Light Intensity: A Key Ecological Factor in Determining the Growth of Pseudolarix amabilis Seedlings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials and Treatments
2.2. Experimental Methods
2.2.1. Determination of Morphological Characteristics of P. amabilis Seedlings
2.2.2. Determination of Biomass in P. amabilis Seedlings
2.2.3. Determination of Soil Physical and Chemical Properties
2.3. Statistical Analysis
3. Results
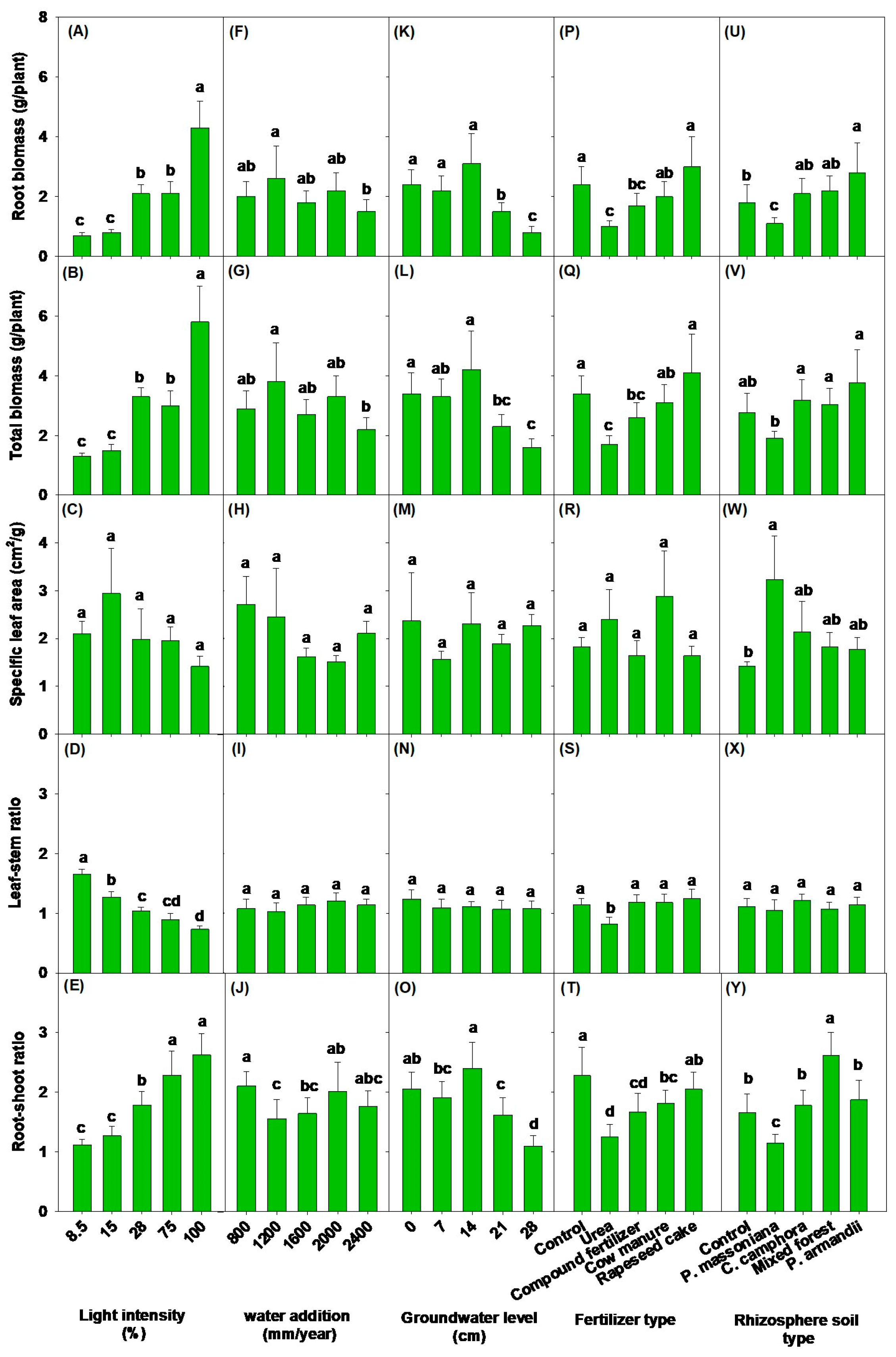
3.1. Effects of Light Intensity on Soil Physicochemical Properties, Aboveground Morphological Parameters, and Biomass
3.2. Impacts of Different Water Addition and Groundwater Levels on Soil Physicochemical Properties, Aboveground Morphological Parameters, and Biomass
3.3. Impacts of Different Fertilizer Types and Rhizosphere Soil Types on Soil Physicochemical Properties, Aboveground Morphological Parameters, and Biomass
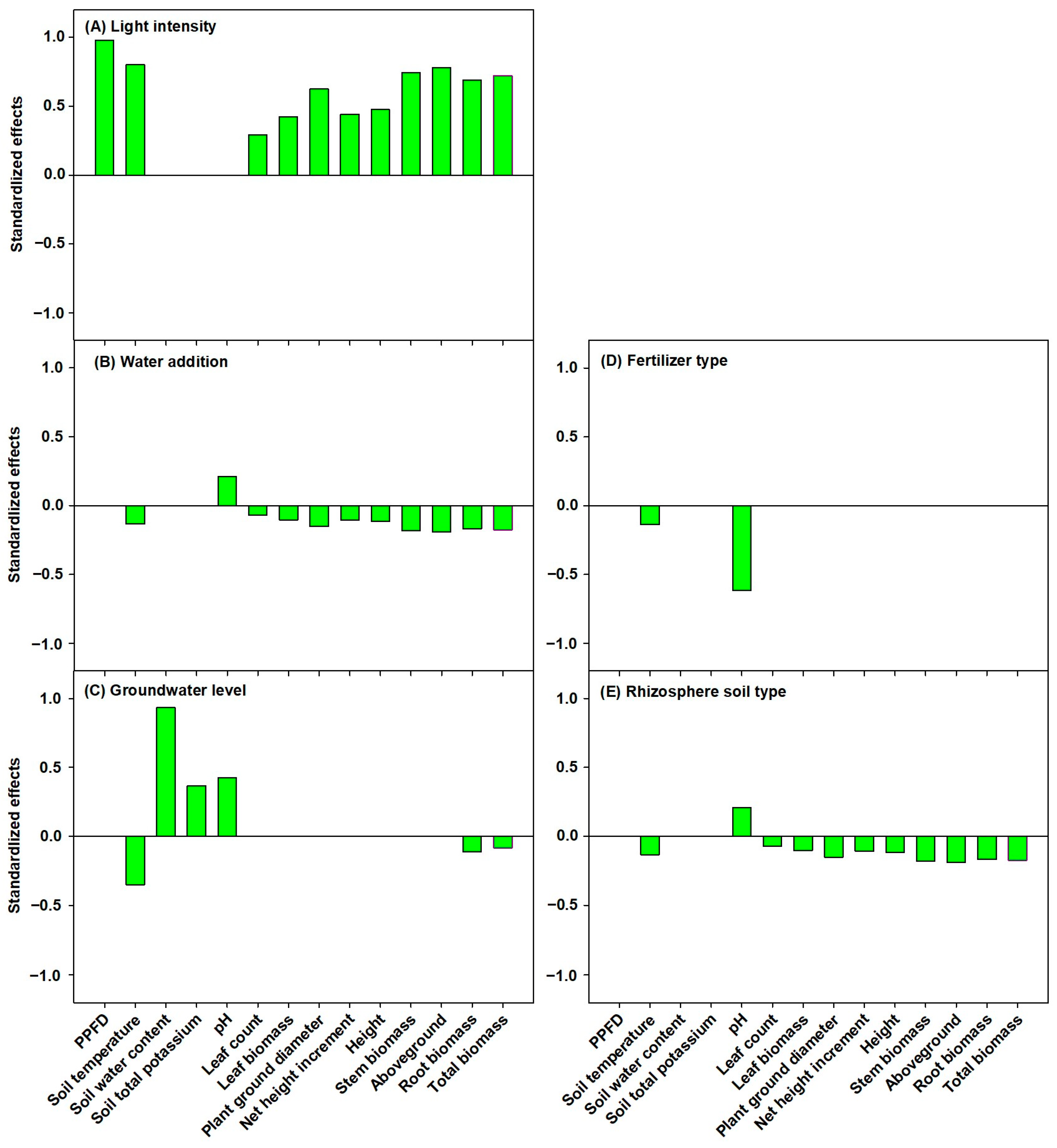
3.4. Path Analysis of Ecological Factors Influencing P. amabilis Seedlings
4. Discussion
4.1. P. amabilis Seedling Growth in Response to Light Intensity
4.2. P. amabilis Seedling Growth in Response to Water Heterogeneity
4.3. P. amabilis Seedling Growth in Response to the Fertilizer Type
4.4. P. amabilis Seedling Growth in Response to the Type of Added Rhizosphere Soil
5. Conclusions and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Green, P.T.; Harms, K.E.; Connell, J.H. Nonrandom, Diversifying Processes Are Disproportionately Strong in the Smallest Size Classes of a Tropical Forest. Proc. Natl. Acad. Sci. USA 2014, 111, 18649–18654. [Google Scholar] [CrossRef] [PubMed]
- Marchin, R.M.; Yuan, Z. Editorial: Natural Tree Seedling Establishment and Forest Regeneration under Climate Change. Front. For. Glob. Chang. 2023, 6, 1256577. [Google Scholar] [CrossRef]
- Dodd, A.N.; Salathia, N.; Hall, A.; Kévei, E.; Tóth, R.; Nagy, F.; Hibberd, J.M.; Millar, A.J.; Webb, A.A.R. Plant Circadian Clocks Increase Photosynthesis, Growth, Survival, and Competitive Advantage. Science 2005, 309, 630–633. [Google Scholar] [CrossRef]
