Soil Environments Regulate Dominant Soil Fungal Communities along an Elevational Gradient in Subtropical Forests
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Location
2.2. Sample Plot Setting
2.3. Microenvironment Detection
2.4. Soil Sample Collection
2.5. Soil Samples Measurement
2.5.1. Determination of Soil Physical and Chemical Properties
2.5.2. DNA Extraction, Sequencing and Bioinformatics Analysis
2.6. Data Analysis
3. Results
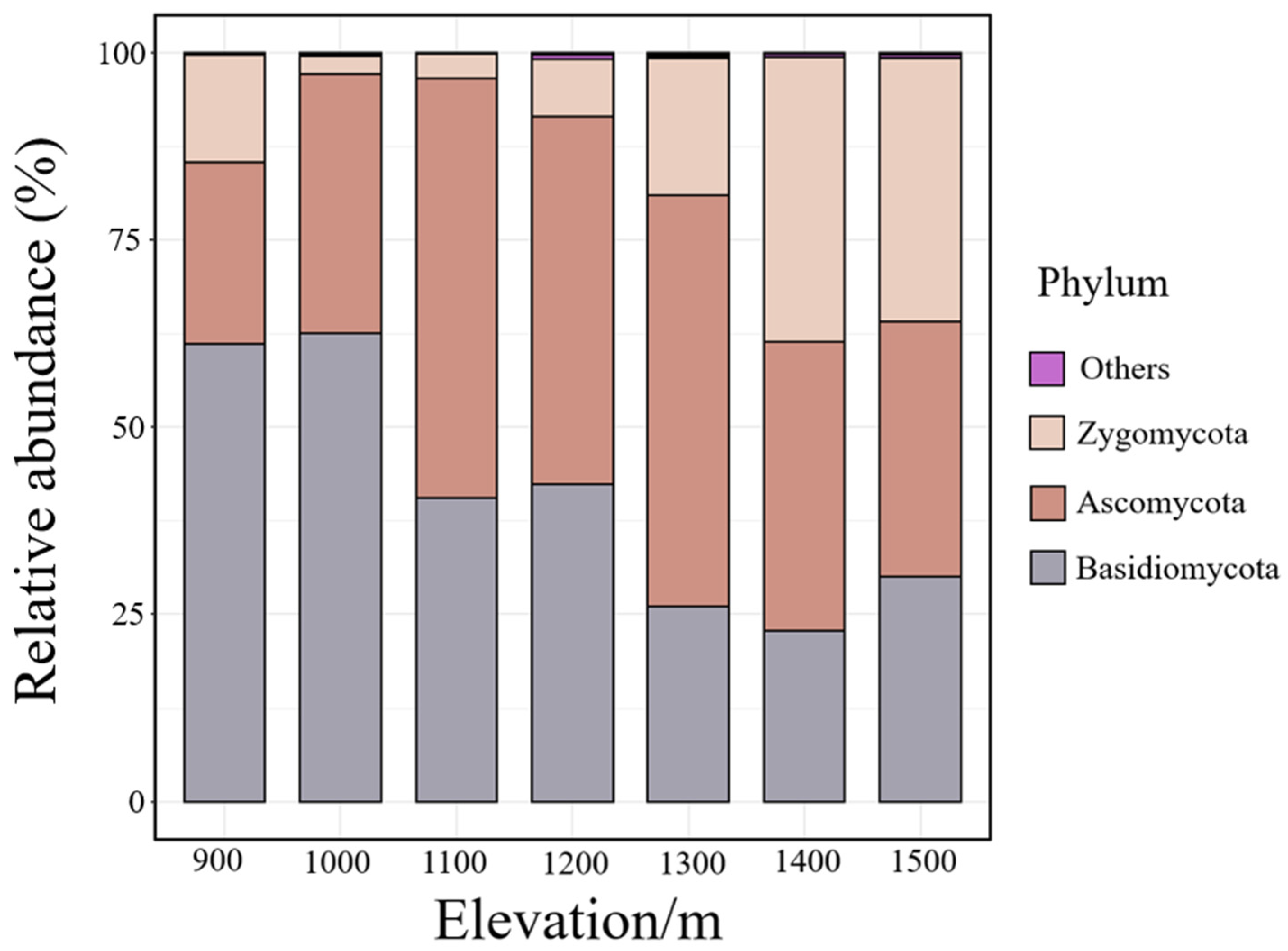
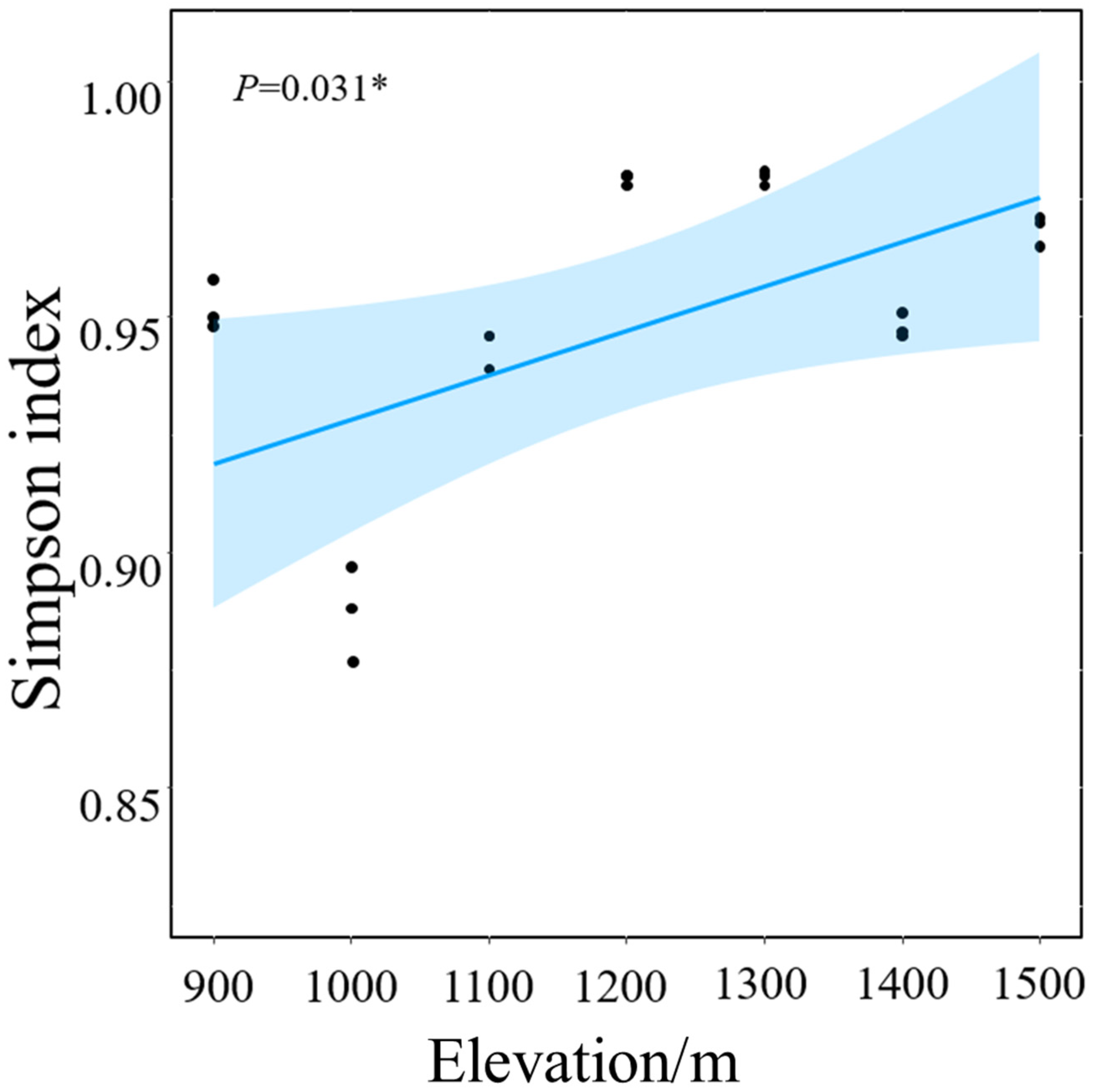
3.1. Soil Fungal Community Structure and α Diversity
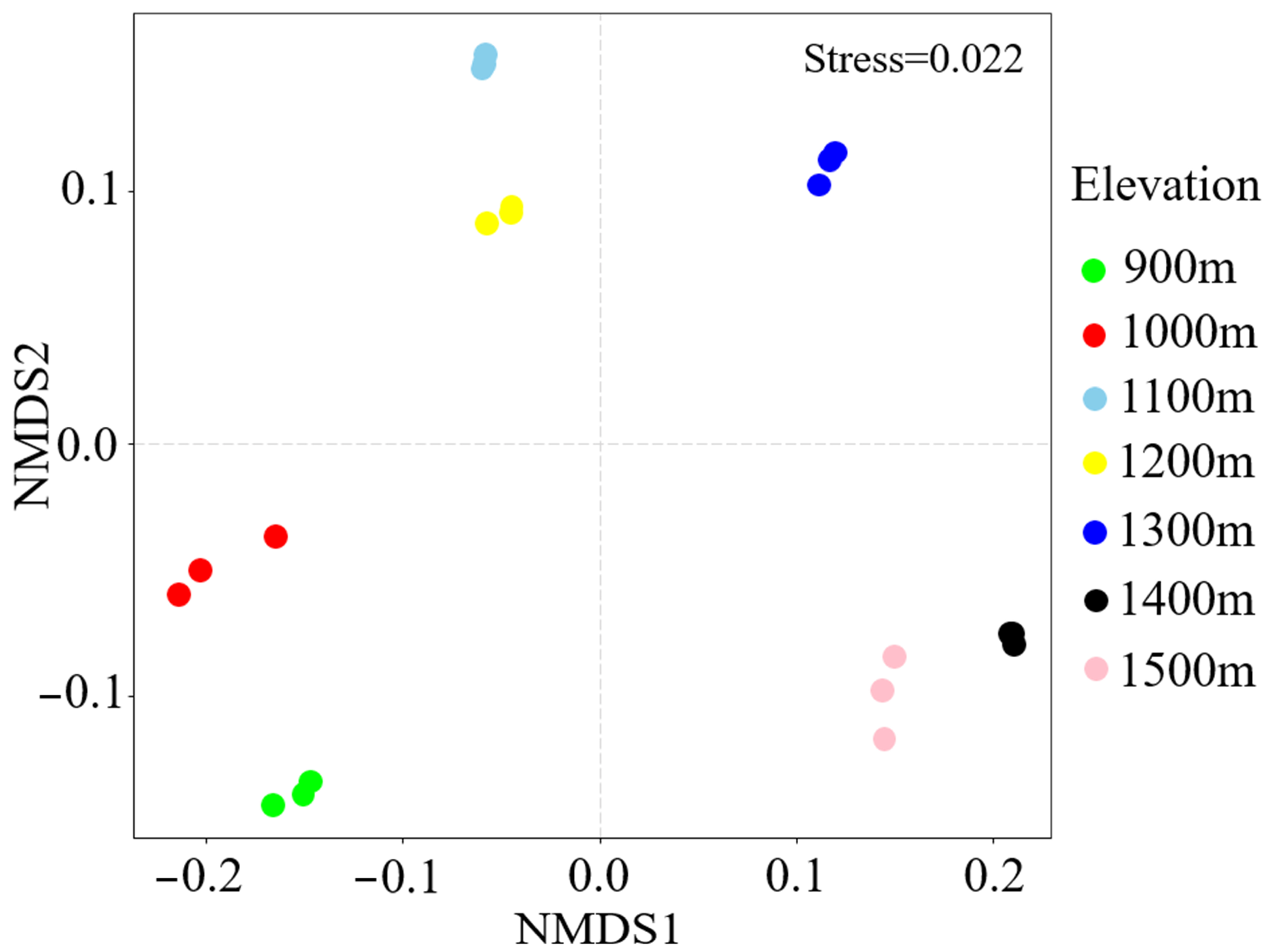
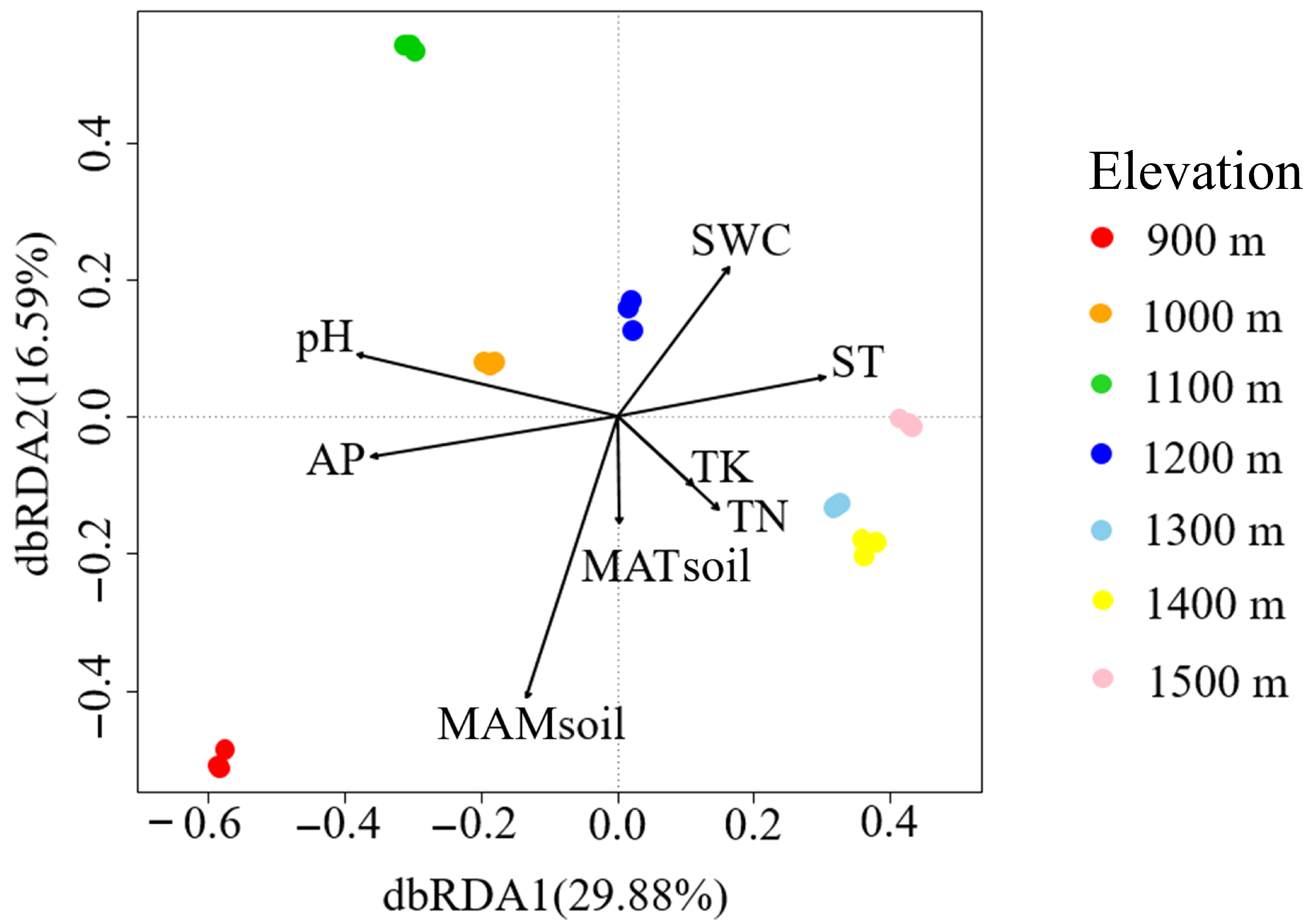
3.2. β-Diversity of Soil Fungal Community
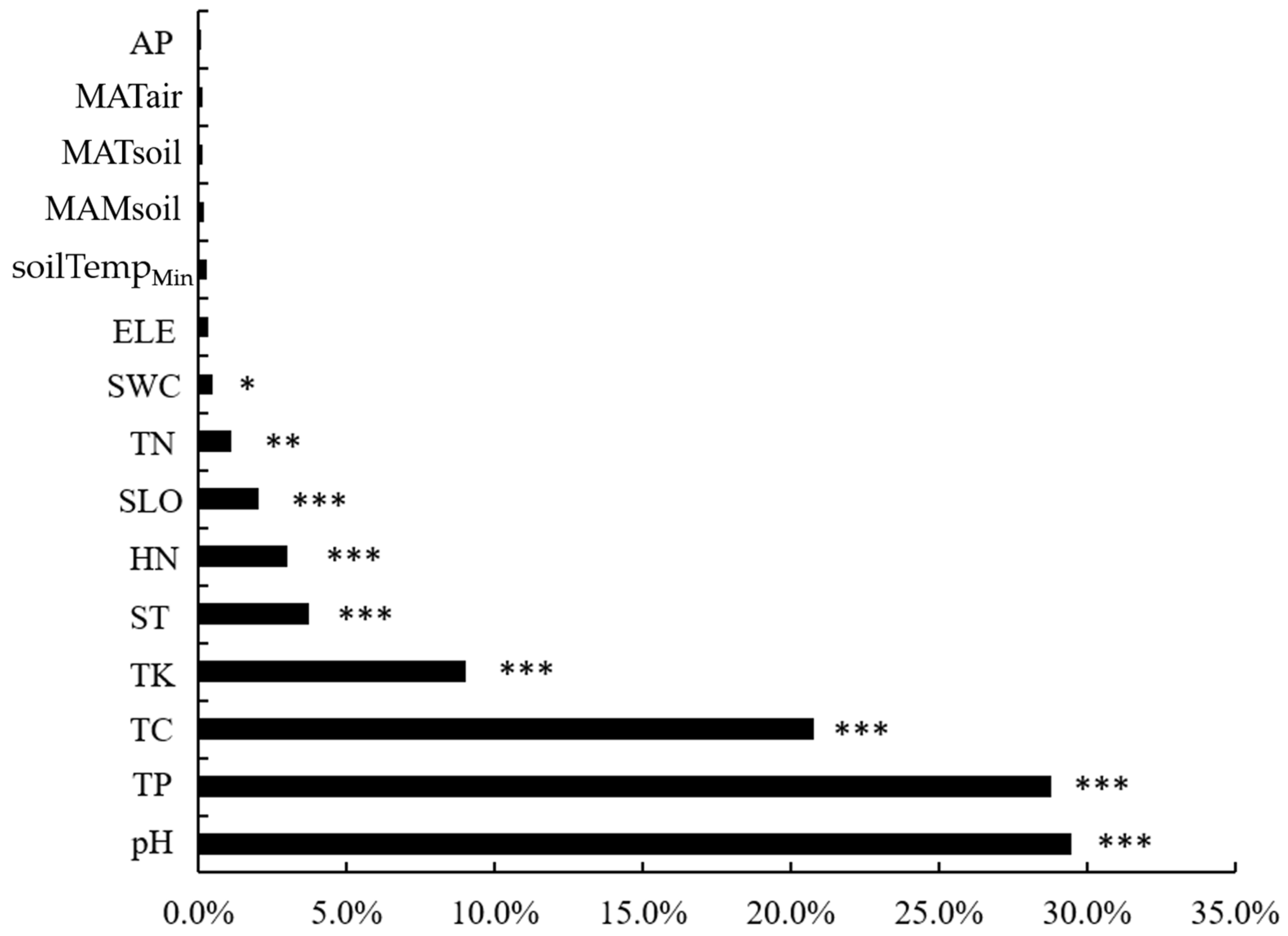
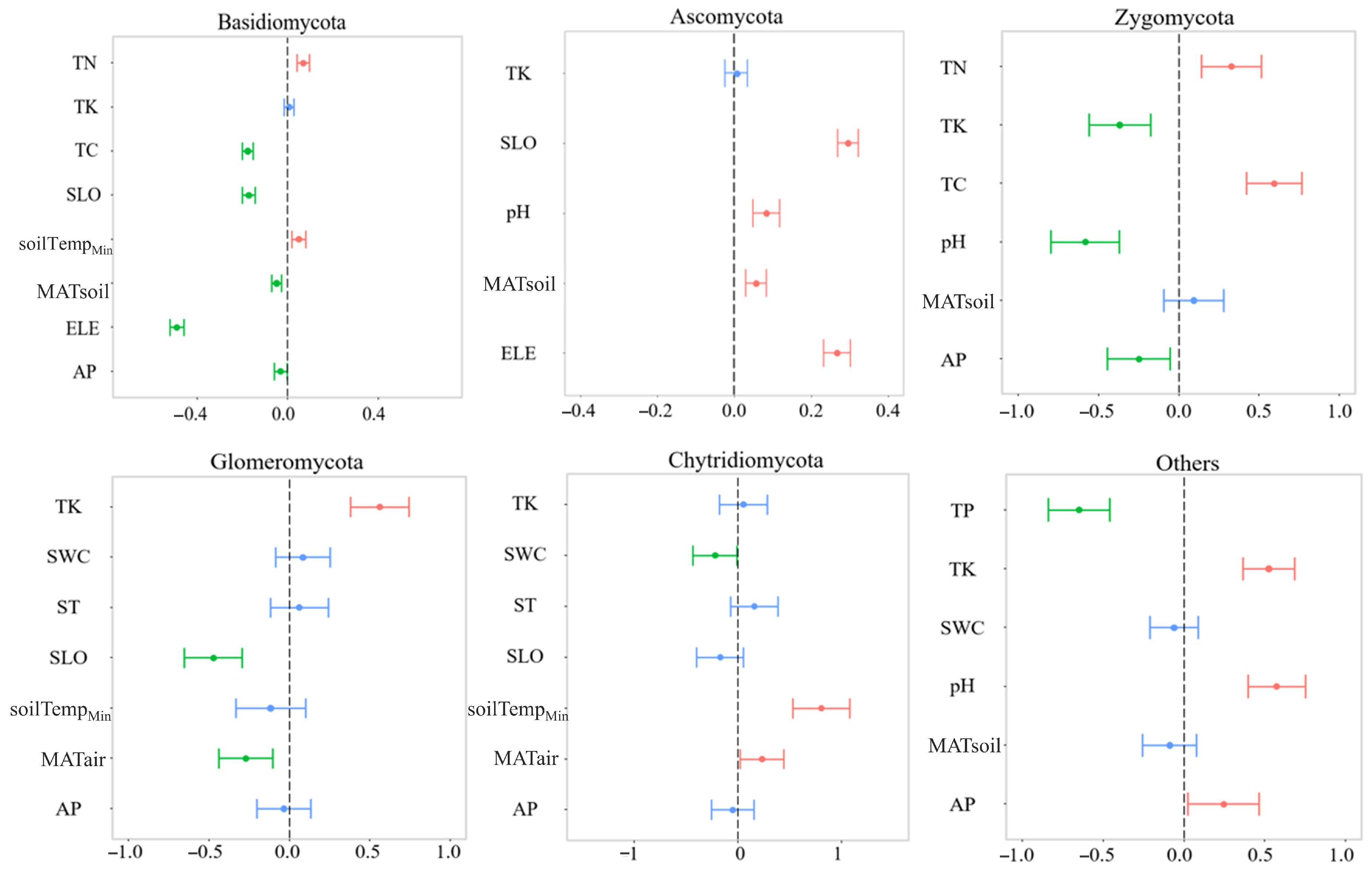
3.3. Dominant Soil Fungal Community and Soil Environmental Factors Relationship
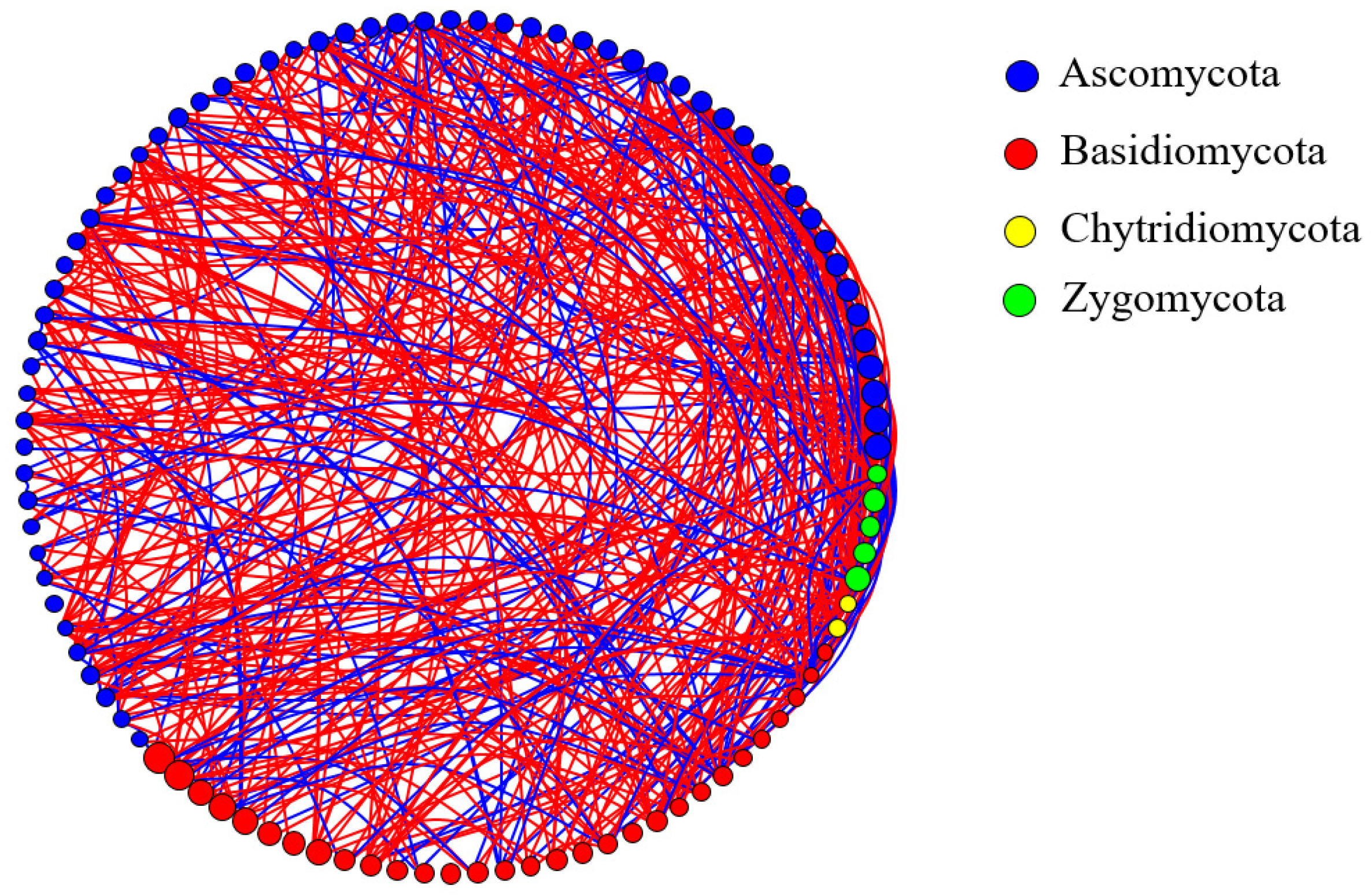
3.4. Co-Occurrence Network Analysis of Dominant Soil Fungal Communities
4. Discussion
4.1. Composition and Diversity of Soil Fungal Communities at Various Elevations
4.2. Factors Influencing the Soil Fungal Community along an Elevational Gradient
4.3. Dominant Soil Fungal Community and Network Interaction Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, Y.L.; Deng, Y.; Ding, J.Z.; Hu, H.W.; Xu, T.L.; Li, F.; Yang, G.B.; Yang, Y.H. Distinct microbial communities in the active and permafrost layers on the Tibetan Plateau. Mol. Ecol. 2017, 26, 6608–6620. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.X.; Bing, H.J.; Fang, L.C.; Wu, Y.H.; Yu, J.L.; Shen, G.T.; Jiang, M.; Wang, X.; Zhang, X.C. Diversity patterns of the rhizosphere and bulk soil microbial communities along an elevational gradient in an alpine ecosystem of the eastern Tibetan Plateau. Geoderma 2019, 338, 118–127. [Google Scholar] [CrossRef]
