Response of Soil Physicochemical Properties and Microbial Community Composition in Larix olgensis Plantations to Disturbance by a Large Outbreak of Bark Beetle
Abstract
1. Introduction
2. Materials and Methods
2.1. Overview of the Study Area and Sample Plots
2.2. Sample Collection and Analysis
2.3. Data Processing and Analysis
3. Results
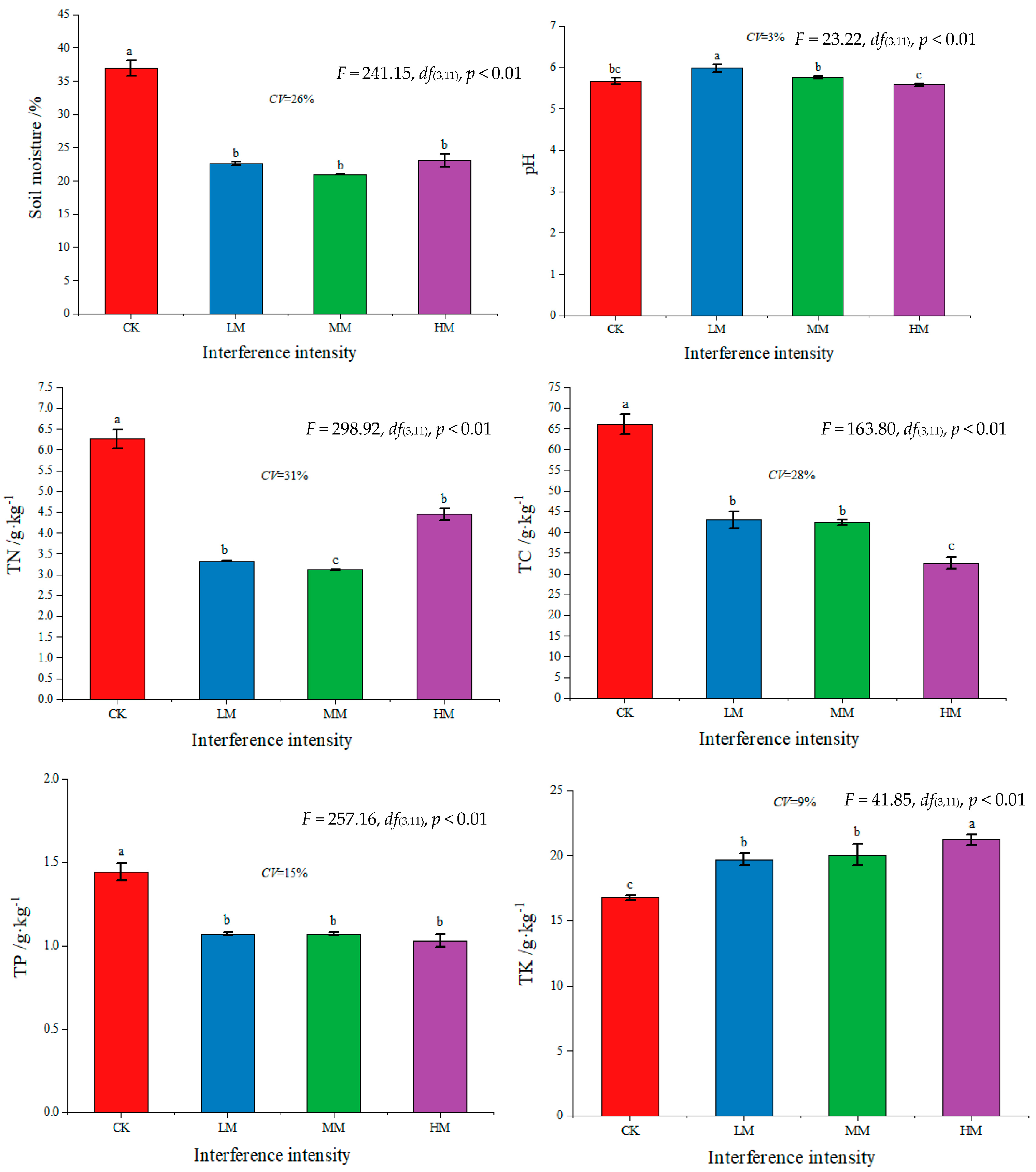
3.1. Changes in Physical and Chemical Properties of Soil with Different Levels of Disturbance
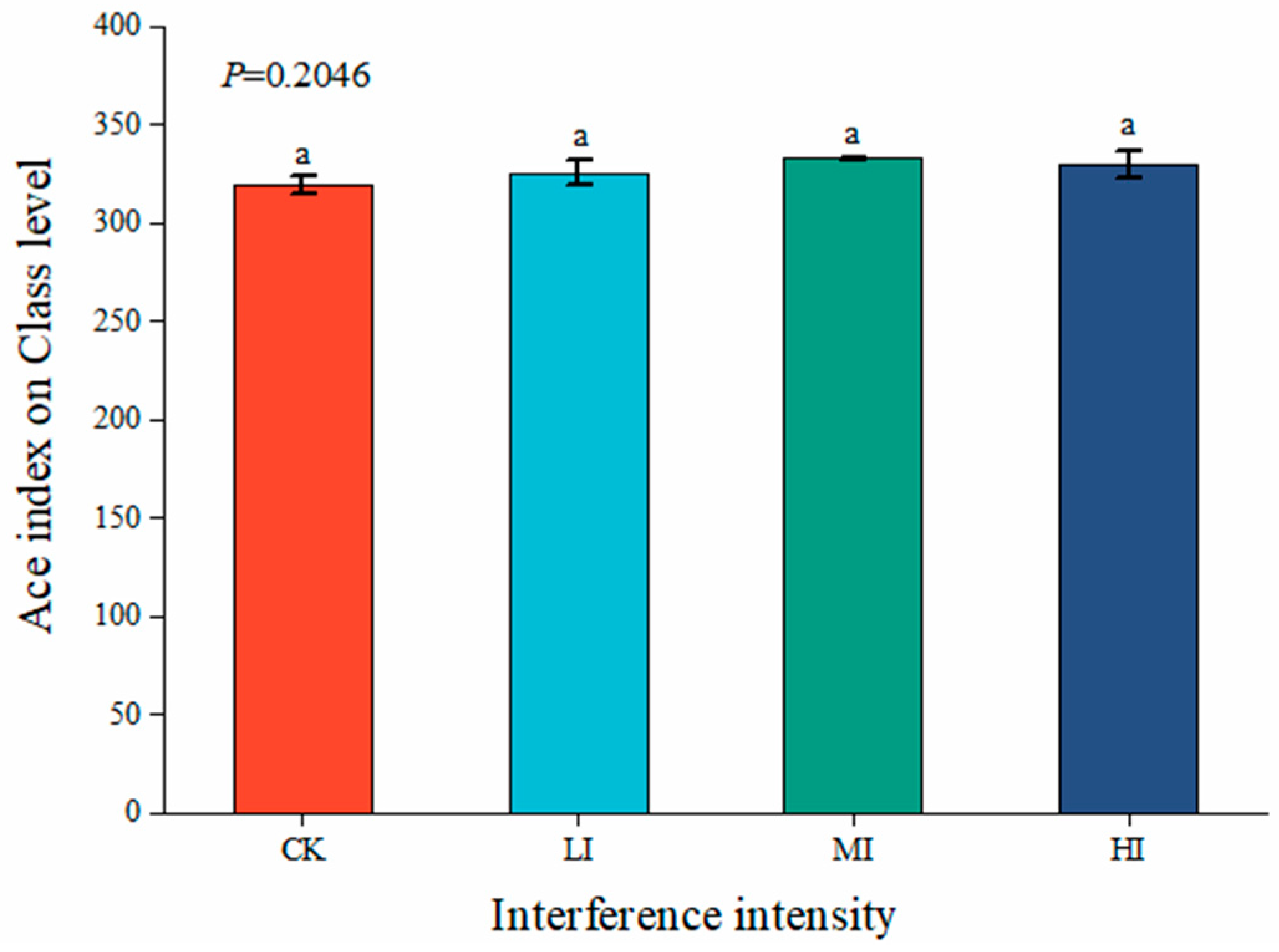
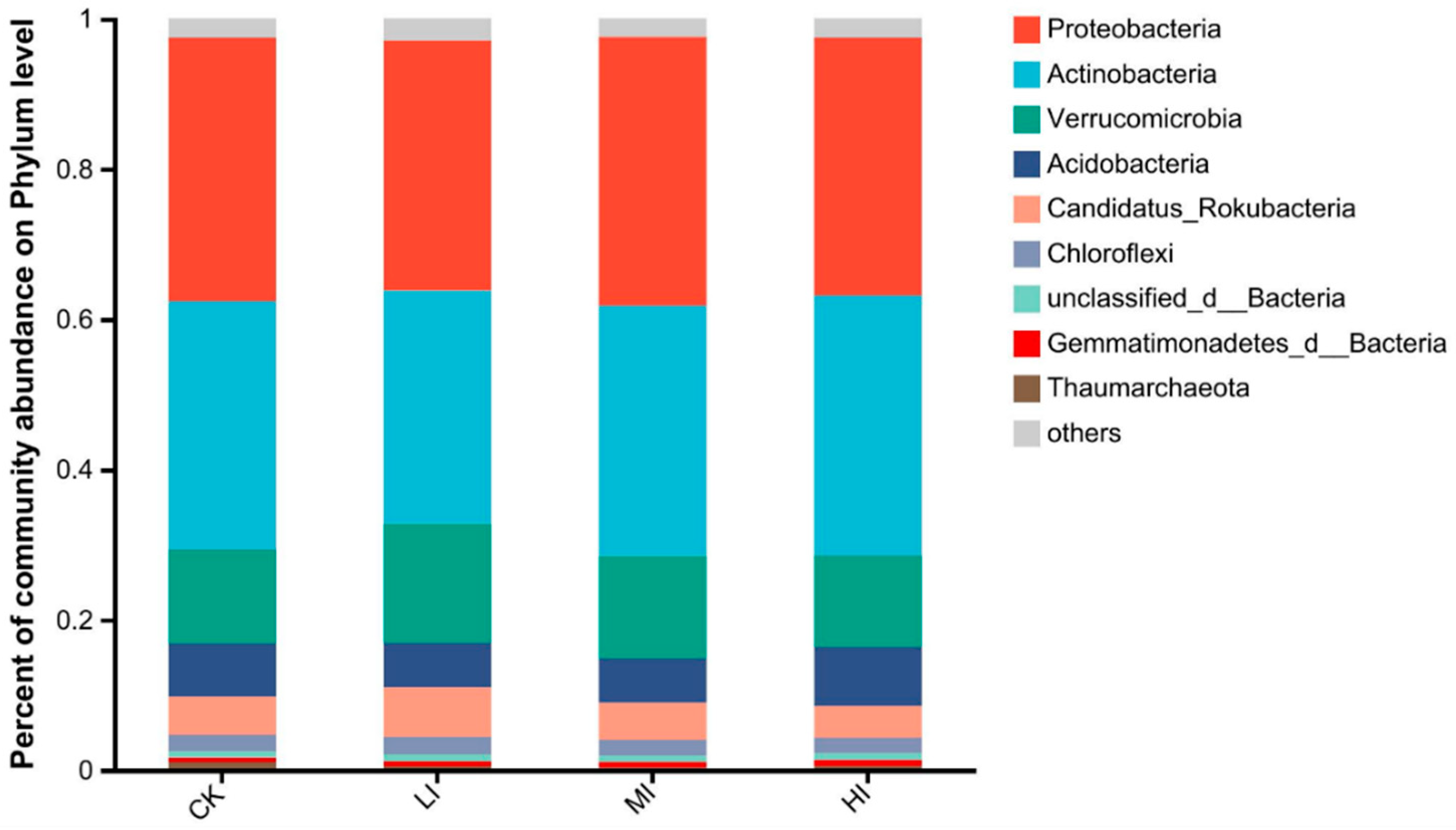
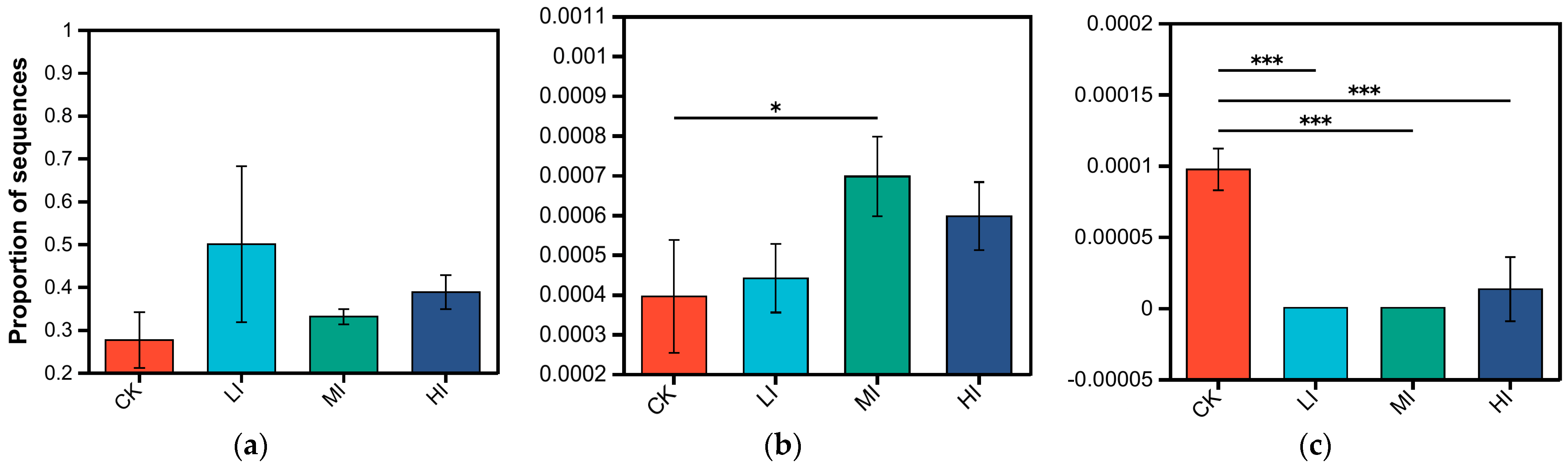
3.2. Characterization of Soil Microbial Community Structure at Different Levels of Disturbance
4. Discussion
4.1. Soil Response to Bark Beetle Disturbance
4.2. Relationships between Soil Physical and Chemical Properties at Different Levels of Disturbance
4.3. Changes in Soil Microbial Community Structure at Different Levels of Disturbance
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bonan, G.B. Forests and Climate Change: Forcings, Feedbacks, and the Climate Benefits of Forests. Science 2008, 320, 1444–1449. [Google Scholar] [CrossRef] [PubMed]
- Pankratov, T.A.; Ivanova, A.O.; Dedysh, S.N.; Liesack, W. Bacterial populations and environmental factors controlling cellulose degradation in an acidic Sphagnum peat. Environ. Microbiol. 2011, 13, 1800–1814. [Google Scholar] [CrossRef] [PubMed]
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; Hogg, E.H.; et al. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For. Ecol. Manag. 2009, 259, 660–684. [Google Scholar] [CrossRef]
- Jolly, W.M.; Cochrane, M.A.; Freeborn, P.H.; Holden, Z.A.; Brown, T.J.; Williamson, G.J.; Bowman, D.M.J.S. Climate-induced variations in global wildfire danger from 1979 to 2013. Nat. Commun. 2015, 6, 7537. [Google Scholar] [CrossRef]
- Patacca, M.; Lindner, M.; Lucas-Borja, M.E.; Cordonnier, T.; Fidej, G.; Gardiner, B.; Hauf, Y.; Jasinevicius, G.; Labonne, S.; Linkevicius, E.; et al. Significant increase in natural disturbance impacts on European forests since 1950. Glob. Chang. Biol. 2022, 23, 1359–1376. [Google Scholar] [CrossRef] [PubMed]
