The Short-Term Effects of Heavy Thinning on Selected Soil Carbon Pools and Microbial Activity in a Young Aleppo Pine Forest
Abstract
:1. Introduction
2. Materials and Methods
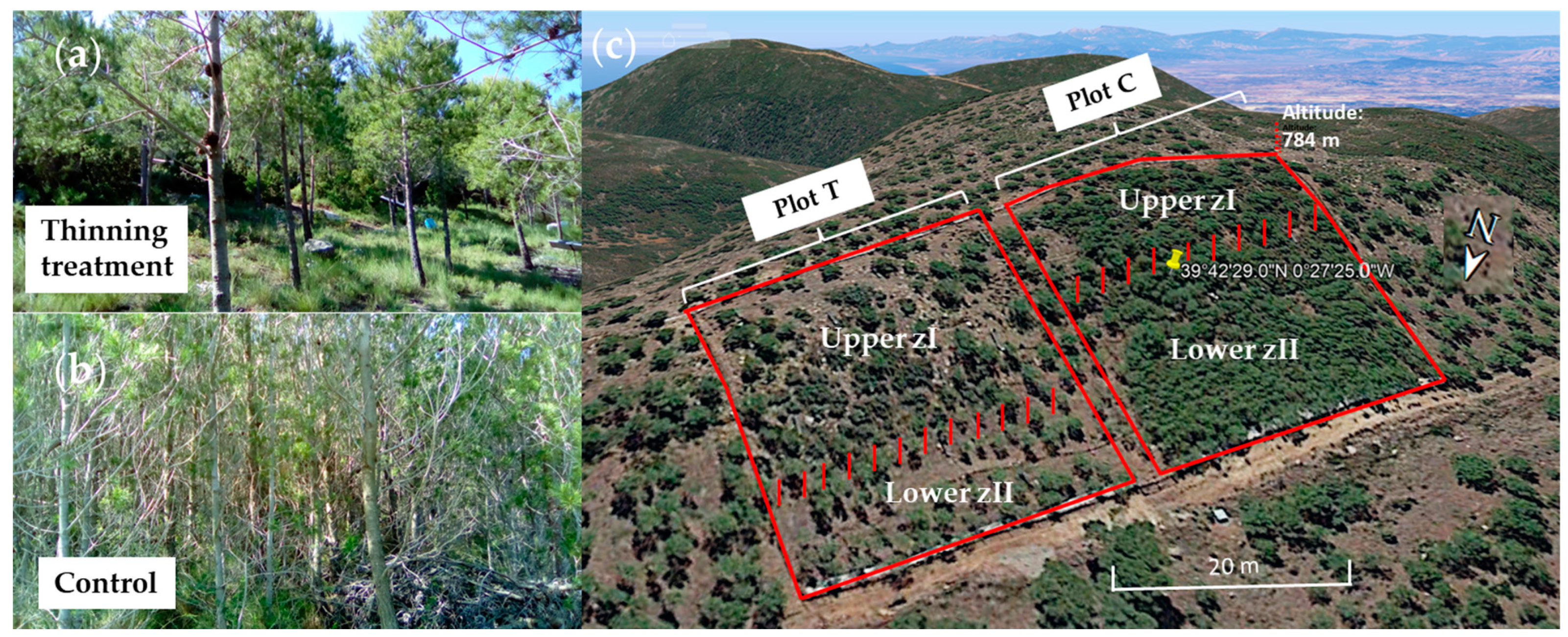
2.1. Study Site and Experimental Design
2.2. Soil Characterisation
2.3. Environmental Conditions
2.4. Sampling for Selected Soil Carbon Pools and Microbiological Activity
2.5. Microbial Biomass, Soil Respiration, and Soil Enzyme Activities
2.6. Statistical Analysis
3. Results
3.1. Experimental Plots Soil Characterisation
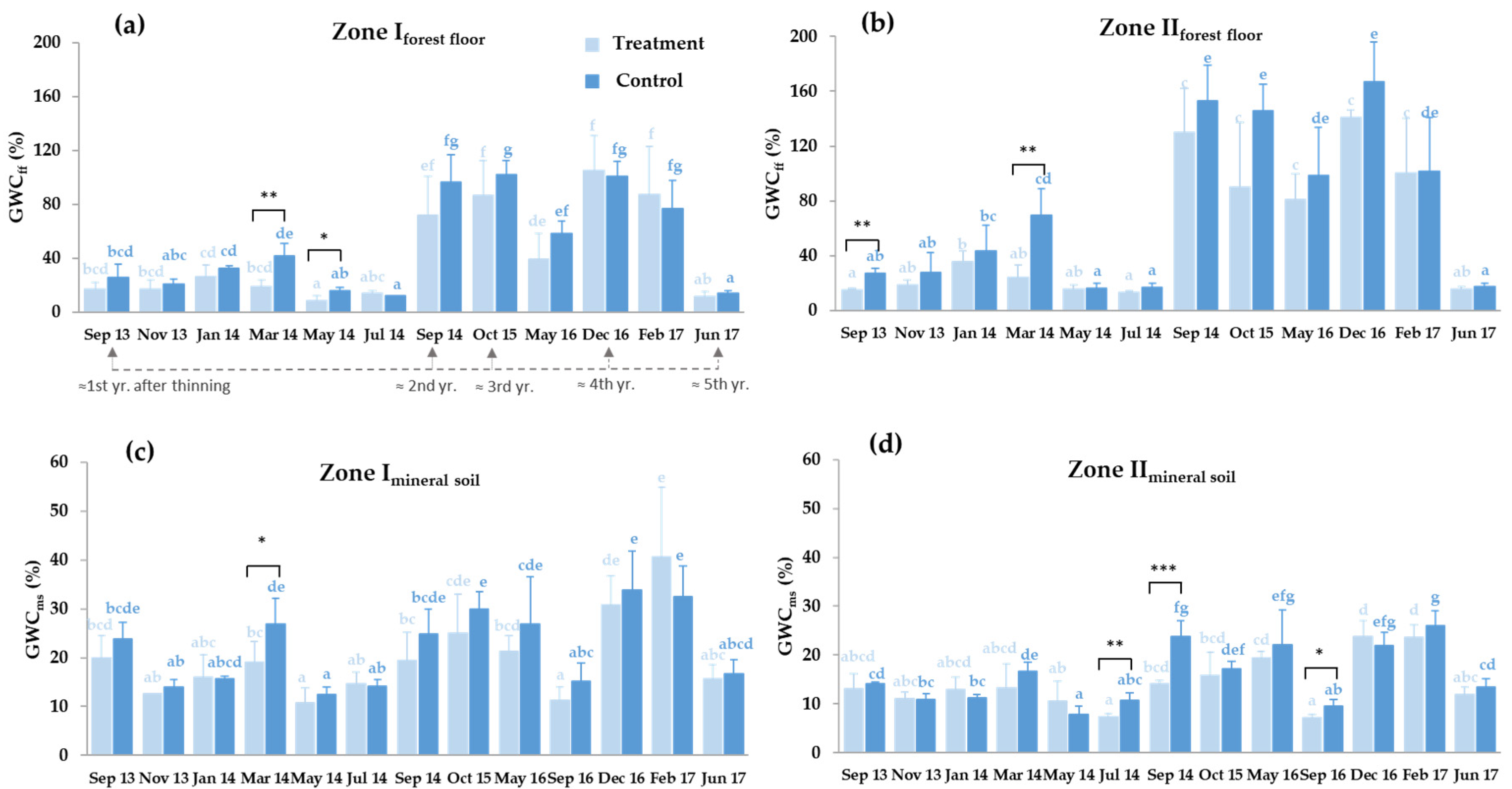
3.2. Forest Floor and Mineral Soil Moisture
3.3. Soil Carbon Pools and Microbiological Activity
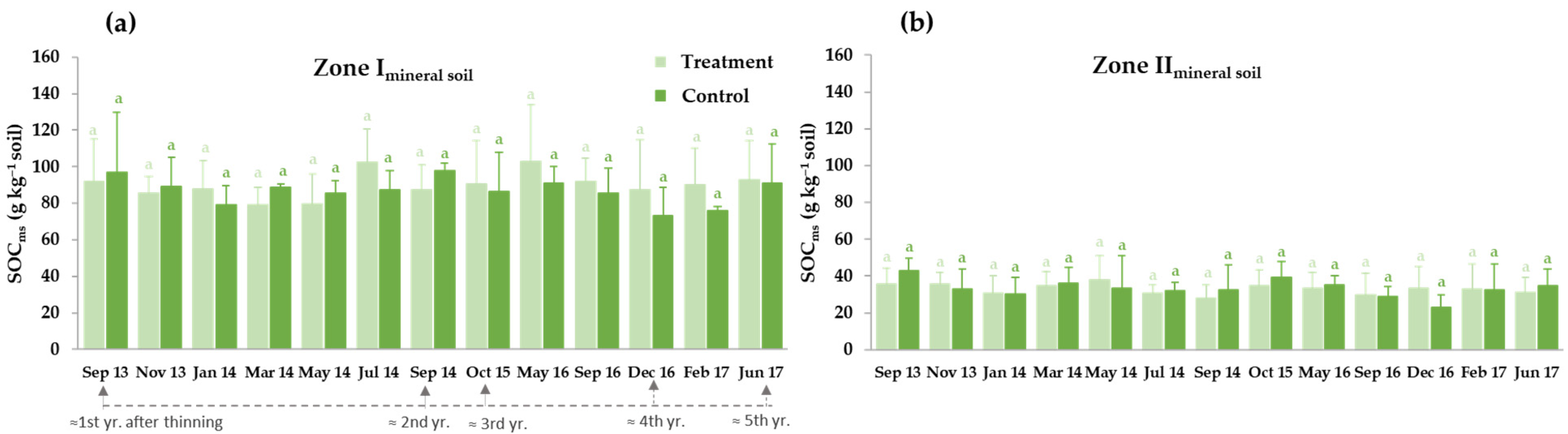
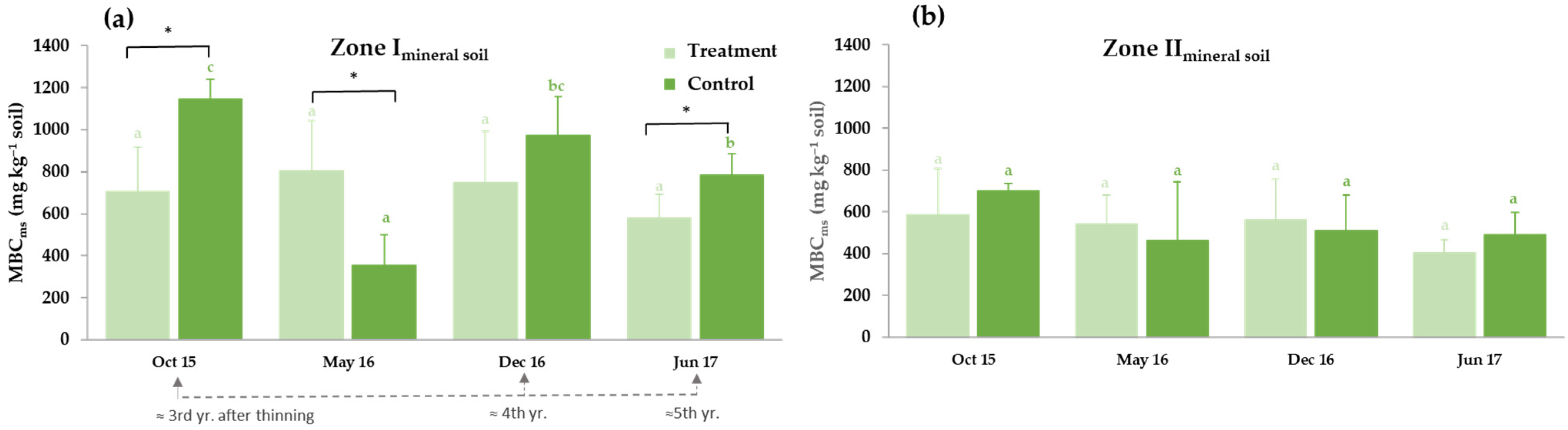
3.3.1. Thinning Effects on Selected Soil Carbon Fractions: Soil Organic Carbon (SOC), Water-Soluble Organic Carbon (WSOC) and Microbial Biomass Carbon (MBC)
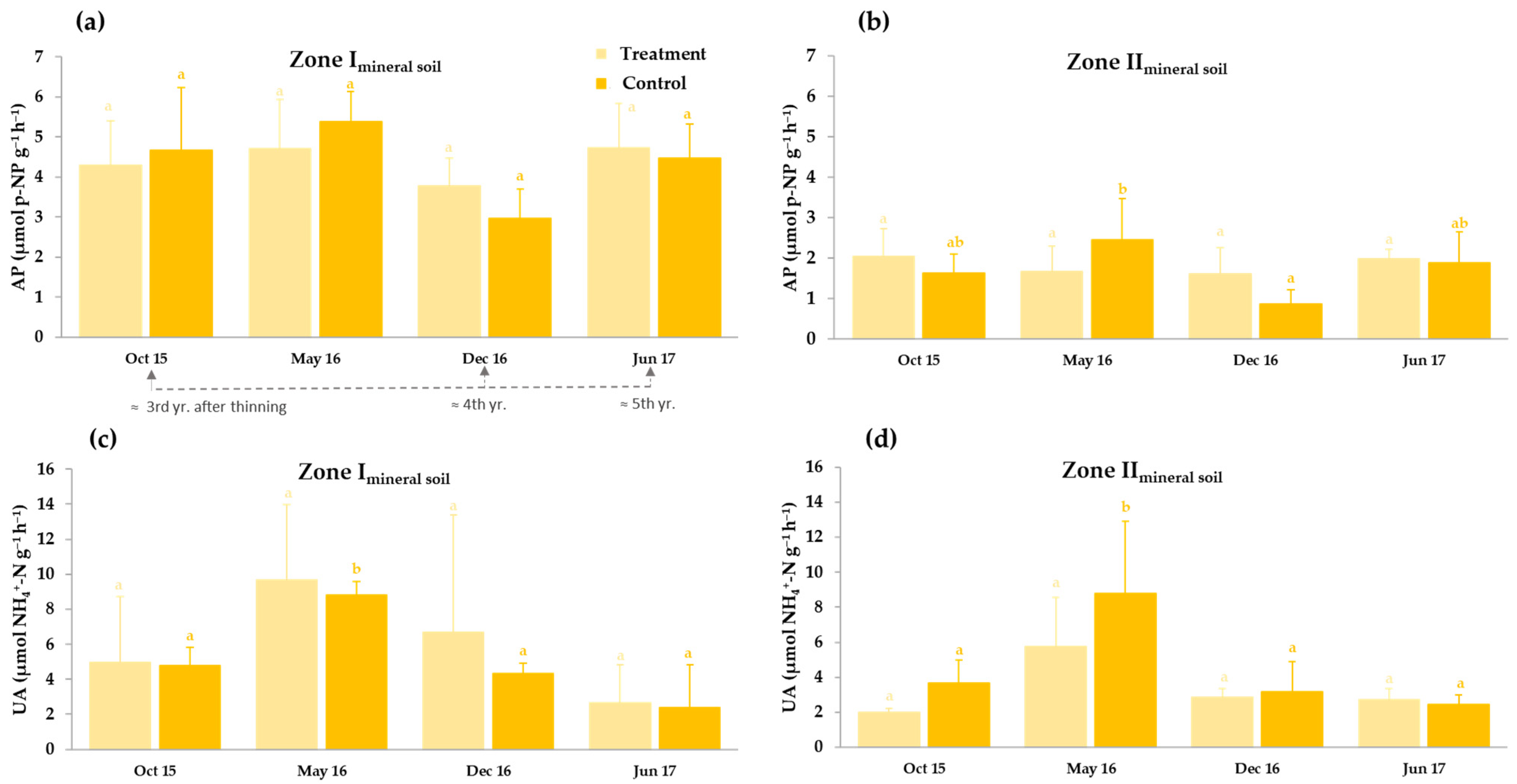
3.3.2. Thinning Effects on Basal Soil Respiration and Enzyme Activities
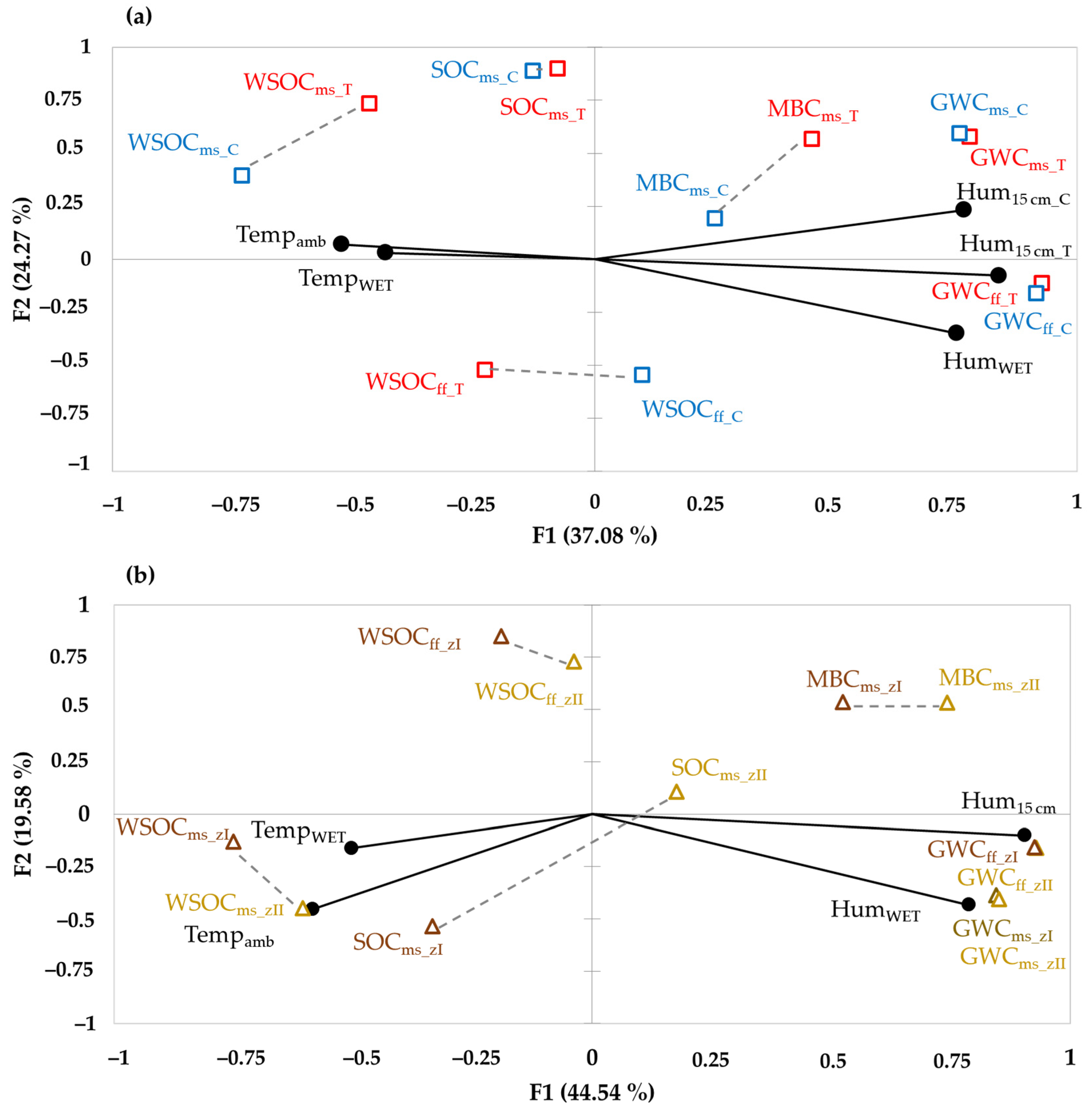
3.4. Correlations between Soil Carbon Fractions and Climate Using Principal Component Analysis (PCA)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Parameter 2 | Factor/Interaction | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment (T) | Sampling Date (S) | T × S | Residuals | ||||||||
| Zonehorizon | F | df | p-Value 1 | F | df | p-Value 1 | F | df | p-Value 1 | df | |
| GWC (Log10-Transformed) | Iff | 16.28 | 1 | 0.0001 *** | 45.61 | 11 | < 0.0001 *** | 1.47 | 11 | 0.1600 ns | 80 |
| IIff | 23.63 | 1 | <0.0001 *** | 74.08 | 11 | < 0.0001 *** | 2.13 | 11 | 0.0288 * | 71 | |
| Ims | 7.50 | 1 | 0.0074 ** | 19.33 | 12 | < 0.0001 *** | 0.91 | 12 | 0.5406 | 91 | |
| IIms | 9.94 | 1 | 0.0023 ** | 32.43 | 12 | < 0.0001 *** | 2.77 | 12 | 0.0035 ** | 78 | |
| SOC (Log10-Transformed) | Ims | 0.57 | 1 | 0.4531 ns | 0.79 | 12 | 0.6572 ns | 0.57 | 12 | 0.8609 ns | 91 |
| IIms | 0.01 | 1 | 0.9241 ns | 0.73 | 12 | 0.7169 ns | 0.48 | 12 | 0.9222 ns | 78 | |
| WSOC (Log10-Transformed) | Iff | 5.96 | 1 | 0.0169 * | 3.15 | 11 | 0.0014 ** | 0.68 | 11 | 0.7559 ns | 79 |
| IIff | 2.13 | 1 | 0.1490 ns | 11.35 | 11 | < 0.0001 *** | 0.94 | 11 | 0.5117 ns | 65 | |
| Ims | 2.62 | 1 | 0.1097 ns | 7.19 | 11 | < 0.0001 *** | 1.03 | 11 | 0.4292 ns | 82 | |
| IIms | 0.00 | 1 | 0.9502 ns | 12.97 | 11 | < 0.0001 *** | 0.60 | 11 | 0.8239 ns | 72 | |
| MBC | Ims | 2.39 | 1 | 0.1335 ns | 5.56 | 3 | 0.0040 ** | 8.07 | 3 | 0.0005 *** | 28 |
| IIms | 0.08 | 1 | 0.7737 ns | 1.72 | 3 | 0.1901 ns | 0.61 | 3 | 0.6143 ns | 24 | |
| BSR (Log10-Transformed) | Ims | 0.00 | 1 | 0.9663 ns | 11.61 | 1 | 0.0043 ** | 0.01 | 1 | 0.9403 ns | 14 |
| IIms | 3.53 | 1 | 0.0847 ns | 0.48 | 1 | 0.5014 ns | 0.00 | 1 | 0.9578 ns | 12 | |
| AP | Ims | 0.00 | 1 | 0.9840 ns | 3.75 | 3 | 0.0222 * | 0.80 | 3 | 0.5018 ns | 28 |
| IIms | 0.25 | 1 | 0.6247 ns | 2.27 | 3 | 0.1057 ns | 1.86 | 3 | 0.1641 ns | 24 | |