- Liu, D.; Wang, H.L.; An, S.S.; Bhople, P.; Davlatbekov, F. Geographic distance and soil microbial biomass carbon drive biogeographical distribution of fungal communities in Chinese Loess Plateau soils. Sci. Total Environ. 2019, 660, 1058–1069. [Google Scholar] [CrossRef]
- Margesin, R.; Niklinska, M.A. Elevation gradients: Microbial indicators of climate change? Front. Microbiol. 2019, 10, 2405. [Google Scholar] [CrossRef]
- Bahram, M.; Hildebrand, F.; Forslund, S.K.; Anderson, J.L.; Soudzilovskaia, N.A.; Bodegom, P.M.; Bengtsson-Palme, J.; Anslan, S.; Coelho, L.P.; Harend, H.; et al. Structure and function of the global topsoil microbiome. Nature 2018, 560, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Siles, J.A.; Cajthaml, T.; Filipová, A.; Minerbi, S.; Margesin, R. Altitudinal, seasonal and interannual shifts in microbial communities and chemical composition of soil organic matter in Alpine forest soils. Soil Biol. Biochem. 2017, 112, 1–13. [Google Scholar] [CrossRef]
- Zhong, Z.M.; Zhang, G.Y.; Fu, G. Effect of Experiment Warming on Soil Fungal Community of Medicago sativa, Elymus nutans and Hordeum vulgare in Tibet. J. Fungi 2023, 9, 885. [Google Scholar] [CrossRef]
- Li, G.X.; Xu, G.R.; Shen, C.C.; Tang, Y.; Zhang, Y.X.; Ma, K.M. Contrasting elevational diversity patterns for soil bacteria between two ecosystems divided by the treeline. Sci. China—Life Sci. 2016, 59, 1177–1186. [Google Scholar] [CrossRef]
- Ren, C.J.; Liu, W.C.; Zhao, F.Z.; Zhong, Z.K.; Deng, J.; Han, X.H.; Yang, G.H.; Feng, Y.Z.; Ren, G.X. Soil bacterial and fungal diversity and compositions respond differently to forest development. Catena 2019, 181, 104071. [Google Scholar] [CrossRef]
- Crowther, T.W.; Hoogen, J.v.D.; Wan, J.; Mayes, M.A.; Keiser, A.D.; Mo, L.; Averill, C.; Maynard, D.S. The global soil community and its influence on biogeochemistry. Science 2019, 365, eaav0550. [Google Scholar] [CrossRef]
