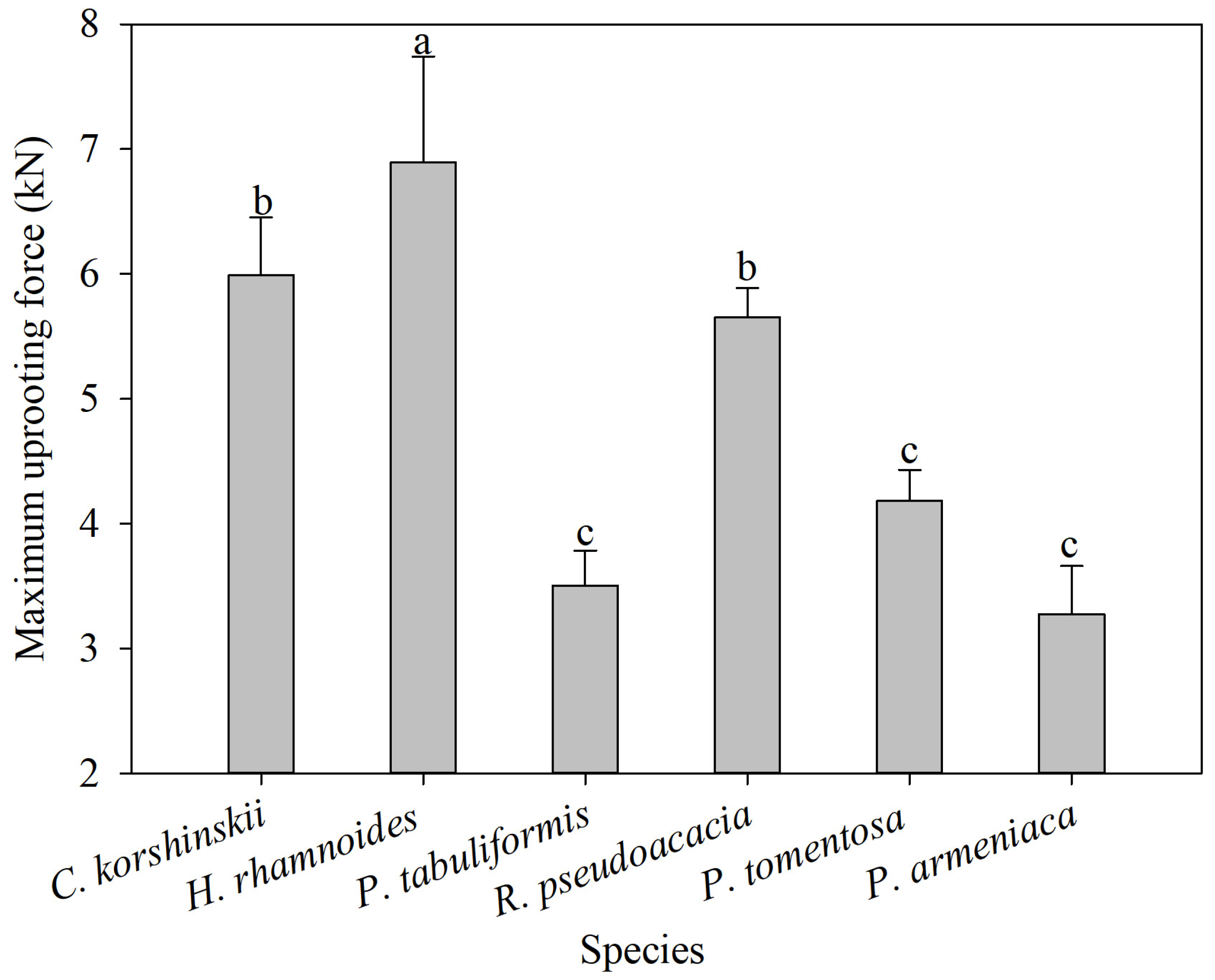
3.4. Maximum Vertical Pull-Out Force of Root System
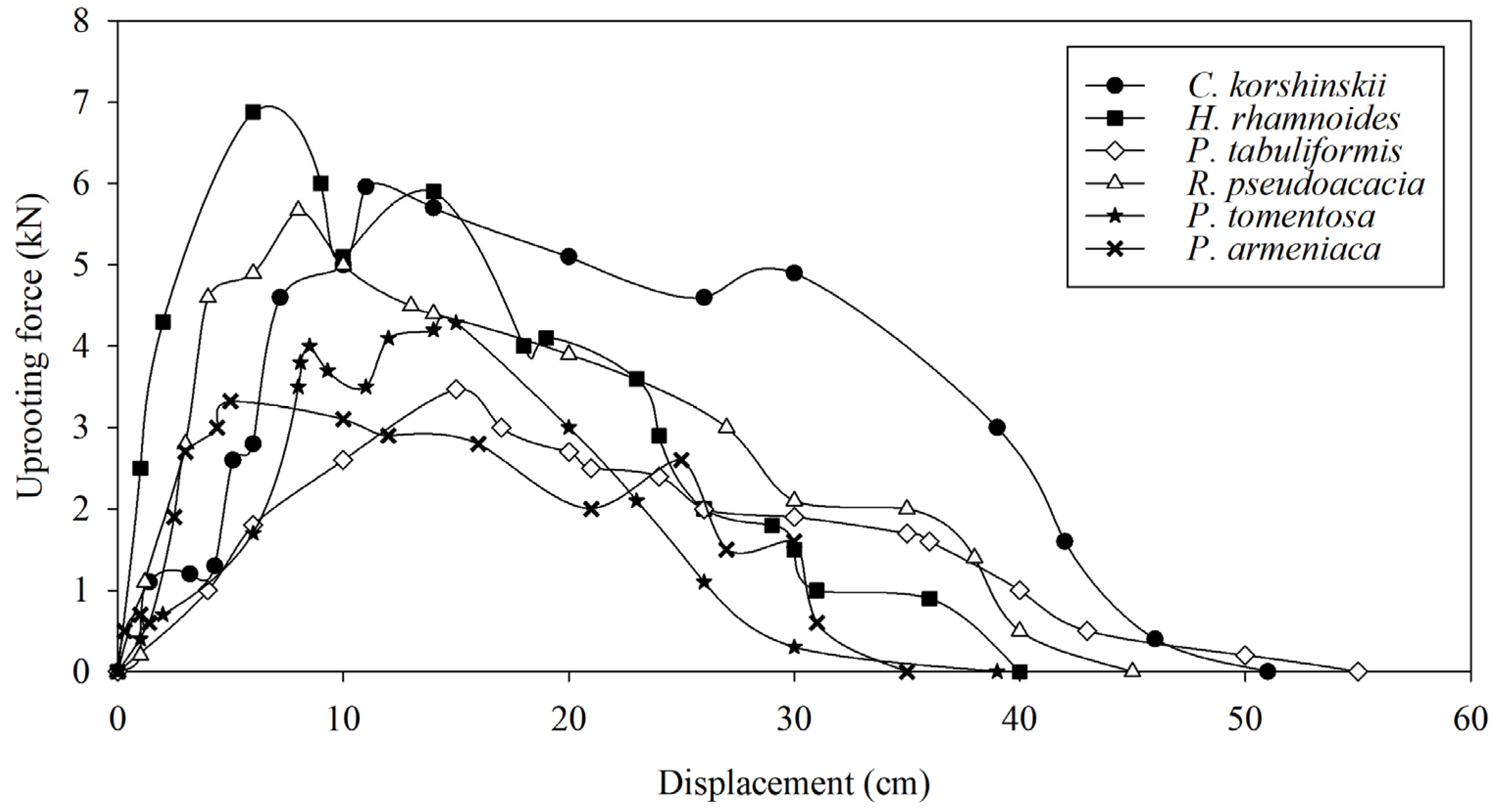
The maximum vertical pull-out force of the entire root system is an important parameter for characterizing the mechanical soil-anchoring properties of plant root systems. The greater the pull-out force, the more favorable the root system is for slope stability. The range of maximum vertical pull-out force is 6.05–7.74 kN for H. rhamnoides, 5.53–6.45 kN for C. korshinskii, 3.93–4.43 kN for P. tomentosa, 5.41–5.89 kN for R. pseudoacacia, 2.89–3.66 kN for P. armeniaca, and 3.22–3.79 kN for P. tabulaeformis. The average maximum vertical pull-out force of H. rhamnoides is significantly higher than those of other tree species, and the average maximum vertical pull-out force of C. korshinskii and R. pseudoacacia is significantly higher than those of P. tomentosa, P. armeniaca, and P. tabulaeformis (Figure 6). From the process of pulling force and root displacement, the root displacement of the maximum pulling force of C. korshinskii, H. rhamnoides, P. tabuliformis, R. pseudoacacia, P. tomentosa, and P. armeniaca appeared at 11 cm, 6 cm, 15 cm, 8 cm, 15 cm, and 5 cm, respectively (Figure 7). The correlation analysis between the plant-root-system-configuration parameters and the average maximum vertical pull-out force of the whole-root system shows that the total-root length, the total-root surface area, and the number of inclined roots are significantly positively correlated with the average maximum vertical pull-out force of the root system. In other words, the total-root length, the total-root surface area, and the number of inclined roots are the main root-system-configuration factors that cause differences in the soil-anchoring ability of the whole-root system among the tested tree species (Table 3).
