Effects of Thinning on Carbon Storage in a Mixed Broadleaved Plantation in a Subtropical Area of China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site and Experimental Design
2.2. Understory Vegetation Survey and Index Calculations
2.3. Litter, Soil Sampling, and Measuring Methods
2.4. Leaf Area INDEX Measurement
2.5. Biomass Carbon, Litter Carbon, and Soil Carbon Calculations
| Species | Biomass (kg) | C Content Percentage | References | |
|---|---|---|---|---|
| Schima superba | Aboveground | W = 0.5373 × D1.903 (R2 = 0.91) | C = 0.5077 | [30] |
| Roots | W = 0.5759 × D1.4093 (R2 = 0.79) | C = 0.5487 | ||
| Castanopsis hystrix | Trunk | W = 0.0641 × (D2H)0.8699 (R2 = 0.99) | C = 0.4902 | [31,32] |
| Bark | W = 0.0105 × (D2H)0.8246 (R2 = 0.92) | C = 0.4491 | ||
| Branches | W = 0.0001 × (D2H)1.3949 (R2 = 0.81) | C = 0.4912 | ||
| Leaves | W = 0.0000028 × (D2H)1.6052 (R2 = 0.91) | C = 0.5018 | ||
| Roots | W = 0.1210 × (D2H)0.6495 (R2 = 0.81) | C = 0.4775 | ||
| Michelia macclurei | Trunk with bark | W = 0.033232 × (D2H)0.97166 (R2 = 0.98) | C = 0.5059 | [33,34] |
