Site Quality for Araucaria angustifolia Plantations with Subtropical Cambisol Is Driven by Soil Organism Assemblage and the Litter and Soil Compartments
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design Overview
2.2. Litter Material and Greenhouse Conditions to Prepare Standard Litter Material
2.3. Field Conditions, Litter Collection, and Soil Sampling
2.4. Soil Organism Assemblage
2.5. Litter Decomposition Assay
2.6. Statistical Analyses
3. Results
3.1. Litter Deposition and Litter Nutrient Content from the Studied Site Quality Treatments
3.2. Influence of Araucaria’s Site Quality on Soil Chemical Properties
3.3. Soil Organism Collection in A. angustifolia Plantations
3.4. Multivariate Analysis
3.5. k-Factor Low and High C:N Ratio Residues in A. angustifolia Plantations
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Banerjee, S.; van der Heijden, M.G.A. Soil microbiomes and one health. Nat. Rev. Microbiol. 2023, 21, 6–20. [Google Scholar] [CrossRef] [PubMed]
- Dobner, M., Jr.; Paixão, C.A.; Costa, E.A.; Finger, C.A.G. Effect of site and competition on diameter growth of Araucaria angustifolia. Floresta 2019, 49, 717–724. [Google Scholar] [CrossRef]
- Jones, A.; Smith, B.; Johnson, C. The role of decomposers in ecosystem nutrient cycling. Ecol. Lett. 2023, 45, 210–225. [Google Scholar]
- Deng, Y.; Bai, Y.; Cao, R.; Jiang, Y.; Wang, Z.; Li, F.; Gong, H.; Yang, W. Key drivers of soil arthropod community shift across a subalpine forest series vary greatly with litter and topsoil layers. Eur. J. Soil Biol. 2022, 111, 103421. [Google Scholar] [CrossRef]
- Yin, R.; Liu, Q.; Tian, S.; Potapov, A.; Zhu, B.; Yang, K.; Li, Z.; Zhuang, L.; Tan, B.; Zhang, L.; et al. Nitrogen deposition stimulates decomposition via changes in the structure and function of litter food webs. Soil Biol. Biochem. 2022, 166, 108522. [Google Scholar] [CrossRef]
- Araujo, P.I.; Grasso, A.A.; González-Arzac, A.; Méndez, M.S.; Austin, A.T. Sunlight and soil biota accelerate decomposition of crop residues in the Argentine Pampas. Agric. Ecosyst. Environ. 2022, 330, 107908. [Google Scholar] [CrossRef]
- Dudas, M.; Pjevac, P.; Kotianová, M.; Gančarčíková, K.; Rozmoš, M.; Hršelová, H.; Bukovská, P.; Jansa, J. Arbuscular mycorrhiza and nitrification: Disentangling processes and players by using synthetic nitrification inhibitors. Appl. Environ. Microbiol. 2022, 88, 20. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.; Johnson, E.; Williams, D.; Brown, S.; Martinez, M. Advances in soil quality research: Implications for litter dynamics, nutrient cycling, and soil organism abundance. Soil Sci. Soc. Am. J. 2023, 87, 310–325. [Google Scholar]