- Yukich Clendon, O.M.M.; Carpenter, J.K.; Kelly, D.; Timoti, P.; Burns, B.R.; Boswijk, G.; Monks, A. Global Change Explains Reduced Seeding in a Widespread New Zealand Tree: Indigenous Tūhoe Knowledge Informs Mechanistic Analysis. Front. For. Glob. Chang. 2023, 6, 1172326. [Google Scholar] [CrossRef]
- Soubeyrand, M.; Gennaretti, F.; Blarquez, O.; Bergeron, Y.; Taylor, A.R.; D’Orangeville, L.; Marchand, P. Competitive Interactions under Current Climate Allow Temperate Tree Species to Grow and Survive in Boreal Mixedwood Forest. Ecography 2023, 2023, e06525. [Google Scholar] [CrossRef]
- Zhou, L.; Thakur, M.P.; Jia, Z.; Hong, Y.; Yang, W.; An, S.; Zhou, X. Light Effects on Seedling Growth in Simulated Forest Canopy Gaps Vary across Species from Different Successional Stages. Front. For. Glob. Chang. 2023, 5, 1088291. [Google Scholar] [CrossRef]
- Sylvester, O.; Avalos, G. Influence of Light Conditions on the Allometry and Growth of the Understory Palm Geonoma undata Subsp. Edulis (Arecaceae) of Neotropical Cloud Forests. Am. J. Bot. 2013, 100, 2357–2363. [Google Scholar] [CrossRef]
- Westerband, A.C.; Horvitz, C.C. Interactions between Plant Size and Canopy Openness Influence Vital Rates and Life-History Tradeoffs in Two Neotropical Understory Herbs. Am. J. Bot. 2015, 102, 1290–1299. [Google Scholar] [CrossRef]
- Caselli, M.; Urretavizcaya, M.F.; Loguercio, G.Á.; Contardi, L.; Gianolini, S.; Defossé, G.E. Effects of Canopy Cover and Neighboring Vegetation on the Early Development of Planted Austrocedrus chilensis and Nothofagus dombeyi in North Patagonian Degraded Forests. For. Ecol. Manag. 2021, 479, 118543. [Google Scholar] [CrossRef]
- Xi, W.; Peet, R.K.; Lee, M.T.; Urban, D.L. Hurricane Disturbances, Tree Diversity, and Succession in North Carolina Piedmont Forests, USA. J. For. Res. 2019, 30, 219–231. [Google Scholar] [CrossRef]
- Wright, J.S. Plant Diversity in Tropical Forests: A Review of Mechanisms of Species Coexistence. Oecologia 2002, 130, 1–14. [Google Scholar] [CrossRef]
- Kneeshaw, D.D.; Bergeron, Y. Canopy Gap Characteristics and Tree Replacement in the Southeastern Boreal Forest. Ecology 1998, 79, 783–794. [Google Scholar] [CrossRef]