| Branches | W = 0.022721 × (D2H)0.84435 (R2 = 0.96) | C = 0.5050 | ||
| Leaves | W = 0.079679 × (D2H)0.59671 (R2 = 0.96) | C = 0.5077 | ||
| Roots | W = 0.039307 × (D2H)0.86499 (R2 = 0.96) | C = 0.4232 |
2.6. Data Analysis
3. Results
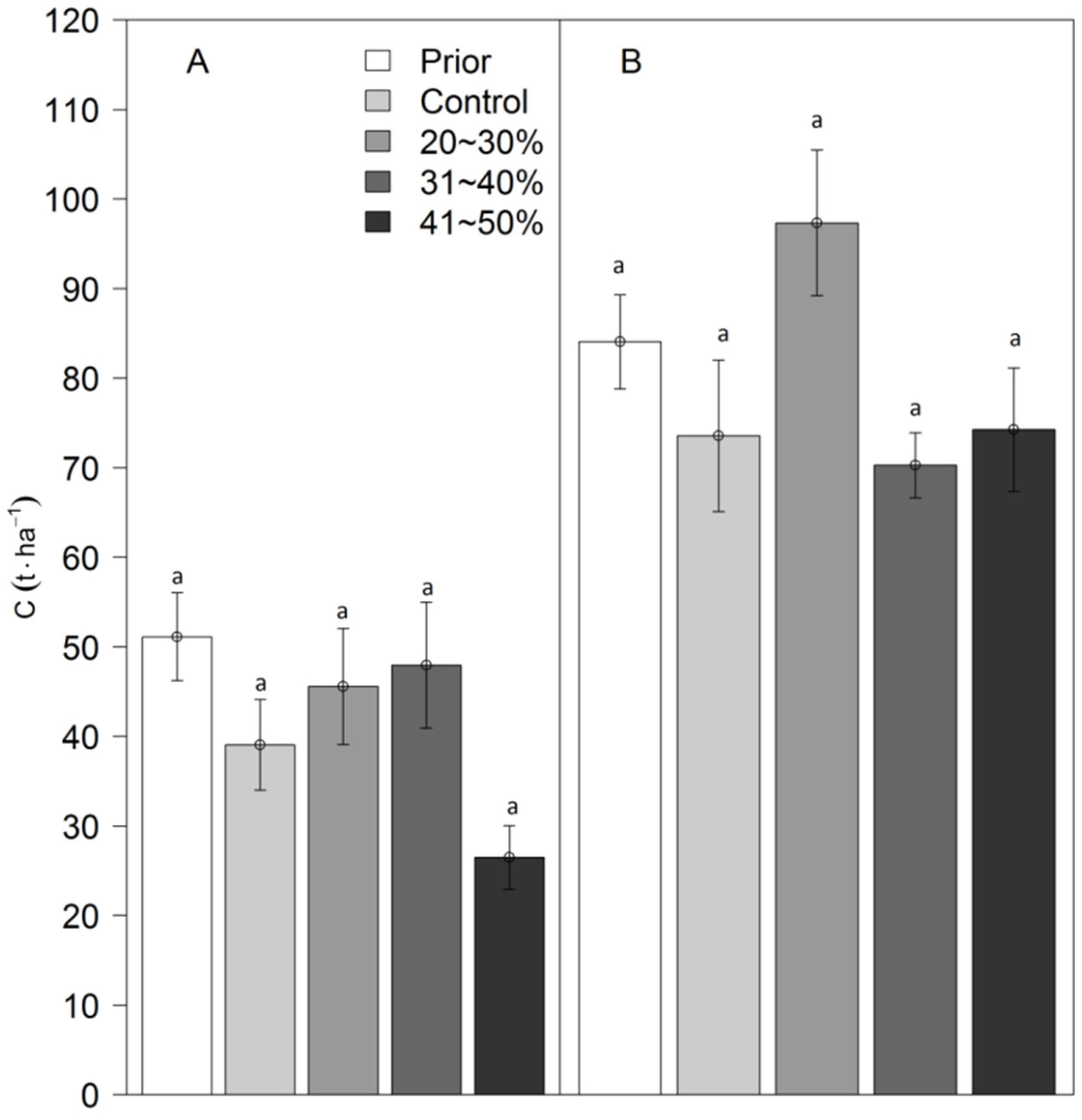
3.1. Carbon Storage in the Tree Layer
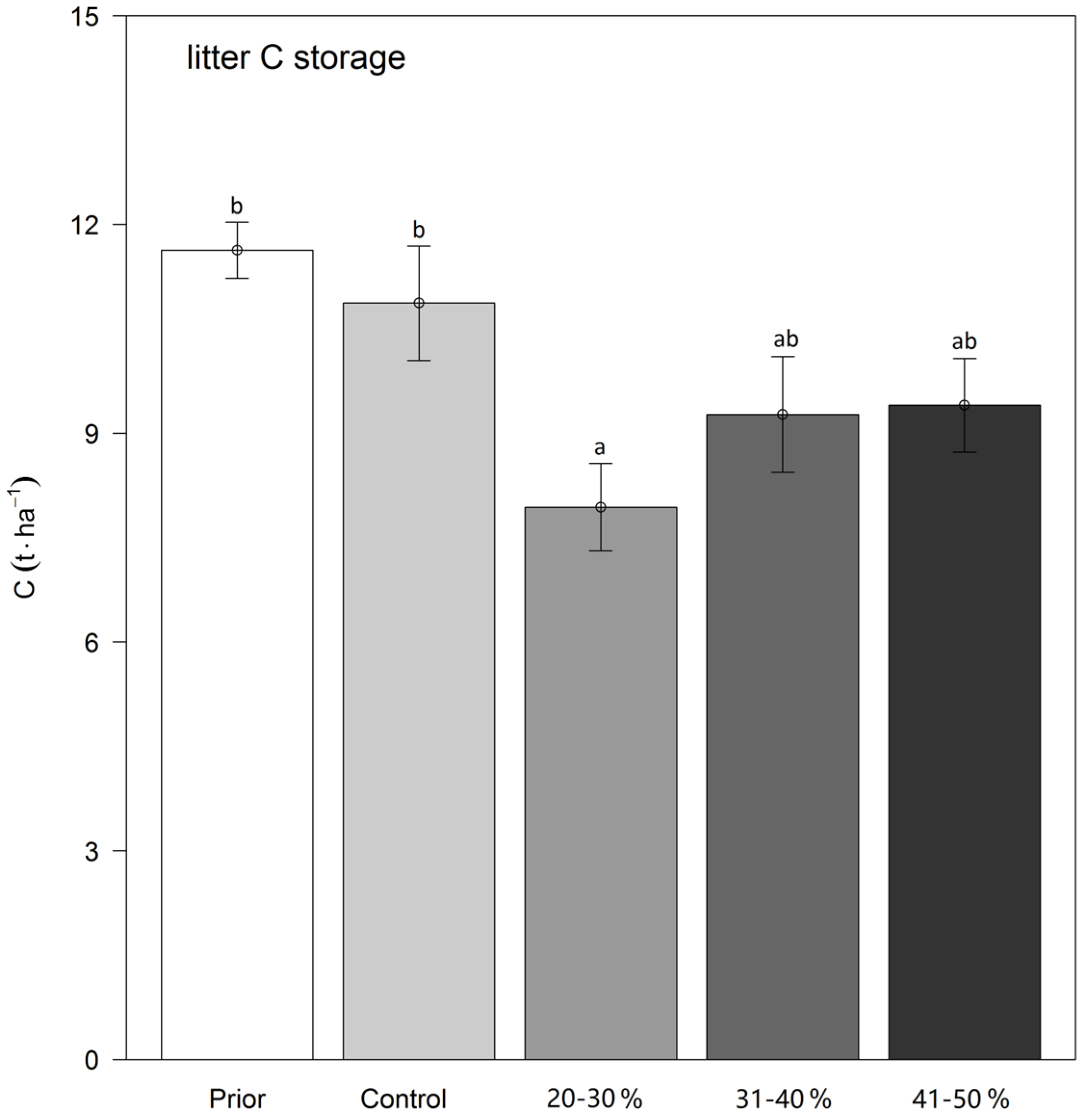
3.2. Carbon Storage in the Litter Layer
3.3. Carbon Storage in Soil
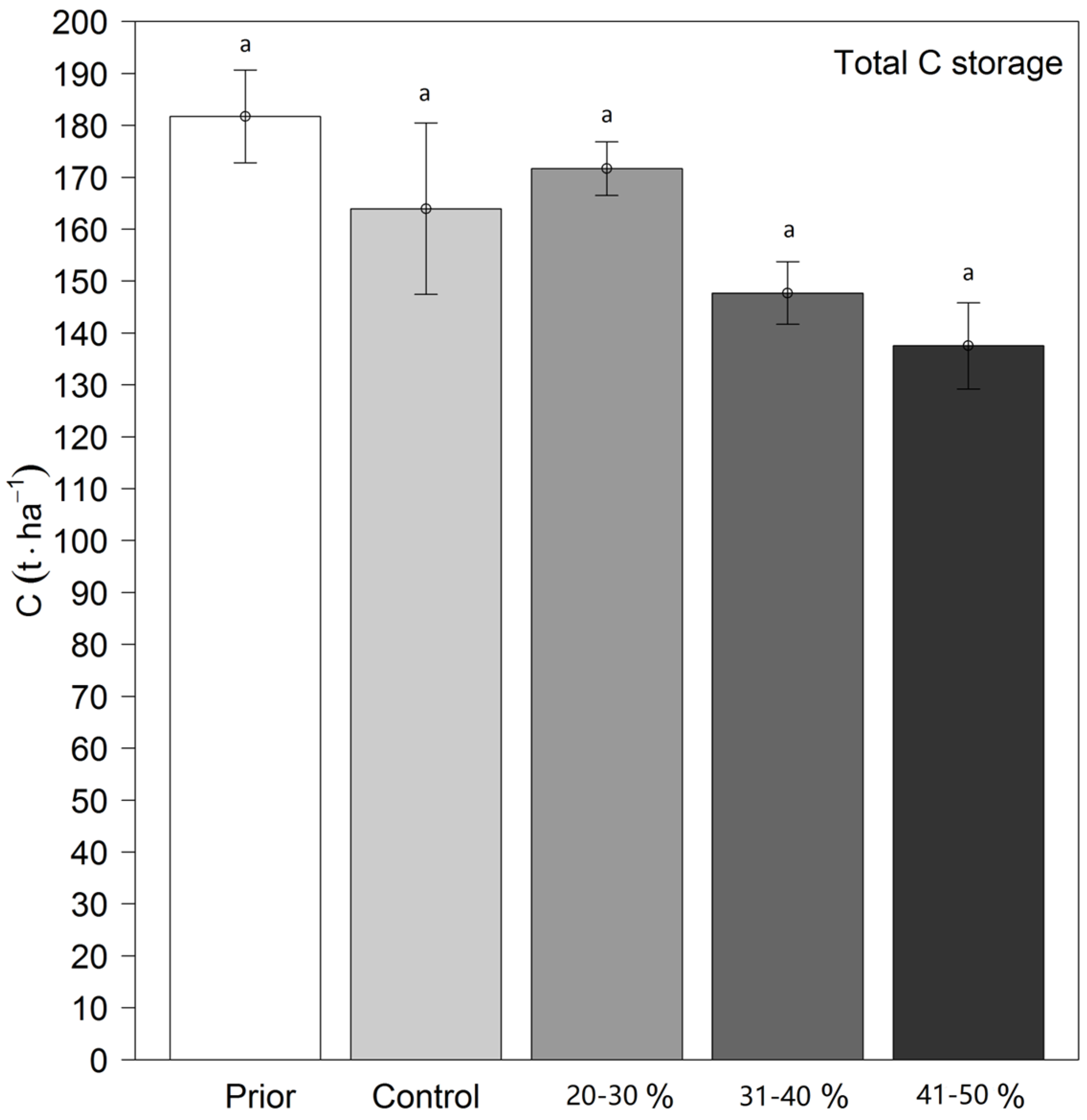
3.4. The Total Carbon Storage in the Mixed Broadleaved Plantation
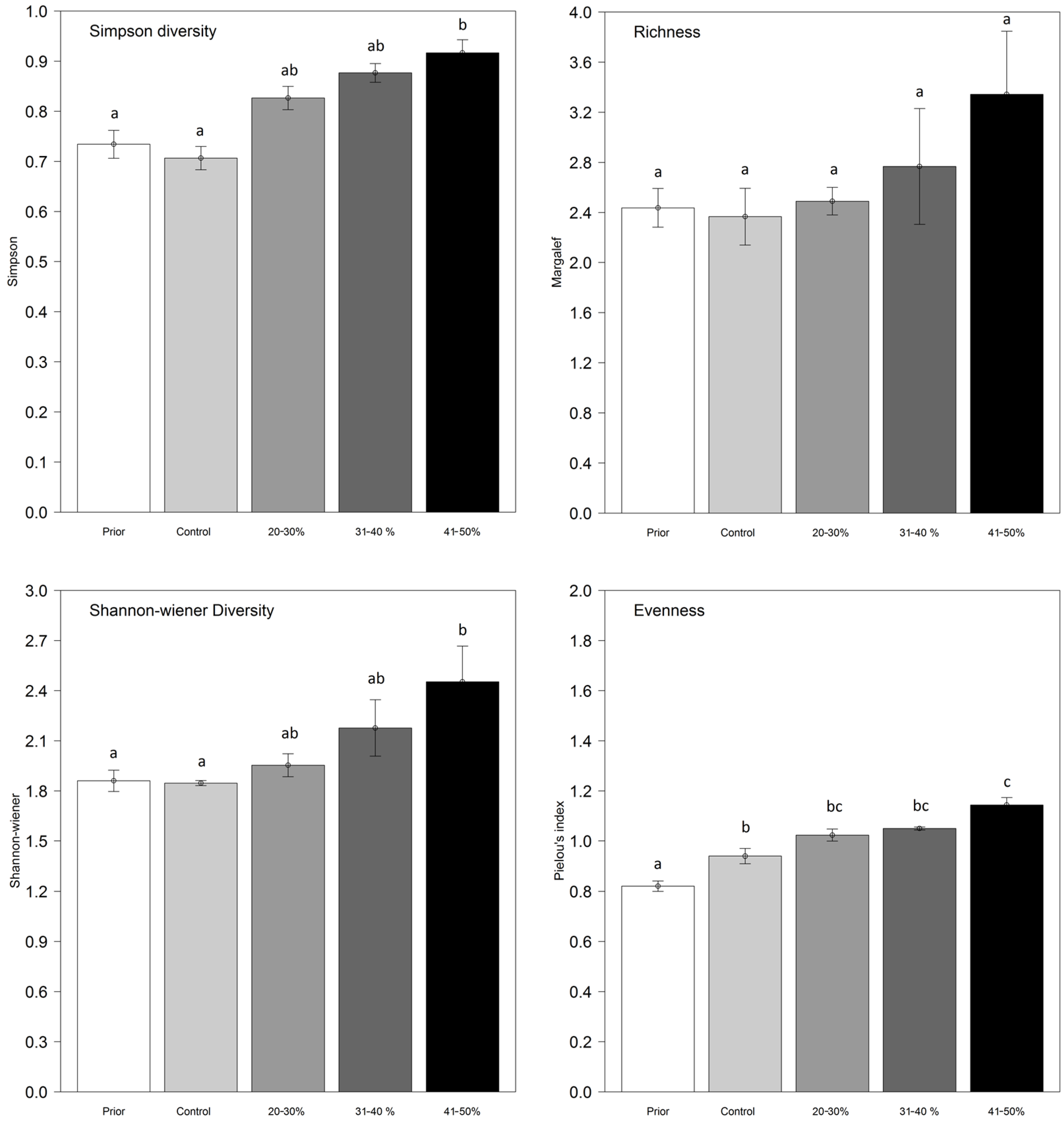
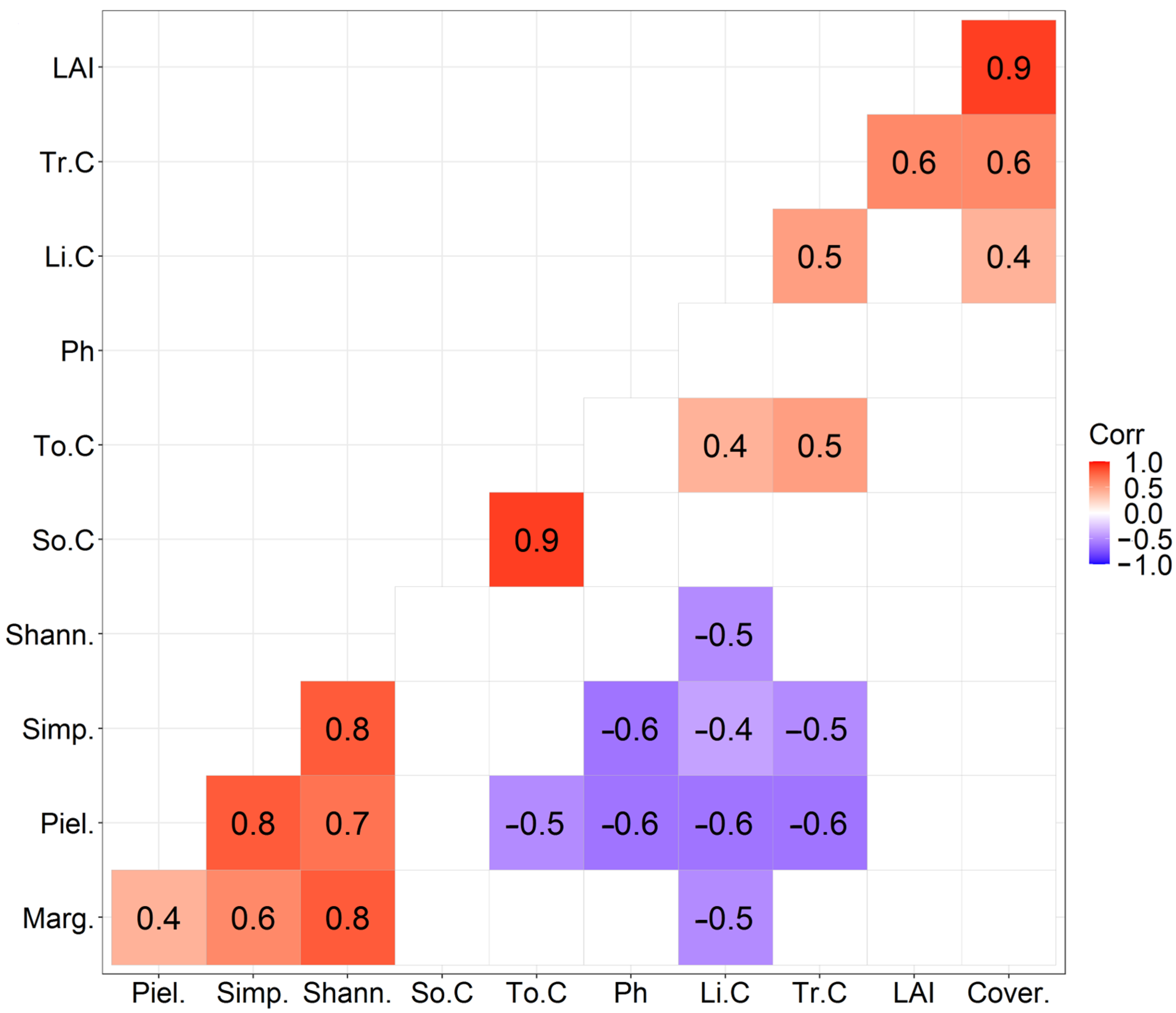
3.5. The Development of Understory Plants
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, Y.; Chen, Y.; Castro-Izaguirre, N.; Baruffol, M.; Brezzi, M.; Lang, A.N.; Li, Y.; Haerdtle, W.; Oheimb, G.; Yang, X.; et al. Impacts of species richness on productivity in a large-scale subtropical forest experiment. Science 2018, 362, 80–83. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.C.; Piao, S.L.; Myneni, R.B.; Huang, M.T.; Zeng, Z.Z.; Canadell, J.G.; Ciais, P.; Sitch, S.; Friedlingstei, P.; Arneth, A.; et al. Greening of the Earth and its drivers. Nat. Clim. Chang. 2016, 6, 791–795. [Google Scholar] [CrossRef]