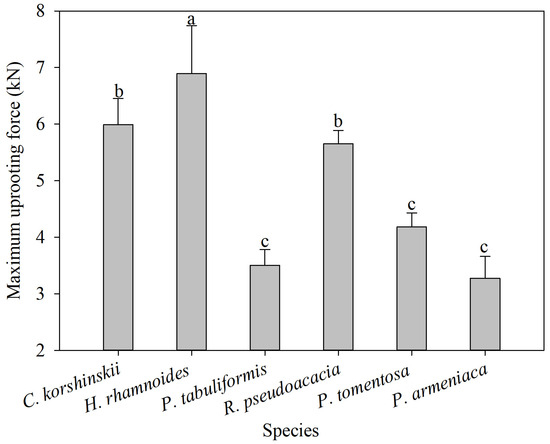
Figure 6.
Average maximum uprooting force of different species of shrubs and trees. Different letters meant significant difference among different tree species at 0.05 level.
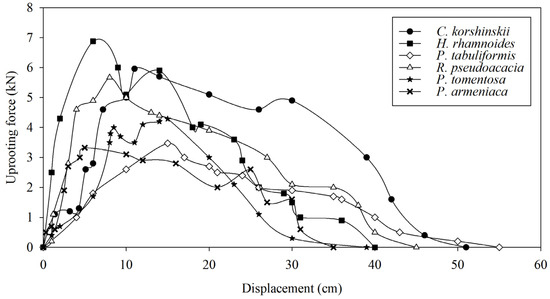
Figure 7.
The curves of pull-out force versus displacement of different species of shrubs and trees.

Table 3.
The relationships between parameters of root architecture and the maximum force required for uprooting for six species of shrubs and trees (n = 27).
4. Discussion
4.1. Differences in Root Architecture among Different Tree Species
The spatial distribution of roots determines the range and ability of different plant root networks to stabilize soil. This study found that the root system of H. rhamnoides consists mainly of oblique roots, which dominate the vertical distribution of the root system and can stabilize deeper soil layers. P. tomentosa, C. korshinskii, R. pseudoacacia, and P. armeniaca have a dominant horizontal root system, which is advantageous for reinforcing shallow soil layers. P. tabuliformis has a main root with deeper vertical distribution and more horizontal lateral roots, which are relatively balanced in horizontal and vertical distribution. The main root plays an anchoring role in deep soil layers, while the horizontal lateral roots have a pulling effect in shallow soil layers. The reason for these differences is that each plant has an independent “growth strategy” that is controlled by genetic factors. Jian et al. [40] indicates a significant positive correlation between the fine-root length of six major afforestation tree species on the Loess Plateau and soil nitrogen content as well as organic matter. Moreover, it shows a significant negative correlation with soil moisture and soil bulk density. However, there is no significant correlation observed between fine-root length and soil particle size composition. In addition, to adapt to different environmental water and nutrient conditions, the distribution of the root system changes with the environment, showing a high degree of plasticity [48,49].
Geometric parameters of the root system, such as root length, root surface area, and root volume, can reflect the morphology and function of roots in the soil, and are of great importance in quantitatively describing root architecture [50]. In this study, except for the total-root surface area of P. tomentosa and total-root length of P. armeniaca, shrub species had significantly larger total-root length and total-root surface area than tree species. This may be because shrubs and trees belong to different plant life forms, and there are differences in their light, heat, water, and other aspects under the same environment, which leads to differences in the geometric characteristics of their root systems. It was also found that roots with diameters less than 2 mm were the main contributors to the total-root length and total-root surface area, and had the highest proportion of root length and root surface area. From the perspective of soil-erosion resistance, fine roots play a reinforcing role in soil retention, while coarse roots play an anchoring role. Therefore, C. korshinskii and H. rhamnoides have abundant fine roots, resulting in larger root length and root surface area, and a stronger reinforcing effect on the soil. While P. tabuliformis and P. armeniaca have fewer fine roots, resulting in a smaller root length and root surface area, and a weaker reinforcing effect on the soil, but P. tabuliformis has a developed taproot, which has a stronger anchoring effect on the root system in the soil. P. tomentosa and R. pseudoacacia fall somewhere in between. Many studies have shown that the fractal dimension of root systems increases as the number of fine roots increases, and the fractal abundance of root systems is positively correlated with the extension range and extension ability of root systems [51,52,53].