| UA | Ims | 0.47 | 1 | 0.5001 ns | 4.40 | 3 | 0.0121 * | 0.13 | 3 | 0.9383 ns | 27 |
| IIms | 2.47 | 1 | 0.1294 ns | 8.73 | 3 | 0.0004 *** | 0.96 | 3 | 0.4254 ns | 24 | |
References
- Mauri, A.; Di Leo, M.; de Rigo, D.; Caudullo, G. Pinus halepensis and Pinus brutia in Europe: Distribution, habitat, usage and threats. In European Atlas of Forest Tree Species; San-Miguel-Ayanz, J., de Rigo, D., Caudullo, G., Houston-Durrant, T., Mauri, A., Eds.; Publication Office of the European Union: Luxembourg, 2016; pp. 122–123. [Google Scholar]
- Picornell-Gelabert, L.; Servera-Vives, G.; Carrión Marco, Y.; Burjachs, F.; Currás, A.; Llergo, Y.; Dufraisse, A.; De Luís Arrillaga, M.; Mus Amézquita, M. Late Holocene Aleppo pine (Pinus halepensis Miller) woodlands in Mallorca (Balearic Islands, Western Mediterranean): Investigation of their distribution and the role of human management based on anthracological, dendro-anthracological and archaeopalynological data. Quat. Int. 2021, 593–594, 346–363. [Google Scholar] [CrossRef]
- Ruiz-Navarro, A.; Barberá, G.G.; Navarro-Cano, J.A.; Albaladejo, J.; Castillo, V.M. Soil dynamics in Pinus halepensis reforestation: Effect of microenvironments and previous land use. Geoderma 2009, 153, 353–361. [Google Scholar] [CrossRef]
- Maestre, F.T.; Cortina, J. Are Pinus halepensis plantations useful as a restoration tool in semiarid Mediterranean areas? For. Ecol. Manag. 2004, 198, 303–317. [Google Scholar] [CrossRef]
- Moreno, M.; Bertolín, C.; Arlanzón, D.; Ortiz, P.; Ortiz, R. Climate change, large fires, and cultural landscapes in the mediterranean basin: An analysis in southern Spain. Heliyon 2023, 9, e16941. [Google Scholar] [CrossRef] [PubMed]
- Nunes, A.; Oliveira, G.; Cabral, M.S.; Branquinho, C.; Correia, O. Beneficial effect of pine thinning in mixed plantations through changes in the understory functional composition. Ecol. Eng. 2014, 70, 387–396. [Google Scholar] [CrossRef]
- Hernandez-Tecles, E.; Osem, Y.; Alfaro Sánchez, R.; Heras, J. Vegetation structure of planted versus natural Aleppo pine stands along a climatic gradient in Spain. Ann. For. Sci. 2015, 72, 641–650. [Google Scholar] [CrossRef]
- Kaarakka, L.; Cornett, M.; Domke, G.; Ontl, T.; Dee, L. Improved forest management as a natural climate solution: A review. Ecol. Solut. Evid. 2021, 2, e12090. [Google Scholar] [CrossRef]
- Zhang, X.; Guan, D.; Li, W.; Sun, D.; Jin, C.; Yuan, F.; Wang, A.; Wu, J. The effects of forest thinning on soil carbon stocks and dynamics: A meta-analysis. For. Ecol. Manag. 2018, 429, 36–43. [Google Scholar] [CrossRef]
- Li, X.; Li, Y.; Zhang, J.; Peng, S.; Chen, Y.; Cao, Y. The effects of forest thinning on understory diversity in China: A meta-analysis. Land. Degrad. Dev. 2020, 31, 1225–1240. [Google Scholar] [CrossRef]
- Zhou, T.; Wang, C.; Zhou, Z. Impacts of forest thinning on soil microbial community structure and extracellular enzyme activities: A global meta-analysis. Soil Biol. Biochem. 2020, 149, 107915. [Google Scholar] [CrossRef]
