Composition of Attractant Semiochemicals of North American Species of Dendroctonus Bark Beetles: A Review
Abstract
1. Introduction
2. Natural Sources of Attractants in Dendroctonus
3. Analytical Techniques for Identification of Dendroctonus Attractants
4. Behavioral Bioassay Techniques for Identification of Dendroctonus Attractants
5. Mechanistic Classifications for Attractants Based on Experimental Data
6. Functional Classifications for Attractants Based on Experimental Data
7. Pitfalls of Executing and Interpreting Trapping Experiments
8. Composition of Attractive Pheromones of Dendroctonus
9. Composition of Attractive Host Odors for Dendroctonus
10. Differences in Responses between the Sexes
11. Composition of Attractants for North American Species of Dendroctonus
11.1. Dendroctonus frontalis Zimmermann—Southern Pine Beetle
11.2. Dendroctonus mesoamericanus Armendáriz-Toledano & Sullivan
11.3. Dendroctonus vitei Wood
11.4. Dendroctonus mexicanus Hopkins—Smaller Mexican Pine Beetle
11.5. Dendroctonus approximatus Dietz—Larger Mexican Pine Beetle
11.6. Dendroctonus brevicomis LeConte—Western Pine Beetle
11.7. Dendroctonus barberi Hopkins—Southwestern Pine Beetle
11.8. Dendroctonus adjunctus Blandford—Roundheaded Pine Beetle
11.9. Dendroctonus parallelocollis Chapuis
11.10. Dendroctonus ponderosae Hopkins—Mountain Pine Beetle
11.11. Dendroctonus jeffreyi Hopkins—Jeffrey Pine Beetle
11.12. Dendroctonus rhizophagus Thomas & Bright
11.13. Dendroctonus valens LeConte—Red Turpentine Beetle
11.14. Dendroctonus terebrans (Olivier)—Black Turpentine Beetle
11.15. Dendroctonus murrayanae Hopkins—Lodgepole Pine Beetle
11.16. Dendroctonus punctatus LeConte —Boreal Spruce Beetle
11.17. Dendroctonus rufipennis (Kirby)—Spruce Beetle
11.18. Dendroctonus simplex LeConte—Eastern Larch Beetle
11.19. Dendroctonus pseudotsugae Hopkins—Douglas-fir beetle
12. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wood, S.L. The bark and ambrosia beetles of North and Central America (Coleoptera: Scolytidae), a taxonomic monograph. Great Basin Nat. Mem. 1982, 6, 1–1359. [Google Scholar]
- Six, D.; Bracewell, R. Dendroctonus. In Bark Beetles: Biology and Ecology of Native and Invasive Species; Vega, F.E., Hofstetter, R., Eds.; Academic Press: Cambridge, MA, USA, 2015; pp. 305–350. [Google Scholar]
- Meddens, A.J.; Hicke, J.A.; Ferguson, C.A. Spatiotemporal patterns of observed bark beetle-caused tree mortality in British Columbia and the western United States. Ecol. Appl. 2012, 22, 1876–1891. [Google Scholar] [CrossRef] [PubMed]
- Gomez, D.F.; Sathyapala, S.; Hulcr, J. Towards sustainable forest management in Central America: Review of Southern Pine Beetle (Dendroctonus frontalis Zimmermann) outbreaks, their causes, and solutions. Forests 2020, 11, 173. [Google Scholar] [CrossRef]
- Pye, J.M.; Holmes, T.P.; Prestemon, J.P.; Wear, D.N. Economic impacts of the southern pine beetle. In Southern Pine Beetle II; US Forest Service Southern Research Station General Technical Report SRS-140; US Forest Service Southern Research Station: Asheville, NC, USA, 2011; pp. 213–234. [Google Scholar]
- Prestemon, J.P.; Abt, K.L.; Potter, K.M.; Koch, F.H. An economic assessment of mountain pine beetle timber salvage in the west. West. J. Appl. For. 2013, 28, 143–153. [Google Scholar] [CrossRef][Green Version]
- Corbett, L.J.; Withey, P.; Lantz, V.; Ochuodho, T. The economic impact of the mountain pine beetle infestation in British Columbia: Provincial estimates from a CGE analysis. Forestry 2016, 89, 100–105. [Google Scholar] [CrossRef]
- Holmes, T.; Koch, F. Bark beetle epidemics, life satisfaction, and economic well-being. Forests 2019, 10, 696. [Google Scholar] [CrossRef]
- Dhar, A.; Parrott, L.; Heckbert, S. Consequences of mountain pine beetle outbreak on forest ecosystem services in western Canada. Can. J. For. Res. 2016, 46, 987–999. [Google Scholar] [CrossRef]
- Morris, J.L.; Cottrell, S.; Fettig, C.J.; DeRose, R.J.; Mattor, K.M.; Carter, V.A.; Clear, J.; Clement, J.; Hansen, W.D.; Hicke, J.A. Bark beetles as agents of change in social–ecological systems. Front. Ecol. Environ. 2018, 16, S34–S43. [Google Scholar] [CrossRef]
- Boyd, I.L.; Freer-Smith, P.; Gilligan, C.A.; Godfray, H.C.J. The consequence of tree pests and diseases for ecosystem services. Science 2013, 342, 1235773. [Google Scholar] [CrossRef] [PubMed]
- Negrón, J.F.; Cain, B. Mountain pine beetle in Colorado: A story of changing forests. J. For. 2019, 117, 144–151. [Google Scholar] [CrossRef]
- Grégoire, J.-C.; Raffa, K.F.; Lindgren, B.S. Economics and politics of bark beetles. In Bark Beetles; Vega, F.E., Hofstetter, R.W., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 585–613. [Google Scholar]
- Wood, D.L. The role of pheromones, kairomones, and allomones in the host selection and colonization behavior of bark beetles. Annu. Rev. Entomol. 1982, 27, 411–446. [Google Scholar] [CrossRef]
- Lindgren, B.; Raffa, K. Evolution of tree killing in bark beetles (Coleoptera: Curculionidae): Trade-offs between the maddening crowds and a sticky situation. Can. Entomol. 2013, 145, 471–495. [Google Scholar] [CrossRef]
- Kirkendall, L.R. The evolution of mating systems in bark and ambrosia beetles (Coleoptera: Scolytidae and Platypodidae). Zool. J. Linn. Soc. 1983, 77, 293–352. [Google Scholar] [CrossRef]
- Raffa, K.; Andersson, M.N.; Schlyter, F. Chapter One—Host selection by bark beetles: Playing the odds in a high-stakes game. In Advances in Insect Physiology; Elsevier: Amsterdam, The Netherlands, 2016; Volume 50, pp. 1–74. [Google Scholar] [CrossRef]
- Borden, J.H. Aggregation pheromones. In Bark Beetles in North American Conifers: A System for the Study of Evolutionary Biology; Mitton, J.B., Sturgeon, K.B., Eds.; University of Texas Press: Austin, TX, USA, 1982; pp. 74–139. [Google Scholar]
- Byers, J.A. Chemical ecology of bark beetles. Experientia 1989, 45, 271–283. [Google Scholar] [CrossRef]
- Raffa, K.F. Mixed messages across multiple trophic levels: The ecology of bark beetle chemical communication systems. Chemoecology 2001, 11, 49–65. [Google Scholar] [CrossRef]
- Raffa, K.F.; Gregoire, J.C.; Lindgren, B.S. Natural history and ecology of bark beetles. In Bark Beetles; Vega, F.E., Hofstetter, R.W., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 1–40. [Google Scholar]
- Seybold, S.J.; Bentz, B.J.; Fettig, C.J.; Lundquist, J.E.; Progar, R.A.; Gillette, N.E. Management of western North American bark beetles with semiochemicals. Annu. Rev. Entomol. 2018, 63, 407–432. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, B.T.; Clarke, S.R. Semiochemicals for management of the southern pine beetle (Coleoptera: Curculionidae: Scolytinae): Successes, failures, and obstacles to progress. Can. Entomol. 2021, 153, 36–61. [Google Scholar] [CrossRef]
- Fettig, C.J.; Audley, J.P.; Homicz, C.S.; Progar, R.A. Applied chemical ecology of the western pine beetle, an important pest of ponderosa pine in western North America. Forests 2023, 14, 757. [Google Scholar] [CrossRef]
- Dodds, K.J.; Aoki, C.F.; Arango-Velez, A.; Cancelliere, J.; D’Amato, A.W.; DiGirolomo, M.F.; Rabaglia, R.J. Expansion of southern pine beetle into northeastern forests: Management and impact of a primary bark beetle in a new region. J. For. 2018, 116, 178–191. [Google Scholar] [CrossRef]
- Faccoli, M.; Gallego, D.; Branco, M.; Brockerhoff, E.G.; Corley, J.; Coyle, D.R.; Hurley, B.P.; Jactel, H.; Lakatos, F.; Lantschner, V. A first worldwide multispecies survey of invasive Mediterranean pine bark beetles (Coleoptera: Curculionidae, Scolytinae). Biol. Invasions 2020, 22, 1785–1799. [Google Scholar] [CrossRef]
- Hayes, C.J.; DeGomez, T.E.; Clancy, K.M.; Williams, K.K.; McMillin, J.D.; Anhold, J.A. Evaluation of funnel traps for characterizing the bark beetle (Coleoptera: Scolytidae) communities in ponderosa pine forests of north-central Arizona. J. Econ. Entomol. 2008, 101, 1253–1265. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, K.J.; Cognato, A.I.; Lightle, D.M.; Mosley, B.J.; Nielsen, D.G.; Herms, D.A. Species composition, seasonal activity, and semiochemical response of native and exotic bark and ambrosia beetles (Coleoptera: Curculionidae: Scolytinae) in northeastern Ohio. J. Econ. Entomol. 2010, 103, 1187–1195. [Google Scholar] [CrossRef] [PubMed]
- Pfammatter, J.A.; Coyle, D.R.; Journey, A.M.; Pahs, T.L.; Luhman, J.C.; Cervenka, V.J.; Koch, R.L. Bark beetle (Coleoptera: Curculionidae: Scolytinae) community structure in northeastern and central Minnesota. Gt. Lakes Entomol. 2011, 44, 3. [Google Scholar] [CrossRef]
- Ostrauskas, H.; Ferenca, R. Beetles (Coleoptera) caught in traps baited with pheromones for Dendroctonus rufipennis (Kirby) (Curculionidae: Scolytinae) in Lithuania. Ekologija 2010, 56, 41–46. [Google Scholar] [CrossRef]
- Brockerhoff, E.G.; Jones, D.C.; Kimberley, M.O.; Suckling, D.M.; Donaldson, T. Nationwide survey for invasive wood-boring and bark beetles (Coleoptera) using traps baited with pheromones and kairomones. For. Ecol. Manag. 2006, 228, 234–240. [Google Scholar] [CrossRef]
- Rassati, D.; Faccoli, M.; Petrucco Toffolo, E.; Battisti, A.; Marini, L. Improving the early detection of alien wood-boring beetles in ports and surrounding forests. J. Appl. Entomol. 2015, 52, 50–58. [Google Scholar] [CrossRef]
- Rabaglia, R.J.; Cognato, A.I.; Hoebeke, E.R.; Johnson, C.W.; LaBonte, J.R.; Carter, M.E.; Vlach, J.J. Early detection and rapid response: A 10-year summary of the USDA Forest Service program of surveillance for non-native bark and ambrosia beetles. Am. Entomol. 2019, 65, 29–42. [Google Scholar] [CrossRef]
- Billings, R.F.; Upton, W.W. A methodology for assessing annual risk of southern pine beetle outbreaks across the southern region using pheromone traps. In Advances in Threat Assessment and Their Application to Forest and Rangeland Management; Pye, J.M., Rauscher, H.M., Sands, Y., Lee, D.C., Beatty, J.S., Eds.; USDA Forest Service Pacific Northwest and Southern Research Stations Gen. Tech. Rep. PNW-GTR-802; USDA Forest Service Pacific Northwest and Southern Research Stations: Portland, OR, USA, 2010; Volume 1, pp. 73–85. [Google Scholar]
- Faccoli, M.; Stergulc, F. Ips typographus (L.) pheromone trapping in south Alps: Spring catches determine damage thresholds. J. Appl. Entomol. 2004, 128, 307–311. [Google Scholar] [CrossRef]
- Faccoli, M.; Stergulc, F. A practical method for predicting the short-time trend of bivoltine populations of Ips typographus (L.) (Col., Scolytidae). J. Appl. Entomol. 2006, 130, 61–66. [Google Scholar] [CrossRef]
- Schlyter, F.; Zhang, Q.H.; Liu, G.T.; Ji, L.Z. A successful case of pheromone mass trapping of the bark beetle Ips duplicatus in a forest island, analysed by 20-year time-series data. Integr. Pest Manag. Rev. 2001, 6, 185–196. [Google Scholar] [CrossRef]
- Faccoli, M.; Stergulc, F. Damage reduction and performance of mass trapping devices for forest protection against the spruce bark beetle, Ips typographus (Coleoptera Curculionidae Scolytinae). Ann. For. Sci 2008, 65, 309. [Google Scholar] [CrossRef]