4.2. The Impact of Root Configuration on Soil Stability
The vertical traction force of the entire root system of a plant is influenced not only by factors such as soil texture, structure, and root–soil-interface friction, but also by changes in root morphology, number, and other characteristics. In the Loess Plateau region, the average maximum vertical traction forces of the entire root systems of H. rhamnoides, C. korshinskii, and R. pseudoacacia are significantly greater than those of P. tomentosa, P. armeniaca, and P. tabulaeformis. Research has shown that the greater the root length and surface area, the greater the friction between the root system and the soil, and the greater the ability of the root system to stabilize the soil [54]. Therefore, it can be concluded that H. rhamnoides, C. korshinskii, and R. pseudoacacia have a significant advantage in total-root length and surface area, resulting in greater root–soil-interface friction and stronger soil stability. The study also found that, unlike total-root surface area, total-root volume is not significantly correlated with the average maximum vertical pull of the entire root system. This is mainly because for roots of the same diameter, greater root volume corresponds to greater root surface area, which satisfies a significant linear relationship. However, for the entire root system, the total-root surface area and total-root volume are the weighted sum of each root diameter, which may cause the total-root length and surface area to have a non-linear relationship. The results of the correlation analysis in Table 3 also confirm this.
Sun et al. [55] believed that when the main lateral root angle of a plant is between 45° and 60°, the effect of the root system on soil stabilization is better. Tao et al. [56] found that the closer the lateral tilt angle of the plant root system is to 60°, the greater the shear failure force it can withstand and the stronger the soil stability. In this study, the number of tilted roots of H. rhamnoides was significantly greater than those of other tree species, and the number of tilted roots of R. pseudoacacia was significantly greater than those of P. armeniaca and P. tabulaeformis. Therefore, the ability of H. rhamnoides and R. pseudoacacia to resist shear failure and stabilize the soil is greater. The differences in total-root length, total-root surface area, and the number of tilted roots between P. tomentosa and R. pseudoacacia are not significant, but the root depth, vertical root quantity, and total number of primary roots of R. pseudoacacia are significantly greater than those of P. tomentosa, which may be the main reason why the maximum vertical traction force of the entire root system of R. pseudoacacia is stronger than that of P. tomentosa.
The mechanical characteristics of the root–soil composite are influenced not only by plant factors but also by soil factors. Osman and Barakbah [57] conducted experiments on five slopes with varying degrees of destabilization along the North–South Expressway in Malaysia. Their findings suggest that soil-moisture content and root-length density, as indicative parameters of slope stability, can predict whether slope failure is likely to occur. Wang et al. [58] conducted direct shear tests on the subsoil of slopes planted with two different herbaceous plants. Their results indicate that both herbaceous plants enhance the shear strength of the slope’s subsoil. However, as soil-moisture content increases, the soil’s shear strength decreases, showing a negative correlation. This study exclusively focused on investigating the impact of vegetation root systems on soil-stabilization capacity. We aimed to identify vegetation that demonstrates excellent soil-stabilization capabilities within a specific research area. Understanding how soil-stabilization capacity varies with climatic zone, soil type, topography, and other factors will be a key focus of our future research.
The Loess Plateau region experiences low precipitation, high evaporation, and a dry climate, resulting in a low soil-moisture content, which poses great challenges to afforestation efforts. Selecting tree species for afforestation that can prevent soil erosion while also providing ecological benefits is a difficult problem we currently face. This study has found that shrub species, such as H. rhamnoides and L. japonica, have better soil-stabilization abilities than tree species. From a water-balance perspective, previous research in this study area has shown that shrubs generate less canopy interception [40] and evapotranspiration compared to trees. Moreover, the decline in soil moisture in shrublands is significantly lower than in forests dominated by trees [40]. In terms of soil improvement and increased water infiltration, a survey of 17 soil profiles conducted in the Ziwuling of the Loess Plateau by Zhao et al. [59] revealed that the organic matter content in the topsoil of shrublands ranged from 2.558% to 7.997%, which is 1.4–3.7 times and 1.9–6.0 times that of grasslands and farmlands, respectively. Regarding its impact on runoff and sediment, Zuo et al. [60] found that the surface runoff rate in shrublands was only 6.16%–11.41% of that in bare land, and sediment reduction reached 81% compared to bare land. These findings demonstrate the significant influence of shrubs on surface runoff. In summary, shrubs exhibit better soil-stabilization effects and provide good soil- and water-conservation benefits. Choosing shrubs for afforestation and slope protection may be more effective for soil- and water-conservation efforts in the Loess Plateau.
4.3. Limitations
In this study, the soil-reinforcement capacity of different vegetation was analyzed only from the aspect of the influence of root-structure characteristics on the pull-out force of vegetation. As the intersection of different disciplines such as ecology, soil science, botany, hydrology, soil mechanics, and material mechanics, the mechanism of soil fixation of vegetation roots needs to be further studied. The distribution morphology of the root system is considerably related to the range of soil consolidation, and the shear and tensile strength of the taproot and lateral roots are different, and the rigidity and flexibility are also different. In the process of the research, the soil-fixing capacity of vegetation should be comprehensively analyzed in combination with the shear strength of different vegetation roots. At the same time, the understanding of the mechanism of the root–soil interface is insufficient, and the numerical simulation of root soil consolidation is still relatively weak in model selection, and the model is relatively simple, so strengthening the research on the root–soil-consolidation foundation is a premise for numerical simulation development. In addition, slope greening and mountain ecological restoration mostly adopt a composite plant system combining grass, shrubs, and trees, and different plant root systems may have complementary effects on soil fixation. On the basis of the research on the soil consolidation of single plant roots, it will be a future development trend to carry out research on the soil-consolidation mechanism of grass–shrub–tree composite roots.