- del Campo, A.D.; Otsuki, K.; Serengil, Y.; Blanco, J.A.; Yousefpour, R.; Wei, X. A global synthesis on the effects of thinning on hydrological processes: Implications for forest management. For. Ecol. Manag. 2022, 519, 120324. [Google Scholar] [CrossRef]
- Xu, M.; Liu, H.; Zhang, Q.; Zhang, Z.; Ren, C.; Feng, Y.; Yang, G.; Han, X.; Zhang, W. Effect of forest thinning on soil organic carbon stocks from the perspective of carbon-degrading enzymes. Catena 2022, 218, 106560. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, L.; Wang, Y.; Jiang, P.; Hu, Y.; Ouyang, S.; Wu, H.; Lei, P.; Kuzyakov, Y.; Xiang, W. Plantations thinning: A meta-analysis of consequences for soil properties and microbial functions. Sci. Total Environ. 2023, 877, 162894. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, S.; Yu, J.; Li, J.; Shangguan, Z.; Deng, L. Thinning increases forest ecosystem carbon stocks. For. Ecol. Manag. 2024, 555, 121702. [Google Scholar] [CrossRef]
- Olivar, J.; Bogino, S.; Rathgeber, C.; Bonnesoeur, V.; Bravo, F. Thinning has a positive effect on growth dynamics and growth-climate relationships in Aleppo pine (Pinus halepensis) trees of different crown classes. Ann. For. Sci. 2014, 71, 395–404. [Google Scholar] [CrossRef]
- Calev, A.; Zoref, C.; Tzukerman, M.; Moshe, Y.; Zangy, E.; Osem, Y. High-intensity thinning treatments in mature Pinus halepensis plantations experiencing prolonged drought. Eur. J. For. Res. 2016, 135, 551–563. [Google Scholar] [CrossRef]
- Sohn, J.A.; Saha, S.; Bauhus, J. Potential of forest thinning to mitigate drought stress: A meta-analysis. For. Ecol. Manag. 2016, 380, 261–273. [Google Scholar] [CrossRef]
- Gavinet, J.; Ourcival, J.M.; Gauzere, J.; García de Jalón, L.; Limousin, J.M. Drought mitigation by thinning: Benefits from the stem to the stand along 15 years of experimental rainfall exclusion in a holm oak coppice. For. Ecol. Manag. 2020, 473, 118266. [Google Scholar] [CrossRef]
- Navarro-Cerrillo, R.M.; Cachinero-Vivar, A.M.; Pérez-Priego, Ó.; Aspizua Cantón, R.; Begueria, S.; Camarero, J.J. Developing alternatives to adaptive silviculture: Thinning and tree growth resistance to drought in a Pinus species on an elevated gradient in Southern Spain. For. Ecol. Manag. 2023, 537, 120936. [Google Scholar] [CrossRef]
- Tonelli, E.; Vitali, A.; Brega, F.; Gazol, A.; Colangelo, M.; Urbinati, C.; Camarero, J.J. Thinning improves growth and resilience after severe droughts in Quercus subpyrenaica coppice forests in the Spanish Pre-Pyrenees. Dendrochronologia 2023, 77, 126042. [Google Scholar] [CrossRef]
- Dang, P.; Gao, Y.; Liu, J.; Yu, S.; Zhao, Z. Effects of thinning intensity on understory vegetation and soil microbial communities of a mature Chinese pine plantation in the Loess Plateau. Sci. Total Environ. 2018, 630, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Bautista, I.; Lidón, A.; Lull, C.; González-Sanchis, M.; del Campo, A.D. Thinning decreased soil respiration differently in two dryland Mediterranean forests with contrasted soil temperature and humidity regimes. Eur. J. For. Res. 2021, 140, 1469–1485. [Google Scholar] [CrossRef]