- Looby, C.I.; Martin, P.H. Diversity and function of soil microbes on montane gradients: The state of knowledge in a changing world. FEMS Microbiol. Ecol. 2020, 96, fiaa122. [Google Scholar] [CrossRef] [PubMed]
- Schoenborn, A.A.; Yannarell, S.M.; MacVicar, C.T.; Barriga-Medina, N.N.; Bonham, K.S.; Leon-Reyes, A.; Riveros-Iregui, D.; Klepac-Ceraj, V.; Shank, E.A. Microclimate is a strong predictor of the native and invasive plant-associated soil microbiome on San Cristóbal Island, Galápagos archipelago. Environ. Microbiol. 2023, 25, 1377–1392. [Google Scholar] [CrossRef] [PubMed]
- Frąc, M.; Jędryczka, M.; Hannula, E.S. Editorial: Soil fungal biodiversity for plant and soil health, volume II. Front. Microbiol. 2023, 14, 1170312. [Google Scholar] [CrossRef] [PubMed]
- Tedersoo, L.; Bahram, M.; Põlme, S.; Kõljalg, U.; Yorou, N.S.; Wijesundera, R.; Ruiz, L.V.; Vasco–Palacios, A.M.; Thu, P.Q.; Suija, A.; et al. Global diversity and geography of soil fungal. Science 2014, 346, 1256688. [Google Scholar] [CrossRef] [PubMed]
- Sui, X.; Li, M.S.; Frey, B.; Dai, G.H.; Yang, L.B.; Li, H.M. Effect of elevation on composition and diversity of fungal in the rhizosphere of a population of Deyeuxia angustifolia on Changbai Mountain, northeastern China. Front. Microbiol. 2023, 14, 1087475. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Cáceres, D.; Stokes, A.; Angeles–Alvarez, G.; Abadie, J.; Anthelme, F.; Bounous, M.; Freschet, G.T.; Roumet, C.; Weemstra, M.; Merino–Martín, L.; et al. Vegetation creates microenvironments that influence soil microbial activity and functional diversity along an elevation gradient. Soil Biol. Biochem. 2021, 165, 108485. [Google Scholar] [CrossRef]
- Jiang, L.; Zhang, X.; Zhu, J.; Wei, X.; Chen, B.; Liu, J.F.; Zheng, S.Q.; He, Z.S. Environmental heterogeneity determines beta diversity and species turnover for woody plants along an elevation gradient in subtropical forests of China. For. Res. 2023, 3, 26. [Google Scholar] [CrossRef]