- Rasmussen, P.U.; Bennet, A.E.; Tack, A.J.M. The impact of elevated temperature and drought on the ecology and evolution of plant-soil microbe interactions. J. Ecol. 2019, 108, 337–352. [Google Scholar] [CrossRef]
- Barbieri, S.F.; Lima, L.T.F.; Baum, J.C.S.; Mischiatti, K.L.; Godoy, R.C.B.; Silveir, J.L.M. Polysaccharides from pinhão seeds of Araucaria angustifolia: Extraction, isolation and structural characterization. J. Food Compost. Anal. 2023, 115, 104888. [Google Scholar] [CrossRef]
- de Mattos, P.P.; Curto, R.A.; Braz, E.M.; Netto, S.P. How do Araucaria angustifolia trees grow in overstocked stands? Dendrochronologia 2022, 74, 125976. [Google Scholar] [CrossRef]
- Garcia, L.; Silva, M.; Martinez, J.; Lopez, A.; Hernandez, C. Ecological considerations for the establishment of plantations of the endangered tree species A. angustifolia. Environ. Conserv. 2023, 45, 567–582. [Google Scholar]
- Souza, T.A.F.; da Silva, L.J.R.; Nascimento, G.S. Amazonian deforestation and its influence on soil biotic factors and abiotic properties. Pedobiologia 2023, 97–98, 150865. [Google Scholar] [CrossRef]
- da Silva, L.J.R.; Souza, T.A.F.; Laurindo, L.K.; Freitas, H.; Campos, M.C.C. Decomposition rate of organic residues and soil organisms’ abundance in a Subtropical Pyrus pyrifolia field. Agronomy 2022, 12, 263. [Google Scholar] [CrossRef]
- Laurindo, L.K.; Souza, T.A.F.; da Silva, L.J.R.; Nascimento, G.S.; Cruz, S.P. Pinus taeda L. changes arbuscular mycorrhizal fungi communities in a Brazilian subtropical ecosystem. Symbiosis 2022, 87, 269–279. [Google Scholar] [CrossRef]
- Skovsgaard, J.P.; Vanclay, J.K. Forest site productivity: A review of the evolution of dendrometric concepts for even-aged stands. Forestry 2013, 81, 13–31. [Google Scholar] [CrossRef]
- WRB—IUSS Working Group. World Reference Base for Soil; World Soil Resources Reports; WRB: Rome, Italy, 2015. [Google Scholar]
- Alvares, C.A.; Stape, J.L.; Sentelhas, P.C.; Moraes, G.; Leonardo, J.; Sparovek, G. Köppen’s climate classification map for Brazil. Meteorol. Zeitschrift 2013, 22, 711–728. [Google Scholar] [CrossRef] [PubMed]
- Tedesco, M.J.; Gianello, C.; Bissani, C.A.; Bohnen, H.; Volkweiss, S.J. Análise do Solo, Planta e Outros Materiais, 2nd ed.; UFRGS: Porto Alegre, Brazil, 1995; 174p. [Google Scholar]
- Morais, J.P.S.; Rosa, M.F.; Marconcini, J.M. Procedimentos Para Análise Lignocelulósica; Embrapa Algodão: Campina Grande, Brazil, 2010. [Google Scholar]
- Black, C.A. (Ed.) Methods of Soil Analysis, Part 2. In Agronomy Monograph; American Society of Agronomy: Madison, WI, USA, 1965; Volume 9, pp. 771–1572. [Google Scholar]
- Okalebo, J.R.; Gathua, K.W.; Woomer, P.L. Laboratory Methods of Plant and Soil Analysis: A Working Manual; Technical Bulletin n. 1. Nairobi; Tropical Soil Biology and Fertility Programme. Soil Science Society East Africa; UNESCO: Naiorobi, Kenya, 1993. [Google Scholar]