- Deganutti, L.; Biscontin, F.; Bernardinelli, I.; Faccoli, M. The semiochemical push-and-pull technique can reduce bark beetle damage in disturbed Norway spruce forests affected by the Vaia storm. Agric. For. Entomol. 2023, 26, 115–125. [Google Scholar] [CrossRef]
- Bentz, B.; Munson, A. Spruce beetle population suppression in northern Utah. West. J. Appl. For. 2000, 15, 122–128. [Google Scholar] [CrossRef]
- Borden, J.H.; Birmingham, A.L.; Burleigh, J.S. Evaluation of the push-pull tactic against the mountain pine beetle using verbenone and non-host volatiles in combination with pheromone-baited trees. For. Chron. 2006, 82, 579–590. [Google Scholar] [CrossRef]
- Hansen, M.E.; Munson, S.A.; Blackford, D.C.; Wakarchuk, D.; Baggett, S.L. Lethal trap trees and semiochemical repellents as area host protection strategies for spruce beetle (Coleoptera: Curculionidae, Scolytinae) in Utah. J. Econ. Entomol. 2016, 109, 2137–2144. [Google Scholar] [CrossRef] [PubMed]
- Dodds, K.J.; Hanavan, R.P.; Wansleben, T. Enhancing stand structure through snag creation in northeastern US forests: Using ethanol injections and bark beetle pheromones to artificially stress red maple and white pine. Forests 2016, 7, 124. [Google Scholar] [CrossRef]
- Ross, D.W.; Niwa, C.G. Using aggregation and antiaggregation pheromones of the Douglas-fir beetle to produce snags for wildlife habitat. West. J. Appl. For. 1997, 12, 52–54. [Google Scholar] [CrossRef]
- Tittiger, C.; Blomquist, G. Pheromone production in pine bark beetles. Adv. Insect Physiol. 2016, 50, 235–263. [Google Scholar] [CrossRef]
- Zethner-Møller, O.; Rudinsky, J. Studies on the site of sex pheromone production in Dendroctonus pseudotsugae (Coleoptera: Scolytidae). Ann. Entomol. Soc. Am. 1967, 60, 575–582. [Google Scholar] [CrossRef]
- Byers, J.A. Influence of sex, maturity and host substances on pheromones in the guts of the bark beetles, Ips paraconfusus and Dendroctonus brevicomis. J. Insect Physiol. 1983, 29, 5–13. [Google Scholar] [CrossRef]
- Byers, J.A.; Wood, D.L.; Craig, J.; Hendry, L.B. Attractive and inhibitory pheromones produced in the bark beetle, Dendroctonus brevicomis, during host colonization: Regulation of inter- and intraspecific competition. J. Chem. Ecol. 1984, 10, 861–877. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, B.T. Electrophysiological and behavioral responses of Dendroctonus frontalis (Coleoptera: Curculionidae) to volatiles isolated from conspecifics. J. Econ. Entomol. 2005, 98, 2067–2078. [Google Scholar] [CrossRef] [PubMed]
- Phillips, T.W.; Nation, J.L.; Wilkinson, R.C.; Foltz, J.L. Secondary attraction and field activity of beetle-produced volatiles in Dendroctonus terebrans. J. Chem. Ecol. 1989, 15, 1513–1533. [Google Scholar] [CrossRef] [PubMed]
- Pureswaran, D.S.; Gries, R.; Borden, J.H.; Pierce, H.D., Jr. Dynamics of pheromone production and communication in the mountain pine beetle, Dendroctonus ponderosae Hopkins, and the pine engraver, Ips pini (Say)(Coleoptera: Scolytidae). Chemoecology 2000, 10, 153–168. [Google Scholar] [CrossRef]
- Pureswaran, D.S.; Sullivan, B.T.; Ayres, M.P. High individual variation in pheromone production by tree-killing bark beetles (Coleoptera: Curculionidae: Scolytinae). Naturwissenschaften 2008, 95, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, B.T.; Niño, A.; Moreno, B.; Brownie, C.; Macías-Sámano, J.; Clarke, S.R.; Kirkendall, L.R.; Zúñiga, G. Biochemical evidence that Dendroctonus frontalis consists of two sibling species in Belize and Chiapas, Mexico. Ann. Entomol. Soc. Am. 2012, 105, 817–831. [Google Scholar] [CrossRef]
- Isitt, R.; Bleiker, K.; Pureswaran, D.; Hillier, N.; Huber, D. The effect of feeding and mate presence on the pheromone production of the spruce beetle (Coleoptera: Curculionidae). Environ. Entomol. 2018, 47, 1293–1299. [Google Scholar] [CrossRef] [PubMed]
- Wood, D.L.; Browne, L.E.; Silverstein, R.M.; Rodin, J.O. Sex pheromones of bark beetles—I. Mass production, bio-assay, source, and isolation of the sex pheromone of Ips confusus (Lec.). J. Insect Physiol. 1966, 12, 523–536. [Google Scholar] [CrossRef] [PubMed]
- Silverstein, R.M.; Brownlee, R.G.; Bellas, T.E.; Wood, D.L.; Browne, L.E. Brevicomin: Principal sex attractant in the frass of the female western pine beetle. Science 1968, 159, 889–891. [Google Scholar] [CrossRef]
- Smith, M.T.; Payne, T.L.; Birch, M.C. Olfactory-based behavioral interactions among five species in the southern pine bark beetle group. J. Chem. Ecol. 1990, 16, 3317–3331. [Google Scholar] [CrossRef]
- Pitman, G.B.; Vité, J.P.; Kinzer, G.W.; Fentiman, A.F., Jr. Specificity of population-aggregating pheromones in Dendroctonus. J. Insect Physiol. 1969, 15, 363–366. [Google Scholar] [CrossRef]
- Pureswaran, D.S.; Hofstetter, R.W.; Sullivan, B.T.; Grady, A.M.; Brownie, C. Western pine beetle populations in Arizona and California differ in the composition of their aggregation pheromones. J. Chem. Ecol. 2016, 42, 404–413. [Google Scholar] [CrossRef] [PubMed]
- Hofstetter, R.W.; Gaylord, M.L.; Martinson, S.; Wagner, M.R. Attraction to monoterpenes and beetle-produced compounds by syntopic Ips and Dendroctonus bark beetles and their predators. Agric. For. Entomol. 2012, 14, 207–215. [Google Scholar] [CrossRef]
- Birch, M.C.; Svihra, P.; Paine, T.D.; Miller, J.C. Influence of chemically mediated behavior on host tree colonization by four cohabitating species of bark beetle. J. Chem. Ecol. 1980, 6, 395–414. [Google Scholar] [CrossRef]
- Miller, D.; Asaro, C.; Berisford, C. Attraction of southern pine engravers and associated bark beetles (Coleoptera: Scolytidae) to ipsenol, ipsdienol, and lanierone in southeastern United States. J. Econ. Entomol. 2005, 98, 2058–2066. [Google Scholar] [CrossRef] [PubMed]
- Erbilgin, N.; Raffa, K.F. Opposing effects of host monoterpenes on responses by two sympatric species of bark beetles to their aggregation pheromones. J. Chem. Ecol. 2000, 26, 2527–2548. [Google Scholar] [CrossRef]
- Seybold, S.J.; Huber, D.P.W.; Lee, J.C.; Graves, A.D.; Bohlmann, J. Pine monoterpenes and pine bark beetles: A marriage of convenience for defense and chemical communication. Phytochem. Rev. 2006, 5, 143–178. [Google Scholar] [CrossRef]
- Phillips, M.A.; Croteau, R.B. Resin-based defenses in conifers. Trends Plant Sci. 1999, 4, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Gijzen, M.; Lewinsohn, E.; Savage, T.J.; Croteau, R.B. Conifer monoterpenes: Biochemistry and bark beetle chemical ecology. In Bioactive Volatile Compounds from Plants; Teranishi, R., Buttery, R.G., Sugisawa, H., Eds.; Am. Chemical Soc.: Washington, DC, USA, 1993; pp. 8–22. [Google Scholar]
- Haapanala, S.; Hakola, H.; Hellén, H.; Vestenius, M.; Levula, J.; Rinne, J. Is forest management a significant source of monoterpenes into the boreal atmosphere? Biogeosciences 2012, 9, 1291–1300. [Google Scholar] [CrossRef]
- Schade, G.W.; Goldstein, A.H. Increase of monoterpene emissions from a pine plantation as a result of mechanical disturbances. Geophys. Res. Lett. 2003, 30. [Google Scholar] [CrossRef]
- Strömvall, A.M.; Petersson, G. Conifer monoterpenes emitted to air by logging operations. Scand. J. For. Res. 1991, 6, 253–258. [Google Scholar] [CrossRef]
- Zamora-Ballesteros, C.; Martín-García, J.; Fernández-Fernández, M.; Diez, J. Pine pitch canker (PPC): An introduction, an overview. In Forest Microbiology; Elsevier: Amsterdam, The Netherlands, 2022; pp. 325–342. [Google Scholar] [CrossRef]
- Davis, T.S.; Jarvis, K.; Parise, K.; Hofstetter, R.W. Oleoresin exudation quantity increases and viscosity declines following a fire event in a ponderosa pine ecosystem. J. Ariz.-Nev. Acad. Sci. 2011, 43, 6–11. [Google Scholar] [CrossRef]
- Janson, R.W. Monoterpene emissions from Scots pine and Norwegian spruce. J. Geophys. Res. Atmos. 1993, 98, 2839–2850. [Google Scholar] [CrossRef]
- Niño-Domínguez, A.; Sullivan, B.T.; Lopez-Urbina, J.H.; Macías-Sámano, J.E. Discrimination of odors associated with conspecific and heterospecific frass by sibling species Dendroctonus frontalis (Coleoptera: Curculionidae: Scolytinae) and Dendroctonus mesoamericanus (Coleoptera: Curculionidae: Scolytinae). Environ. Entomol. 2018, 47, 1532–1540. [Google Scholar] [CrossRef]
- Pureswaran, D.S.; Sullivan, B.T. Semiochemical emission from individual galleries of the southern pine beetle, (Coleoptera: Curculionidae: Scolytinae), attacking standing trees. J. Econ. Entomol. 2012, 105, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Geron, C.D.; Arnts, R.R. Seasonal monoterpene and sesquiterpene emissions from Pinus taeda and Pinus virginiana. Atmos. Environ. 2010, 44, 4240–4251. [Google Scholar] [CrossRef]
- Christiansen, E.; Waring, R.H.; Berryman, A.A. Resistance of conifers to bark beetle attack: Searching for general relationships. For. Ecol. Manag. 1987, 22, 89–106. [Google Scholar] [CrossRef]
- Franceschi, V.R.; Krokene, P.; Christiansen, E.; Krekling, T. Anatomical and chemical defenses of conifer bark against bark beetles and other pests. New Phytol. 2005, 167, 353–376. [Google Scholar] [CrossRef]
- Chiu, C.C.; Keeling, C.I.; Bohlmann, J. Toxicity of pine monoterpenes to mountain pine beetle. Sci. Rep. 2017, 7, 8858. [Google Scholar] [CrossRef]
- Miller, D.R.; Borden, J.H. Dose-dependent and species-specific responses of pine bark beetles (Coleoptera: Scolytidae) to monoterpenes in association with pheromones. Can. Entomol. 2000, 132, 183–195. [Google Scholar] [CrossRef]
- Fatzinger, C.W. Attraction of the black turpentine beetle (Coleoptera: Scolytidae) and other forest Coleoptera to turpentine-baited traps. Environ. Entomol. 1985, 14, 768–775. [Google Scholar] [CrossRef]
- Billings, R.F. Southern pine bark beetles and associated insects: Effects of rapidly-released host volatiles on response to aggregation pheromones. J. Appl. Entomol. 1985, 99, 483–491. [Google Scholar] [CrossRef]
- Pureswaran, D.S.; Borden, J.H. Primary attraction and kairomonal host discrimination in three species of Dendroctonus (Coleoptera: Scolytidae). Agric. For. Entomol. 2005, 7, 219–230. [Google Scholar] [CrossRef]
- Erbilgin, N.; Powell, J.S.; Raffa, K.F. Effect of varying monoterpene concentrations on the response of Ips pini (Coleoptera: Scolytidae) to its aggregation pheromone: Implications for pest management and ecology of bark beetles. Agric. For. Entomol. 2003, 5, 269–274. [Google Scholar] [CrossRef]
- Emerick, J.J.; Snyder, A.I.; Bower, N.W.; Snyder, M.A. Mountain pine beetle attack associated with low levels of 4-allylanisole in ponderosa pine. Environ. Entomol. 2008, 37, 871–875. [Google Scholar] [CrossRef] [PubMed]
- Pureswaran, D.S.; Gries, R.; Borden, J.H. Quantitative variation in monoterpenes in four species of conifers. Biochem. Syst. Ecol. 2004, 32, 1109–1136. [Google Scholar] [CrossRef]
- Kelsey, R.G.; Joseph, G. Ethanol in ponderosa pine as an indicator of physiological injury from fire and its relationship to secondary beetles. Can. J. For. Res. 2003, 33, 870–884. [Google Scholar] [CrossRef]