- He, Z.S.; Wang, Z.W.; Zhu, J.; Chen, J.J.; Gu, X.G.; Jiang, L.; Chen, B.; Wu, Z.Y.; Liu, J.F.; Chen, W.W. Characteristics of microbial functional diversity and its influencing factors of forest soils at different elevations on the southern slope of Daiyun Mountain. Acta Ecol. Sin. 2022, 42, 3504–3515. (In Chinese) [Google Scholar]
- Chen, R.Z.; Zhu, J.; Jiang, L.; Liu, L.; Gao, C.; Chen, B.; Xu, D.W.; Liu, J.F.; He, Z.S. Soil phosphorus availability controls deterministic and stochastic processes of soil microbial community along an elevational gradient in subtropical forests. Forests 2023, 14, 1475. [Google Scholar] [CrossRef]
- Luo, X.Y.; Li, Q.; Huang, X.L. DNA barcode reference dataset for flower-visiting insects in Daiyun Mountain National Nature Reserve. Biodivers. Sci. 2023, 31, 23236. (In Chinese) [Google Scholar]
- Su, S.J.; Liu, J.F.; He, Z.S.; Zheng, S.Q.; Hong, W.; Xu, D.W. Ecological species groups and interspecific association of dominant tree species in Daiyun Mountain National Nature Reserve. J. Mt. Sci. 2015, 12, 637–646. [Google Scholar] [CrossRef]
- Wild, J.; Kopecký, M.; Macek, M.; Šanda, M.; Jankovec, J.; Haase, T. Climate at ecologically relevant scales: A new temperature and soil moisture logger for long-term microclimate measurement. Agric. For. Meteorol. 2019, 268, 40–47. [Google Scholar] [CrossRef]
- Wang, X.L.; Liu, J.F.; He, Z.S.; Xing, C.; Zhu, J.; Gu, X.G.; Lan, Y.Q.; Wu, Z.Y.; Liao, P.C.; Zhu, D.H. Forest gaps mediate the structure and function of the soil microbial community in a Castanopsis kawakamii forest. Ecol. Indic. 2021, 122, 107288. [Google Scholar] [CrossRef]
- State Forestry Administration. Forest Soil Analysis Method; China Standards Press: Beijing, China, 1999. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing (Version 4.2.3); R Foundation for Statistical Computing: Vienna, Austria. Available online: https://www.R-project.org/ (accessed on 26 June 2023).