- Elkin, C.; Giuggiola, A.; Rigling, A.; Bugmann, H. Short- and long-term efficacy of forest thinning to mitigate drought impacts in mountain forests in the European Alps. Ecol. Appl. 2015, 25, 1083–1098. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Niu, S.; Tian, D.; Zhang, C.; Liu, W.; Yu, Z.; Yan, T.; Yang, W.; Zhao, X.; Wang, J. A global synthesis reveals increases in soil greenhouse gas emissions under forest thinning. Sci. Total Environ. 2022, 804, 150225. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Birdsey, R.A.; Fang, J.; Houghton, R.; Kauppi, P.E.; Kurz, W.A.; Phillips, O.L.; Shvidenko, A.; Lewis, S.L.; Canadell, J.G.; et al. A large and persistent carbon sink in the world’s forests. Science 2011, 333, 988–993. [Google Scholar] [CrossRef] [PubMed]
- Stockmann, U.; Adams, M.A.; Crawford, J.W.; Field, D.J.; Henakaarchchi, N.; Jenkins, M.; Minasny, B.; McBratney, A.B.; Courcelles, V.d.R.d.; Singh, K.; et al. The knowns, known unknowns and unknowns of sequestration of soil organic carbon. Agric. Ecosyst. Environ. 2013, 164, 80–99. [Google Scholar] [CrossRef]
- Cheng, X.; Yu, M.; Wang, G. Effects of thinning on soil organic carbon fractions and soil properties in Cunninghamia lanceolata stands in Eastern China. Forests 2017, 8, 198. [Google Scholar] [CrossRef]
- Ma, J.; Kang, F.; Cheng, X.; Han, H. Moderate thinning increases soil organic carbon in Larix principis-rupprechtii (Pinaceae) plantations. Geoderma 2018, 329, 118–128. [Google Scholar] [CrossRef]
- Mayer, M.; Baltensweiler, A.; James, J.; Rigling, A.; Hagedorn, F. A global synthesis and conceptualization of the magnitude and duration of soil carbon losses in response to forest disturbances. Glob. Ecol. Biogeogr. 2024, 33, 141–150. [Google Scholar] [CrossRef]
- Sądej, W.; Żołnowski, C.A. Comparison of the effect of various long-term fertilization systems on the content and fractional composition of humic compounds in Lessive soil. Plant Soil Environ. 2019, 65, 172–180. [Google Scholar] [CrossRef]
- Adamczyk, B.; Adamczyk, S.; Kukkola, M.; Tamminen, P.; Smolander, A. Logging residue harvest may decrease enzymatic activity of boreal forest soils. Soil Biol. Biochem. 2015, 82, 74–80. [Google Scholar] [CrossRef]
- Gong, C.; Tan, Q.; Liu, G.; Xu, M. Forest thinning increases soil carbon stocks in China. For. Ecol. Manag. 2021, 482, 118812. [Google Scholar] [CrossRef]
- Lull, C.; Bautista, I.; Lidón, A.; del Campo, A.D.; González-Sanchis, M.; García-Prats, A. Temporal effects of thinning on soil organic carbon pools, basal respiration and enzyme activities in a Mediterranean Holm oak forest. For. Ecol. Manag. 2020, 464, 118088. [Google Scholar] [CrossRef]
- Yang, L.; Wang, J.; Geng, Y.; Niu, S.; Tian, D.; Yan, T.; Liu, W.; Pan, J.; Zhao, X.; Zhang, C. Heavy thinning reduces soil organic carbon: Evidence from a 9-year thinning experiment in a pine plantation. Catena 2022, 211, 106013. [Google Scholar] [CrossRef]