- Rodriguez, A.; Martinez, S.; Garcia, J.; Perez, M.; Lopez, A. Functional group classification of soil organisms: Integrating taxonomic units into ecological frameworks. Soil Biol. Biochem. 2023, 105, 210–225. [Google Scholar]
- Gomez, R.; Martinez, S.; Rodriguez, A.; Garcia, J.; Lopez, A. Assessing model fit in structural equation modeling: Guidelines and recommendations. J. Appl. Stat. 2023, 50, 325–340. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018; Available online: https://www.R-project.org/ (accessed on 16 February 2023).
- Jones, R.S.; Smith, P.L. Tree species diversity enhances ecosystem multifunctionality in temperate forests. J. Appl. Ecol. 2020, 57, 319–328. [Google Scholar]
- Brown, A.B.; Green, C.D. Tree species composition influences litter decomposition rates and nutrient dynamics in temperate forests. Ecology 2019, 100, e02563. [Google Scholar]
- Garcia, M.A.; Martinez, S.G.; Lopez, J.R. Impact of plant species composition on litter decomposition and nutrient release in Mediterranean forests. Plant Soil 2020, 447, 193–206. [Google Scholar]
- Smith, J.K.; Johnson, R.M.; Brown, A.L. Effects of site quality on soil chemical properties and ecosystem functioning in managed forests. For. Ecol. Manag. 2020, 275, 123–135. [Google Scholar]
- Borges, W.L.B.; Calonego, J.C.; Rosolem, C.A. Impact of crop-livestock-forest integration on soil quality. Agroforest Syst. 2019, 93, 2111–2119. [Google Scholar] [CrossRef]
- Yang, K.; Zhu, J.; Zhang, W.; Zhang, Q.; Lu, D.; Zhang, Y.; Zheng, X.; Xu, S.; Wang, G.G. Litter decomposition and nutrient release from monospecific and mixed litters: Comparisons of litter quality, fauna and decomposition site effects. J. Ecol. 2022, 110, 1673–1686. [Google Scholar] [CrossRef]
- Manral, V.; Bargali, K.; Bargali, S.S.; Jhariya, M.K.; Padalia, K. Relationships between soil and microbial biomass properties and annual flux of nutrients in Central Himalaya forests, India. Land Degrad. Dev. 2022, 33, 2014–2025. [Google Scholar] [CrossRef]
- Martinez, S.; Rodriguez, A.; Garcia, J.; Lopez, A.; Hernandez, M. Effects of decreased litter deposition on soil properties and biota abundance. Soil Ecol. Lett. 2023, 78, 210–225. [Google Scholar]
- Johnson, L.K.; Smith, R.J.; Brown, A.M. Effects of site quality on soil conditions, plant productivity, and litter deposition rates in temperate forests. For. Ecol. Manag. 2019, 245, 123–135. [Google Scholar]
- Garcia, M.A.; Martinez, S.G.; Lopez, J.R. Effects of site quality on litter quality and nutrient contents in Mediterranean forests. For. Ecol. Manag. 2020, 350, 123–135. [Google Scholar]