- Grubb, P.J. The Maintenance of Species-Richness in Plant Communities: The Importance of the Regeneration Niche. Biol. Rev. 1977, 52, 107–145. [Google Scholar] [CrossRef]
- Hu, J.; Herbohn, J.; Chazdon, R.L.; Baynes, J.; Wills, J.; Meadows, J.; Sohel, M.d.S.I. Recovery of Species Composition over 46 years in a Logged Australian Tropical Forest Following Different Intensity Silvicultural Treatments. For. Ecol. Manag. 2018, 409, 660–666. [Google Scholar] [CrossRef]
- Fu, L.K.; Jin, J.M. China Plant Red Data Book—Rare and Endangered Plants 1; Science Press: Beijing, China, 1992. [Google Scholar]
- Christian, E.T.; Yang, Y. IUCN Red List of Threatened Species: Pseudolarix amabilis; International Union for Conservation of Nature (IUCN): Cambridge, UK, 2010. [Google Scholar]
- Wang, G.; Gen, Q.; Xiao, M.; Zhang, M.; Zhang, Y.; Wang, Z. Predicting Pseudolarix Amabilis Potential Habitat Based on Four Niche Models. Acta Ecol. Sin. 2020, 40, 6096–6104. [Google Scholar] [CrossRef]
- Zhou, L.; Huang, L.; Jin, C.; Hu, S.; Long, Y.; Qian, S.; Shang, K.; Song, K.; Song, Y.-B.; Lin, D.; et al. Fengshui Forests as a Conservation Paradigm of the Golden Larch in China. For. Ecol. Manag. 2022, 520, 120358. [Google Scholar] [CrossRef]
- Xie, C.P.; Liu, D.W.; Huang, C.Y.; He, J. Study on the Intraspecific and Interspecific Competition of Pseudolarix amabilis in Changxing County, Zhejiang Province. Pol. J. Environ. Stud. 2021, 30, 3317–3345. [Google Scholar] [CrossRef] [PubMed]
- Freschet, G.T.; Violle, C.; Bourget, M.Y.; Scherer-Lorenzen, M.; Fort, F. Allocation, Morphology, Physiology, Architecture: The Multiple Facets of Plant above- and below-Ground Responses to Resource Stress. New Phytol. 2018, 219, 1338–1352. [Google Scholar] [CrossRef]
- Wang, B. Seed Density Affects Post-Dispersal Seed Predation: Evidence from a Seed Removal Experiment of 62 Species. Integr. Zool. 2020, 15, 135–143. [Google Scholar] [CrossRef]
- Zhang, H.; Yan, C.; Wu, S.; Si, J.; Yi, X.; Li, H.; Zhang, Z. Effects of Masting on Seedling Establishment of a Rodent-Dispersed Tree Species in a Warm-Temperate Region, Northern China. Integr. Zool. 2021, 16, 97–108. [Google Scholar] [CrossRef]