- Kelsey, R.G.; Gallego, D.; Sánchez-García, F.; Pajares, J. Ethanol accumulation during severe drought may signal tree vulnerability to detection and attack by bark beetles. Can. J. For. Res. 2014, 44, 554–561. [Google Scholar] [CrossRef]
- Kelsey, R.G.; Beh, M.M.; Shaw, D.C.; Manter, D.K. Ethanol attracts scolytid beetles to Phytophthora ramorum cankers on coast live oak. J. Chem. Ecol. 2013, 39, 494–506. [Google Scholar] [CrossRef]
- Gara, R.I.; Littke, W.R.; Rhoades, D.F. Emission of ethanol and monoterpenes by fungal infected lodgepole pine trees. Phytochemistry 1993, 34, 987–990. [Google Scholar] [CrossRef]
- Ranger, C.M.; Reding, M.E.; Schultz, P.B.; Oliver, J.B. Influence of flood-stress on ambrosia beetle host-selection and implications for their management in a changing climate. Agric. For. Entomol. 2013, 15, 56–64. [Google Scholar] [CrossRef]
- Brand, J.M.; Bracke, J.W.; Markovetz, A.J.; Wood, D.L.; Browne, L.L. Production of verbenol pheromone by a bacterium isolated from bark beetles. Nature 1975, 254, 136–137. [Google Scholar] [CrossRef]
- Brand, J.M.; Schultz, J.; Barras, S.J.; Edson, L.J.; Payne, T.L.; Hedden, R.L. Bark-beetle pheromones—Enhancement of Dendroctonus frontalis (Coleoptera: Scolytidae) aggregation pheromone by yeast metabolites in laboratory bioassays. J. Chem. Ecol. 1977, 3, 657–666. [Google Scholar] [CrossRef]
- Kandasamy, D.; Zaman, R.; Nakamura, Y.; Zhao, T.; Hartmann, H.; Andersson, M.N.; Hammerbacher, A.; Gershenzon, J. Conifer-killing bark beetles locate fungal symbionts by detecting volatile fungal metabolites of host tree resin monoterpenes. PLoS Biol. 2023, 21, e3001887. [Google Scholar] [CrossRef] [PubMed]
- Kandasamy, D.; Gershenzon, J.; Hammerbacher, A. Volatile organic compounds emitted by fungal associates of conifer bark beetles and their potential in bark beetle control. J. Chem. Ecol. 2016, 42, 952–969. [Google Scholar] [CrossRef]
- Byers, J.A.; Wood, D.L. Antibiotic-induced inhibition of pheromone synthesis in a bark beetle. Science 1981, 213, 763–764. [Google Scholar] [CrossRef] [PubMed]
- Hunt, D.W.A.; Borden, J.H.; Lindgren, B.S.; Gries, G. The role of autoxidation of alpha-pinene in the production of pheromones of Dendroctonus ponderosae (Coleoptera: Scolytidae). Can. J. For. Res. 1989, 19, 1275–1282. [Google Scholar] [CrossRef]
- Zhao, T.; Ganji, S.; Schiebe, C.; Bohman, B.; Weinstein, P.; Krokene, P.; Borg-Karlson, A.K.; Unelius, C.R. Convergent evolution of semiochemicals across Kingdoms: Bark beetles and their fungal symbionts. ISME J. 2019, 13, 1535–1545. [Google Scholar] [CrossRef]
- Pitman, G.B.; Vité, J.P.; Kinzer, G.W.; Fentiman, A.F., Jr. Bark beetle attractants: Trans-verbenol isolated from Dendroctonus. Nature 1968, 218, 168–169. [Google Scholar] [CrossRef]
- Vité, J.; Gara, R.; Von Scheller, H. Field observations on the response to attractants of bark beetles infesting southern pines. Contrib. Boyce Thompson Inst. 1964, 22, 461–470. [Google Scholar]
- Vité, J.P.; Renwick, J.A.A. Insect and host factors in the aggregation of the southern pine beetle. Contrib. Boyce Thompson Inst. 1968, 24, 61–63. [Google Scholar]
- Rudinsky, J. Host selection and invasion by the Douglas-fir beetle, Dendroctonus pseudotsugae Hopkins, in coastal Douglas-fir forests. Can. Entomol. 1966, 98, 98–111. [Google Scholar] [CrossRef]
- Mccambridge, W.F. Nature of induced attacks by the Black Hills beetle, Dendroctonus ponderosae (Coleoptera: Scolytidae). Ann. Entomol. Soc. Am. 1967, 60, 920–928. [Google Scholar] [CrossRef]
- Pitman, G.B.; Vité, J.P. Aggregation behavior of Dendroctonus ponderosae (Coleoptera: Scolytidae) in response to chemical messengers. Can. Entomol. 1969, 101, 143–149. [Google Scholar] [CrossRef]
- Gara, R.I.; Vité, J.P.; Cramer, H.H. Manipulation of Dendroctonus frontalis by use of a population aggregation pheromone. Contrib. Boyce Thompson Inst. 1965, 23, 55–66. [Google Scholar]
- Mcmullen, L.H.; Atkins, M.D. On the flight and host selection of the Douglas-fir beetle, Dendroctonus pseudotsugae Hopk. (Coleoptera: Scolytidae). Can. Entomol. 1962, 94, 1309–1325. [Google Scholar] [CrossRef]
- Chapman, J.A. Field selection of different log odors by scolytid beetles. Can. Entomol. 1963, 95, 673–676. [Google Scholar] [CrossRef]
- Borden, J.; Silverstein, R.; Brownlee, R. Sex pheromone of Dendroctonus pseudotsugae (Coleoptera: Scolytidae): Production, bio-assay, and partial isolation. Can. Entomol. 1968, 100, 597–603. [Google Scholar] [CrossRef]
- Niño-Domínguez, A.; Sullivan, B.T.; López-Urbina, J.H.; Macías-Sámano, J.E. Pheromone-mediated mate location and discrimination by two syntopic sibling species of Dendroctonus bark beetles in Chiapas, Mexico. J. Chem. Ecol. 2015, 41, 746–756. [Google Scholar] [CrossRef]
- Byers, J.; Wood, D. Interspecific inhibition of the response of the bark beetles, Dendroctonus brevicomis and Ips paraconfusus, to their pheromones in the field. J. Chem. Ecol. 1980, 6, 149–164. [Google Scholar] [CrossRef]
- Liu, Z.; Xu, B.; Miao, Z.; Sun, J. The pheromone frontalin and its dual function in the invasive bark beetle Dendroctonus valens. Chem. Senses 2013, 38, 485–495. [Google Scholar] [CrossRef] [PubMed]
- Rudinsky, J.A.; Morgan, M.; Libbey, L.M.; Michael, R.R. Sound production in Scolytidae: 3-methyl-2-cyclohexen-1-one released by the female Douglas-fir beetle in response to male sonic signal. Environ. Entomol. 1973, 2, 505–509. [Google Scholar] [CrossRef]
- Barkawi, L.S.; Francke, W.; Blomquist, G.J.; Seybold, S.J. Frontalin: De novo biosynthesis of an aggregation pheromone component by Dendroctonus spp. bark beetles (Coleoptera: Scolytidae). Insect Biochem. Mol. Biol. 2003, 33, 773–788. [Google Scholar] [CrossRef]
- Vité, J.; Pitman, G. Insect and host odors in the aggregation of the western pine beetle. Can. Entomol. 1969, 101, 113–117. [Google Scholar] [CrossRef]
- Kinzer, G.W.; Fentiman, A.F., Jr.; Page, T.F., Jr.; Foltz, R.L.; Vité, J.P. Bark beetle attractants: Identification, synthesis and field bioassay of a new compound isolated from Dendroctonus. Nature 1969, 221, 477–478. [Google Scholar] [CrossRef]
- Vité, J.P.; Crozier, R.G. Studies on the attack behavior of the southern pine beetle. IV. Influence of host condition on aggregation pattern. Contrib. Boyce Thompson Inst. 1968, 24, 87–93. [Google Scholar]
- Byers, J.A. Active space of pheromone plume and its relationship to effective attraction radius in applied models. J. Chem. Ecol 2008, 34, 1134–1145. [Google Scholar] [CrossRef]
- Shi, Z.H.; Sun, J.H. Quantitative variation and biosynthesis of hindgut volatiles associated with the red turpentine beetle, Dendroctonus valens LeConte, at different attack phases. Bull. Entomol. Res. 2010, 100, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Cano-Ramírez, C.; Armendáriz-Toledano, F.; Macías-Sámano, J.E.; Sullivan, B.T.; Zúñiga, G. Electrophysiological and behavioral responses of the bark beetle Dendroctonus rhizophagus to volatiles from host pines and conspecifics. J. Chem. Ecol. 2012, 38, 512–524. [Google Scholar] [CrossRef] [PubMed]
- Hunt, D.W.A.; Borden, J.H. Response of mountain pine beetle, Dendroctonus ponderosae Hopkins, and pine engraver, Ips pini (Say), to ipsdienol in southwestern British Columbia. J. Chem. Ecol. 1988, 14, 277–293. [Google Scholar] [CrossRef]
- Bedard, W.D.; Tilden, P.E.; Wood, D.L.; Silverstein, R.M.; Brownlee, R.G.; Rodin, J.O. Western pine beetle: Field response to its sex pheromone and a synergistic host terpene, myrcene. Science 1969, 164, 1284–1285. [Google Scholar] [CrossRef] [PubMed]
- Werner, R. Response of the beetle, Ips grandicollis, to combinations of host and insect produced attractants. J. Insect Physiol. 1972, 18, 1403–1412. [Google Scholar] [CrossRef]
- Gries, G.; Pierce, H.D.; Lindgren, B.S.; Borden, J.H. New techniques for capturing and analyzing semiochemicals for scolytid beetles (Coleoptera, Scolytidae). J. Econ. Entomol. 1988, 81, 1715–1720. [Google Scholar] [CrossRef]
- Birgersson, G.; Bergstrom, G. Volatiles released from individual spruce bark beetle entrance holes—Quantitative variations during the first week of attack. J. Chem. Ecol. 1989, 15, 2465–2483. [Google Scholar] [CrossRef] [PubMed]
- Cale, J.A.; Taft, S.; Najar, A.; Klutsch, J.G.; Hughes, C.C.; Sweeney, J.D.; Erbilgin, N. Mountain pine beetle (Dendroctonus ponderosae) can produce its aggregation pheromone and complete brood development in naïve red pine (Pinus resinosa) under laboratory conditions. Can. J. For. Res. 2016, 45, 1873–1877. [Google Scholar] [CrossRef]
- Browne, L.E.; Wood, D.L.; Bedard, W.D.; Silverstein, R.M.; West, J.R. Quantitative estimates of the western pine beetle attractive pheromone components, exo-brevicomin, frontalin, and myrcene in nature. J. Chem. Ecol. 1979, 5, 397–414. [Google Scholar] [CrossRef]
- Berisford, C.W.; Payne, T.L.; Berisford, Y.C. Geographical variation in response of southern pine beetle (Coleoptera: Scolytidae) to aggregating pheromones in laboratory bioassays. Environ. Entomol. 1990, 19, 1671–1674. [Google Scholar] [CrossRef]
- Stock, A.J.; Borden, J.H. Secondary attraction in the western balsam bark beetle, Dryocoetes confusus (Coleoptera: Scolytidae). Can. Entomol. 1983, 115, 539–550. [Google Scholar] [CrossRef]
- Baker, B.H.; Hostetler, B.B.; Furniss, M.M. Response of eastern larch beetle (Coleoptera: Scolytidae) in Alaska to its natural attractant and to Douglas-fir beetle pheromones. Can. Entomol. 1977, 109, 289–294. [Google Scholar] [CrossRef]
- Niño-Domínguez, A.; Sullivan, B.T.; López-Urbina, J.H.; Macías-Sámano, J. Responses by Dendroctonus frontalis and Dendroctonus mesoamericanus (Coleoptera: Curculionidae) to semiochemical lures in Chiapas, Mexico: Possible roles of pheromones during joint host attacks. J. Econ. Entomol. 2016, 50, 129–193. [Google Scholar] [CrossRef]
- Billings, R.F.; Gara, R.I.; Hrutfiord, B.F. Influence of ponderosa pine resin volatiles on the response of Dendroctonus ponderosae to synthetic trans-verbenol. Environ. Entomol. 1976, 5, 171–179. [Google Scholar] [CrossRef]
- Rudinsky, J.A. Scolytid beetles associated with Douglas fir: Response to terpenes. Science 1966, 152, 218–219. [Google Scholar] [CrossRef] [PubMed]
- Lindgren, B.; Gries, G.; Pierce, H.; Mori, K. Dendroctonus pseudotsugae Hopkins (Coleoptera: Scolytidae): Production of and response to enantiomers of 1-methylcyclohex-2-en-1-ol. J. Chem. Ecol 1992, 18, 1201–1208. [Google Scholar] [CrossRef] [PubMed]
- Libbey, L.M.; Ryker, L.C.; Yandell, K.L. Laboratory and field studies of volatiles released by Dendroctonus ponderosae Hopkins (Coleoptera: Scolytidae). Z. Angew. Entomol. 1985, 100, 381–392. [Google Scholar] [CrossRef]
- Rahmani, R.; Hedenström, E.; Schroeder, M. SPME collection and GC-MS analysis of volatiles emitted during the attack of male Polygraphus poligraphus (Coleoptera, Curcolionidae) on Norway spruce. Z. Naturforsch. C 2015, 70, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Liu, B.; Sun, Y.; Wang, Y.; Dai, L.; Chen, H. Presence and roles of myrtenol, myrtanol and myrtenal in Dendroctonus armandi (Coleoptera: Curculionidae: Scolytinae) and Pinus armandi (Pinales: Pinaceae: Pinoideae). Pest Manag. Sci. 2020, 76, 188–197. [Google Scholar] [CrossRef]