- Wang, X.; Li, Y.; Zhang, Z. Influence of litter quality on decomposition rates in subtropical forests: A meta-analysis. For. Ecol. Manag. 2018, 407, 123–135. [Google Scholar]
- Zhang, Q.; Zhang, Y.; Fu, S.; Liu, C. Effects of site quality on soil microbial communities and litter decomposition in subtropical forests. Soil Biol. Biochem. 2019, 131, 123–135. [Google Scholar]
- Li, B.; Li, Y.; Fanin, N.; Han, X.; Du, X.; Liu, H.; Li, Q. Adaptation of soil micro-food web to elemental limitation: Evidence from the forest-steppe ecotone. Soil Biol. Biochem. 2022, 170, 108698. [Google Scholar] [CrossRef]
- Li, R.; Yang, Q.; Guan, X.; Chen, L.; Wang, Q.; Wang, S.; Zhang, W. High quality litters with faster initial decomposition produce more stable residue remaining in a subtropical forest ecosystem. Catena 2022, 213, 106134. [Google Scholar] [CrossRef]
- Long, C.; Zhang, Q.; Chen, Q.; Cheng, X. Divergent controls on leaf and root litter decay linking to soil C, N, and P pools under a Subtropical land-use change. Ecosystems 2023, 26, 1209–1223. [Google Scholar] [CrossRef]
- Sadeghi, S.H.R.; Khazayi, M.; Mirnia, S.K. Effect of soil surface disturbance on overland flow, sediment yield, and nutrient loss in a hyrcanian deciduous forest stand in Iran. Catena 2022, 218, 106546. [Google Scholar] [CrossRef]
- Auclerc, A.; Beaumelle, L.; Barantal, S.; Chauvat, M.; Cortet, J.; Almeida, T.D.; Dulaurent, A.M.; Dutoit, T.; Joimel, S.; Séré, G.; et al. Fostering the use of soil invertebrate traits to restore ecosystem functioning. Geoderma 2022, 424, 116019. [Google Scholar] [CrossRef]
- Guhra, T.; Stolze, K.; Totsche, K.U. Pathways of biogenically excreted organic matter into soil aggregates. Soil Biol. Biochem. 2022, 164, 108483. [Google Scholar] [CrossRef]
- Gruss, I.; Twardowski, J.; Nebeská, D.; Trögl, J.; Stefanovska, T.; Pidlisnyuk, V.; Machová, I. Microarthropods and vegetation as biological indicators of soil quality studied in poor sandy sites at former military facilities. Land Degrad. Dev. 2021, 33, 358–367. [Google Scholar] [CrossRef]
- Manu, M.; Băncilă, R.I.; Mountford, O.J.; Onete, M. Soil invertebrate communities as indicator of ecological conservation status of some fertilised grasslands from Romania. Diversity 2022, 14, 1031. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, C.; Ai, N.; Tuo, X.; Zhang, Z.; Gao, R.; Qin, J.; Yuan, C. Characteristics of soil macrofauna and its coupling relationship with environmental factors in the loess area of Northern Shaanxi. Sustainability 2022, 14, 2484. [Google Scholar] [CrossRef]
- Lü, Y.; Xue, W.F.; Wan, X. Changes in the soil biotic community are associated with variation in Illicium verum productivity. Plant Soil 2023, 486, 323–336. [Google Scholar] [CrossRef]