- Palow, D.T.; Oberbauer, S.F. Soil Type Affects Seedling Shade Response at Low Light for Two Inga Species from Costa Rica. Plant Soil 2009, 319, 25–35. [Google Scholar] [CrossRef]
- Felsmann, K.; Baudis, M.; Kayler, Z.E.; Puhlmann, H.; Ulrich, A.; Gessler, A. Responses of the Structure and Function of the Understory Plant Communities to Precipitation Reduction across Forest Ecosystems in Germany. Ann. For. Sci. 2018, 75, 3. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X. Effects of Shading on Physiological Characteristics of Pseudolarix kaempferi Seedlings. North. Hortic. 2012, 2012, 81–84. [Google Scholar]
- Xie, X.-F.; Hu, Y.-K.; Pan, X.; Liu, F.-H.; Song, Y.-B.; Dong, M. Biomass Allocation of Stoloniferous and Rhizomatous Plant in Response to Resource Availability: A Phylogenetic Meta-Analysis. Front. Plant Sci. 2016, 7, 186064. [Google Scholar] [CrossRef] [PubMed]
- Qian, H.; Zhang, J.; Song, J.; Zhang, T.; Yan, J.; Shi, J. Effects of transplant measurements on growth of seedlings Pseudolarix amabilis (Nelson) Rehd. J. For. Eng. 2005, 19, 58–60. [Google Scholar] [CrossRef]
- Jani, A.D.; Meadows, T.D.; Eckman, M.A.; Ferrarezi, R.S. Automated Ebb-and-Flow Subirrigation Conserves Water and Enhances Citrus Liner Growth Compared to Capillary Mat and Overhead Irrigation Methods. Agric. Water Manag. 2021, 246, 106711. [Google Scholar] [CrossRef]
- Chen, D.; Li, J.; Lan, Z.; Hu, S.; Bai, Y. Soil Acidification Exerts a Greater Control on Soil Respiration than Soil Nitrogen Availability in Grasslands Subjected to Long-Term Nitrogen Enrichment. Funct. Ecol. 2016, 30, 658–669. [Google Scholar] [CrossRef]
- Guo, J.H.; Liu, X.J.; Zhang, Y.; Shen, J.L.; Han, W.X.; Zhang, W.F.; Christie, P.; Goulding, K.W.T.; Vitousek, P.M.; Zhang, F.S. Significant Acidification in Major Chinese Croplands. Science 2010, 327, 1008–1010. [Google Scholar] [CrossRef]
- Li, J.; Huang, Y.; Xu, F.; Wu, L.; Chen, D.; Bai, Y. Responses of Growing-Season Soil Respiration to Water and Nitrogen Addition as Affected by Grazing Intensity. Funct. Ecol. 2018, 32, 1890–1901. [Google Scholar] [CrossRef]