- Paine, T.; Millar, J.; Hanlon, C.; Hwang, J.S. Identification of semiochemicals associated with Jeffrey pine beetle, Dendroctonus jeffreyi. J. Chem. Ecol. 1999, 25, 433–453. [Google Scholar] [CrossRef]
- Peacock, J.W.; Cuthbert, R.A.; Gore, W.E.; Lanier, G.N.; Pearce, G.T.; Silverstein, R.M. Collection on Porapak Q of the aggregation pheromone of Scolytus multistriatus (Coleoptera: Scolytidae). J. Chem. Ecol. 1975, 1, 149–160. [Google Scholar] [CrossRef]
- de Groot, P.; DeBarr, G.; Birgersson, G.; Pierce, H.; Borden, J.; Berisford, Y.; Berisford, C. Evidence for a female-produced pheromone in the white pine cone beetle, Conophthorus coniperda (Schwarz), and in the red pine cone beetle, C. resinosae Hopkins (Coleoptera: Scolytidae). Can. Entomol. 1991, 123, 1057–1064. [Google Scholar] [CrossRef]
- Pierce, H.D., Jr.; Conn, J.E.; Oehlschlager, A.C.; Borden, J.H. Monoterpene metabolism in female mountain pine beetles, Dendroctonus ponderosae Hopkins, attacking ponderosa pine. J. Chem. Ecol. 1987, 13, 1455–1480. [Google Scholar] [CrossRef]
- Rudinsky, J.A.; Morgan, M.E.; Libbey, L.M.; Putnam, T.B. Antiaggregative-rivalry pheromone of the mountain pine beetle, and a new arrestant of the southern pine beetle. Environ. Entomol. 1974, 3, 90–97. [Google Scholar] [CrossRef]
- Byers, J.A.; Birgersson, G.; Löfqvist, J.; Appelgren, M.; Bergström, G. Isolation of pheromone synergists of bark beetle, Pityogenes chalcographus, from complex insect-plant odors by fractionation and subtractive-combination bioassay. J. Chem. Ecol. 1990, 16, 861–876. [Google Scholar] [CrossRef] [PubMed]
- Bjostad, L.B. Electrophysiological methods. In Methods in Chemical Ecology, Volume 1: Chemical Methods; Millar, J.G., Haynes, K.F., Eds.; Kluwer Academic Publishers: Boston, MA, USA, 1998; pp. 339–375. [Google Scholar]
- Struble, D.L.; Arn, H. Combined gas chromatography and electroantennogram recording of insect olfactory responses. In Techniques in Pheromone Research; Springer: New York, NY, USA, 1984; pp. 161–178. [Google Scholar]
- Renwick, J.A.A.; Hughes, P.R.; Vité, J.P. The aggregation pheromone system of a Dendroctonus bark beetle in Guatemala. J. Insect Physiol. 1975, 21, 1097–1100. [Google Scholar] [CrossRef]
- Giesbrecht, F.G.; Gumpertz, M.L. Planning, Construction, and Analysis of Comparative Experiments; John Wiley & Sons: Hoboken, NJ, USA, 2004. [Google Scholar]
- Borden, J.H.; Conn, J.E.; Friskie, L.M.; Scott, B.E.; Chong, L.J.; Pierce, H.D., Jr. Semiochemicals for the mountain pine beetle, Dendroctonus ponderosae (Coleoptera: Scolytidae), in British Columbia: Baited-tree studies. Can. J. For. Res. 1983, 13, 325–333. [Google Scholar] [CrossRef]
- Furniss, M.M.; Kline, L.N.; Schmitz, R.F.; Rudinsky, J.A. Tests of three pheromones to induce or disrupt aggregation of Douglas-fir beetles (Coleoptera: Scolytidae) on live trees. Ann. Entomol. Soc. Am. 1972, 65, 1227–1232. [Google Scholar] [CrossRef]
- Knopf, J.; Pitman, G. Aggregation pheromone for manipulation of the Douglas-fir beetle. J. Econ. Entomol. 1972, 65, 723–726. [Google Scholar] [CrossRef]
- Borden, J.H.; Chong, L.J.; Lindgren, B.S. Redundancy in the semiochemical message required to induce attack on lodgepole pines by the mountain pine beetle, Dendroctonus ponderosae Hopkins (Coleoptera: Scolytidae). Can. Entomol. 1990, 122, 769–777. [Google Scholar] [CrossRef]
- Chatelain, M.P.; Schenk, J.A. Evaluation of frontalin and exo-brevicomin as kairomones to control mountain pine beetle (Coleoptera: Scolytidae) in lodgepole pine. Environ. Entomol. 1984, 13, 1666–1674. [Google Scholar] [CrossRef]
- Shore, T.L.; Safranyak, L.; Lindgren, B.S. The response of mountain pine beetle (Dendroctonus ponderosae) to lodgepole pine trees baited with verbenone and exo-brevicomin. J. Chem. Ecol. 1992, 18, 533–541. [Google Scholar] [CrossRef]
- Furniss, M.M.; Baker, B.H.; Hostetler, B.B. Aggregation of spruce beetles (Coleoptera) to seudenol and repression of attraction by methylcyclohexenone in Alaska. Can. Entomol. 1976, 108, 1297–1302. [Google Scholar] [CrossRef]
- Rudinsky, J.A.; Furniss, M.M.; Kline, L.N.; Schmitz, R.F. Attraction and repression of Dendroctonus pseudotsugae (Coleoptera: Scolytidae) by three synthetic pheromones in traps in Oregon and Idaho. Can. Entomol. 1972, 104, 815–822. [Google Scholar] [CrossRef]
- Lindgren, B.S. A multiple funnel trap for scolytid beetles. Can. Entomol. 1983, 115, 299–302. [Google Scholar] [CrossRef]
- Czokajlo, D.; Ross, D.; Kirsch, P.; Forster, B.; Grodzki, W. Intercept panel trap, a novel trap for monitoring forest Coleoptera. J. For. Sci. 2001, 47, 63–65. [Google Scholar]
- Miller, D.R.; Crowe, C.M. Relative performance of Lindgren multiple-funnel, intercept panel, and colossus pipe traps in catching Cerambycidae and associated species in the southeastern United States. J. Econ. Entomol. 2011, 104, 1934–1941. [Google Scholar] [CrossRef] [PubMed]
- Payne, T.L.; Hart, E.R.; Edson, L.J.; McCarty, F.A.; Billings, P.M.; Coster, J.E. Olfactometer for assay of behavioral chemicals for the southern pine beetle, Dendroctonus frontalis (Coleoptera: Scolytidae). J. Chem. Ecol. 1976, 2, 411–419. [Google Scholar] [CrossRef]
- Browne, L.E.; Birch, M.C.; Wood, D.L. Novel trapping and delivery systems for airborne insect pheromones. J. Insect Physiol. 1974, 20, 183–193. [Google Scholar] [CrossRef]
- Zhang, L.W.; Sun, J.H. Electrophysiological and behavioral responses of Dendroctonus valens (Coleoptera: Curculionidae: Scolytinae) to candidate pheromone components identified in hindgut extracts. Environ. Entomol. 2006, 35, 1232–1237. [Google Scholar] [CrossRef]
- Jantz, O.K.; Rudinsky, J.A. Laboratory and field methods for assaying olfactory responses of the Douglas-fir beetle, Dendroctonus pseudotsugae Hopkins. Can. Entomol. 1965, 97, 935–941. [Google Scholar] [CrossRef]
- Sullivan, B.T.; Shepherd, W.P.; Pureswaran, D.S.; Tashiro, T.; Mori, K. Evidence that (+)-endo-brevicomin is a male-produced component of the southern pine beetle aggregation pheromone. J. Chem. Ecol. 2007, 33, 1510–1527. [Google Scholar] [CrossRef]
- Payne, T.L.; Andryszak, N.A.; Wieser, H.; Dixon, E.A.; Ibrahim, N.; Coers, J. Antennal olfactory and behavioral response of southern pine beetle, Dendroctonus frontalis, to analogs of its aggregation pheromone frontalin. J. Chem. Ecol. 1988, 14, 1217–1225. [Google Scholar] [CrossRef]
- Zhang, L.W.; Clarke, S.R.; Sun, J.H. Electrophysiological and behavioral responses of Dendroctonus valens (Coleoptera: Curculionidae: Scolytinae) to four bark beetle pheromones. Environ. Entomol. 2009, 38, 472–477. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.R.; Lindgren, B.S.; Borden, J.H. Dose-dependent pheromone responses of mountain pine beetle in stands of lodgepole pine. Environ. Entomol. 2005, 34, 1019–1027. [Google Scholar] [CrossRef]
- Sullivan, B.T.; Munro, H.L.; Shepherd, W.P.; Gandhi, K.J.K. 4-allylanisole as a lure adjuvant for Dendroctonus frontalis (Coleoptera: Curculionidae: Scolytinae) and two associated beetles. J. Appl. Entomol. 2022, 146, 813–822. [Google Scholar] [CrossRef]
- Tilden, P.E.; Bedard, W.D. Field response of Dendroctonus brevicomis to exo-brevicomin, frontalin, and myrcene released at two proportions and three levels. J. Chem. Ecol. 1985, 11, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Rudinsky, J.A.; Ryker, L.C. Multifunctionality of Douglas-fir beetle pheromone 3,2-MCH confirmed with solvent dibutyl phthalate. J. Chem. Ecol. 1980, 6, 193–201. [Google Scholar] [CrossRef]
- Rudinsky, J.A. Multiple functions of the Douglas-fir beetle pheromone 3-methyl-2-cyclohexen-1-one. Environ. Entomol. 1973, 2, 579–585. [Google Scholar] [CrossRef][Green Version]
- Borden, J.H. Disruption of semiochemical-mediated aggregation in bark beetles. In Insect Pheromone Research: New Directions; Cardé, R.T., Minks, A.K., Eds.; Chapman and Hall: New York, NY, USA, 1997; pp. 421–438. [Google Scholar]
- Symonds, M.R.E.; Elgar, M.A. The mode of pheromone evolution: Evidence from bark beetles. Proc. R. Soc. Lond. B 2004, 271, 839–846. [Google Scholar] [CrossRef] [PubMed]
- Werner, R.A. Research on the use of semiochemicals to manage spruce beetles in Alaska. In Proceedings of the Symposium on Management of Western Bark Beetles with Pheromones: Research and Development, Kailua-Kona, HI, USA, 22–25 June 1992; General Technical Report PSW-GTR-150. USDA Forest Service, Pacific Southwest Research Station: Albany, CA, USA, 1994; pp. 15–21. [Google Scholar]
- Byers, J.A. Behavioral mechanisms involved in reducing competition in bark beetles. Holarct. Ecol. 1989, 12, 466–476. [Google Scholar] [CrossRef]
- Byers, J.A. Sex-specific responses to aggregation pheromone: Regulation of colonization density in the bark beetle Ips paraconfusus. J. Chem. Ecol. 1983, 9, 129–142. [Google Scholar] [CrossRef]
- Schlyter, F.; Byers, J.A.; Löfqvist, J. Attraction to pheromone sources of different quantity, quality, and spacing: Density-regulation mechanisms in bark beetle Ips typographus. J. Chem. Ecol. 1987, 13, 1503–1523. [Google Scholar] [CrossRef]
- Sullivan, B.T. Host odour alpha-pinene increases or reduces response of Ips avulsus (Coleoptera: Curculionidae: Scolytinae) to its aggregation pheromone depending on separation of release points. Can. Entomol. 2023, 155, e4. [Google Scholar] [CrossRef]
- Zhang, Q.H.; Schlyter, F. Redundancy, synergism, and active inhibitory range of non-host volatiles in reducing pheromone attraction in European spruce bark beetle Ips typographus. Oikos 2003, 101, 299–310. [Google Scholar] [CrossRef]
- Shepherd, W.P.; Sullivan, B.T. Southern pine beetle (Coleoptera: Curculionidae: Scolytinae) pheromone component trans-verbenol: Enantiomeric specificity and potential as a lure adjuvant. Environ. Entomol. 2018, 48, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Payne, T.L.; Coster, J.E.; Richerson, J.V.; Edson, L.J.; Hart, E.R. Field response of the southern pine beetle to behavioral chemicals. Environ. Entomol. 1978, 7, 578–582. [Google Scholar] [CrossRef]
- Renwick, J.A.A.; Vité, J.P. Systems of chemical communication in Dendroctonus. Contrib. Boyce Thompson Inst. 1970, 24, 283–292. [Google Scholar]
- Cardé, R.T. Defining attraction and aggregation pheromones: Teleological versus functional perspectives. J. Chem. Ecol. 2014, 40, 519. [Google Scholar] [CrossRef] [PubMed]
- Blum, M.S. Semiochemical parsimony in the Arthropoda. Annu. Rev. Entomol. 1996, 41, 353–374. [Google Scholar] [CrossRef] [PubMed]
- Rudinsky, J.A.; Morgan, M.E.; Libbey, L.M.; Putnam, T.B. Additional components of the Douglas-fir beetle (Col., Scolytidae) aggregative pheromone and their possible utility in pest control. J. Appl. Entomol. 1974, 76, 65–77. [Google Scholar] [CrossRef]
- Michael, R.R.; Rudinsky, J.A. Sound production in Scolytidae: Specificity in male Dendroctonus beetles. J. Insect Physiol. 1972, 18, 2189–2201. [Google Scholar] [CrossRef]
- Symonds, M.R.E.; Elgar, M.A. Species overlap, speciation and the evolution of aggregation pheromones in bark beetles. Ecol. Lett. 2004, 7, 202–212. [Google Scholar] [CrossRef]