- Sánchez, A.C.; Jones, S.K.; Purvis, A.; Estrada-Carmona, N.; Palma, A.D. Landscape complexity and functional groups moderate the effect of diversified farming on biodiversity: A global meta-analysis. Agric. Ecosyst. Environ. 2022, 332, 107933. [Google Scholar] [CrossRef]
- Aguilar-Paredes, A.; Valdés, G.; Araneda, N.; Valdebenito, E.; Hansen, F.; Nuti, M. Microbial community in the composting process and its positive impact on the soil biota in sustainable agriculture. Agronomy 2023, 13, 542. [Google Scholar] [CrossRef]
- Vaupel, A.; Bednar, Z.; Herwig, N.; Hommel, B.; Moran-Rodas, V.E.; Beule, L. Tree-distance and tree-species effects on soil biota in a temperate agroforestry system. Plant Soil 2023, 487, 355–372. [Google Scholar] [CrossRef]
- Cappelli, S.L.; Domeignoz-Horta, L.A.; Loaiza, V.; Laine, A.L. Plant biodiversity promotes sustainable agriculture directly and via belowground effects. Trends Plant Sci. 2022, 27, 674–687. [Google Scholar] [CrossRef]
- Lozano, Y.M.; Aguilar-Trigueros, C.A.; Ospina, J.M.; Rillig, M.C. Drought legacy effects on root morphological traits and plant biomass via soil biota feedback. New Phytol. 2022, 236, 222–234. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Deng, D.; Feng, Q.; Xu, Z.; Pan, H.; Li, H. Changes in litter input exert divergent effects on the soil microbial community and function in stands of different densities. Sci. Total Environ. 2022, 845, 57297. [Google Scholar] [CrossRef]
- Swart, R.C.; Samways, M.J.; Roets, F. Interspecific green leaf-litter selection by ground detritivore arthropods indicates generalist over specialist detritivore communities. Appl. Soil Ecol. 2022, 174, 104439. [Google Scholar] [CrossRef]
- Greff, B.; Szigeti, J.; Nagy, A.; Lakatos, E.; Varga, L. Influence of microbial inoculants on co-composting of lignocellulosic crop residues with farm animal manure: A review. J. Environ. Manag. 2022, 302, 114088. [Google Scholar] [CrossRef]
- Marco, A.; Vittozzi, P.; Santo, A.V. Elements dynamics, from leaf to stable leaf litter residue and soil, for two functional types of tree planted on volcanic deposits. Plant Soil 2022, 482, 127–140. [Google Scholar] [CrossRef]
- Jones, A.; Smith, B.; Johnson, C.; Martinez, M.; Garcia, L. Investigating potential drivers of habitat quality and nutrient availability hypotheses in ecosystem dynamics. Ecol. Lett. 2023, 45, 210–225. [Google Scholar]
- Wang, P.; Yang, F.; Chen, X.; Li, J.; Zhou, X.; Guo, H. Long-term fertilization effects on soil biotic communities are mediated by plant diversity in a Tibetan alpine meadow. Plant Soil 2022, 474, 525–540. [Google Scholar] [CrossRef]