- Fang, X.; Zhang, X.; Chen, F.; Zong, Y.; Bu, W.; Wan, S.; Luo, Y.; Wang, H. Phosphorus Addition Alters the Response of Soil Organic Carbon Decomposition to Nitrogen Deposition in a Subtropical Forest. Soil Biol. Biochem. 2019, 133, 119–128. [Google Scholar] [CrossRef]
- Marinari, S.; Masciandaro, G.; Ceccanti, B.; Grego, S. Influence of Organic and Mineral Fertilisers on Soil Biological and Physical Properties. Bioresour. Technol. 2000, 72, 9–17. [Google Scholar] [CrossRef]
- Lal, B.; Sharma, S.C.; Meena, R.L.; Sarkar, S.; Sahoo, A.; Balai, R.C.; Gautam, P.; Meena, B.P. Utilization of Byproducts of Sheep Farming as Organic Fertilizer for Improving Soil Health and Productivity of Barley Forage. J. Environ. Manag. 2020, 269, 110765. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Chen, L.; Wang, W.; Xu, Y.; Zhang, W.; Zhang, H.; Liu, L.; Wang, Z.; Gu, J.; Yang, J. Effects of Application of Rapeseed Cake as Organic Fertilizer on Rice Quality at High Yield Level. J. Sci. Food Agric. 2022, 102, 1832–1841. [Google Scholar] [CrossRef] [PubMed]
- Parniske, M. Arbuscular Mycorrhiza: The Mother of Plant Root Endosymbioses. Nat. Rev. Microbiol. 2008, 6, 763–775. [Google Scholar] [CrossRef] [PubMed]
- Bonfante, P.; Genre, A. Mechanisms Underlying Beneficial Plant–Fungus Interactions in Mycorrhizal Symbiosis. Nat. Commun. 2010, 1, 48. [Google Scholar] [CrossRef] [PubMed]
- van Der Heijden, M.G.; Francis, M.M.; Marc-André, S.; Ian, R.S. Mycorrhizal Ecology and Evolution: The Past, the Present, and the Future. New Phytol. 2015, 205, 1406–1423. [Google Scholar] [CrossRef] [PubMed]
- Koide, R.T. Nutrient Supply, Nutrient Demand and Plant Response to Mycorrhizal Infection. New Phytol. 1991, 117, 365–386. [Google Scholar] [CrossRef]
- Chen, F.-S.; Niklas, K.J.; Liu, Y.; Fang, X.-M.; Wan, S.-Z.; Wang, H. Nitrogen and Phosphorus Additions Alter Nutrient Dynamics but Not Resorption Efficiencies of Chinese Fir Leaves and Twigs Differing in Age. Tree Physiol. 2015, 35, 1106–1117. [Google Scholar] [CrossRef] [PubMed]