- Seidl, R.; Thom, D.; Kautz, M.; Martin-Benito, D.; Peltoniemi, M.; Vacchiano, G.; Wild, J.; Ascoli, D.; Petr, M.; Honkaniemi, J.; et al. Forest disturbances under climate change. Nat. Clim. Chang. 2017, 7, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Kurz, W.A.; Dymond, C.C.; Stinson, G.; Rampley, G.J.; Neilson, E.T.; Carroll, A.L.; Ebata, T.; Safranyik, L. Mountain pine beetle and forest carbon feedback to climate change. Nature 2008, 452, 987–990. [Google Scholar] [CrossRef]
- Reed, C.C.; Hood, S.M.; Cluck, D.R.; Smith, S.L. Fuels change quickly after California drought and bark beetle outbreaks with implications for potential fire behavior and emissions. Fire Ecol. 2023, 19, 16. [Google Scholar] [CrossRef]
- Woolley, T.; Shaw, C.D.; Hollingsworth, T.L.; Michelle, C.; Agne; Fitzgerald, S. Beyond red crowns: Complex changes in surface and crown fuels and their interactions 32 years following mountain pine beetle epidemics in south-central Oregon, USA. Fire Ecol. 2019, 15, 4. [Google Scholar] [CrossRef]
- Lutz, J.A.; Halpern, C.B. Tree Mortality during Early Forest Development: A Long-Term Study of Rates, Causes, and Consequences. Ecol. Monogr. 2006, 76, 257–275. [Google Scholar] [CrossRef]
- Zhang, X.Q.; Cao, Q.V.; Duan, A.G.; Zhang, J.G. Modeling tree mortality in relation to climate, initial planting density and competition in chinese fir plantations using a bayesian logistic multilevel method. Can. J. For. Res. 2017, 47, 1278–1285. [Google Scholar] [CrossRef]
- Lugo, A.E.; Scatena, F.N. Background and catastrophic tree mortality in tropical moist, wet, and rain forests. Biotropica 1996, 28, 585–599. [Google Scholar] [CrossRef]
- Michael, C.; Dietze, P.R.; Moorcroft, S. Tree mortality in the eastern and central United States: Patterns and drivers. Glob. Chang. Biol. 2011, 35, 3312–3326. [Google Scholar]
- Collins, B.J.; Rhoades, C.C.; Hubbard, R.M.; Battaglia, M.A. Tree regeneration and future stand development after bark beetle infestation and harvesting in Colorado lodgepole pine stands. For. Ecol. Manag. 2011, 261, 2168–2175. [Google Scholar] [CrossRef]
- Diskin, M.; Rocca, M.E.; Nelson, K.N.; Aoki, C.F.; Romme, W.H. Forest developmental trajectories in mountain pine beetle disturbed forests of Rocky Mountain National Park, Colorado. Can. J. For. Res. 2011, 41, 782–792. [Google Scholar] [CrossRef]