- Zhang, Z.; Yan, J.; Han, X.; Zou, W.; Chen, X.; Lu, X.; Feng, Y. Labile organic carbon fractions drive soil microbial communities after long-term fertilization. Glob. Ecol. Conserv. 2021, 32, e01867. [Google Scholar] [CrossRef]
- Scaglia, B.; Adani, F. Biodegradability of soil water soluble organic carbon extracted from seven different soils. J. Environ. Sci. 2009, 21, 641–646. [Google Scholar] [CrossRef]
- Chen, X.; Chen, H.Y.H.; Chen, X.; Wang, J.; Chen, B.; Wang, D.; Guan, Q. Soil labile organic carbon and carbon-cycle enzyme activities under different thinning intensities in Chinese fir plantations. Appl. Soil. Ecol. 2016, 107, 162–169. [Google Scholar] [CrossRef]
- Wang, D.; Chen, X.; Chen, H.Y.H.; Olatunji, O.A.; Guan, Q. Contrasting effects of thinning on soil CO2 emission and above- and belowground carbon regime under a subtropical Chinese fir plantation. Sci. Total Environ. 2019, 690, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Lei, L.; Xiao, W.; Zeng, L.; Zhu, J.; Huang, Z.; Cheng, R.; Gao, S.; Li, M.-H. Thinning but not understory removal increased heterotrophic respiration and total soil respiration in Pinus massoniana stands. Sci. Total Environ. 2018, 621, 1360–1369. [Google Scholar] [CrossRef]
- Sullivan, B.W.; Kolb, T.E.; Hart, S.C.; Kaye, J.P.; Dore, S.; Montes-Helu, M. Thinning reduces soil carbon dioxide but not methane flux from southwestern USA ponderosa pine forests. For. Ecol. Manag. 2008, 255, 4047–4055. [Google Scholar] [CrossRef]
- Yang, L.; Qin, J.; Geng, Y.; Zhang, C.; Pan, J.; Niu, S.; Tian, D.; Zhao, X.; Wang, J. Long-term effects of forest thinning on soil respiration and its components in a pine plantation. For. Ecol. Manag. 2022, 513, 120189. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, C.; Jin, Y.; Sun, Z. Impacts of thinning on soil carbon and nutrients and related extracellular enzymes in a larch plantation. For. Ecol. Manag. 2019, 450, 117523. [Google Scholar] [CrossRef]
- Lull, C.; Gil-Ortiz, R.; Bautista, I.; Lidón, A. Seasonal variation and soil texture-related thinning effects on soil microbial and enzymatic properties in a semi-arid pine forest. Forests 2023, 14, 1674. [Google Scholar] [CrossRef]
- Zeng, L.; Xiao, W.; Liu, C.; Lei, L.; Jian, Z.; Shen, Y.; Li, M.-H. Effects of thinning and understorey removal on soil extracellular enzyme activity vary over time during forest recovery after treatment. Plant Soil 2023, 492, 457–469. [Google Scholar] [CrossRef]
- del Campo, A.D.; González-Sanchis, M.; Lidón, A.; Ceacero, C.J.; García-Prats, A. Rainfall partitioning after thinning in two low-biomass semiarid forests: Impact of meteorological variables and forest structure on the effectiveness of water-oriented treatments. J. Hydrol. 2018, 565, 74–86. [Google Scholar] [CrossRef]
- Liu, Y.; Hao, L.; Zhou, D.; Pan, C.; Liu, P.; Xiong, Z.; Sun, G. Identifying a transition climate zone in an arid river basin using the evaporative stress index. Nat. Hazards Earth Syst. Sci. 2019, 19, 2281–2294. [Google Scholar] [CrossRef]