- Bremner, J.M.; Tabatabai, M.A. Use of an Ammonia Electrode for Determination of Ammonium in Kjeldahl Analysis of Soils. Commun. Soil Sci. Plant Anal. 1972, 3, 159–165. [Google Scholar] [CrossRef]
- Murphy, J.; Riley, J.P. A Modified Single Solution Method for the Determination of Phosphate in Natural Waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Sevillano, I.; Short, I.; Grant, J.; O’Reilly, C. Effects of Light Availability on Morphology, Growth and Biomass Allocation of Fagus sylvatica and Quercus robur Seedlings. For. Ecol. Manag. 2016, 374, 11–19. [Google Scholar] [CrossRef]
- Zhou, Q.; Feng, Z.; Zhang, H.; Zhu, Z. Responses of the Growth, Photosynthetic Characteristics, Endogenous Hormones and Antioxidant Activity of Carpinus betulus L. Seedlings to Different Light Intensities. Front. Plant Sci. 2022, 13, 1055984. [Google Scholar] [CrossRef]
- Sheng, H.; Li, W.; Chang, J. Comparison of Plasticity of Seedling Growth of Two Umbellaceae Species in Response to Light Intensity. Acta Ecol. Sin. 2006, 26, 1854–1861. [Google Scholar] [CrossRef]
- Deng, X.; Shi, Z.; Xiao, F.; Zeng, L.; Lei, L. Effects of drought and shading on growth and photosynthetic characteristics of Pinus massoniana seedlings. Acta Ecol. Sin. 2019, 40, 2735–2742. [Google Scholar] [CrossRef]
- Feng, Y.; Yan, J.; Guo, Y.; Zhao, Y.; Dong, Y.; Liang, W.; Wei, X.; Bi, H. Effect of thinning intensity on natural regeneration of Larix principis-rupprechtii. Chin. J. Appl. Ecol. 2023, 34, 1169–1177. [Google Scholar] [CrossRef]
- Zhou, T.; Wang, L.; Li, S.; Gao, Y.; Du, Y.; Zhao, L.; Liu, W.; Yang, W. Interactions Between Light Intensity and Phosphorus Nutrition Affect the P Uptake Capacity of Maize and Soybean Seedling in a Low Light Intensity Area. Front. Plant Sci. 2019, 10, 183. [Google Scholar] [CrossRef] [PubMed]
- Allan Powelson, R.; Lieffers, V.J. Effect of Light and Nutrients on Biomass Allocation in Calamagrostis canadensis. Ecography 1992, 15, 31–36. [Google Scholar] [CrossRef]
- Bailey-Serres, J.; Lee, S.C.; Brinton, E. Waterproofing Crops: Effective Flooding Survival Strategies. Plant Physiol. 2012, 160, 1698–1709. [Google Scholar] [CrossRef] [PubMed]