- Brouillard, B.M.; Mikkelson, K.M.; Bokman, C.M.; Berryman, E.M.; Sharp, J.O. Extent of localized tree mortality influences soil biogeochemical response in a beetle-infested coniferous forest. Soil Biol. Biochem. 2017, 114, 309–318. [Google Scholar] [CrossRef]
- Young, D.J.N.; Stevens, J.T.; Earles, J.M.; Moore, J.; Ellis, A.; Jirka, A.L.; Latimer, A.M. Long-term climate and competition explain forest mortality patterns under extreme drought. Ecol. Lett. 2017, 20, 78–86. [Google Scholar] [CrossRef]
- Anderegg, W.R.L.; Chegwidden, O.S.; Badgley, G.; Trugman, A.T.; Cullenward, D.; Abatzoglou, J.T.; Hicke, J.A.; Freeman, J.; Hamman, J.J. Future climate risks from stress, insects and fire across US forests. Ecol. Lett. 2022, 25, 1510–1520. [Google Scholar] [CrossRef]
- Perret, D.L.; Bell, D.M.; Gray, A.N.; Shaw, J.D.; Zald, H.S.J. Range-wide population assessments for subalpine fir indicate widespread disturbance-driven decline. For. Ecol. Manag. 2023, 542, 121128. [Google Scholar] [CrossRef]
- Williams, A.P.; Allen, C.D.; Millar, C.I.; Swetnam, T.W.; Michaelsen, J.; Still, C.J.; Leavitt, S.W. Forest responses to increasing aridity and warmth in the southwestern United States. Proc. Natl. Acad. Sci. USA 2010, 107, 21289–21294. [Google Scholar] [CrossRef]
- Kopácek, J.; Capek, P.; Choma, M.; Cudlín, P.; Kana, J.; Kopácek, M.; Porcal, P.; Santrucková, H.; Tahovská, K.; Turek, J. Long-term changes in soil composition in unmanaged central European mountain spruce forests after decreased acidic deposition and a bark beetle outbreak. Catena 2023, 222, 106839. [Google Scholar] [CrossRef]
- Morehouse, K.; Johns, T.; Kaye, J.; Kaye, A. Carbon and nitrogen cycling immediately following bark beetle outbreaks in southwestern ponderosa pine forests. For. Ecol. Manag. 2008, 255, 2698–2708. [Google Scholar] [CrossRef]
- Griffin, J.M.; Turner, M.G.; Simard, M. Nitrogen cycling following mountain pine beetle disturbance in lodgepole pine forests of Greater Yellowstone. For. Ecol. Manag. 2010, 261, 1077–1089. [Google Scholar] [CrossRef]
- Sandén, H.; Mayer, M.; Stark, S.; Sandén, T.; Nilsson, L.O.; Jepsen, J.U.; Wäli, P.R.; Rewald, B. Moth Outbreaks Reduce Decomposition in Subarctic Forest Soils. Ecosystems 2020, 23, 151–163. [Google Scholar] [CrossRef]