5. Conclusions
H. rhamnoides has a larger number of oblique roots, giving it an advantage in the vertical spatial distribution of its root system. C. korshinskii, P. tomentosa, R. pseudoacacia, and P. armeniaca have higher proportions of horizontal roots, resulting in a significant advantage in the horizontal spatial distribution of their root systems. P. tabuliformis, on the other hand, achieves a more balanced distribution of its root system in both horizontal and vertical spaces through its well-developed taproot and numerous lateral roots. H. rhamnoides and C. korshinskii have a greater number of fine roots, with a complex branching pattern, higher total-root length, total-root surface area, and root fractal dimension. P. armeniaca and P. tabuliformis have relatively fewer fine roots, simpler branching patterns, and the lowest values for total-root length, total-root surface area, and root fractal dimension. P. tabuliformis and P. tomentosa fall between these two categories. H. rhamnoides, C. korshinskii, and R. pseudoacacia exhibit significantly higher average maximum vertical pull forces for their entire root systems compared to P. tomentosa, P. armeniaca, and P. tabuliformis. This indicates that H. rhamnoides, C. korshinskii, and R. pseudoacacia possess strong soil-stabilization capabilities, with H. rhamnoides exhibiting the strongest ability. Therefore, it is preferable to utilize shrubs in soil and water conservation as well as slope-protection efforts in the Loess Plateau region. Further research on a wider range of vegetation species is needed to enrich knowledge for the selection of slope-protecting vegetation.
Author Contributions
Conceptualization, S.J.; methodology, X.Z.; software, Y.F.; validation, X.Z.; formal analysis, Y.F.; investigation, Q.P.; resources, S.J.; data curation, Y.F.; writing—original draft preparation, Q.P.; writing—review and editing, Y.F.; visualization, Q.P.; supervision, S.J.; project administration, S.J.; funding acquisition, J.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Key Research Priorities Program of China (2022YFC300340502) (Guo Jinjun); Qian Kehe Zhicheng [2023] Yiban 206 (Jian Shengqi).
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Xing, S.K.; Zhang, G.H.; Zhu, P.Z.; Wang, L.L.; Wang, Z.G.; Wang, C.S. Variation in shear strength of soil-root system under five typical land use types on the Loess Plateau of China. Catena 2023, 222, 13. [Google Scholar] [CrossRef]
- Zhao, F.B.; Ma, S.; Wu, Y.P.; Qiu, L.J.; Wang, W.K.; Lian, Y.Q.; Chen, J. The role of climate change and vegetation greening on evapotranspiration variation in the Yellow River Basin, China. Agric. For. Meteorol. 2022, 316, 14. [Google Scholar] [CrossRef]
- Xing, S.K.; Zhang, G.H.; Zhu, P.Z.; Wang, L.L.; Wang, Z.G.; Wang, C.S. Shear strength of soil-root system under different vegetation restoration modes on the Loess Plateau of China. Soil. Tillage Res. 2023, 228, 12. [Google Scholar] [CrossRef]
- An, S.-S.; Darboux, F.; Cheng, M. Revegetation as an efficient means of increasing soil aggregate stability on the Loess Plateau (China). Geoderma 2013, 209–210, 75–85. [Google Scholar] [CrossRef]
- Alam, S.; Manzur, T.; Borquist, E.; Williams, J.; Rogers, C.; Hall, D.; Patterson, W.B. In-situ assessment of soil-root bonding strength to aid in preventing soil erosion. Soil. Tillage Res. 2021, 213, 8. [Google Scholar] [CrossRef]
- Zhang, S.L.; Zhao, H.B.; Wang, X.Z.; Dong, J.X.; Zhao, P.F.; Yang, F.F.; Chen, X.H. Discrete element modeling and shear properties of the maize stubble-soil complex. Comput. Electron. Agric. 2023, 204, 14. [Google Scholar] [CrossRef]
- Amiri Khaboushan, E.; Emami, H.; Mosaddeghi, M.R.; Astaraei, A.R. Estimation of unsaturated shear strength parameters using easily-available soil properties. Soil Tillage Res. 2018, 184, 118–127. [Google Scholar] [CrossRef]