- Tong, X.W.; Brandt, M.; Yue, Y.M.; Ciais, P.; Jepsen, M.R.; Penuelas, J.; Wigneron, J.-P.; Xiao, X.M.; Song, X.-P.; Horion, S.; et al. Forest management in southern China generates short term extensive carbon sequestration. Nat. Commun. 2020, 11, 129. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, H.; Luan, J.W.; Chang, S.X.; Gao, B.; Wang, Y.; Liu, S.R. Functional diversity dominates positive species mixture effects on ecosystem multifunctionality in subtropical plantations. For. Ecosyst. 2022, 9, 100039. [Google Scholar] [CrossRef]
- Pretzsch, H.; Schutze, G. Effect of tree species mixing on the size structure, density, and yield of forest stands. Eur. J. For. Res. 2016, 135, 1–22. [Google Scholar] [CrossRef]
- Coll, L.; Ameztegui, A.; Collet, C.; Lof, M.; Mason, B.; Pach, M.; Verheyen, K.; Abrudan, L.; Barbati, A.; Barreiro, S.; et al. Knowledge gaps about mixed forests: What do European forest managers want to know and what answers can science provide? For. Ecol. Manag. 2018, 407, 106–115. [Google Scholar] [CrossRef]
- Liu, C.L.C.; Kuchma, O.; Krutovsky, K.V. Mixed-species versus monocultures in plantation forestry: Development, benefits, ecosystem services and perspectives for the future. Glob. Ecol. Conserv. 2018, 15, 13. [Google Scholar] [CrossRef]
- Baldocchi, D.; Penuelas, J. The physics and ecology of mining carbon dioxide from the atmosphere by ecosystems. Glob. Chang. Biol. 2019, 25, 1191–1197. [Google Scholar] [CrossRef] [PubMed]
- Luyssaert, S.; Marie, G.; Valade, A.; Chen, Y.Y.; Djomo, S.N.; Ryder, J.; Otto, J.; Naudts, K.; LansØ, A.S.; Ghattas, J.; et al. Trade-offs in using European forests to meet climate objectives. Nature 2018, 562, 259. [Google Scholar] [CrossRef] [PubMed]
- Mehrabi, Z.; Ellis, E.C.; Ramankutty, N. The challenge of feeding the world while conserving half the planet. Nat. Sustain. 2018, 1, 409. [Google Scholar] [CrossRef]
- Paris Agreement, FCCC/CP/2015/10/Add.1. United Nations Framework Convention on Climate Change, 2015. Available online: https://unfccc.int/process-and-meetings/the-paris-agreement (accessed on 4 November 2016).