- Trahan, N.A.; Dynes, E.L.; Pugh, E.; Moore, D.J.P.; Monson, R.K. Changes in soil biogeochemistry following disturbance by girdling and mountain pine beetles in subalpine forests. Oecologia 2015, 177, 981–995. [Google Scholar] [CrossRef] [PubMed]
- Marschner, P.; Crowley, D.; Rengel, Z. Rhizosphere interactions between microorganisms and plants govern iron and phosphorus acquisition along the root axis—Model and research methods. Soil Biol. Biochem. 2011, 43, 883–894. [Google Scholar] [CrossRef]
- Zhao, J.; Ni, T.; Li, Y.; Xiong, W.; Ran, W. Responses of bacterial communities in arable soils in a rice-wheat cropping system to different fertilizer regimes and sampling times. PLoS ONE 2018, 9, e85301. [Google Scholar] [CrossRef] [PubMed]
- Ngosong, C.; Jarosch, M.; Raupp, J.; Neumann, E.; Ruess, L. The impact of farming practice on soil microorganisms and arbuscular mycorrhizal fungi: Crop type versus long-term mineral and organic fertilization. Appl. Soil Ecol. 2010, 46, 134–142. [Google Scholar] [CrossRef]
- Shen, J.P.; Zhang, L.M.; Guo, J.F.; Ray, J.L.; He, J.Z. Impact of long-term fertilization practices on the abundance and composition of soil bacterial communities in Northeast China. Appl. Soil Ecol. 2010, 46, 119–124. [Google Scholar] [CrossRef]
- Nkongolo, K.K.; Narendrula-Kotha, R. Advances in monitoring soil microbial community dynamic and function. J. Appl. Genet. 2020, 61, 249–263. [Google Scholar] [CrossRef]
- Wang, J.X.; Gao, J.; Zhang, H.Q.; Tang, M.; Chen, H. How does tree mortality caused by bark beetle (Trypophloeus klimeschi) outbreaks affect changes in soil fungal communities? Catena 2022, 217, 106516. [Google Scholar] [CrossRef]
- Mayer, M.; Rosinger, C.; Gorfer, M.; Berger, H.; Deltedesco, E.; Bässler, C.; Müller, J.; Seifert, L.; Rewald, B.; Godbold, D.L. Surviving trees and deadwood moderate changes in soil fungal communities and associated functioning after natural forest disturbance and salvage logging. Soil Biol. Biochem. 2022, 166, 108558. [Google Scholar] [CrossRef]
- Fernández-Calviño, D.; Rousk, J.; Brookes, P.C.; Bååth, E. Bacterial pH-optima for growth track soil pH, but are higher than expected at low pH. Soil Biol. Biochem. 2011, 43, 1569–1575. [Google Scholar] [CrossRef]