- Bešťáková, Z.; Strnad, F.; Vargas Godoy, M.R.; Singh, U.; Markonis, Y.; Hanel, M.; Máca, P.; Kyselý, J. Changes of the aridity index in Europe from 1950 to 2019. Theor. Appl. Climatol. 2023, 151, 587–601. [Google Scholar] [CrossRef]
- del Campo, A.D.; González-Sanchis, M.; Molina, A.J.; García-Prats, A.; Ceacero, C.J.; Bautista, I. Effectiveness of water-oriented thinning in two semiarid forests: The redistribution of increased net rainfall into soil water, drainage and runoff. For. Ecol. Manag. 2019, 438, 163–175. [Google Scholar] [CrossRef]
- Walkley, A.; Black, I.A. An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Forster, J.C. Soil physical analysis. Determination of the gravimetric water content and soil dry mass. In Methods in Applied soil Microbiology and Biochemistry; Alef, K., Nannipieri, P., Eds.; Academic Press: London, UK, 1995; pp. 105–116. [Google Scholar]
- Bouyoucos, G.J. The hydrometer as a new method for the mechanical analysis of soils. Soil Sci. 1927, 23, 343–354. [Google Scholar] [CrossRef]
- Topp, G.C.; Ferré, T.P.A. Measuring Soil Water Content. In Encyclopedia of Hydrological Sciences; Anderson, M.G., McDonnell, J.J., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2005. [Google Scholar] [CrossRef]
- MAPAMA. Available online: https://eportal.mapa.gob.es/websiar/SeleccionParametrosMap.aspx?dst=1 (accessed on 1 January 2024).
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Yakovchenko, V.; Sikora, L.J. Modified dichromate method for determining low concentrations of extractable organic carbon in soil. Commun. Soil Sci. Plant Anal. 1998, 29, 421–433. [Google Scholar] [CrossRef]
- Tabatabai, M.A.; Bremner, J.M. Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Biol. Biochem. 1969, 1, 301–307. [Google Scholar] [CrossRef]
- Nannipieri, P.; Ceccanti, B.; Cervelli, S.; Matarese, E. Extraction of phosphatase, urease, proteases, organic carbon, and nitrogen from soil. Soil Sci. Soc. Am. J. 1980, 44, 1011–1016. [Google Scholar] [CrossRef]
- Obour, P.B.; Jensen, J.L.; Lamandé, M.; Watts, C.W.; Munkholm, L.J. Soil organic matter widens the range of water contents for tillage. Soil Tillage Res. 2018, 182, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Voroney, P. Chapter 4—Soils for Horse Pasture Management. In Horse Pasture Management; Sharpe, P., Ed.; Academic Press, Inc.: London, UK, 2019; pp. 65–79. [Google Scholar] [CrossRef]
- Aleš, K.; Pavel, S.; Aleš, B.; Keith Ronald, S.; Tomáš, V.; Valerie, V.; Ram Swaroop, M.; Rahul, D. Forest soil water in landscape context. In Soil Moisture Importance; Ram Swaroop, M., Rahul, D., Eds.; IntechOpen: Rijeka, Croatia, 2020; Chapter 3. [Google Scholar]
- Wu, H.; Hu, B.; Yan, J.; Cheng, X.; Yi, P.; Kang, F.; Han, H. Mixed plantation regulates forest floor water retention and temperature sensitivity in restored ecosystems on the Loess Plateau, China. Catena 2023, 222, 106838. [Google Scholar] [CrossRef]