- Anderson, M.J. Permutational Multivariate Analysis of Variance (PERMANOVA); Balakrishnan, N., Colton, T., Everitt, B., Piegorsch, W., Ruggeri, F., Teugels, J.L., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2017; pp. 1–15. [Google Scholar]
- Albano, A.; García–Lapresta, J.L.; Plaia, A.; Sciandra, M. Clustering alternatives in preference-approvals via novel pseudometrics. Stat. Methods Appl. 2023, 1–27. [Google Scholar] [CrossRef]
- Shen, C.C.; Gunina, A.; Luo, Y.; Wang, J.J.; He, J.Z.; Kuzyakov, Y.; Hemp, A.; Classen, A.T.; Ge, Y. Contrasting patterns and drivers of soil bacterial and fungal diversity across a mountain gradient. Environ. Microbiol. 2020, 22, 3287–3301. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.W.; Liu, L.; Lu, Y.S.; Chen, L.; Zhang, Z.C.; Zhang, H.W.; Wang, X.R.; Shu, L.; Yang, Q.P.; Song, Q.N.; et al. When microclimates meet soil microbes: Temperature controls soil microbial diversity along an elevational gradient in subtropical forests. Soil Biol. Biochem. 2022, 166, 108566. [Google Scholar] [CrossRef]
- Nie, S.A.; Lei, X.M.; Zhao, L.X.; Brookes, P.C.; Wang, F.; Chen, C.R.; Yang, W.H.; Xing, S.H. Fungal communities and functions response to long-term fertilization in paddy soils. Appl. Soil Ecol. 2018, 130, 251–258. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, C.; Wang, M.L. Linkages of C: N: P stoichiometry between soil and leaf and their response to climatic factors along elevational gradients. J. Soils Sediments 2019, 19, 1820–1829. [Google Scholar] [CrossRef]
- Thakur, M.P.; Milcu, A.; Manning, P.; Niklaus, P.A.; Roscher, C.; Power, S.; Reich, P.B.; Scheu, S.; Tilman, D.; Ai, F.X.; et al. Plant diversity drives soil microbial biomass carbon in grasslands irrespective of global environmental change factors. Glob. Change Biol. 2015, 21, 4076–4085. [Google Scholar] [CrossRef]