- Symonds, M.; Gitau-Clarke, C. Chapter five-The evolution of aggregation pheromone diversity in bark beetles. Adv. Insect Physiol. 2016, 50, 195–234. [Google Scholar] [CrossRef]
- Tilden, P.E.; Bedard, W.D.; Lindahl, K.Q., Jr.; Wood, D.L. Trapping Dendroctonus brevicomis: Changes in attractant release rate, dispersion of attractant, and silhouette. J. Chem. Ecol. 1983, 9, 311–321. [Google Scholar] [CrossRef]
- Pitman, G.B.; Hedden, R.L.; Gara, R.I. Synergistic effects of ethyl alcohol on the aggregation of Dendroctonus pseudotsugae (Col., Scolytidae) in response to pheromones. Z. Angew. Entomol. 1975, 78, 203–208. [Google Scholar] [CrossRef]
- Byers, J.A. Optimal fractionation and bioassay plans for isolation of synergistic chemicals: The subtractive-combination method. J. Chem. Ecol. 1992, 18, 1603–1621. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, B.T.; Mori, K. Spatial displacement of release point can enhance activity of an attractant pheromone synergist of a bark beetle. J. Chem. Ecol. 2009, 35, 1222–1233. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sullivan, B.T. Chapter Four—Semiochemicals in the natural history of southern pine beetle Dendroctonus frontalis Zimmermann and their role in pest management. Adv. Insect Physiol. 2016, 50, 129–193. [Google Scholar] [CrossRef]
- Sullivan, B.T.; Brownie, C. Some effects of endo-brevicomin background on southern pine beetle (Coleoptera: Curculionidae: Scolytinae) aggregation behavior. Environ. Entomol. 2021, 50, 1304–1310. [Google Scholar] [CrossRef]
- Sullivan, B.T.; Dalusky, M.J.; Mori, K.; Brownie, C. Variable responses by southern pine beetle, Dendroctonus frontalis Zimmermann, to the pheromone component endo-brevicomin: Influence of enantiomeric composition, release rate, and proximity to infestations. J. Chem. Ecol. 2011, 37, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Borden, J.H.; Pureswaran, D.S.; Lafontaine, J.P. Synergistic blends of monoterpenes for aggregation pheromones of the mountain pine beetle (Coleoptera: Curculionidae). J. Econ. Entomol. 2008, 101, 1266–1275. [Google Scholar] [CrossRef]
- Hansen, E.M.; Bentz, B.J.; Munson, A.S.; Vandygriff, J.C.; Turner, D.L. Evaluation of funnel traps for estimating tree mortality and associated population phase of spruce beetle in Utah. Can. J. For. Res. 2006, 36, 2574–2584. [Google Scholar] [CrossRef]
- Wood, D.L.; Browne, L.E.; Ewing, B.; Lindahl, K.; Bedard, W.D.; Tilden, P.E.; Mori, K.; Pitman, G.B.; Hughes, P.R. Western pine beetle—Specificity among enantiomers of male and female components of an attractant pheromone. Science 1976, 192, 896–898. [Google Scholar] [CrossRef] [PubMed]
- Pureswaran, D.S.; Hofstetter, R.W.; Sullivan, B.T. Attraction of the southern pine beetle, Dendroctonus frontalis, to pheromone components of the western pine beetle, Dendroctonus brevicomis (Coleoptera: Curculionidae: Scolytinae), in an allopatric zone. Environ. Entomol. 2008, 37, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, B.T.; Grady, A.M.; Hofstetter, R.W.; Pureswaran, D.S.; Brownie, C.; Cluck, D.; Coleman, T.W.; Graves, A.; Willhite, E.; Spiegel, L. Evidence for semiochemical divergence between sibling bark beetle species: Dendroctonus brevicomis and Dendroctonus barberi. J. Chem. Ecol. 2021, 47, 10–27. [Google Scholar] [CrossRef] [PubMed]
- Kostyk, B.C.; Borden, J.H.; Gries, G. Photoisomerization of antiaggregation pheromone verbenone: Biological and practical implications with respect to the mountain pine beetle, Dendroctonus ponderosae Hopkins (Coleoptera: Scolytidae). J. Chem. Ecol. 1993, 19, 1749–1759. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.R.; Borden, J.H.; Slessor, K.N. Enantiospecific pheromone production and response profiles for populations of pine engraver, Ips pini (Say) (Coleoptera: Scolytidae), in British Columbia. J. Chem. Ecol. 1996, 22, 2157–2172. [Google Scholar] [CrossRef] [PubMed]
- Lanier, G.N.; Birch, M.C.; Schmitz, R.F.; Furniss, M.M. Pheromones of Ips pini (Coleoptera: Scolytidae): Variation in response among three populations. Can. Entomol. 1972, 104, 1917–1923. [Google Scholar] [CrossRef]
- Erbilgin, N.; Mori, S.R.; Sun, J.H.; Stein, J.D.; Owen, D.R.; Merrill, L.D.; Bolanos, R.C.; Raffa, K.F.; Montiel, T.M.; Wood, D.L.; et al. Response to host volatiles by native and introduced populations of Dendroctonus valens (Coleoptera: Curculionidae, Scolytinae) in North America and China. J. Chem. Ecol. 2007, 33, 131–146. [Google Scholar] [CrossRef] [PubMed]
- Borden, J.; Gries, G.; Chong, L.; Werner, R.; Holsten, E.; Wieser, H.; Dixon, E.; Cerezke, H. Regionally-specific bioactivity of two new pheromones for Dendroctonus rufipennis (Kirby) (Col., Scolytidae). J. Appl. Entomol. 1996, 120, 321–326. [Google Scholar] [CrossRef]
- Sullivan, B.T.; Brownie, C.; Barrett, J.P. Intra-annual variation in responses by flying southern pine beetles (Coleoptera: Curculionidae: Scolytinae) to pheromone component endo-brevicomin. J. Econ. Entomol. 2016, 109, 1720–1728. [Google Scholar] [CrossRef][Green Version]
- Teale, S.A.; Lanier, G.N. Seasonal variability in response of Ips pini (Coleoptera: Scolytidae) to ipsdienol in New York. J. Chem. Ecol. 1991, 17, 1145–1158. [Google Scholar] [CrossRef]
- Sullivan, B.T.; Shepherd, W.P.; Nowak, J.T.; Clarke, S.R.; Merten, P.R.; Billings, R.F.; Upton, W.W.; Riggins, J.J.; Brownie, C. Alternative formulations of trap lures for operational detection, population monitoring, and outbreak forecasting of southern pine beetle in the United States. J. Econ. Entomol. 2021, 114, 1189–1200. [Google Scholar] [CrossRef]
- Steed, B.E.; Wagner, M.R. Seasonal pheromone response by Ips pini in northern Arizona and western Montana, USA. Agric. For. Entomol. 2008, 10, 189–203. [Google Scholar] [CrossRef]
- Keeling, C.I.; Tittiger, C.; MacLean, M.; Blomquist, G.J. Pheromone production in bark beetles. In Insect Pheromone Biochemistry and Molecular Biology; Elsevier: Amsterdam, The Netherlands, 2021; pp. 123–162. [Google Scholar]
- Renwick, J.A.A.; Hughes, P.R. Oxidation of unsaturated cyclic hydrocarbons by Dendroctonus frontalis. Insect Biochem. 1975, 5, 459–463. [Google Scholar] [CrossRef]
- Renwick, J.A.A.; Hughes, P.R.; Ty, T.D. Oxidation products of pinene in the bark beetle Dendroctonus frontalis. J. Insect Physiol. 1973, 19, 1735–1740. [Google Scholar] [CrossRef]
- Renwick, J.A.A.; Hughes, P.R.; Pitman, G.B.; Vité, J.P. Oxidation products of terpenes identified from Dendroctonus and Ips bark beetles. J. Insect Physiol. 1976, 22, 725–727. [Google Scholar] [CrossRef]
- Vité, J.P.; Islas, S.F.; Renwick, J.A.A.; Hughes, P.R.; Kliefoth, R.A. Biochemical and biological variation of southern pine beetle populations in North and Central America. Z. Angew. Entomol. 1974, 75, 422–435. [Google Scholar] [CrossRef]
- Hughes, P.R.; Renwick, J.A.A.; Vité, J.P. The identification and field bioassay of chemical attractants in the roundheaded pine beetle. Environ. Entomol. 1976, 5, 1165–1168. [Google Scholar] [CrossRef]
- Francke, W.; Bartels, J.; Meyer, H.; Schröder, F.; Kohnle, U.; Baader, E.; Vité, J. Semiochemicals from bark beetles: New results, remarks, and reflections. J. Chem. Ecol 1995, 21, 1043–1063. [Google Scholar] [CrossRef]
- Hunt, D.W.A.; Borden, J.H.; Pierce, H.D., Jr.; Slessor, K.N.; King, G.G.S.; Czy, E.K. Sex-specific production of ipsdienol and myrcenol by Dendroctonus ponderosae (Coleoptera: Scolytidae) exposed to myrcene vapors. J. Chem. Ecol. 1986, 12, 1579–1586. [Google Scholar] [CrossRef]
- Byers, J.A. Male-specific conversion of the host plant compound, myrcene, to the pheromone, (+)- ipsdienol, in the bark beetle, Dendroctonus brevicomis. J. Chem. Ecol. 1982, 8, 363–371. [Google Scholar] [CrossRef]
- Wang, Y.; Lim, L.; Madilao, L.; Lah, L.; Bohlmann, J.; Breuil, C. Gene discovery for enzymes involved in limonene modification or utilization by the mountain pine beetle-associated pathogen Grosmannia clavigera. Appl. Environ. Microbiol. 2014, 80, 4566–4576. [Google Scholar] [CrossRef] [PubMed]
- Hunt, D.W.A.; Borden, J.H. Conversion of verbenols to verbenone by yeasts isolated from Dendroctonus ponderosae (Coleoptera: Scolytidae). J. Chem. Ecol. 1990, 16, 1385–1397. [Google Scholar] [CrossRef] [PubMed]
- Leufven, A.; Bergstrom, G.; Falsen, E. Interconversion of verbenols and verbenone by identified yeasts isolated from the spruce bark beetle Ips typographus. J. Chem. Ecol. 1984, 10, 1349–1361. [Google Scholar] [CrossRef] [PubMed]
- Cognato, A.I.; Seybold, S.J.; Wood, D.L.; Teale, S.A. A cladistic analysis of pheromone evolution in Ips bark beetles (Coleoptera: Scolytidae). Evolution 1997, 51, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Fettig, C.J.; Borys, R.R.; Dabney, C.P.; McKelvey, S.R.; Cluck, D.R.; Smith, S.L. Disruption of red turpentine beetle attraction to baited traps by the addition of California fivespined Ips pheromone components. Can. Entomol. 2005, 137, 748–752. [Google Scholar] [CrossRef]
- Gaylord, M.L.; Kolb, T.E.; Wallin, K.F.; Wagner, M.R. Seasonality and lure preference of bark beetles (Curculionidae: Scolytinae) and associates in a northern Arizona ponderosa pine forest. Environ. Entomol. 2006, 35, 37–47. [Google Scholar] [CrossRef]
- Økland, B.; Skarpaas, O.; Kausrud, K. Threshold facilitations of interacting species. Popul. Ecol. 2009, 51, 513–523. [Google Scholar] [CrossRef]
- Svihra, P.; Paine, T.D.; Birch, M.C. Interspecific olfactory communications in southern pine beetles. Naturwissenschaften 1980, 67, 518–520. [Google Scholar] [CrossRef]
- Pureswaran, D.S.; Hofstetter, R.W.; Sullivan, B.T.; Potter, K.A. The role of multimodal signals in species recognition between tree-killing bark beetles in a narrow sympatric zone. Environ. Entomol. 2016, 45, 582–591. [Google Scholar] [CrossRef] [PubMed]
- Payne, T.L.; Richerson, J.V.; Dickens, J.C.; West, J.R.; Mori, K.; Berisford, C.W.; Hedden, R.L.; Vité, J.P.; Blum, M.S. Southern pine beetle: Olfactory receptor and behavior discrimination of enantiomers of the attractant pheromone frontalin. J. Chem. Ecol. 1982, 8, 873–881. [Google Scholar] [CrossRef]
- Vité, J.P.; Billings, R.F.; Ware, C.W.; Mori, K. Southern pine beetle: Enhancement or inhibition of aggregation response mediated by enantiomers of endo-brevicomin. Naturwissenschaften 1985, 72, 99–100. [Google Scholar] [CrossRef]
- Phillips, T.W.; Nation, J.L.; Wilkinson, R.C.; Foltz, J.L.; Pierce, H.D., Jr.; Oehlschlager, A.C. Response specificity of Dendroctonus terebrans (Coleoptera: Scolytidae) to enantiomers of its sex pheromones. Ann. Entomol. Soc. Am. 1990, 83, 251–257. [Google Scholar] [CrossRef]
- Mirov, N.T. Composition of Gum Turpentines of Pines; USDA Forest Service Pacific Southwest Forest and Range Experiment Station Technical Bulletin 1239; USDA Forest Service Pacific Northwest and Southern Research Stations: Portland, OR, USA, 1961; 158p.
- Smith, R.H. Monoterpenes of Ponderosa Pine Xylem Resin in Western United States; U.S. Department of Agriculture Forest Service Technical Bulletin; U.S. Department of Agriculture Forest Service: Washington, DC, USA, 1977; Volume 1532.