- Amiri, E.; Emami, H.; Mosaddeghi, M.R.; Astaraei, A.R. Shear strength of an unsaturated loam soil as affected by vetiver and polyacrylamide. Soil Tillage Res. 2019, 194, 104331. [Google Scholar] [CrossRef]
- Meng, S.Y.; Zhao, G.Q.; Yang, Y.Y. Impact of Plant Root Morphology on Rooted-Soil Shear Resistance Using Triaxial Testing. Adv. Civ. Eng. 2020, 2020, 8825828. [Google Scholar] [CrossRef]
- Giadrossich, F.; Schwarz, M.; Cohen, D.; Cislaghi, A.; Vergani, C.; Hubble, T.; Phillips, C. Methods to measure the mechanical behaviour of tree roots: A review. Ecol. Eng. 2017, 109, 256–271. [Google Scholar] [CrossRef]
- Gonzalez-Ollauri, A.; Mickovski, S.B. Plant-soil reinforcement response under different soil hydrological regimes. Geoderma 2017, 285, 141–150. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, X.M.; Tian, J.Y. Effectiveness of plant roots to increase the anti-scourability of soil on the loess plateau. Chin. Sci. Bull. 1991, 36, 2077–2082. [Google Scholar]
- Li, Z.W.; Liu, C.; Dong, Y.T.; Chang, X.F.; Nie, X.D.; Liu, L.; Xiao, H.B. Response of soil organic carbon and nitrogen stocks to soil erosion and land use types in the Loess hilly-gully region of China. Soil. Tillage Res. 2017, 166, 1–9. [Google Scholar] [CrossRef]
- Pallewattha, M.; Indraratna, B.; Heitor, A.; Rujikiatkamjorn, C. Shear strength of a vegetated soil incorporating both root reinforcement and suction. Transp. Geotech. 2019, 18, 72–82. [Google Scholar] [CrossRef]
- Yildiz, A.; Graf, F.; Rickli, C.; Springma, S.M. Determination of the shearing behaviour of root-permeated soils with a large-scale direct shear apparatus. Catena 2018, 166, 98–113. [Google Scholar] [CrossRef]
- Xu, Y.Z.; Guo, Y.Y.; Huang, Z.; Liu, D.Z.; Huang, Q.E.; Tang, H. Study on Cynodon dactylon root system affecting dry-wet cracking behavior and shear strength characteristics of expansive soil. Sci. Rep. 2023, 13, 17. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.B.; Wu, Y.P.; Yin, X.W.; Alexandrov, G.; Qiu, L.J. Toward Sustainable Revegetation in the Loess Plateau Using Coupled Water and Carbon Management. Engineering 2022, 15, 143–153. [Google Scholar] [CrossRef]
- Zhu, P.Z.; Zhang, G.H.; Zhang, B.J.; Wang, H.X. Variation in soil surface roughness under different land uses in a small watershed on the Loess Plateau, China. Catena 2020, 188, 10. [Google Scholar] [CrossRef]
- Sui, Z.F.; Yi, W.; Lu, Y.A.; Deng, L. Experimental and Numerical Simulation Study on the Shear Strength Characteristics of Magnolia multiflora Root-Soil Composites. Adv. Civ. Eng. 2021, 2021, 6642594. [Google Scholar] [CrossRef]
- Zhu, P.Z.; Zhang, G.H.; Wang, H.X.; Zhang, B.J.; Liu, Y.N. Soil moisture variations in response to precipitation properties and plant communities on steep gully slope on the Loess Plateau. Agric. Water Manag. 2021, 256, 14. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, G.H.; Yang, Y.F.; Li, P.P.; Liu, J.X. Response of soil detachment capacity to plant root and soil properties in typical grasslands on the Loess Plateau. Agric. Ecosyst. Environ. 2018, 266, 68–75. [Google Scholar] [CrossRef]
- Zhu, W.; Zhao, D.H.; Di, N.; Li, D.D.; Zhou, O.; Sun, Y.M.; Jia, L.M.; Xi, B.Y. Matching root water uptake patterns to fine root and soil water distributions. Plant Soil 2024, 495, 499–516. [Google Scholar] [CrossRef]
- Burylo, M.; Dutoit, T.; Rey, F. Species Traits as Practical Tools for Ecological Restoration of Marly Eroded Lands. Restor. Ecol. 2014, 22, 633–640. [Google Scholar] [CrossRef]
- Burylo, M.; Rey, F.; Mathys, N.; Dutoit, T. Plant root traits affecting the resistance of soils to concentrated flow erosion. Earth Surf. Process Landf. 2012, 37, 1463–1470. [Google Scholar] [CrossRef]
- De Baets, S.; Poesen, J. Empirical models for predicting the erosion-reducing effects of plant roots during concentrated flow erosion. Geomorphology 2010, 118, 425–432. [Google Scholar] [CrossRef]