- Zheng, J.; Li, S.; Wang, H.; Dai, X.; Meng, S.; Jiang, L.; Ma, N.; Yan, H.; Fu, X.; Kou, L. Home-field advantage meets priming effect in root decomposition: Implications for belowground carbon dynamics. Funct. Ecol. 2022, 37, 676–689. [Google Scholar] [CrossRef]
- Remke, M.J.; Johnson, N.C.; Bowker, M.A. Sympatric soil biota mitigate a warmer-drier climate for Bouteloua gracilis. Glob. Chang. Biol. 2022, 28, 6280–6292. [Google Scholar] [CrossRef] [PubMed]
- Long, J.R.; Heinen, R.; Heinze, J.; Morriën, E.; Png, G.K.; Sapsford, S.J.; Teste, F.P.; Fry, E.L. Plant-soil feedback: Incorporating untested influential drivers and reconciling terminology. Plant Soil 2022, 485, 7–43. [Google Scholar] [CrossRef]
- Oliva, R.L.; Veen, G.F.; Tanaka, M.O. Soil nutrient dissimilarity and litter nutrient limitation as major drivers of home field advantage in riparian tropical forests. Biotropica 2023, 55, 628–638. [Google Scholar] [CrossRef]
- Fan, Z.; Wu, Y.; Zhao, L.; Fu, L.; Deng, L.; Deng, J.; Ding, D.; Xiao, S.; Deng, X.; Peng, S.; et al. MYB308-mediated transcriptional activation of plasma membrane H+-ATPase 6 promotes iron uptake in citrus. Hortic. Res. 2022, 9, uhac088. [Google Scholar] [CrossRef] [PubMed]
- Ulbrich, T.C.; Rivas-Ubach, A.; Tiemann, L.K.; Friesen, M.L.; Evans, S.E. Plant root exudates and rhizosphere bacterial communities shift with neighbor context. Soil Biol. Biochem. 2022, 172, 108753. [Google Scholar] [CrossRef]
- Chen, W.; Zhou, H.; Wu, Y.; Wang, J.; Zhao, Z.; Li, Y.; Qiao, L.; Yang, B.; Chen, K.; Liu, G.; et al. Loss of plant functional groups impacts soil carbon flow by changing multitrophic interactions within soil micro-food webs. Appl. Soil Ecol. 2022, 178, 104566. [Google Scholar] [CrossRef]
- Hopkins, J.R.; Semenova-Nelsen, T.A.; Sikes, B.A. Land management drives dynamic changes to microbial function through edaphic factors and soil biota. Pedobiologia 2023, 96, 150859. [Google Scholar] [CrossRef]
- Kang, L.; Chen, L.; Zhang, D.; Peng, Y.; Song, Y.; Kou, D.; Deng, Y.; Yang, Y. Stochastic processes regulate belowground community assembly in alpine grasslands on the Tibetan Plateau. Environ. Microbiol. 2021, 24, 179–194. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, E.A.; Schoereder, J.H.; Cornelissen, T.G.; Brando, P.M.; Maracahipes, L.; Paolucci, L.N. Reduced predation by arthropods and higher herbivory in burned Amazonian forests. Biotropica 2022, 54, 1052–1060. [Google Scholar] [CrossRef]

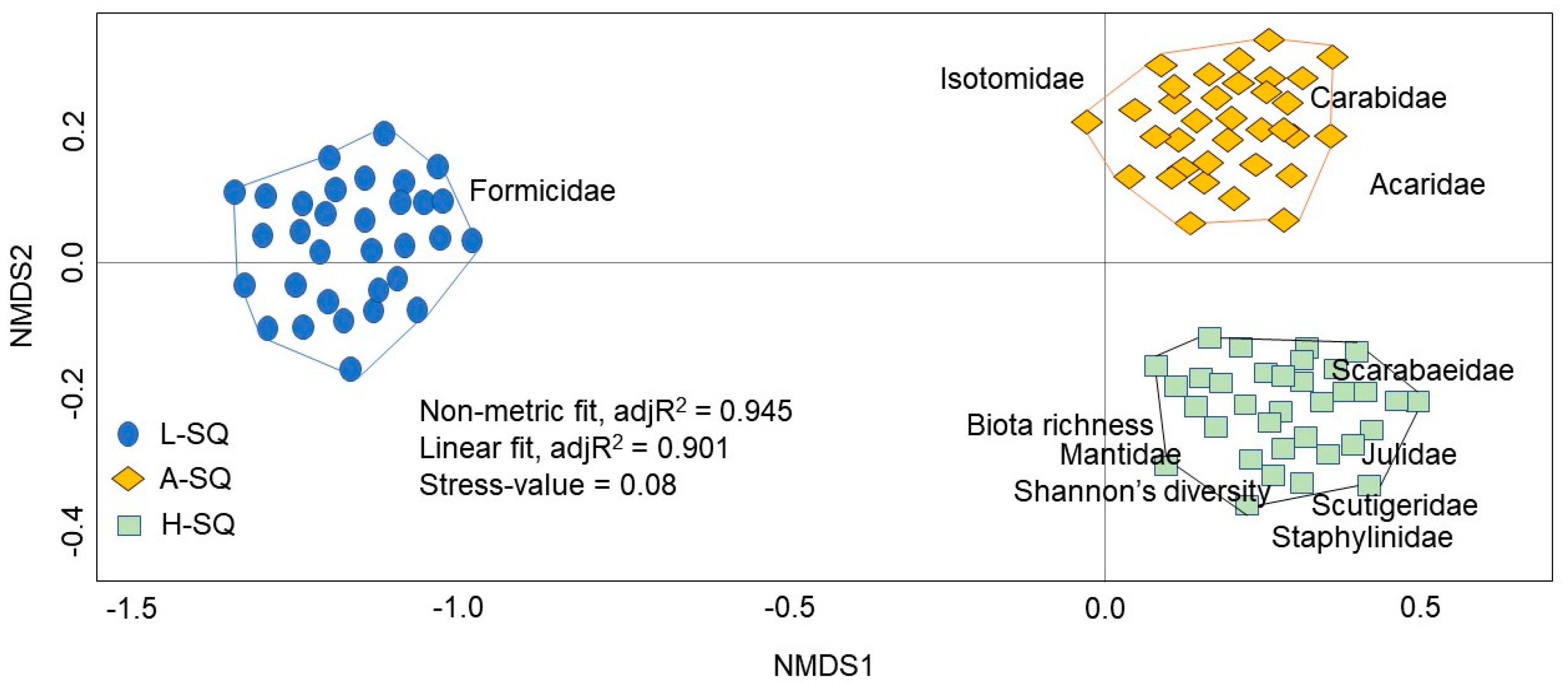

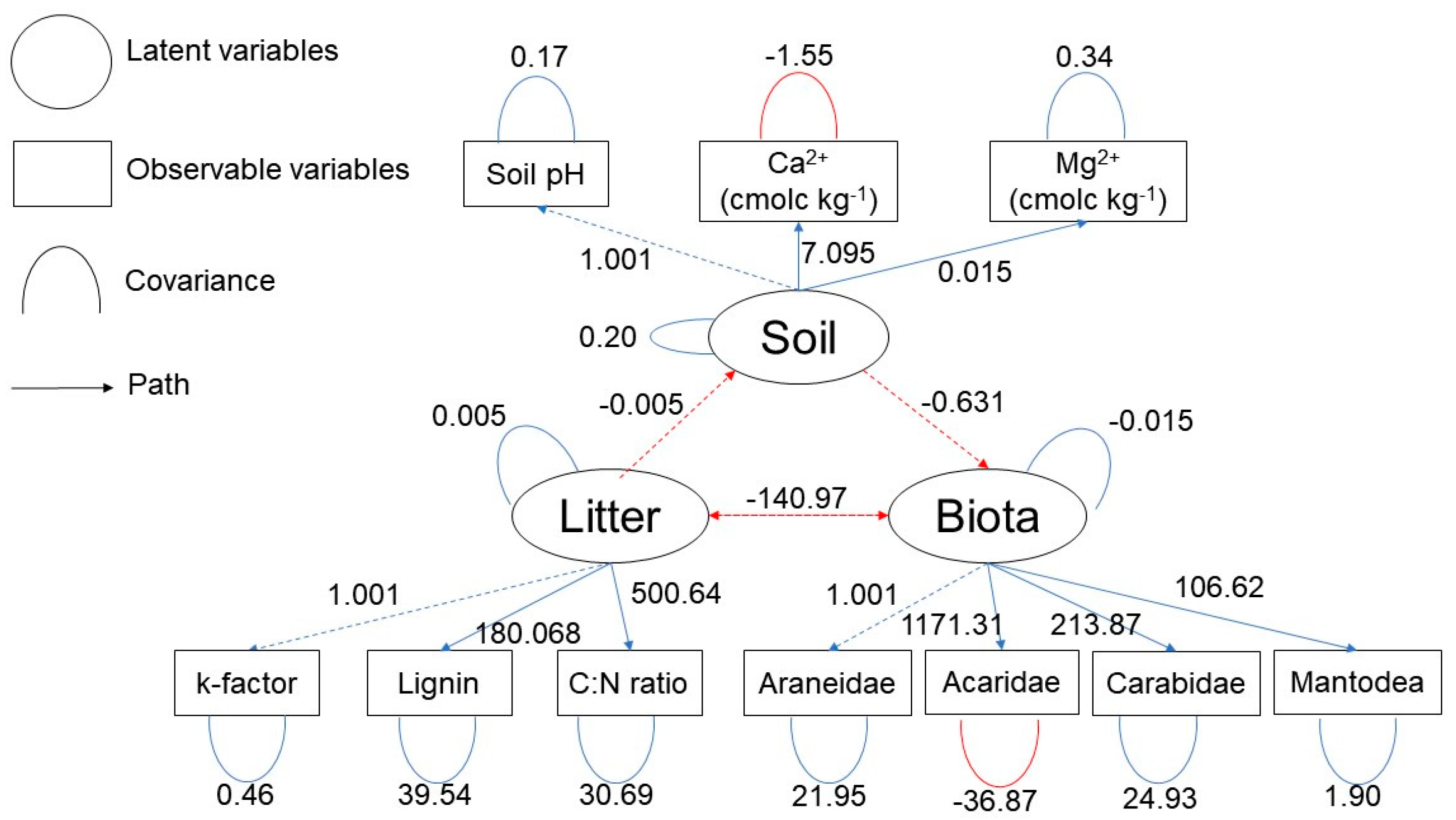
| Site | Altitude (m) | Slope (%) | Clay Content (g kg−1) | Silt Content (g kg−1) | Sand Content (g kg−1) | Stand Age (Years) | Tree Dominant Height (m) |
|---|---|---|---|---|---|---|---|