- Tian, J.; McCormack, L.; Wang, J.; Guo, D.; Wang, Q.; Zhang, X.; Yu, G.; Blagodatskaya, E.; Kuzyakov, Y. Linkages between the soil organic matter fractions and the microbial metabolic functional diversity within a broad-leaved Korean pine forest. Eur. J. Soil Biol. 2015, 66, 57–64. [Google Scholar] [CrossRef]
- Bahadori, M.; Chen, C.; Lewis, S.; Boyd, S.; Rashti, M.R.; Esfandbod, M.; Garzon-Garcia, A.; Van Zwieten, L.; Kuzyakov, Y. Soil organic matter formation is controlled by the chemistry and bioavailability of organic carbon inputs across different land uses. Sci. Total Environ. 2021, 770, 145307. [Google Scholar] [CrossRef] [PubMed]
- Mayer, M.; Prescott, C.E.; Abaker, W.E.A.; Augusto, L.; Cécillon, L.; Ferreira, G.W.D.; James, J.; Jandl, R.; Katzensteiner, K.; Laclau, J.P.; et al. Influence of forest management activities on soil organic carbon stocks: A knowledge synthesis. For. Ecol. Manag. 2020, 466, 118127. [Google Scholar] [CrossRef]
- Nave, L.E.; Vance, E.D.; Swanston, C.W.; Curtis, P.S. Harvest impacts on soil carbon storage in temperate forests. For. Ecol. Manag. 2010, 259, 857–866. [Google Scholar] [CrossRef]
- Han, S.H.; An, J.Y.; Hernandez, J.O.; Yang, H.M.; Kim, E.-S.; Noh, N.J.; Seo, J.M.; Park, B.B. Effects of thinning intensity on litterfall production, soil chemical properties, and fine root distribution in Pinus koraiensis plantation in Republic of Korea. Plants 2023, 12, 3614. [Google Scholar] [CrossRef] [PubMed]
- del Río, M.; Bravo-Oviedo, A.; Pretzsch, H.; Löf, M.; Ruiz-Peinado, R. A review of thinning effects on scots pine stands: From growth and yield to new challenges under global change. For. Syst. 2017, 26, eR03S. [Google Scholar] [CrossRef]
- Kim, S.; Li, G.; Han, S.H.; Kim, H.-J.; Kim, C.; Lee, S.-T.; Son, Y. Thinning affects microbial biomass without changing enzyme activity in the soil of Pinus densiflora Sieb. et Zucc. forests after 7 years. Ann. For. Sci. 2018, 75, 13. [Google Scholar] [CrossRef]
- Das, S.; Deb, S.; Sahoo, S.S.; Sahoo, U.K. Soil microbial biomass carbon stock and its relation with climatic and other environmental factors in forest ecosystems: A review. Acta Ecol. Sin. 2023, 43, 933–945. [Google Scholar] [CrossRef]
- Wu, R.; Cheng, X.; Han, H. The effect of forest thinning on soil microbial community structure and function. Forests 2019, 10, 352. [Google Scholar] [CrossRef]
- Mukhtar, H.; Wunderlich, R.F.; Muzaffar, A.; Ansari, A.; Shipin, O.V.; Cao, T.N.-D.; Lin, Y.-P. Soil microbiome feedback to climate change and options for mitigation. Sci. Total Environ. 2023, 882, 163412. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Wang, C.; Luo, Y. Meta-analysis of the impacts of global change factors on soil microbial diversity and functionality. Nat. Commun. 2020, 11, 3072. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Cheng, S.; Fang, H.; Guo, Y.; Li, Y.; Zhou, Y. Interactions between soil organic matter chemical structure and microbial communities determine the spatial variation of soil basal respiration in boreal forests. Appl. Soil. Ecol. 2023, 183, 104743. [Google Scholar] [CrossRef]