- Aldea, J.; Bravo, F.; Bravo-Oviedo, A.; Ruiz-Peinada, R.; Rodríguez, F.; del Río, M. Thinning enhances the species-specific radial increment response to drought in Mediterranean pine-oak stands. Agric. For. Meteorol. 2017, 237–238, 371–383. [Google Scholar] [CrossRef]
- Bellassen, V.; Luyssaert, S. Carbon sequestration: Managing forests in uncertain times. Nature 2014, 506, 153–155. [Google Scholar] [CrossRef] [PubMed]
- Lavigne, M.B. Growth and net assimilation rates in thinned and unthinned stands of balsam fir. Can. J. For. Res. 1988, 18, 1205–1210. [Google Scholar] [CrossRef]
- Brix, H. Effects of thinning and nitrogen fertilization on growth of Douglasfir: Relative contribution of foliage quantity and efficiency. Can. J. For. Res. 1983, 13, 167–175. [Google Scholar] [CrossRef]
- Omari, K.; MacLean, D.A.; Lavigne, M.B.; Kershaw, J.A., Jr.; Adams, G.W. Effects of local stand structure on leaf area, growth, and growth efficiency following thinning of white spruce. For. Ecol. Manag. 2016, 368, 55–62. [Google Scholar] [CrossRef]
- Lin, N.; Deng, N.L.; Lu, D.H.; Xie, H.Y.; Feng, M.C.; Chen, S.Q. Short-term effects of thinning on tree growth and soil nutrients in the Middle-aged Chinese fir (Cunninghamia lanceolata (Lamb.) Hook.) plantations. Forests 2023, 14, 74. [Google Scholar] [CrossRef]
- Adamczyk, B.; Adamczyk, S.; Kukkola, M.; Tamminen, P.; Smolander, A. Logging residue harvest may decrease enzymatic activity of boreal forest soils. Soil Biol. Biochem. 2015, 82, 74–80. [Google Scholar] [CrossRef]
- Xu, X.; Wang, X.; Hu, Y.; Wang, P.; Saeed, S.; Sun, Y. Short-term effects of thinning on the development and communities of understory vegetation of Chinese fir plantations in Southeastern China. Peer J. 2020, 8, e8536. [Google Scholar] [CrossRef] [PubMed]
- Misson, L.; Tang, J.; Xu, M.; Mckay, M.; Goldstein, A. Influence of recovery from clear-cut, climate variability, and thinning on the carbon balance of a young ponderosa pine plantation. Agric. For. Meteorol. 2005, 130, 207–222. [Google Scholar] [CrossRef]
- Bai, S.H.; Dempsey, R.; Reverchon, F.; Blumfield, T.J.; Ryan, S.; Cernusak, L.A. Effects of forest thinning on soil-plant carbon and nitrogen dynamics. Plant Soil 2017, 411, 437–449. [Google Scholar] [CrossRef]
- Högberg, P. Environmental science: Nitrogen impacts on forest carbon. Nature 2007, 447, 781–782. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Chen, C.; He, J.; Liu, J. Trends and challenges in soil research 2009: Linking global climate change to local long-term forest productivity. J. Soils Sediments 2009, 9, 83–88. [Google Scholar] [CrossRef]
- Huang, X.M.; You, Y.M.; Lan, J.C.; Liu, S.R.; Jia, H.Y.; Zhu, H.G.; Wen, Y.G. The effects of carbon storage and its allocation in Cunninghamia lanceolata plantations with different thinning intensities. Acta Ecol. Sin. 2016, 36, 156–163. [Google Scholar]
- Yin, B.; Dong, P.F.; Dang, K.L. Effects of thinning on carbon density of Betula albo-sinensis ecosystem. J. Northwest Univ. 2019, 34, 105–112. [Google Scholar]