- Yao, Q.M.; Li, Z.; Song, Y.; Wright, S.J.; Guo, X.; Tringe, S.G.; Tfaily, M.M.; Pasa-Tolic, L.; Hazen, T.C.; Turner, B.L.; et al. Community proteogenomics reveals the systemic impact of phosphorus availability on microbial functions in tropical soil. Nat. Ecol. Evol. 2018, 2, 499–509. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.N.; Qian, Z.Y.; Li, D.J. Effects of tree diversity on soil microbial community in a subtropical forest in Southwest China. Eur. J. Soil Biol. 2023, 116, 103490. [Google Scholar] [CrossRef]
- Reinhart, K.O.; Callaway, R.M. Soil biota and invasive plants. New Phytol. 2006, 170, 445–457. [Google Scholar] [CrossRef] [PubMed]
- Ng, E.-L.; Patti, A.F.; Rose, M.T.; Schefe, C.R.; Wilkinson, K.; Smernik, R.J.; Cavagnaro, T.R. Does the chemical nature of soil carbon drive the structure and functioning of soil microbial communities? Soil Biol. Biochem. 2014, 70, 54–61. [Google Scholar] [CrossRef]
- Zheng, W.; Ya, L.; Huimin, W.; Xianjing, M.; Xuewei, L. Ophiostomatoid fungi associated with Ips subelongatus, including eight new species from northeastern China. IMA Fungus 2020, 11, 3. [Google Scholar]
- Yue, Y.; Men, X.; Sun, Z.; Chen, X. Exploring the Role of Stumps in Soil Ecology: A Study of Microsite Organic Carbon and Enzyme Activities in a Larix olgensis Henry Plantation. Forests 2023, 14, 1027. [Google Scholar] [CrossRef]
- Song, U.; Zuo, Z.; Zhao, X.; Qiao, X.; Ya, J.; Li, H.; Yue, X.; Chen, P.; Wang, M.; Medina-Roldán, S.K. Plant functional traits mediate the response magnitude of plant-litter-soil microbial C: N: P stoichiometry to nitrogen addition in a desert steppe. Cience Total Environ. 2024, 915, 169915. [Google Scholar] [CrossRef]
- Jofre, C.; Marta, C.; Miquel, N.; Xavier, P.; Gerardo, S. Widespread crown condition decline, food web disruption, and amplified tree mortality with increased climate change-type drought. Proc. Natl. Acad. Sci. USA 2011, 108, 1474–1478. [Google Scholar]
- Johnson, W.D.; Curtis, S.P. Effects of forest management on soil C and N storage: Meta analysis. For. Ecol. Manag. 2001, 140, 227–238. [Google Scholar] [CrossRef]