| Low quality | 1200 (53) | 12.1 (1.4) | 29.5 (1.7) | 331.6 (9.2) | 638.9 (12.8) | 27.5 (2.8) | 12.7 (1.2) |
| Average quality | 1172 (102) | 11.9 (2.1) | 31.5 (3.2) | 326.6 (5.2) | 641.9 (15.7) | 29.0 (3.2) | 16.0 (2.0) |
| High quality | 1135 (89) | 12.0 (0.9) | 29.6 (2.1) | 339.7 (11.4) | 630.7 (18.2) | 28.8 (2.6) | 20.8 (3.2) |
| Plant Material | Lignin (%) | Total Organic Carbon (%) | N (%) | P (mg kg−1) |
|---|---|---|---|---|
| Araucaria angustifolia | 58.6 ± 3.4 | 43.6 ± 1.4 | 0.65 ± 0.04 | 670.0 ± 45.8 |
| Mimosa scabrella | 34.3 ± 1.4 | 20.9 ± 1.2 | 3.95 ± 0.23 | 134.8 ± 14.7 |
| Properties | L-SQ | A-SQ | H-SQ |
|---|---|---|---|
| Litter deposition (g m−2) | 4416.28 (626.46) c | 6518.22 (652.34) b | 8922.70 (797.89) a |
| Lignin content (%) | 63.50 (2.96) a | 51.95 (1.19) b | 43.48 (0.61) c |
| N content (g kg−1) | 10.46 (3.02) a | 8.96 (1.73) b | 10.53 (1.72) a |
| P content (g kg−1) | 0.82 (0.18) a | 0.74 (0.11) b | 0.69 (0.12) c |
| K content (g kg−1) | 0.56 (0.02) b | 0.93 (0.20) a | 1.11 (0.40) a |
| Ca content (g kg−1) | 17.18 (3.62) b | 15.67 (5.05) b | 19.50 (1.97) a |
| Mg content (g kg−1) | 2.00 (0.69) c | 2.48 (0.35) b | 3.60 (1.26) a |
| S content (g kg−1) | 2.43 (0.78) a | 2.09 (0.57) b | 2.42 (0.36) a |
| C content (g kg−1) | 384.30 (4.24) a | 369.05 (4.14) b | 383.60 (4.15) a |
| C:N ratio | 38.45 (1.52) b | 41.80 (1.09) a | 36.86 (0.98) c |
| Properties | L-SQ | A-SQ | H-SQ |
|---|---|---|---|
| Soil pH | 5.30 (0.42) a | 4.97 (0.17) b | 5.56 (0.87) a |
| Soil organic matter (%) | 5.85 (1.48) b | 5.70 (1.22) b | 7.36 (1.21) a |
| P (mg kg−1) | 10.90 (1.25) b | 9.72 (1.30) b | 12.35 (1.21) a |
| K (mg kg−1) | 44.00 (1.27) b | 107.80 (5.09) a | 113.35 (4.12) a |
| Exchangeable Ca (cmolc kg−1) | 5.60 (0.30) b | 4.60 (0.37) b | 8.74 (0.28) a |
| Exchangeable Mg (cmolc kg−1) | 2.40 (0.56) a | 1.47 (0.49) b | 1.70 (0.62) b |
| Exchangeable Al (cmolc kg−1) | 0.40 (0.05) b | 1.50 (0.17) a | 0.38 (0.05) b |
| H+ + Al3+ (cmolc kg−1) | 9.50 (0.51) b | 14.92 (0.56) a | 10.74 (0.78) b |
| S (mg kg−1) | 20.65 (1.29) a | 18.87 (0.81) b | 16.20 (0.43) c |
| B (mg kg−1) | 0.61 (0.04) a | 0.42 (0.05) b | 0.43 (0.03) b |
| Cu (mg kg−1) | 4.05 (0.12) a | 2.57 (0.13) b | 3.30 (0.16) a |
| Mn (mg kg−1) | 50.60 (1.32) b | 56.97 (0.42) a | 57.68 (1.23) a |
| Zn (mg kg−1) | 2.55 (0.63) c | 8.07 (0.75) a | 4.08 (0.25) b |
| Taxonomic Group | L-SQ | A-SQ | H-SQ | F-Value |
|---|---|---|---|---|
| Araneae | ||||
| Araneidae | 15.0 (1.7) a | 11.2 (2.1) b | 5.2 (1.0) c | 7.83 ** |
| Filistatidae | 0.0 (0.0) b | 15.0 (2.1) a | 15.8 (3.8) a | 9.85 *** |
| Acari | ||||
| Acaridae | 0.0 (0.0) c | 18.7 (3.1) b | 55.0 (4.7) a | 8.63 ** |
| Collembola | ||||
| Isotomidae | 16.5 (0.7) b | 24.0 (1.4) a | 16.0 (4.5) b | 7.08 * |