- Ming, A.G.; Zhang, Z.J.; Chen, H.H.; Zhang, X.Q.; Tao, L.; Su, Y. Effects of thinning on the biomass and carbon storage in Pinus massoniana plantation. Sci. Silvae Sin. 2013, 49, 1–6. [Google Scholar]
- Meiwes, K.J.; Khanna, P.K.; Ulrich, B. Parameters for describing soil acidification and their relevance to stability of forest ecosystems. For. Ecol. Manag. 1986, 15, 161–179. [Google Scholar] [CrossRef]
- Cheng, X.Q.; Han, H.R.; Kang, F.F.; Liu, K.; Song, Y.L. Short-term effects of thinning on soil respiration in a pine (Pinus tabulaeformis) plantation. Biol. Fertil. Soils 2015, 50, 357–367. [Google Scholar] [CrossRef]
- Frazer, G.W.; Canham, C.D.; Lertzman, K.P. Gap Light Analyzer (GLA): Imaging Software to Extract Canopy Structure and Gap Light Transmission Indices from True-Colour Fisheye Photographs, Users Manual and Program Documentation; Simon Fraser University: Burnaby, BC, Canada; Institute of Ecosystem Studies: Millbrook, NY, USA, 1999. [Google Scholar]
- He, X.; Lei, Y.C.; Xue, C.Q.; Xu, Q.H.; Li, H.K.; Cao, L. Carbon density uncertainty estimates for Schima superba in Guangdong Province. Sci. Silvae Sin. 2019, 55, 163–171. (In Chinese) [Google Scholar]
- Qin, L.; He, Y.J.; Li, Z.Y.; Shao, M.X.; Liang, X.Y.; Tan, L. Allocation pattern of biomass and productivity for three plantations of Catanopsisi hystrix, Pinus massoniana and their mixture in South subtropical area of Guangxi, China. Sci. Silvae Sin. 2011, 47, 17–21. (In Chinese) [Google Scholar]
- Zhao, Z. Biomass and Carbon Storage of Cunninghamia lanceolata and Castanopsis hystrix Plantations in South Subtropical China. Master’s Thesis, Guangxi University, Nanning, China, 2017. (In Chinese). [Google Scholar]
- Qi, Z.Y.; Ma, J.X.; Li, S.M. Studies on the biomass and productivity of artificial Michelia macclurei Var. sublancea forest. J. Ecol. 1985, 2, 17–19. (In Chinese) [Google Scholar]
- Liu, L.Y.; Zheng, X.Y.; Xue, L. Distribution pattern of nutrient accumulation in Michelia macclurei plantation. J. Northeast. For. Univ. 2019, 47, 25–29. (In Chinese) [Google Scholar]
- Pan, Y.D.; Birdsey, R.A.; Fang, J.Y.; Houghton, R.; Kauppi, P.; Kurz, W.A.; Phillips, O.L.; Shvidenko, A.; Lewis, S.L.; Canadell, J.G.; et al. A large and persistent carbon sink in the world’s forests. Science 2011, 333, 988–993. [Google Scholar] [CrossRef]
- Knott, J.; Domke, G.; Woodall, C.; Walters, B.; Jenkins, M.; Fei, S.L. Shifting forests and carbon: Linking community composition and aboveground carbon attributes. Ecosystems 2023, 26, 412–427. [Google Scholar] [CrossRef]
- Zheng, L.; Cai, D.X.; Lu, L.H.; Ming, A.G.; Li, Z.Y. Carbon pool of different species plantation ecosystems in lower subtropical area of China. J. Cent. South Univ. For. Technol. 2014, 34, 110–116. (In Chinese) [Google Scholar]
- Canadell, J.G.; Raupach, M.R. Managing forests for climate change mitigation. Science 2008, 320, 1456–1457. [Google Scholar] [CrossRef] [PubMed]
- Georgiou, K.; Jackson, R.B.; Vindušková, O.; Abramoff, R.Z.; Ahlström, A.; Feng, W.T.; Harden, J.W.; Pellegrini, A.F.A.; Polley, H.W.; Soong, J.L.; et al. Global stocks and capacity of mineral-associated soil organic carbon. Nat. Commun. 2022, 13, 1797. [Google Scholar] [CrossRef] [PubMed]