- Sterkenburg, E.; Bahr, A.; Durling, M.B.; Clemmensen, K.E.; Lindahl, B.D. Changes in fungal communities along a boreal forest soil fertility gradient. New Phytol. 2015, 207, 1145–1158. [Google Scholar] [CrossRef]
- Tong, S.; Cao, G.C.; Zhang, Z.; Zhang, J.H.; Yan, X. Soil microbial community diversity and distribution characteristics under three vegetation types in the Qilian Mountains, China. J. Arid Land 2023, 15, 359–376. [Google Scholar] [CrossRef]
- Lundell, T.K.; Mäkelä, M.R.; Hildén, K. Lignin-modifying enzymes in filamentous basidiomycetes–ecological, functional and phylogenetic review. J. Basic Microbiol. 2010, 50, 5–20. [Google Scholar] [CrossRef] [PubMed]
- Egidi, E.; Delgado–Baquerizo, M.; Plett, J.M.; Wang, J.; Eldridge, D.J.; Bardgett, R.D.; Maestre, F.T.; Singh, B.K. A few Ascomycota taxa dominate soil fungal communities worldwide. Nat. Commun. 2019, 10, 2369. [Google Scholar] [CrossRef]
- Donhauser, J.; Frey, B. Alpine soil microbial ecology in a changing world. FEMS Microbiol. Ecol. 2018, 94, fiy099. [Google Scholar] [CrossRef]
- Siles, J.A.; Margesin, R. Abundance and diversity of bacterial, archaeal, and fungal communities along an elevationtudinal gradient in alpine forest soils: What are the driving factors? Microb. Ecol. 2016, 72, 207–220. [Google Scholar] [CrossRef]
- Peng, S.L.; Ge, Z.W.; Liu, G.C.; Mao, L.F. Environmental drivers of soil microbial activity and diversity along an elevational gradient. J. Mt. Sci. 2022, 19, 1336–1347. [Google Scholar] [CrossRef]
- Zhao, W.; Wang, D.D.; Huang, K.C.; Liu, S.; Reyila, M.; Sun, Y.F.; Li, J.N.; Cui, B.K. Seasonal variation in the soil fungal community structure of Larix gmelinii forests in Northeast China. Front. Microbiol. 2023, 14, 1106888. [Google Scholar] [CrossRef]