- Hammer, A.J.; Bower, N.W.; Snyder, A.I.; Snyder, Z.N.; Archila, F.L.; Snyder, M.A. Longitudinal study of Caribbean pine elucidates the role of 4-allylanisole in patterns of chemical resistance to bark beetle attack. J. Trop. Ecol. 2020, 36, 43–46. [Google Scholar] [CrossRef]
- Bookwalter, J.D.; Riggins, J.J.; Dean, J.F.D.; Mastro, V.C.; Schimleck, L.R.; Sullivan, B.T.; Gandhi, K.J.K. Colonization and development of Sirex noctilio (Hymenoptera: Siricidae) in bolts of a native pine host and six species of pine grown in the southeastern United States. J. Entomol. Sci. 2019, 54, 1–18. [Google Scholar] [CrossRef]
- Ott, D.S.; Yanchuk, A.D.; Huber, D.P.; Wallin, K.F. Genetic variation of lodgepole pine, Pinus contorta var. latifolia, chemical and physical defenses that affect mountain pine beetle, Dendroctonus ponderosae, attack and tree mortality. J. Chem. Ecol 2011, 37, 1002–1012. [Google Scholar] [CrossRef] [PubMed]
- Clark, E.L.; Carroll, A.L.; Huber, D.P. Differences in the constitutive terpene profile of lodgepole pine across a geographical range in British Columbia, and correlation with historical attack by mountain pine beetle. Can. Entomol. 2010, 142, 557–573. [Google Scholar] [CrossRef]
- Fischer, M.J.; Waring, K.M.; Hofstetter, R.W.; Kolb, T.E. Ponderosa pine characteristics associated with attack by the roundheaded pine beetle. For. Sci. 2010, 56, 473–483. [Google Scholar]
- Kimmerer, T.W.; Kozlowski, T.T. Ethylene, ethane, acetaldehyde, and ethanol production by plants under stress. Plant Physiol. 1982, 69, 840–847. [Google Scholar] [CrossRef] [PubMed]
- Klimetzek, D.; Kohler, J.; Vité, J.P.; Kohnle, U. Dosage response to ethanol mediates host selection by “secondary” bark beetles. Naturwissenschaften 1986, 73, 270–272. [Google Scholar] [CrossRef]
- Schroeder, L.M.; Lindelow, A. Attraction of scolytids and associated beetles by different absolute amounts and proportions of α-pinene and ethanol. J. Chem. Ecol. 1989, 15, 807–817. [Google Scholar] [CrossRef]
- Kelsey, R.G.; Joseph, G.; Peck, R.W.; Niwa, C.G. Response of some scolytids and their predators to ethanol and 4-allylanisole in pine forests of central Oregon. J. Chem. Ecol. 2001, 27, 697–715. [Google Scholar] [CrossRef]
- Petrakis, P.V.; Tsitsimpikou, C.; Tzakou, O.; Couladis, M.; Vagias, C.; Roussis, V. Needle volatiles from five Pinus species growing in Greece. Flavour Fragr. J. 2001, 16, 249–252. [Google Scholar] [CrossRef]
- Ioannou, E.; Koutsaviti, A.; Tzakou, O.; Roussis, V. The genus Pinus: A comparative study on the needle essential oil composition of 46 pine species. Phytochem. Rev. 2014, 13, 741–768. [Google Scholar] [CrossRef]
- Pureswaran, D.S.; Borden, J.H. Test of semiochemical mediated host specificity in four species of tree killing bark beetles (Coleoptera: Scolytidae). Environ. Entomol. 2003, 32, 963–969. [Google Scholar] [CrossRef][Green Version]
- Snyder, M.A.; Bower, N.W. Resistance to bark beetle attack in Caribbean pine: Potential role of 4-Allylanisole. Biotropica 2005, 37, 702–705. [Google Scholar] [CrossRef]
- Hayes, J.; Ingram, L.; Strom, B.; Roton, L.; Boyette, M.; Walsh, M. Identification of a host compound and its practical applications: 4-allylanisole as a bark beetle repellent. In Proceedings of the 4th Southern Station Chemical Sciences Meeting, Starkville, MS, USA, 1–2 February 1994; USDA Forest Service General Technical Report SO-104. pp. 69–80. [Google Scholar]
- Munro, H.L.; Gandhi, K.J.K.; Barnes, B.F.; Montes, C.R.; Nowak, J.T.; Shepherd, W.P.; Villari, C.; Sullivan, B.T. Electrophysiological and behavioral responses Dendroctonus frontalis and D. terebrans (Coleoptera: Curculionidae) to resin odors of host pines (Pinus spp.). Chemoecology 2020, 30, 215–231. [Google Scholar] [CrossRef]
- Hobson, K.R.; Wood, D.L.; Cool, L.G.; White, P.R.; Ohtsuka, T.; Kubo, I.; Zavarin, E. Chiral specificity in responses by the bark beetle Dendroctonus valens to host kairomones. J. Chem. Ecol. 1993, 19, 1837–1846. [Google Scholar] [CrossRef]
- Staeben, J.C.; Sullivan, B.T.; Nowak, J.T.; Gandhi, K.J. Enantiospecific responses of southern pine beetle (Dendroctonus frontalis) and its clerid predator, Thanasimus dubius, to α-pinene. Chemoecology 2015, 25, 73–83. [Google Scholar] [CrossRef]
- Cronin, J.T.; Hayes, J.L.; Turchin, P. Evaluation of traps used to monitor southern pine beetle aerial populations and sex ratios. Agric. For. Entomol. 2000, 2, 69–76. [Google Scholar] [CrossRef]
- Payne, T.L.; Billings, R.F.; Delorme, J.D.; Andryszak, N.A.; Bartels, J.; Francke, W.; Vité, J.P. Kairomonal-pheromonal system in the black turpentine beetle, Dendroctonus terebrans (Ol.). J. Appl. Entomol. 1987, 103, 15–22. [Google Scholar] [CrossRef]
- Hughes, P.R. Response of female southern pine beetles to the aggregation pheromone frontalin. Z. Angew. Entomol. 1976, 81, 463–466. [Google Scholar]
- Pureswaran, D.S.; Sullivan, B.T.; Ayres, M.P. Fitness consequences of pheromone production and host selection strategies in a tree-killing bark beetle (Coleoptera: Curculionidae: Scolytinae). Oecologia 2006, 148, 720–728. [Google Scholar] [CrossRef] [PubMed]
- Coster, J.E.; Vité, J.P. Effects of feeding and mating on pheromone release in the southern pine beetle. Ann. Entomol. Soc. Am. 1972, 65, 263–266. [Google Scholar] [CrossRef]
- Coster, J.E. Production of aggregating pheromones in re-emerged parent females of the southern pine beetle. Ann. Entomol. Soc. Am. 1970, 63, 1186–1187. [Google Scholar] [CrossRef] [PubMed]
- Stewart, T.E.; Plummer, E.L.; McCanless, L.L.; West, J.R.; Silverstein, R.M. Determination of enantiomer composition of several bicyclic ketal insect pheromone components. J. Chem. Ecol. 1977, 3, 27–43. [Google Scholar] [CrossRef]
- Grosman, D.M.; Salom, S.M.; Ravlin, F.W.; Young, R.W. Geographic and gender differences in semiochemicals in emerging adult southern pine beetle (Coleoptera: Scolytidae). Ann. Entomol. Soc. Am. 1997, 90, 438–446. [Google Scholar] [CrossRef]
- Redlich, H.; Bruns, W.; Francke, W.; Schurig, V.; Payne, T.L.; Vite, J.P. Chiral building units from carbohydrates-XII.: 8. Identification of the absolute configuration of endo-brevicomin from Dendroctonus frontalis and synthesis of both enantiomers from D-ribose. Tetrahedron 1987, 43, 2029–2034. [Google Scholar] [CrossRef]
- Salom, S.M.; Billings, R.F.; Upton, W.W.; Dalusky, M.J.; Grosman, D.M.; Payne, T.L.; Berisford, C.W.; Shaver, T.N. Effect of verbenone enantiomers and racemic endo-brevicomin on response of Dendroctonus frontalis (Coleoptera, Scolytidae) to attractant-baited traps. Can. J. For. Res. 1992, 22, 925–931. [Google Scholar] [CrossRef]
- Hayes, J.L.; Strom, B.L.; Roton, L.M.; Ingram, L.L. Repellent properties of the host compound 4-allylanisole to the southern pine beetle. J. Chem. Ecol. 1994, 20, 1595–1615. [Google Scholar] [CrossRef]
- Strom, B.L.; Roton, L.M.; Goyer, R.A.; Meeker, J.R. Visual and semiochemical disruption of host finding in the southern pine beetle. Ecol. Appl. 1999, 9, 1028–1038. [Google Scholar] [CrossRef]
- Sánchez-Martínez, G.; Reséndiz-Martínez, J.F. Respuesta de Dendroctonus frontalis Zimmerman y Dendroctonus mexicanus Hopkins a dos atrayentes semioquímicos en la Sierra Gorda de Querétaro, México. Southwest. Entomol. 2020, 45, 511–520. [Google Scholar] [CrossRef]
- Díaz-Núñez, V.; Sánchez-Martínez, G.; Gillette, N.E. Respuesta de Dendroctonus mexicanus (Hopkins) a dos isómeros ópticos de verbenona. Agrociencia 2006, 40, 349–354. [Google Scholar]
- Soto-Correa, J.C.; Hernández-Muñoz, G.; Cambrón-Sandoval, V.H. Efecto de las variables climáticas en Dendroctonus mexicanus Hopkins (Coleoptera: Curculionidae) en bosques de Hidalgo. Rev. Mex. Cienc. For. 2022, 13, 112–131. [Google Scholar] [CrossRef]
- Mendoza-Villa, O.N.; Obregón-Zúñiga, J.A. Cambio en la abundancia de Dendroctonus frontalis Zimmerman, 1868 y Dendroctonus mexicanus Hopkins, 1909 (Coleoptera: Curculionidae: Scolytinae) en un gradiente altitudinal en el cerro “La Pingüica”, Pinal de Amoles, Querétaro. Entomol. Mex. 2016, 3, 644–648. [Google Scholar]
- Moser, J.C.; Fitzgibbon, B.A.; Klepzig, K.D. The mexican pine beetle, Dendroctonus mexicanus: First record in the United States and co-occurrence with the southern pine beetle—Dendroctonus frontalis (Coleoptera: Scolytidae or Curculionidae: Scolytinae). Entomol. News 2005, 116, 235–244. [Google Scholar]
- Bedard, W.D.; Wood, D.L.; Tilden, P.E.; Lindahl Jr, K.Q.; Silverstein, R.M.; Rodin, J.O. Field responses of the western pine beetle and one of its predators to host-and beetle-produced compounds. J. Chem. Ecol. 1980, 6, 625–641. [Google Scholar] [CrossRef]
- Bedard, W.D.; Silverstein, R.M.; Wood, D.L. Bark beetle pheromones. Science 1970, 167, 1638–1639. [Google Scholar] [CrossRef] [PubMed]
- Vité, J.P.; Pitman, G.B. Aggregation behaviour of Dendroctonus brevicomis in response to synthetic pheromones. J. Insect Physiol. 1969, 15, 1617–1622. [Google Scholar] [CrossRef]
- Moeck, H.A.; Wood, D.L.; Lindahl, K.Q., Jr. Host selection behavior of bark beetles (Coleoptera: Scolytidae) attacking Pinus ponderosa, with special emphasis on the western pine beetle, Dendroctonus brevicomis. J. Chem. Ecol. 1981, 7, 49–83. [Google Scholar] [CrossRef]
- Shepherd, W.P.; Huber, D.P.W.; Seybold, S.J.; Fettig, C.J. Antennal responses of the western pine beetle, Dendroctonus brevicomis (Coleoptera: Curculionidae), to stem volatiles of its primary host, Pinus ponderosa, and nine sympatric nonhost angiosperms and conifers. Chemoecology 2007, 17, 209–221. [Google Scholar] [CrossRef]
- Hofstetter, R.W.; Chen, Z.; Gaylord, M.L.; McMillin, J.D.; Wagner, M.R. Synergistic effects of alpha-pinene and exo-brevicomin on pine bark beetles and associated insects in Arizona. J. Appl. Entomol. 2008, 132, 387–397. [Google Scholar] [CrossRef]
- Castillo, J.V. Atrayentes quimicos en escarabajos descortezadores Dendroctonus mexicanus y D. adjunctus (Col: Scolytidae). Rev. Mex. Cienc. For. 1992, 17, 104–121. [Google Scholar]
- Rodríguez-Ortega, A.; Equihua-Martínez, A.; Cibrián-Tovar, J.; Estrada-Venegas, E.G.; Méndez-Montiel, J.T.; Villa-Castillo, J. Fluctuación de Dendroctonus adjunctus Blandford (Curculioniade: Scolytinae) y sus depredadores atraídos por frontalina + alfa-pineno, la Estación Experimental de Zoquiapan, Edo. De México. Bol. Mus. Entomol. Univ. Val. 2010, 11, 20–27. [Google Scholar]
- Rodríguez-Ortega, A.; Equihua Martínez, A.; Cibrián Tovar, J.; Estrada Venegas, E.; Méndez Montiel, J.; Villa Castillo, J.; Barrón Yánez, R. Population dynamics of Dendroctonus adjunctus (Coleoptera: Curculionidae: Scolytinae) and its predators attracted to frontalin + alpha-pinene in Los Pescados, Veracruz, Mexico. Rev. Chil. Entomol. 2013, 38, 41–50. [Google Scholar]
- Ryker, L.C.; Libbey, L. Frontalin in the male mountain pine beetle. J. Chem. Ecol. 1982, 8, 1399–1409. [Google Scholar] [CrossRef] [PubMed]
- Taft, S.; Najar, A.; Erbilgin, N. Pheromone production by an invasive bark beetle varies with monoterpene composition of its naïve host. J. Chem. Ecol. 2015, 41, 540–549. [Google Scholar] [CrossRef]
- Gries, G.; Leufvén, A.; Lafontaine, J.; Pierce, H.; Borden, J.; Vanderwel, D.; Oehlschlager, A. New metabolites of α-pinene produced by the mountain pine beetle, Dendroctonus ponderosae (Coleoptera: Scolytidae). Insect Biochem. 1990, 20, 365–371. [Google Scholar] [CrossRef]
- Borden, J.H.; Ryker, L.C.; Chong, L.J.; Pierce, H.D., Jr.; Johnston, B.D.; Oehlschlager, A.C. Response of the mountain pine beetle, Dendroctonus ponderosae Hopkins (Coleoptera: Scolytidae), to five semiochemicals in British Columbia lodgepole pine forests. Can. J. For. Res. 1987, 17, 118–128. [Google Scholar] [CrossRef]
- Conn, J.E.; Borden, J.H.; Scott, B.E.; Frieske, L.M.; Pierce, H.D.; Oehlschlager, A.C. Semiochemicals for the mountain pine beetle, Dendroctonus ponderosae (Coleoptera: Scolytidae) in British Columbia: Field trapping studies. Can. J. For. Res. 1983, 13, 320–324. [Google Scholar] [CrossRef]
- Ryker, L.C.; Rudinsky, J.A. Field bioassay of exo- and endo-brevicomin with Dendroctonus ponderosae in lodgepole pine. J. Chem. Ecol. 1982, 8, 701–707. [Google Scholar] [CrossRef]
- Miller, D.R.; Lindgren, B.S. Comparison of alpha-pinene and myrcene on attraction of mountain pine beetle, Dendroctonus ponderosae (Coleoptera: Scolytidae) to pheromones in stands of western white pine. J. Entomol. Soc. Br. Columbia 2000, 97, 41–46. [Google Scholar]
- Miller, D.R.; LaFontaine, J.P. cis-Verbenol: An aggregation pheromone for the mountain pine beetle Dendroctonus ponderosae (Coleoptera: Scolytidae). J. Entomol. Soc. Br. Columbia 1991, 88, 34–38. [Google Scholar]
- Moeck, H.A.; Simmons, C.S. Primary attraction of mountain pine beetle, Dendroctonus ponderosae Hopk.(Coleoptera: Scolytidae), to bolts of lodgepole pine. Can. Entomol. 1991, 123, 299–304. [Google Scholar] [CrossRef]
- Pitman, G.B. trans-Verbenol and alpha-pinene: Their utility in manipulation of the mountain pine beetle. J. Econ. Entomol. 1971, 64, 426–430. [Google Scholar] [CrossRef]
- Strom, B.L.; Smith, S.L.; Wakarchuk, D. An improved synthetic attractant for the mountain pine beetle, Dendroctonus ponderosae Hopkins (Coleoptera: Curculionidae: Scolytinae), in northeastern California. Pan-Pac. Entomol. 2008, 84, 51–56. [Google Scholar] [CrossRef]
- Borden, J.H. Aggregation pheromones in the Scolytidae. Front. Biol. 1974, 32, 135–160. [Google Scholar]
- Renwick, J.; Pitman, G. An attractant isolated from female Jeffrey pine beetles, Dendroctonus jeffreyi. Environ. Entomol. 1979, 8, 40–41. [Google Scholar] [CrossRef]
- Strom, B.; Smith, S.; Brownie, C. Attractant and disruptant semiochemicals for Dendroctonus jeffreyi (Coleoptera: Curculionidae: Scolytinae). Environ. Entomol. 2013, 42, 323–332. [Google Scholar] [CrossRef]
- Sarabia, L.E.; López, M.F.; Obregón-Molina, G.; Cano-Ramírez, C.; Sánchez-Martínez, G.; Zúñiga, G. The differential expression of mevalonate pathway genes in the gut of the bark beetle Dendroctonus rhizophagus (Curculionidae: Scolytinae) is unrelated to the de novo synthesis of terpenoid pheromones. Int. J. Mol. Sci. 2019, 20, 4011. [Google Scholar] [CrossRef]
- Liu, Z.; Xin, Y.; Xu, B.; Raffa, K.F.; Sun, J. Sound-triggered production of antiaggregation pheromone limits overcrowding of Dendroctonus valens attacking pine trees. Chem. Senses 2017, 42, 59–67. [Google Scholar] [CrossRef]
- Kelsey, R.G.; Westlind, D.J. β-Pinene + ethanol attracts more red turpentine beetles than carene + ethanol, with or without traces of frontalin, at prescribed burn or thinned sites. Agric. For. Entomol. 2023, 25, 650–657. [Google Scholar] [CrossRef]
- Kelsey, R.G.; Westlind, D.J. Red turpentine beetle primary attraction increases linearly with (−)-β-pinene+ethanol dose regardless of component ratios, and no change in response with addition of high-release frontalin. Agric. For. Entomol. 2022, 24, 63–71. [Google Scholar] [CrossRef]
- Fettig, C.J.; Borys, R.R.; Cluck, D.R.; Smith, S.L. Field response of Dendroctonus valens (Coleoptera: Scolytidae) and a major predator, Temnochila chlorodia (Coleoptera: Trogositidae), to host kairomones and a Dendroctonus spp. pheromone component. J. Entomol. Sci. 2004, 39, 490–499. [Google Scholar] [CrossRef]
- Sun, J.H.; Miao, Z.W.; Zhang, Z.; Zhang, Z.N.; Gillette, N.E. Red turpentine beetle, Dendroctonus valens LeConte (Coleoptera: Scolytidae), response to host semiochemicals in China. Environ. Entomol. 2004, 33, 206–212. [Google Scholar] [CrossRef]
- Kelsey, R.G.; Westlind, D.J. Attraction of red turpentine beetle and other Scolytinae to ethanol, 3-carene or ethanol + 3-carene in an Oregon pine forest. Agric. For. Entomol. 2018, 20, 272–278. [Google Scholar] [CrossRef]
- Kelsey, R.G.; Westlind, D.J.; Gaylord, M.L. Red turpentine beetle primary attraction to β-pinene or 3-carene (with and without ethanol) varies in western US pine forests. Agric. For. Entomol. 2022, 25, 111–118. [Google Scholar] [CrossRef]
- Kelsey, R.G.; Westlind, D.J. Red turpentine beetle primary attraction to (−)-β-pinene+ethanol in US Pacific Northwest ponderosa pine forests. PLoS ONE 2020, 15, e0236276. [Google Scholar] [CrossRef]
- Munro, H.L.; Sullivan, B.T.; Villari, C.; Gandhi, K.J.K. A review of the ecology and management of black turpentine beetle (Coleoptera: Curculionidae). Environ. Entomol. 2019, 48, 765–783. [Google Scholar] [CrossRef] [PubMed]
- Fatzinger, C.W.; Siegfried, B.D.; Wilkinson, R.C.; Nation, J.L. trans-Verbenol, turpentine, and ethanol as trap baits for the black turpentine beetle, Dendroctonus terebrans, and other forest Coleoptera in North Florida. J. Entomol. Sci. 1987, 22, 201–209. [Google Scholar] [CrossRef]
- Clements, R.W.; Williams, H.G. Attractants, Techniques, and Devices for Trapping Bark Beetles; U.S.D.A. Forest Service Southeastern Forest Experiment Station Research Note; U.S.D.A. Forest Service Southeastern Forest Experiment Station: Asheville, NC, USA, 1981; Volume SE-309, 3p.