- Cheng, F.; Peng, X.; Zhao, P.; Yuan, J.; Zhong, C.; Cheng, Y.; Cui, C.; Zhang, S. Soil microbial biomass, basal respiration and enzyme activity of main forest types in the Qinling Mountains. PLoS ONE 2013, 8, e67353. [Google Scholar] [CrossRef] [PubMed]
- Krämer, S.; Green, D.M. Acid and alkaline phosphatase dynamics and their relationship to soil microclimate in a semiarid woodland. Soil Biol. Biochem. 2000, 32, 179–188. [Google Scholar] [CrossRef]
- Cetin, S.C.; Ekinci, H.; Kavdır, Y.; Yüksel, O. Using soil urease enzyme activity as soil quality indicator for reflecting fire influence in forest ecosystem. Fresenius Environ. Bull. 2009, 18, 2380–2387. [Google Scholar]
- Bueis, T.; Turrión, M.B.; Bravo, F.; Pando, V.; Muscolo, A. Factors determining enzyme activities in soils under Pinus halepensis and Pinus sylvestris plantations in Spain: A basis for establishing sustainable forest management strategies. Ann. For. Sci. 2018, 75, 34. [Google Scholar] [CrossRef]
- Błońska, E.; Piaszczyk, W.; Staszel, K.; Lasota, J. Enzymatic activity of soils and soil organic matter stabilization as an effect of components released from the decomposition of litter. Appl. Soil. Ecol. 2021, 157, 103723. [Google Scholar] [CrossRef]
- Li, Y.; Ajloon, F.H.; Wang, X.; Malghani, S.; Yu, S.; Ma, X.; Li, Y.; Wang, W. Temporal effects of thinning on soil organic carbon and carbon cycling-related enzyme activities in oak-pine mixed forests. For. Ecol. Manag. 2023, 545, 121293. [Google Scholar] [CrossRef]
- Ge, T.; Wei, X.; Razavi, B.S.; Zhu, Z.; Hu, Y.; Kuzyakov, Y.; Jones, D.L.; Wu, J. Stability and dynamics of enzyme activity patterns in the rice rhizosphere: Effects of plant growth and temperature. Soil Biol. Biochem. 2017, 113, 108–115. [Google Scholar] [CrossRef]
- Fan, L.; Tarin, M.W.K.; Zhang, Y.; Han, Y.; Rong, J.; Cai, X.; Chen, L.; Shi, C.; Zheng, Y. Patterns of soil microorganisms and enzymatic activities of various forest types in coastal sandy land. Glob. Ecol. Conserv. 2021, 28, e01625. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lull, C.; Gil-Ortiz, R.; Bautista, I.; del Campo, A.; Lidón, A. The Short-Term Effects of Heavy Thinning on Selected Soil Carbon Pools and Microbial Activity in a Young Aleppo Pine Forest. Forests 2024, 15, 658. https://doi.org/10.3390/f15040658
Lull C, Gil-Ortiz R, Bautista I, del Campo A, Lidón A. The Short-Term Effects of Heavy Thinning on Selected Soil Carbon Pools and Microbial Activity in a Young Aleppo Pine Forest. Forests. 2024; 15(4):658. https://doi.org/10.3390/f15040658
Chicago/Turabian StyleLull, Cristina, Ricardo Gil-Ortiz, Inmaculada Bautista, Antonio del Campo, and Antonio Lidón. 2024. "The Short-Term Effects of Heavy Thinning on Selected Soil Carbon Pools and Microbial Activity in a Young Aleppo Pine Forest" Forests 15, no. 4: 658. https://doi.org/10.3390/f15040658