- Anderegg, W.R.L.; Kane, J.M.; Anderegg, L.D.L. Consequences of Widespread Tree Mortality Triggered by Drought and Temperature Stress. Nat. Clim. Chang 2013, 3, 30–36. [Google Scholar] [CrossRef]
- Ma, Z.; Yin, J.; Yang, Y.; Sun, F.; Yang, Z. Effect of Water and Nitrogen Coupling Regulation on the Growth, Physiology, Yield, and Quality Attributes and Comprehensive Evaluation of Wolfberry (Lycium barbarum L.). Front. Plant Sci. 2023, 14, 1130109. [Google Scholar] [CrossRef]
- Minami, A.; Yano, K.; Gamuyao, R.; Nagai, K.; Kuroha, T.; Ayano, M.; Nakamori, M.; Koike, M.; Kondo, Y.; Niimi, Y.; et al. Time-Course Transcriptomics Analysis Reveals Key Responses of Submerged Deepwater Rice to Flooding. Plant Physiol. 2018, 176, 3081–3102. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.R.; Joiner, J.N.; Crews, C.E. Effects of N, K, and Mg on Growth and Leaf Nutrient Composition of Three Container Grown Woody Ornamentals Inoculated with Mycorrhizae. J. Am. Soc. Hortic. Sci. 1980, 105, 286–288. [Google Scholar] [CrossRef]
- Yahaya, S.M.; Mahmud, A.A.; Abdullahi, M.; Haruna, A. Recent Advances in the Chemistry of Nitrogen, Phosphorus and Potassium as Fertilizers in Soil: A Review. Pedosphere 2023, 33, 385–406. [Google Scholar] [CrossRef]
- Li, W.; Wu, W.; Zhang, Z.; Lu, Z. Introduction Assessment of Pseudolarix amabilis and Psedotsuga menziesii in the Loess Hilly Region of Shaanxi. J. Northwest For. Univ. 2006, 21, 94–96. [Google Scholar]


| Treatment Level | Water Addition (A, mm) | Groundwater Level (B, cm) | Rhizosphere Soil Type (C) | Fertilizer Type (D) | Light Intensity (E, %) |
|---|---|---|---|---|---|
| 1 | 800 | 0 | Control (sterilized river sand) | Control (sterilized river sand) | 100% natural light |
| 2 | 1200 | 7 | Pseudolarix armandii rhizosphere soil | Well-rotted cow manure | 75% natural light |
| 3 | 1600 | 14 | Pinus massoniana rhizosphere soil | Well-rotted rapeseed cake | 28% natural light |
| 4 | 2000 | 21 | Cinnamomum camphora rhizosphere soil | Compound fertilizer | 15% natural light |