- Siegfried, B.D.; Fatzinger, C.W.; Wilkinson, R.C.; Nation, J.L. In-flight responses of the black turpentine beetle (Coleotera: Scolytidae) to individual monoterpenes, turpentine, and paraquat-treated slash pines. Environ. Entomol. 1986, 15, 710–714. [Google Scholar] [CrossRef]
- Phillips, T.W.; Wilkening, A.J.; Atkinson, T.H.; Nation, J.L.; Wilkinson, R.C.; Foltz, J.L. Synergism of turpentine and ethanol as attractants for certain pine-infesting beetles (Coleoptera). Environ. Entomol. 1988, 17, 456–462. [Google Scholar] [CrossRef]
- Gries, G. Ratios of geometrical and optical isomers of pheromones: Irrelevant or important in scolytids? J. Appl. Entomol. 1992, 114, 240–243. [Google Scholar] [CrossRef]
- Gries, G.; Borden, J.; Gries, R.; Lafontaine, J.; Dixon, E.; Wieser, H.; Whitehead, A. 4-Methylene-6, 6-dimethylbicyclo [3.1. 1] hept-2-ene (verbenene): New aggregation pheromone of the scolytid beetle Dendroctonus rufipennis. Naturwissenschaften 1992, 79, 367–368. [Google Scholar] [CrossRef]
- Ryall, K.; Silk, P.; Thurston, G.; Scarr, T.; de Groot, P. Elucidating pheromone and host volatile components attractive to the spruce beetle, Dendroctonus rufipennis (Coleoptera: Curculionidae), in eastern Canada. Can. Entomol. 2013, 145, 406–415. [Google Scholar] [CrossRef]
- Vité, J.; Pitman, G.; Fentiman, A.; Kinzer, G. 3-Methyl-2-cyclohexen-1-ol isolated from Dendroctonus. Naturwissenschaften 1972, 59, 469. [Google Scholar] [CrossRef] [PubMed]
- Isitt, R.; Bleiker, K.; Pureswaran, D.; Hillier, N.; Huber, D. Local, geographical, and contextual variation in the aggregation pheromone blend of the spruce beetle, Dendroctonus rufipennis (Coleoptera: Curculionidae). J. Chem. Ecol. 2020, 46, 497–507. [Google Scholar] [CrossRef] [PubMed]
- Dyer, E.D.A. Spruce beetle aggregated by the synthetic pheromone frontalin. Can. J. For. Res. 1973, 3, 486–494. [Google Scholar] [CrossRef]
- Dyer, E.D.A. Frontalin attractant in stands infested by the spruce beetle, Dendroctonus rufipennis (Coleoptera: Scolytidae). Can. Entomol. 1975, 107, 979–988. [Google Scholar] [CrossRef]
- Poland, T.M.; Borden, J. Disruption of secondary attraction of the spruce beetle, Dendroctonus rufipennis, by pheromones of two sympatric species. J. Chem. Ecol. 1998, 24, 151–166. [Google Scholar] [CrossRef]
- Setter, R.R.; Borden, J.H. Bioactivity and efficacy of MCOL and seudenol as potential attractive bait components for Dendroctonus rufipennis (Coleoptera: Scolytidae). Can. Entomol. 1999, 131, 251–257. [Google Scholar] [CrossRef]
- Ross, D.W.; Daterman, G.E.; Munson, A.S. Spruce beetle (Coleoptera: Scolytidae) response to traps baited with selected semiochemicals in Utah. West. N. Am. Nat. 2005, 65, 123–126. [Google Scholar]
- Maroja, L.S.; Bogdanowicz, S.M.; Wallin, K.F.; Raffa, K.F.; Harrison, R.G. Phylogeography of spruce beetles (Dendroctonus rufipennis Kirby)(Curculionidae: Scolytinae) in Nort208h America. Mol. Ecol. 2007, 16, 2560–2573. [Google Scholar] [CrossRef]
- Graham, E.E.; Storer, A.J. Interrupting the response of Dendroctonus simplex Leconte (Coleoptera: Curculionidae: Scolytinae) to compounds that elicit aggregation of adults. Great Lakes Entomol. 2011, 44, 6. [Google Scholar] [CrossRef]
- Werner, R.A.; Furniss, M.M.; Yarger, L.C.; Ward, T. Effects on Eastern Larch Beetle of Its Natural Attractant and Synthetic Pheromones in Alaska; U.S.D.A. Forest Service Pacific Northwest Forest and Range Experiment Station Research Note; U.S.D.A. Forest Service Pacific Northwest Forest and Range Experiment Station: Portland, OR, USA, 1981; Volume PNW-371, 7p.
- Prendergast, B.F. The Chemical Ecology of the Eastern Larch Beetle, Dendroctonus simplex LeConte, and the Spruce Beetle, D. rufipennis (Kirby). Master’s Thesis, State University of New York College of Environmental Science and Forestry, Syracuse, New York, NY, USA, 1991. [Google Scholar]
- Gandhi, K.J.; Gilmore, D.W.; Haack, R.A.; Katovich, S.A.; Krauth, S.J.; Mattson, W.J.; Zasada, J.C.; Seybold, S.J. Application of semiochemicals to assess the biodiversity of subcortical insects following an ecosystem disturbance in a sub-boreal forest. J. Chem. Ecol. 2009, 35, 1384–1410. [Google Scholar] [CrossRef] [PubMed]
- Pitman, G.; Vité, J. Field response of Dendroctonus pseudotsugae (Coleoptera: Scolytidae) to synthetic frontalin. Ann. Entomol. Soc. Am. 1970, 63, 661–664. [Google Scholar] [CrossRef]
- Madden, J.; Pierce, H.; Borden, J.; Butterfield, A. Sites of production and occurrence of volatiles in Douglas-fir beetle, Dendroctonus pseudotsugae Hopkins. J. Chem. Ecol. 1988, 14, 1305–1317. [Google Scholar] [CrossRef]
- Rudinsky, J.A.; Ryker, L.C.; Michael, R.R.; Libbey, L.M.; Morgan, M.E. Sound production in Scolytidae: Female sonic stimulus of male pheromone release in two Dendroctonus beetles. J. Insect Physiol. 1976, 22, 1675–1681. [Google Scholar] [CrossRef]
- Rudinsky, J.A.; Morgan, M.E.; Libbey, L.M.; Putman, T.B. Limonene released by the scolytid beetle Dendroctonus pseudotsugae. J. Appl. Entomol. 1977, 82, 376–380. [Google Scholar] [CrossRef]
- Ryker, L.C.; Libbey, L.M.; Rudinsky, J.A. Comparison of volatile compounds and stridulation emitted by the Douglas-fir beetle from Idaho and western Oregon populations. Environ. Entomol. 1979, 8, 789–798. [Google Scholar] [CrossRef]
- Rudinsky, J.; Morgan, M.; Libbey, L.; Putnam, T. Release of frontalin by male Douglas-fir beetle. J. Appl. Entomol. 1976, 81, 267–269. [Google Scholar] [CrossRef]
- Pitman, G.B.; Vité, J.P. Biosynthesis of methylocyclohexenone by male Douglas-fir beetle. Environ. Entomol. 1974, 3, 886–887. [Google Scholar] [CrossRef]
- Kinzer, G.W.; Fentiman, A.F., Jr.; Foltz, R.L.; Rudinsky, J.A. Bark beetle attractants: 3-methyl-2-cyclohexen-1-one isolated from Dendroctonus pseudotsugae. J. Econ. Entomol. 1971, 64, 970–971. [Google Scholar] [CrossRef]
- Miller, D.R.; Madden, J.L.; Borden, J.H. Primary attraction of Ips latidens (LeConte) and Hylastes gracilis LeConte (Coleoptera, Scolytidae) to high-girdled lodgepole pine, Pinus contorta var latifolia Engelmann. Can. Entomol. 1986, 118, 85–88. [Google Scholar] [CrossRef]
- Libbey, L.M.; Oehlschlager, A.C.; Ryker, L.C. 1-Methlcyclohex-2-en-1-ol as an aggregation pheromone of Dendroctonus pseudotsugae. J. Chem. Ecol. 1983, 9, 1533–1541. [Google Scholar] [CrossRef] [PubMed]
- Lindgren, B.S. Attraction of Douglas-fir beetle, spruce beetle and a bark beetle predator (Coleoptera: Scolytidae and Cleridae) to enantiomers of frontalin. J. Entomol. Soc. Br. Columbia 1992, 89, 13–17. [Google Scholar]
- Stock, M.W.; Pitman, G.B.; Guenther, J.D. Genetic differences between Douglas-fir beetles (Dendroctonus pseudotsugae) from Idaho and coastal Oregon. Ann. Entomol. Soc. Am. 1979, 72, 394–397. [Google Scholar] [CrossRef]
- Lindgren, B.S.; Miller, D.R.; Lafontaine, J.P. MCOL, frontalin and ethanol: A potential operational trap lure for Douglas-fir beetle in British Columbia. J. Entomol. Soc. Br. Columbia 2012, 109, 72–74. [Google Scholar]
- Pitman, G. Further observations on douglure in a Dendroctonus pseudotsugae management system. Environ. Entomol. 1973, 2, 109–112. [Google Scholar] [CrossRef]
- Ross, D.W.; Daterman, G.E. Response of Dendroctonus pseudotsugae (Coleoptera: Scolytidae) and Thanasimus undatulus (Coleoptera: Cleridae) to traps with different semiochemicals. J. Econ. Entomol. 1995, 88, 106–111. [Google Scholar] [CrossRef]
- Ross, D.W.; Daterman, G.E. Pheromone-baited traps for Dendroctonus pseudotsugae (Coleoptera: Scolytidae): Influence of selected release rates and trap designs. J. Econ. Entomol. 1998, 91, 500–506. [Google Scholar] [CrossRef]
- Rudinsky, J.A.; Kinzer, G.W.; Fentiman, A.F., Jr.; Foltz, R.L. trans-Verbenol isolated from Douglas-fir beetle: Laboratory and field bioassays in Oregon. Environ. Entomol. 1972, 4, 485–488. [Google Scholar] [CrossRef]
- Furniss, M.M.; Schmitz, R.F. Comparative Attraction of Douglas-Fir Beetles to Frontalin and Tree Volatiles; U.S.D.A Forest Service Intermountain Forest and Range Experiment Station Research Paper; U.S.D.A Forest Service Intermountain Forest and Range Experiment Station: Ogden, UT, USA, 1971; Volume INT-96, 16p.