| Paronellidae | 0.0 (0.0) c | 10.5 (1.2) b | 21.4 (3.6) a | 8.63 ** |
| Coleoptera | ||||
| Carabidae | 0.0 (0.0) c | 12.0 (2.9) b | 16.8 (3.2) a | 6.86 * |
| Scarabidae | 0.0 (0.0) b | 0.0 (0.0) b | 28.6 (3.2) a | 8.91 ** |
| Staphylinidae | 0.0 (0.0) b | 0.0 (0.0) b | 13.8 (4.5) a | 8.96 ** |
| Nitidulidae | 10.5 (0.7) b | 18.0 (2.1) a | 12.0 (3.3) b | 6.62 * |
| Chilopoda | ||||
| Scutigeridae | 0.0 (0.0) b | 0.0 (0.0) b | 3.4 (1.1) a | 8.96 ** |
| Diplopoda | ||||
| Julidae | 0.0 (0.0) b | 0.0 (0.0) b | 2.0 (0.7) a | 9.11 ** |
| Hymenoptera | ||||
| Formicidae | 12.5 (0.7) c | 50.2 (2.9) b | 64.0 (8.3) a | 8.63 ** |
| Lepidoptera | ||||
| Larvae | 0.0 (0.0) b | 0.0 (0.0) b | 1.8 (0.8) a | 9.01 ** |
| Dermaptera | ||||
| Forficulidae | 0.0 (0.0) b | 1.7 (0.9) a | 1.8 (0.8) a | 9.86 ** |
| Mantodea | ||||
| Mantidae | 0.0 (0.0) b | 0.0 (0.0) b | 4.4 (1.0) a | 8.91 ** |
| Neuroptera | ||||
| Myrmeleontidae | 0.0 (0.0) b | 2.0 (0.1) a | 1.4 (0.2) b | 9.26 ** |
| Pseudoscorpiones | ||||
| Lechytiidae | 0.0 (0.0) b | 0.0 (0.0) b | 3.0 (0.8) a | 9.11 ** |
| Thysanoptera | ||||
| Halictophagidae | 1.5 (0.7) b | 2.7 (0.9) a | 1.0 (0.2) b | 8.13 ** |
| Ecological indices | ||||
| Biota richness | 5.0 (1.0) c | 11.0 (1.5) b | 18.0 (2.0) a | 19.25 *** |
| Shannon’s diversity | 1.44 (0.25) c | 2.19 (0.51) a | 2.31 (0.33) a | 10.12 *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Souza, T.; Dobner, M., Jr.; Batista, D.S.; Araujo, D.J.; Nascimento, G.d.S.; da Silva, L.J.R. Site Quality for Araucaria angustifolia Plantations with Subtropical Cambisol Is Driven by Soil Organism Assemblage and the Litter and Soil Compartments. Forests 2024, 15, 510. https://doi.org/10.3390/f15030510
Souza T, Dobner M Jr., Batista DS, Araujo DJ, Nascimento GdS, da Silva LJR. Site Quality for Araucaria angustifolia Plantations with Subtropical Cambisol Is Driven by Soil Organism Assemblage and the Litter and Soil Compartments. Forests. 2024; 15(3):510. https://doi.org/10.3390/f15030510
Chicago/Turabian StyleSouza, Tancredo, Mário Dobner, Jr., Diego Silva Batista, Damiana Justino Araujo, Gislaine dos Santos Nascimento, and Lucas Jónatan Rodrigues da Silva. 2024. "Site Quality for Araucaria angustifolia Plantations with Subtropical Cambisol Is Driven by Soil Organism Assemblage and the Litter and Soil Compartments" Forests 15, no. 3: 510. https://doi.org/10.3390/f15030510
APA StyleSouza, T., Dobner, M., Jr., Batista, D. S., Araujo, D. J., Nascimento, G. d. S., & da Silva, L. J. R. (2024). Site Quality for Araucaria angustifolia Plantations with Subtropical Cambisol Is Driven by Soil Organism Assemblage and the Litter and Soil Compartments. Forests, 15(3), 510. https://doi.org/10.3390/f15030510