- Zhou, D.; Zhao, Q.S.; Liu, S.; Oeding, J. A meta-analysis on the impacts of partial cutting on forest structure and carbon storage. Biogeosciences 2013, 10, 3691–3703. [Google Scholar] [CrossRef]
- Holden, S.R.; Treseder, K.K. A meta-analysis of soil microbial biomass responses to forest disturbances. Front. Microbiol. 2013, 41, 63. [Google Scholar] [CrossRef]
- Moore, D.J.P.; Trahan, N.A.; Wilkes, P.; Quaife, T.; Stephens, B.B.; Elder, K.; Desai, A.R.; Negron, J.; Monson, R.K. Persistent reduced ecosystem respiration after insect disturbance in high elevation forests. Ecol. Lett. 2013, 16, 731–737. [Google Scholar] [CrossRef] [PubMed]
- Sallé, A.; Bouget, C. Victims or perpetrators: Contribution and response of insects to forest diebacks and declines. Ann. For. Sci. 2020, 77, 104. [Google Scholar] [CrossRef]
- Leverkus, A.B.; Buma, B.; Wagenbrenner, J.; Burton, P.J.; Lingua, E.; Marzano, R.; Thorn, S. Tamm review: Does salvage logging mitigate subsequent forest disturbances? For. Ecol. Manag. 2021, 481, 118721. [Google Scholar] [CrossRef]
- Johannes, R.; Erland, B. Growth of saprotrophic fungi and bacteria in soil. FEMS Microbiol. Ecol. 2011, 78, 17–30. [Google Scholar]
- Delgado-Baquerizo, M.; Oliverio, M.A.; Brewer, E.T.; Benavent-González, A.; Eldridge, D.J. A global atlas of the dominant bacteria found in soil. Science 2018, 359, 320–325. [Google Scholar] [CrossRef]
- Yan, J.; Wang, L.; Hu, Y.; Tsang, Y.F.; Zhang, Y. Plant litter composition selects different soil microbial structures and in turn drives different litter decomposition pattern and soil carbon sequestration capability. Geoderma 2018, 203, 319194. [Google Scholar] [CrossRef]
- Xue, R.; Zhao, K.; Yu, X.; Liu, E.; Ye, S.; Xu, J. Deciphering sample size effect on microbial biogeographic patterns and community assembly processes at centimeter scale. Soil Biol. Biochem. 2021, 156, 108218. [Google Scholar] [CrossRef]
- Cigan, P.W.; Karst, J.; Cahill, J.F.; Sywenky, A.N.; Pec, G.J.; Erbilgin, N. Influence of bark beetle outbreaks on nutrient cycling in native pine stands in western Canada. Plant Soil 2015, 390, 29–47. [Google Scholar] [CrossRef]