- Feng, J.; Zeng, X.M.; Zhang, Q.G.; Zhou, X.Q.; Liu, Y.R.; Huang, Q.Y. Soil microbial trait-based strategies drive metabolic efficiency along an elevation gradient. ISME Commun. 2021, 1, 71. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Walder, F.; Büchi, L.; Meyer, M.; Held, A.Y.; Gattinger, A.; Keller, T.; Charles, R.; Van der Heijden, M.G.A. Agricultural intensification reduces microbial network complexity and the abundance of keystone taxa in roots. ISME J. 2019, 13, 1722–1736. [Google Scholar] [CrossRef]
- Yang, Y.Z.; Gao, Y.C.; Huang, X.N.; Ni, P.; Wu, Y.N.; Deng, Y.; Zhan, A.B. Adaptive shifts of bacterioplankton communities in response to nitrogen enrichment in a highly polluted river. Environ. Pollut. 2018, 245, 290–299. [Google Scholar] [CrossRef]
- Zhang, J.X.; Yang, Y.Y.; Zhao, L.; Li, Y.Z.; Xie, S.G.; Liu, Y. Distribution of sediment bacterial and archaeal communities in plateau freshwater lakes. Appl. Microbiol. Biotechnol. 2014, 99, 3291–3302. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Hu, Y.T.; Zhang, S.S.; Noll, L.; Böckle, T.; Dietrich, M.; Herbold, C.W.; Eichorst, S.A.; Woebken, D.; Richter, A.; et al. Soil multifunctionality is affected by the soil environment and by microbial community composition and diversity. Soil Biol. Biochem. 2019, 136, 107521. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.Y.; Yang, T.; Zhang, K.P.; Shen, C.C.; Chu, H.Y. Fungal communities along a small-scale elevational gradient in an alpine tundra are determined by soil carbon nitrogen ratios. Front. Microbiol. 2018, 9, 1815. [Google Scholar] [CrossRef]
- Liu, J.Y.; Ding, C.J.; Zhang, W.X.; Wei, Y.W.; Zhou, Y.B.; Zhu, W.X. Litter mixing promoted decomposition rate through increasing diversities of phyllosphere microbial communities. Front. Microbiol. 2022, 13, 1009091. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.T.; Sun, Z.H.; Mishra, S.; Xia, S.W.; Lin, L.X.; Yang, X.D. Body size determines multitrophic soil microbiota community assembly associated with soil and plant attributes in a tropical seasonal rainforest. Mol. Ecol. 2022, 32, 6294–6303. [Google Scholar] [CrossRef] [PubMed]