- Lal, R.; Monger, C.; Nave, L.; Smith, P. The role of soil in regulation of climate. Philos. Trans. R. Soc. B Biol. Sci. 2021, 376, 20210084. [Google Scholar] [CrossRef] [PubMed]
- Hulshof, C.M.; Spasojevic, M.J. The edaphic control of plant diversity. Glob. Ecol. Biogeogr. 2019, 29, 1634–1650. [Google Scholar] [CrossRef]
- Rajakaruna, N.; Whitton, J. Trends in the evolution of edaphicspecialists with an example of parallel evolution in the Lastheniacalifornica complex. In Plant Adaptation Molecular Genetics and Ecology; Cronk, Q.C.B., Whitton, J., Ree, R.H., Taylor, I.E.P., Eds.; NRC Research Press: Ottawa, ON, Canada, 2004; pp. 103–110. [Google Scholar]
- Zverev, A.; Kichko, A.; Shapkin, V.; Pinaev, A.; Provorov, N.; Andronov, E. Does plant diversity determine the diversity of the rhizosphere microbial community? bioRxiv 2021. [Google Scholar] [CrossRef]
- Mori, A.S.; Isbell, F.; Seidl, R. β-diversity, community assembly, and ecosystem functioning. Trends Ecol. Evol. 2018, 33, 549–564. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.F.; Zhai, G.Q.; Zhu, S.S.; Liu, X.J.; Schmid, B.; Wang, Z.H.; Ma, K.P.; Feng, X.J. Plant and microbial pathways driving plant diversity effects on soil carbon accumulation in subtropical forest. Soil Biol. Biochem. 2021, 161, 108375. [Google Scholar] [CrossRef]
- Schober, R. Von der Niederdurchforstung zu Auslesedurchforstungen imHerrschenden. AFJZ 1988, 159, 208–213. [Google Scholar]
- Zeller, L.; Caicoya, A.T.; Pretzsch, H. The effect of silvicultural management on the trade-off between stand structural heterogeneity and productivity over time. Eur. J. For. Res. 2021, 140, 615–634. [Google Scholar] [CrossRef]
- de Prado, D.R.; San Martín, R.; Bravo, F.; de Aza, C.H. Potential climatic influence on maximum stand carrying capacity for 15 Mediterranean coniferous and broadleaf species. For. Ecol. Manag. 2020, 460, 117824. [Google Scholar] [CrossRef]
- Pretzsch, H. Facilitation and competition reduction in tree species mixtures in Central Europe: Consequences for growth modeling and forest management. Ecol. Model. 2022, 464, 109812. [Google Scholar] [CrossRef]
- Huang, K.; Xia, J.Y.; Wang, Y.P.; Ahlström, A.; Chen, J.Q.; Cook, R.B.; Cui, E.Q.; Fang, Y.Y.; Fisher, J.B.; Huntzinger, D.N.; et al. Enhanced peak growth of global vegetation and its key mechanisms. Nat. Ecol. Evol. 2018, 2, 1897. [Google Scholar] [CrossRef] [PubMed]
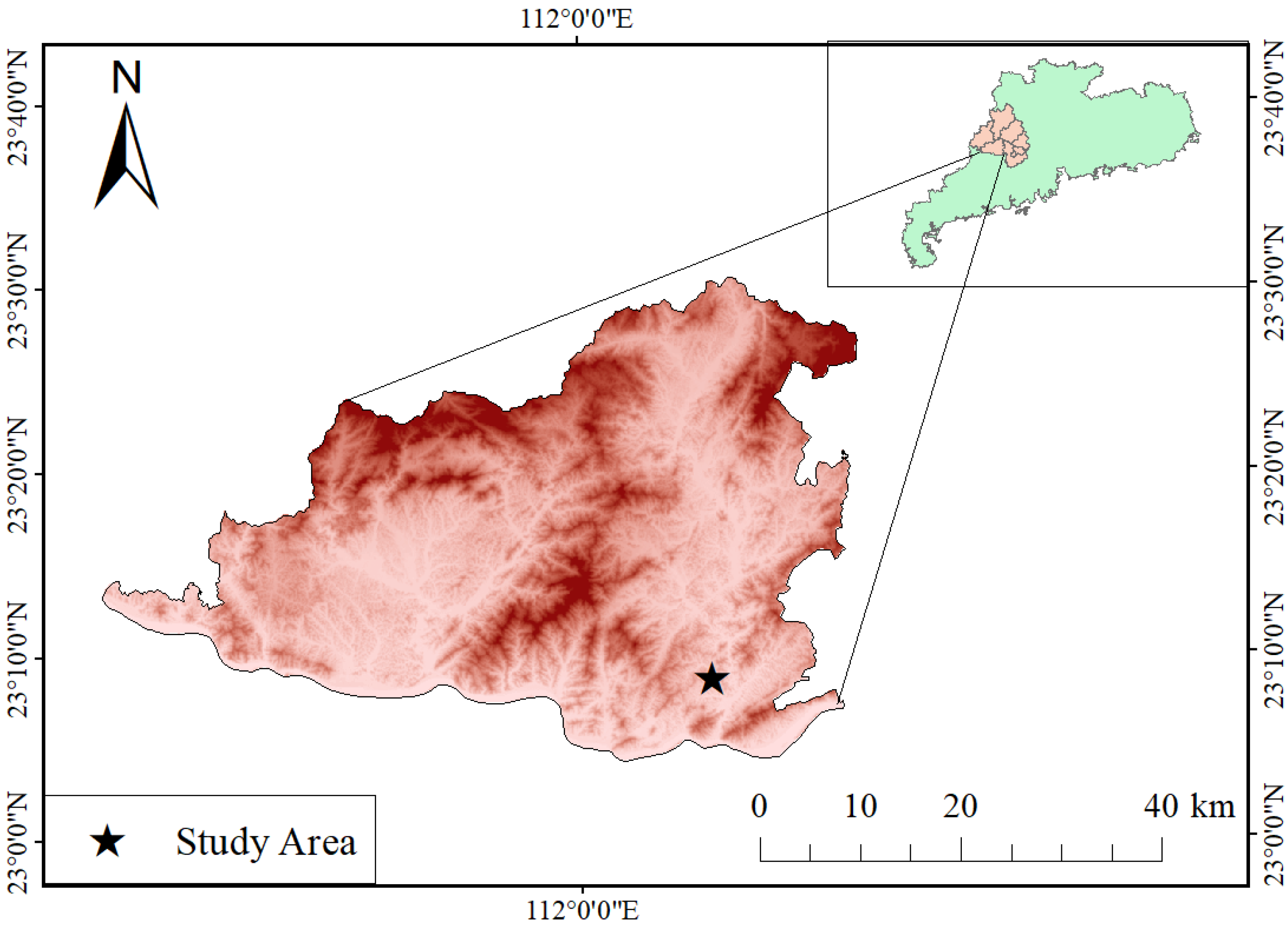

| Plot | General Information | Prior | After Thinning | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Altitude (m) | Slope (°) | Soil Depth (cm) | Density (Tree/hm2) | Canopy Density (%) | Diameter at Breast Height (cm) | Height (m) | Volume (m3/hm2) | Density (Tree/hm2) | Canopy Density (%) | Diameter at Breast Height (cm) | Height (m) | Volume (m3/hm2) | |
| 1 | 61 | 24 | 80 | 1570 | 78 | 9.7 | 9.5 | 56.77 | 1570 | 78 | 9.7 | 9.5 | 55.77 |
| 2 | 46 | 28 | 70 | 1591 | 81 | 9.7 | 8.7 | 61.77 | 1591 | 81 | 9.7 | 8.7 | 62.77 |
| 3 | 60 | 21 | 70 | 1588 | 83 | 8.5 | 7.2 | 56.37 | 1588 | 83 | 8.5 | 7.2 | 56.37 |