- Farahnak, M.; Mitsuyasu, K.; Ide, J.; Chiwa, M.; Enoki, T.; Jeong, S.; Otsuki, K.; Shimizu, K.; Kume, A. Soil pH and divalent cations after clear-cutting on a Japanese cypress plantation. J. For. Res. 2022, 27, 363–370. [Google Scholar] [CrossRef]
- Xiong, Y.M.; D’Atri, J.J.; Fu, S.L.; Xia, H.P.; Seastedt, T.R. Rapid soil organic matter loss from forest dieback in a subalpine coniferous ecosystem. Soil Biol. Biochem. 2011, 43, 2450–2456. [Google Scholar] [CrossRef]
- Griffin, J.M.; Simard, M.; Turner, M.G. Salvage harvest effects on advance tree regeneration, soil nitrogen, and fuels following mountain pine beetle outbreak in lodgepole pine. For. Ecol. Manag. 2013, 291, 228–239. [Google Scholar] [CrossRef]
- Kana, J.; Tahovská, K.; Kopácek, J. Response of soil chemistry to forest dieback after bark beetle infestation. Biogeochemistry 2013, 113, 369–383. [Google Scholar] [CrossRef]
- Fierer, N. Embracing the unknown: Disentangling the complexities of the soil microbiome. Nat. Rev. Microbiol. 2017, 15, 579–590. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Xing, F.; Zhao, H.; Gao, Y.; Bai, Z.; Dong, Y. Response of bacterial community to simulated nitrogen deposition in soils and a unique relationship between plant species and soil bacteria in the Songnen grassland in Northeastern China. J. Soil Sci. Plant Nutr. 2014, 14, 565–580. [Google Scholar] [CrossRef][Green Version]
- Ferrenberg, S.; Knelman, J.E.; Jones, J.M.; Beals, S.C.; Bowman, W.D.; Nemergut, D.R. Soil bacterial community structure remains stable over a 5-year chronosequence of insect-induced tree mortality. Front. Microbiol. 2014, 5, 681. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.Y.H.; Chen, X.L.; Huang, Z.Q. Meta-analysis shows positive effects of plant diversity on microbial biomass and respiration. Nat. Commun. 2019, 10, 1332. [Google Scholar] [CrossRef]
- Janssen, P.H. Identifying the dominant soil bacterial taxa in libraries of 16S rRNA and 16S rRNA genes. Appl. Environ. Microbiol. 2006, 72, 1719–1728. [Google Scholar] [CrossRef] [PubMed]
- Gavande, P.V.; Basak, A.; Sen, S.; Lepcha, K.; Murmu, N.; Rai, V.; Mazumdar, D.; Saha, S.P.; Das, V.; Ghosh, S. Functional characterization of thermotolerant microbial consortium for lignocellulolytic enzymes with central role of Firmicutes in rice straw depolymerization. Sci. Rep. 2021, 11, 3032. [Google Scholar] [CrossRef] [PubMed]