- Faucon, M.P.; Houben, D.; Lambers, H. Plant Functional Traits: Soil and Ecosystem Services. Trends Plant Sci. 2017, 22, 385–394. [Google Scholar] [CrossRef]
- Ma, Z.Q.; Guo, D.L.; Xu, X.L.; Lu, M.Z.; Bardgett, R.D.; Eissenstat, D.M.; McCormack, M.L. Evolutionary history resolves global organization of root functional traits. Nature 2018, 555, 94. [Google Scholar] [CrossRef]
- Du, x.; Jian, J.S.; Du, C.; Dstewart, R. Conservation management decreases surface runoff and soil erosion. Int. Soil. Water Conserv. Res. 2022, 10, 2095–6339. [Google Scholar] [CrossRef]
- Ji, X.D.; Chen, L.H.; Zhang, A. Anchorage properties at the interface between soil and roots with branches. J. For. Res. 2017, 28, 83–93. [Google Scholar] [CrossRef]
- Reubens, B.; Poesen, J.; Danjon, F.; Geudens, G.; Muys, B. The role of fine and coarse roots in shallow slope stability and soil erosion control with a focus on root system architecture: A review. Trees-Struct. Funct. 2007, 21, 385–402. [Google Scholar] [CrossRef]
- Danjon, F.; Reubens, B. Assessing and analyzing 3D architecture of woody root systems, a review of methods and applications in tree and soil stability, resource acquisition and allocation. Plant Soil. 2008, 303, 1–34. [Google Scholar] [CrossRef]
- Zhou, Z.C.; Shangguan, Z.P. Soil anti-scouribility enhanced by plant roots. J. Integr. Plant Biol. 2005, 47, 676–682. [Google Scholar] [CrossRef]
- Helsen, K.; Vannoppen, W.; Honnay, O.; Poesen, J. Both below-ground and above-ground functional traits can help predict levee grassland root length density as a proxy for flow erosion resistance. J. Veg. Sci. 2016, 27, 1254–1263. [Google Scholar] [CrossRef]
- Kervroëdan, L.; Armand, R.; Saunier, M.; Ouvry, J.F.; Faucon, M.P. Plant functional trait effects on runoff to design herbaceous hedges for soil erosion control. Ecol. Eng. 2018, 118, 143–151. [Google Scholar] [CrossRef]
- García-Fayos, P.; Bochet, E.; Cerdá, A. Seed removal susceptibility through soil erosion shapes vegetation composition. Plant Soil. 2010, 334, 289–297. [Google Scholar] [CrossRef]
- Gyssels, G.; Poesen, J.; Bochet, E.; Li, Y. Impact of plant roots on the resistance of soils to erosion by water: A review. Prog. Phys. Geogr. 2005, 29, 189–217. [Google Scholar] [CrossRef]
- Jiao, J.Y.; Zhang, Z.G.; Bai, W.J.; Jia, Y.F.; Wang, N. Assessing the Ecological Success of Restoration by Afforestation on the Chinese Loess Plateau. Restor. Ecol. 2012, 20, 240–249. [Google Scholar] [CrossRef]
- Zhang, D.; Cheng, J.H.; Liu, Y.; Zhang, H.J.; Ma, L.; Mei, X.M.; Sun, Y.H. Spatio-Temporal Dynamic Architecture of Living Brush Mattress: Root System and Soil Shear Strength in Riverbanks. Forests 2018, 9, 493. [Google Scholar] [CrossRef]
- Katuwal, S.; Vermang, J.; Cornelis, W.M.; Gabriels, D.; Moldrup, P.; de Jonge, L.W. Effect of Root Density on Erosion and Erodibility of a Loamy Soil Under Simulated Rain. Soil. Sci. 2013, 178, 29–36. [Google Scholar] [CrossRef]
- Jian, S.Q.; Zhao, C.Y.; Fang, S.M.; Yu, K. Effects of different vegetation restoration on soil water storage and water balance in the Chinese Loess Plateau. Agric. For. Meteorol. 2015, 206, 85–96. [Google Scholar] [CrossRef]
- Yan, H.; Liu, X.L.; Ding, H.; Dai, Z.G.; Niu, X.L.; Zhao, L. Hormonal Balance, Photosynthesis, and Redox Reactions in the Leaves of Caragana korshinskii Kom. under Water Deficit. Plants 2023, 12, 2076. [Google Scholar] [CrossRef] [PubMed]
- He, X.H.; Si, J.H.; Zhu, L.; Zhou, D.M.; Zhao, C.Y.; Jia, B.; Wang, C.L.; Qin, J.; Zhu, X.L. Modeling habitat suitability of Hippophae rhamnoides L. using MaxEnt under climate change in China: A case study of H. r. sinensis and H. r. turkestanica. Front. For. Glob. Change 2023, 5, 1095784. [Google Scholar] [CrossRef]
- Chen, H.; Xiang, Y.; Yao, Z.; Zhang, Q.; Li, H.; Cheng, M. Stability of C:N:P Stoichiometry in the Plant-Soil Continuum along Age Classes in Natural Pinus tabuliformis Carr. Forests of the Eastern Loess Plateau, China. Forests 2023, 14, 44. [Google Scholar] [CrossRef]