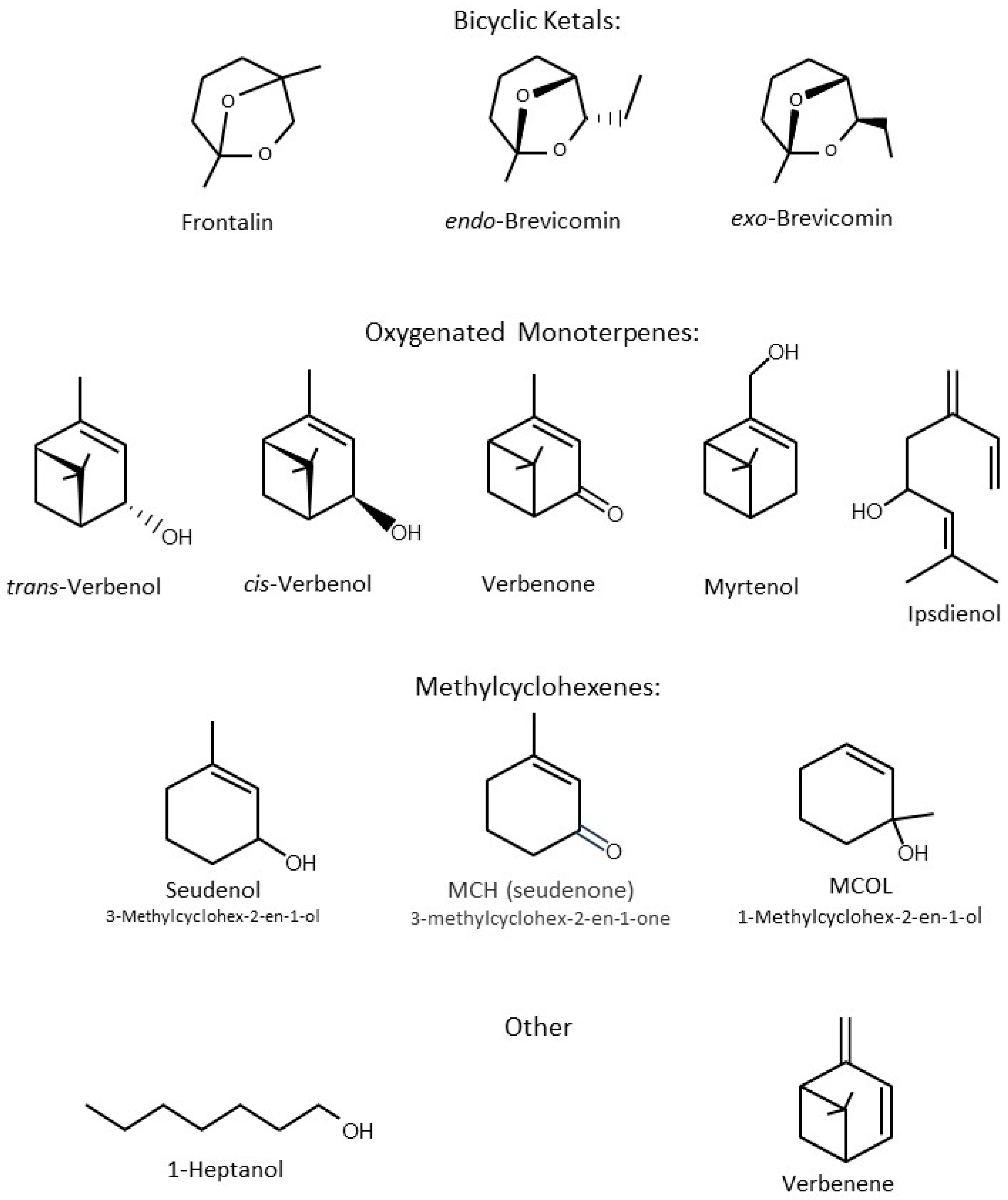
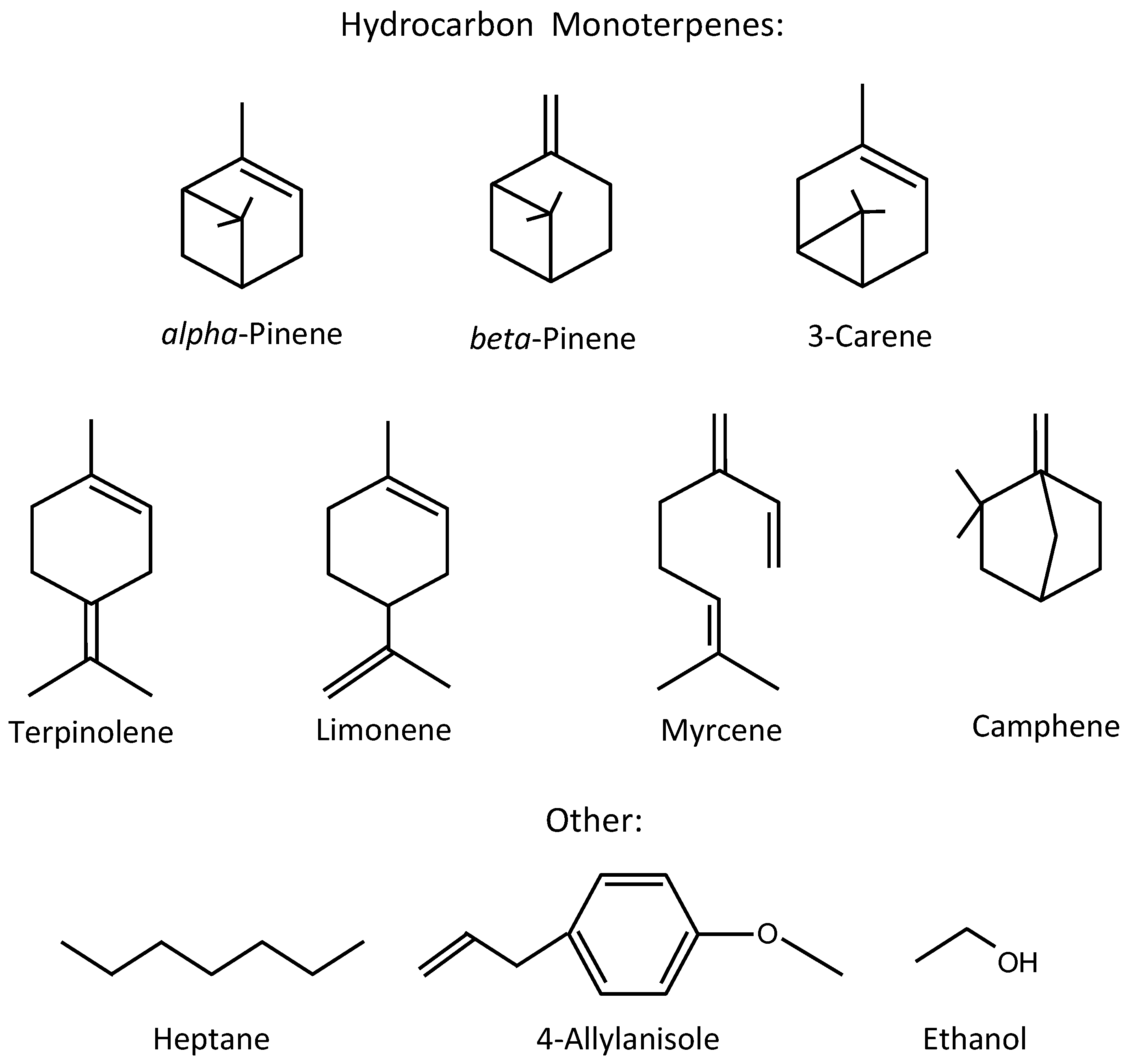
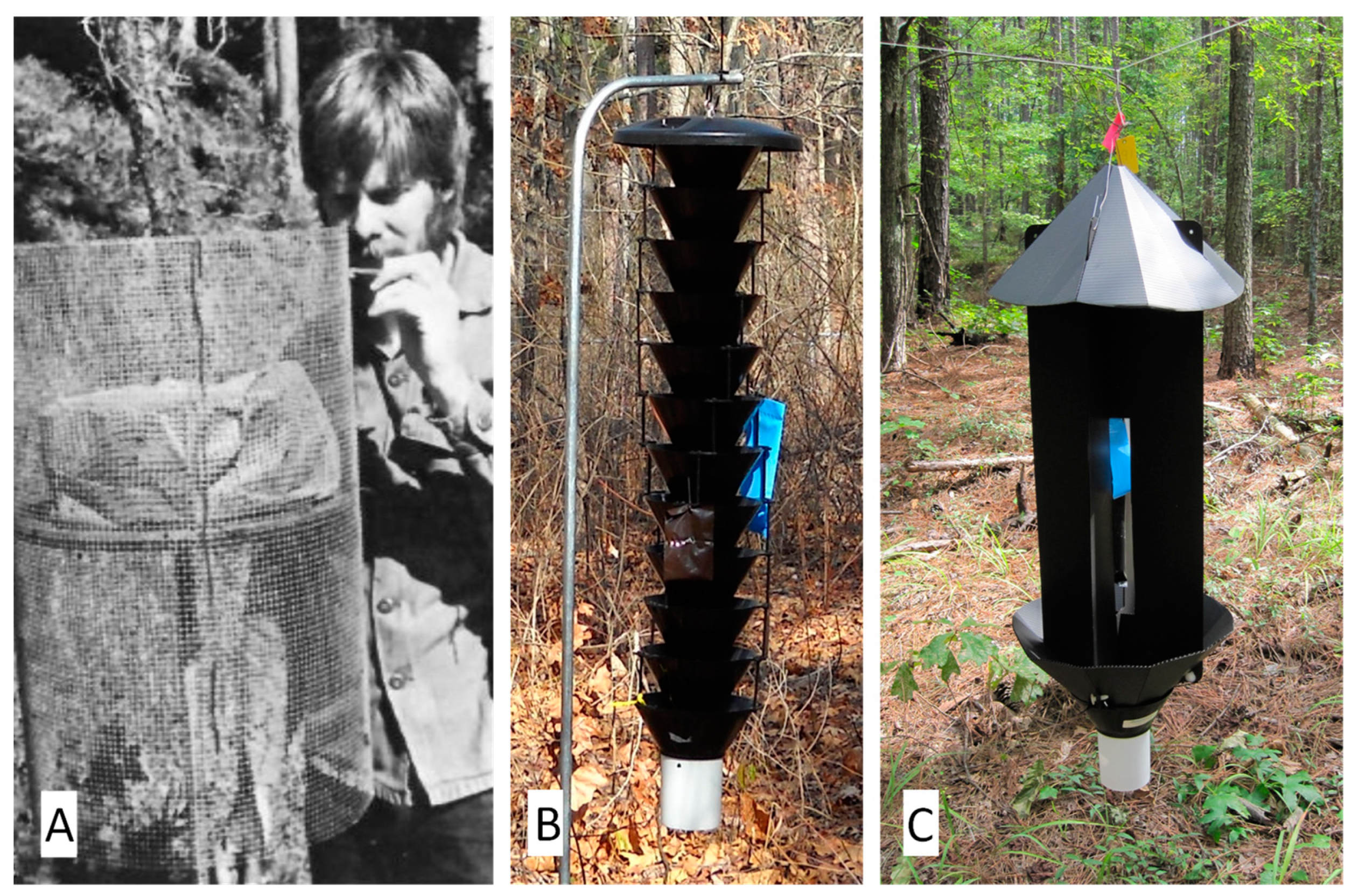
| Females | Males | ||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| frontalin | endo-brevicomin | exo-brevicomin | trans-verbenol | cis-verbenol | verbenone | myrtenol | ipsdienol | seudenol | MCH | MCOL | 1-heptanol | verbenene | frontalin | endo-brevicomin | exo-brevicomin | trans-verbenol | cis-verbenol | verbenone | myrtenol | ipsdienol | seudenol | MCH | MCOL | 1-heptanol | verbenene | ||
| D. frontalis | X | X | X | X1,2 | X | X | |||||||||||||||||||||
| D. mesoamericanus | X | X | X | X1 | |||||||||||||||||||||||
| D. vitei | X | ||||||||||||||||||||||||||
| D. mexicanus | X | X | X | ||||||||||||||||||||||||
| D. brevicomis | X | X1 | X | ||||||||||||||||||||||||
| D. barberi | X1 | X | X | ||||||||||||||||||||||||
| D. adjunctus | X | X | |||||||||||||||||||||||||
| D. ponderosae | X2,3 | X2,3 | X | X | X | X2 | X2 | X | X | X | |||||||||||||||||
| D. jeffreyi | X | X2 | X | ||||||||||||||||||||||||
| D. valens | X2 | X | X | X | X | ||||||||||||||||||||||
| D. terebrans | X | X | X | X | X | X | |||||||||||||||||||||
| D. rufipennis4 | X | X | X | X | X | X | X | ||||||||||||||||||||
| D. simplex | X2 | X | X | ||||||||||||||||||||||||
| D. pseudotsugae | X | X | X2 | X | X | X | X2 | X | |||||||||||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sullivan, B.T. Composition of Attractant Semiochemicals of North American Species of Dendroctonus Bark Beetles: A Review. Forests 2024, 15, 642. https://doi.org/10.3390/f15040642
Sullivan BT. Composition of Attractant Semiochemicals of North American Species of Dendroctonus Bark Beetles: A Review. Forests. 2024; 15(4):642. https://doi.org/10.3390/f15040642
Chicago/Turabian StyleSullivan, Brian T. 2024. "Composition of Attractant Semiochemicals of North American Species of Dendroctonus Bark Beetles: A Review" Forests 15, no. 4: 642. https://doi.org/10.3390/f15040642
APA StyleSullivan, B. T. (2024). Composition of Attractant Semiochemicals of North American Species of Dendroctonus Bark Beetles: A Review. Forests, 15(4), 642. https://doi.org/10.3390/f15040642