- Pester, M.; Schleper, C.; Wagner, M. The Thaumarchaeota: An emerging view of their phylogeny and ecophysiology. Curr. Opin. Microbiol. 2011, 14, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Liu, G.B.; Xue, S.; Wang, G.L. Soil bacterial community dynamics reflect changes in plant community and soil properties during the secondary succession of abandoned farmland in the Loess Plateau. Soil Biol. Biochem. 2016, 97, 40–49. [Google Scholar] [CrossRef]
- Cui, J.Y.; Sun, Z.H.; Wang, Z.X.; Gong, L.F. Effects of the Application of Nutrients on Soil Bacterial Community Composition and Diversity in a Larix olgensis Plantation, Northeast China. Sustainability 2022, 14, 16759. [Google Scholar] [CrossRef]
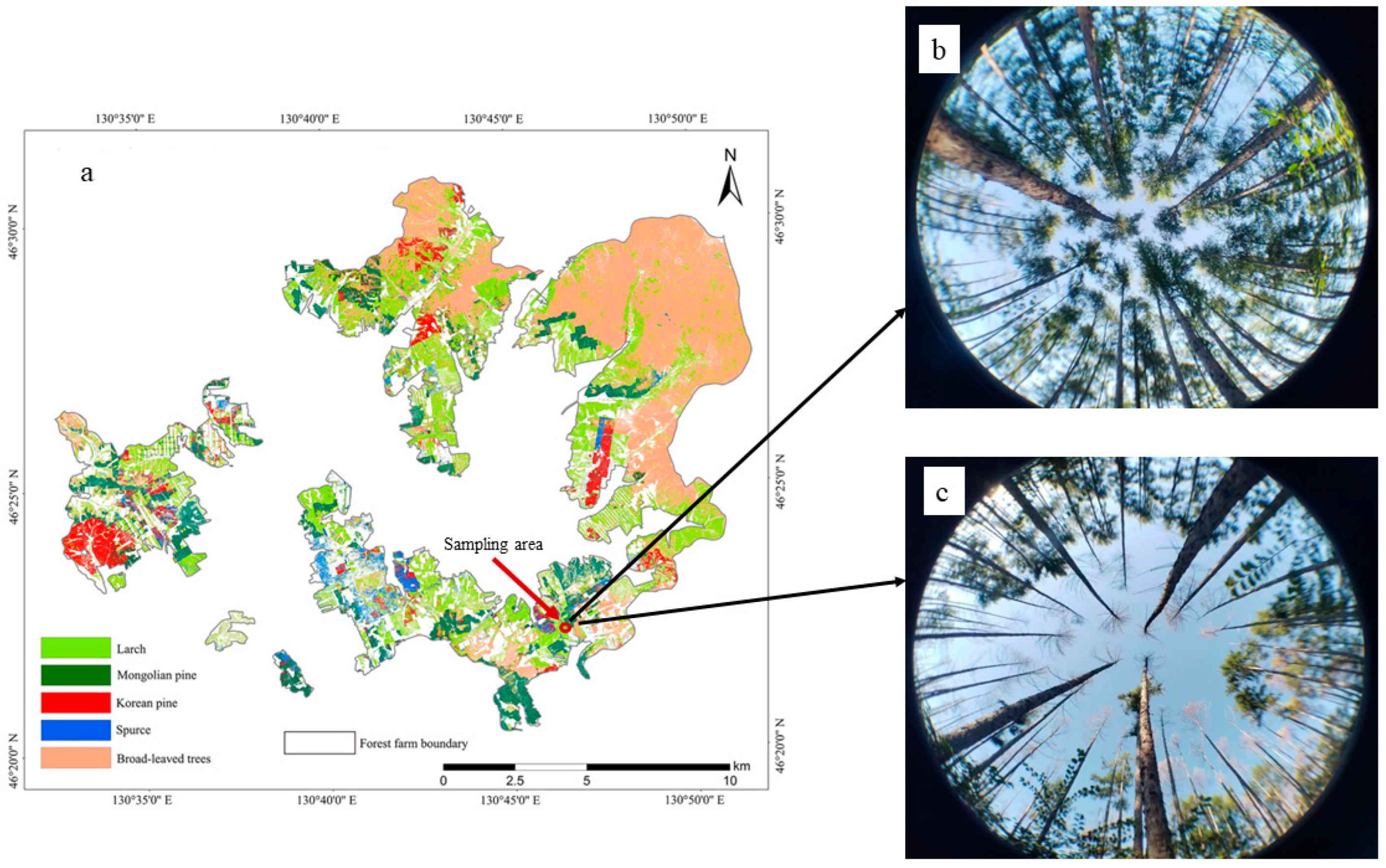

| Damage Intensity | Planting Time | Stand Initial Density (Trees·ha−1) | Stand Density (Trees·ha−1) | Canopy Coverage (%) | Average DBH (cm) | Stand Volume (m3·ha−1) |
|---|---|---|---|---|---|---|
| CK | 1988 | 4440 | 2016 | 88 | 16.21 | 262 |
| LI | 1714 | 74 | 16.42 | 219 | ||
| MI | 1353 | 58 | 17.15 | 189 | ||
| HI | 1263 | 47 | 16.92 | 173 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Sun, Z.; Yin, S. Response of Soil Physicochemical Properties and Microbial Community Composition in Larix olgensis Plantations to Disturbance by a Large Outbreak of Bark Beetle. Forests 2024, 15, 677. https://doi.org/10.3390/f15040677
Zhang Y, Sun Z, Yin S. Response of Soil Physicochemical Properties and Microbial Community Composition in Larix olgensis Plantations to Disturbance by a Large Outbreak of Bark Beetle. Forests. 2024; 15(4):677. https://doi.org/10.3390/f15040677
Chicago/Turabian StyleZhang, Yuqi, Zhihu Sun, and Sainan Yin. 2024. "Response of Soil Physicochemical Properties and Microbial Community Composition in Larix olgensis Plantations to Disturbance by a Large Outbreak of Bark Beetle" Forests 15, no. 4: 677. https://doi.org/10.3390/f15040677
APA StyleZhang, Y., Sun, Z., & Yin, S. (2024). Response of Soil Physicochemical Properties and Microbial Community Composition in Larix olgensis Plantations to Disturbance by a Large Outbreak of Bark Beetle. Forests, 15(4), 677. https://doi.org/10.3390/f15040677