- de Menezes, A.B.; Richardson, A.E.; Thrall, P.H. Linking fungal–bacterial co-occurrences to soil ecosystem function. Curr. Opin. Microbiol. 2017, 37, 135–141. [Google Scholar] [CrossRef]
- Bayranvand, M.; Akbarinia, M.; Salehi Jouzani, G.; Gharechahi, J.; Kooch, Y.; Baldrian, P. Composition of soil bacterial and fungal communities in relation to vegetation composition and soil characteristics along an elevational gradient. FEMS Microbiol. Ecol. 2021, 97, fiaa201. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Wang, Z.; Zhang, W.; Zhu, J.; Chen, B.; Jiang, L.; Xu, D.; Li, W.; Liu, J.; He, Z. Soil Environments Regulate Dominant Soil Fungal Communities along an Elevational Gradient in Subtropical Forests. Forests 2024, 15, 643. https://doi.org/10.3390/f15040643
Li Z, Wang Z, Zhang W, Zhu J, Chen B, Jiang L, Xu D, Li W, Liu J, He Z. Soil Environments Regulate Dominant Soil Fungal Communities along an Elevational Gradient in Subtropical Forests. Forests. 2024; 15(4):643. https://doi.org/10.3390/f15040643
Chicago/Turabian StyleLi, Zhihui, Ziwei Wang, Wenyang Zhang, Jing Zhu, Bo Chen, Lan Jiang, Daowei Xu, Wenzhou Li, Jinfu Liu, and Zhongsheng He. 2024. "Soil Environments Regulate Dominant Soil Fungal Communities along an Elevational Gradient in Subtropical Forests" Forests 15, no. 4: 643. https://doi.org/10.3390/f15040643
APA StyleLi, Z., Wang, Z., Zhang, W., Zhu, J., Chen, B., Jiang, L., Xu, D., Li, W., Liu, J., & He, Z. (2024). Soil Environments Regulate Dominant Soil Fungal Communities along an Elevational Gradient in Subtropical Forests. Forests, 15(4), 643. https://doi.org/10.3390/f15040643