- Danjon, F.; Khuder, H.; Stokes, A. Deep phenotyping of coarse root architecture in R. pseudoacacia reveals that tree root system plasticity is confined within its architectural model. PLoS ONE 2013, 8, e83548. [Google Scholar] [CrossRef]
- Tan, J.; Yu, W.; Liu, Y.; Guo, Y.; Liu, N.; Fu, H.; Di, N.; Duan, J.; Li, X.; Xi, B. Response of fine-root traits of Populus tomentosa to drought in shallow and deep soil. Forests 2023, 14, 951. [Google Scholar] [CrossRef]
- Li, Y.G.; Dong, X.X.; Yao, W.X. C, N, P, K stoichiometric characteristics of the “leaf-root-litter-soil’’ system in dryland plantations. Ecol. Indic. 2022, 143, 109371. [Google Scholar] [CrossRef]
- Zuo, X.A.; Zhao, X.Y.; Zhao, H.L.; Zhang, T.H.; Guo, Y.R.; Li, Y.Q.; Huang, Y.X. Spatial heterogeneity of soil properties and vegetation-soil relationships following vegetation restoration of mobile dunes in Horqin Sandy Land, Northern China. Plant Soil 2009, 318, 153–167. [Google Scholar] [CrossRef]
- Pollen, N.; Simon, A. Estimating the mechanical effects of riparian vegetation on stream bank stability using a fiber bundle model. Water Resour. Res. 2005, 41, 11. [Google Scholar] [CrossRef]
- Prieto, I.; Roumet, C.; Cardinael, R.; Dupraz, C.; Jourdan, C.; Kim, J.H.; Maeght, J.L. Root functional parameters along a land-use gradient: Evidence of a community-level economics spectrum. J. Ecol. 2015, 103, 361–373. [Google Scholar] [CrossRef]
- Chen, A.Q.; Zhang, D.; Peng, H.; Fan, J.R.; Xiong, D.H.; Liu, G.C. Experimental study on the development of collapse of overhanging layers of gully in Yuanmou Valley, China. Catena 2013, 109, 177–185. [Google Scholar] [CrossRef]
- Guo, M.M.; Wang, W.L.; Kang, H.L.; Yang, B. Changes in soil properties and erodibility of gully heads induced by vegetation restoration on the Loess Plateau, China. J. Arid. Land. 2018, 10, 712–725. [Google Scholar] [CrossRef]
- Guo, M.M.; Wang, W.L.; Shi, Q.H.; Chen, T.D.; Kang, H.L.; Li, J.M. An experimental study on the effects of grass root density on gully headcut erosion in the gully region of China’s Loess Plateau. Land. Degrad. Dev. 2019, 30, 2107–2125. [Google Scholar] [CrossRef]
- Ran, Q.H.; Su, D.Y.; Li, P.; He, Z.G. Experimental study of the impact of rainfall characteristics on runoff generation and soil erosion. J. Hydrol. 2012, 424, 99–111. [Google Scholar] [CrossRef]
- Rieke-Zapp, D.H.; Nichols, M.H. Headcut retreat in a semiarid watershed in the southwestern United States since 1935. Catena 2011, 87, 1–10. [Google Scholar] [CrossRef]
- Su, Z.A.; Xiong, D.H.; Dong, Y.F.; Li, J.J.; Yang, D.; Zhang, J.H.; He, G.X. Simulated headward erosion of bank gullies in the Dry-hot Valley Region of southwest China. Geomorphology 2014, 204, 532–541. [Google Scholar] [CrossRef]
- Tao, W.H.; Wu, J.H.; Wang, Q.J. Mathematical model of sediment and solute transport along slope land in different rainfall pattern conditions. Sci. Rep. 2017, 7, 11. [Google Scholar] [CrossRef]
- Osman, N.; Barakbah, S.S. Parameters to predict slope stability—Soil water and root profiles. Ecol. Eng. 2006, 28, 90–95. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, Z.M.; He, L. Experimental research on soil reinforcement of different plant tree types. Railw. Eng. 2009, 11, 61–63. [Google Scholar]
- Hao, S.W.; Su, J.; Yang, Y.H. A fractal method of estimating soil structure changes under different vegetations on Ziwuling Mountains of the Loess Plateau, China. Agric. Sci. China 2006, 5, 530–538. [Google Scholar]
- Zuo, D.P.; Xu, Z.X.; Yao, W.Y.; Jin, S.Y.; Xiao, P.Q.; Ran, D.C. Assessing the effects of changes in land use and climate on runoff and sediment yields from a watershed in the Loess Plateau of China. Sci. Total Environ. 2016, 544, 238–250. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).