| 4 | 49 | 26 | 60 | 1576 | 83 | 9.2 | 7.7 | 58.85 | 1016 | 56 | 9.3 | 8 | 45.29 |
| 5 | 44 | 25 | 70 | 1584 | 78 | 9.5 | 9.6 | 55.68 | 1196 | 57 | 9.6 | 9.6 | 46.39 |
| 6 | 59 | 16 | 80 | 1593 | 82 | 10.2 | 9.4 | 59.91 | 1368 | 57 | 10.3 | 9.3 | 43.79 |
| 7 | 72 | 25 | 60 | 1562 | 85 | 10.2 | 8.1 | 63.16 | 1044 | 48 | 10.2 | 8 | 40.22 |
| 8 | 46 | 28 | 80 | 1628 | 76 | 8.6 | 8.5 | 56.23 | 1012 | 58 | 8.6 | 8.6 | 38.14 |
| 9 | 73 | 22 | 80 | 1589 | 81 | 8.6 | 8.2 | 54.49 | 1004 | 55 | 9 | 8.6 | 38.59 |
| 10 | 55 | 35 | 80 | 1568 | 85 | 10.3 | 9.3 | 53.53 | 846 | 44 | 10.3 | 9.2 | 33.68 |
| 11 | 61 | 24 | 80 | 1570 | 78 | 8.7 | 7.4 | 60.86 | 880 | 43 | 9 | 8.3 | 34.22 |
| 12 | 48 | 20 | 60 | 1577 | 77 | 8.3 | 7.8 | 60.18 | 878 | 44 | 8.5 | 8.7 | 29.98 |
| Prior (t·ha−1) | Thinned (t·ha−1) | |
|---|---|---|
| control | 24.87 ± 1.7 aA | 24.24 ± 1.7 bA |
| 20%~30% | 23.53 ± 0.7 aA | 17.17 ± 1.4 aA |
| 31%~40% | 25.96 ± 1.4 aA | 20.02 ± 1.7 abA |
| 41%~50% | 27.87 ± 1.2 aB | 20.69 ± 1.9 abA |
| Treatment | Total C(t·ha−1) | Soil C(t·ha−1) | Tree C (t·ha−1) | Litter C(t·ha−1) | ||||
|---|---|---|---|---|---|---|---|---|
| Prior | Thinned | Prior | Thinned | Prior | Thinned | Prior | Thinned | |
| Control | 159.24 ± 10.5 aA | 163.95 ± 16.5 aA | 113.72 ± 12.1 aA | 115.98 ± 13.6 aA | 34.38 ± 3.8 aA | 38.86 ± 4.8 aA | 11.15 ± 1.7 aA | 9.10 ± 1.3 aA |
| 20%~30% | 163.65 ± 3.8 aA | 171.66 ± 5.1 aA | 124.00 ± 4.2 aA | 142.92 ± 6.7 aA | 28.77 ± 1.5 aA | 21.55 ± 1.6 aA | 10.88 ± 0.7 aA | 7.18 ± 0.7 aA |
| 31%~40% | 189.89 ± 21.5 aA | 147.69 ± 6.0 aA | 142.30 ± 13.9 aA | 115.71 ± 9.8 aA | 35.58 ± 6.2 aA | 23.44 ± 2.8 aA | 12.01 ± 1.4 aA | 8.54 ± 1.3 aA |
| 41%~50% | 214.02 ± 14.3 aB | 137.54 ± 8.2 aA | 159.24 ± 19.1 aA | 102.11 ± 6.3 aA | 42.30 ± 4.5 aA | 27.40 ± 3.7 aA | 12.47 ± 0.8 aA | 8.02 ± 0.7 aA |
| Tree Biomass C | Litter C | Soil C | |
|---|---|---|---|
| Prior | 19.41 | 6.40 | 74.19 |
| Control | 23.70 | 5.55 | 70.75 |
| 20%~30% | 12.55 | 4.18 | 83.27 |
| 21%~40% | 15.87 | 5.78 | 78.35 |
| 41%~50% | 19.92 | 5.83 | 74.25 |
| Treatment | pH | Coverage (%) | LAI |
|---|---|---|---|
| Prior | 4.12 ± 0.1 b | 70.61 ± 2.8 ab | 1.68 ± 0.1 a |
| Control | 3.99 ± 0.1 ab | 79.19 ± 3.0 b | 2.22 ± 0.2 a |
| 20%~30% | 3.84 ± 0.0 a | 61.39 ± 6.4 ab | 1.13 ± 0.2 a |
| 21%~40% | 3.85 ± 0.1 a | 52.63 ± 10.8 a | 1.13 ± 0.4 a |
| 41%~50% | 3.98 ± 0.10 ab | 69.19 ± 4.5 ab | 1.68 ± 0.3 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, N.; Feng, M.; Huang, H.; Qiu, Z.; Ma, T.; Chen, S. Effects of Thinning on Carbon Storage in a Mixed Broadleaved Plantation in a Subtropical Area of China. Forests 2024, 15, 638. https://doi.org/10.3390/f15040638
Lin N, Feng M, Huang H, Qiu Z, Ma T, Chen S. Effects of Thinning on Carbon Storage in a Mixed Broadleaved Plantation in a Subtropical Area of China. Forests. 2024; 15(4):638. https://doi.org/10.3390/f15040638
Chicago/Turabian StyleLin, Na, Mingchun Feng, Huanqiang Huang, Zhanpeng Qiu, Tao Ma, and Shiqing Chen. 2024. "Effects of Thinning on Carbon Storage in a Mixed Broadleaved Plantation in a Subtropical Area of China" Forests 15, no. 4: 638. https://doi.org/10.3390/f15040638