| 5 | 2400 | 28 | Mixed forest rhizosphere soil | Urea | 8.5% natural light |
| Order | A | B | C | D | E | Vacant Column | Treatment |
|---|---|---|---|---|---|---|---|
| 1 | 1 | 1 | 1 | 1 | 1 | 1 | A1B1C1D1E1 |
| 2 | 2 | 3 | 2 | 3 | 1 | 4 | A2B3C2D3E1 |
| 3 | 3 | 5 | 3 | 5 | 1 | 2 | A3B5C3D5E1 |
| 4 | 4 | 2 | 4 | 2 | 1 | 5 | A4B2C4D2E1 |
| 5 | 5 | 4 | 5 | 4 | 1 | 3 | A5B4C5D4E1 |
| 6 | 1 | 2 | 2 | 5 | 2 | 3 | A1B2C2D5E2 |
| 7 | 2 | 4 | 3 | 2 | 2 | 1 | A2B4C3D2E2 |
| 8 | 3 | 1 | 4 | 4 | 2 | 4 | A3B1C4D4E2 |
| 9 | 4 | 3 | 5 | 1 | 2 | 2 | A4B3C5D1E2 |
| 10 | 5 | 5 | 1 | 3 | 2 | 5 | A5B5C1D3E2 |
| 11 | 1 | 3 | 3 | 4 | 3 | 5 | A1B3C3D4E3 |
| 12 | 2 | 5 | 4 | 1 | 3 | 3 | A2B5C4D1E3 |
| 13 | 3 | 2 | 5 | 3 | 3 | 1 | A3B2C5D3E3 |
| 14 | 4 | 4 | 1 | 5 | 3 | 4 | A4B4C1D5E3 |
| 15 | 5 | 1 | 2 | 2 | 3 | 2 | A5B1C2D2E3 |
| 16 | 1 | 4 | 4 | 3 | 4 | 2 | A1B4C4D3E4 |
| 17 | 2 | 1 | 5 | 5 | 4 | 5 | A2B1C5D5E4 |
| 18 | 3 | 3 | 1 | 2 | 4 | 3 | A3B3C1D2E4 |
| 19 | 4 | 5 | 2 | 4 | 4 | 1 | A4B5C2D4E4 |
| 20 | 5 | 2 | 3 | 1 | 4 | 4 | A5B2C3D1E4 |
| 21 | 1 | 5 | 5 | 2 | 5 | 4 | A1B5C5D2E5 |
| 22 | 2 | 2 | 1 | 4 | 5 | 2 | A2B2C1D4E5 |
| 23 | 3 | 4 | 2 | 1 | 5 | 5 | A3B4C2D1E5 |
| 24 | 4 | 1 | 3 | 3 | 5 | 3 | A4B1C3D3E5 |
| 25 | 5 | 3 | 4 | 5 | 5 | 1 | A5B3C4D5E5 |
| Indexes | F-Value/Treatments | ||||
|---|---|---|---|---|---|
| Light Intensity (%) | Water Addition (mm) | Groundwater Level (cm) | Fertilizer Type | Rhizosphere Soil Type | |
| Physical and chemical indexes | |||||
| PPFD | 323.69 *** | 1.14 ns | 3.50 ** | 2.92 * | 2.00 ns |
| Soil temperature | 23.46 *** | 4.65 ** | 23.01 *** | 0.73 ns | 5.19 ** |
| Soil water content | 3.98 * | 4.59 ** | 409.21 *** | 0.95 ns | 8.22 ** |
| Soil pH value | 6.72 *** | 6.19 ** | 28.94 *** | 65.92 *** | 6.05 ** |
| Soil total nitrogen | 0.58 ns | 5.23 ** | 25.95 *** | 117.05 *** | 11.70 *** |
| Soil total phosphorus | 4.23 ** | 3.19 * | 26.23 *** | 48.98 *** | 12.63 *** |
| Soil total potassium | 7.71 *** | 3.14 * | 16.86 *** | 45.32 *** | 10.34 *** |
| Soil NO3−-N | 5.33 ** | 0.86 ns | 18.53 *** | 32.14 *** | 5.58 ** |
| Soil NH4+-N | 1.74 ns | 0.58 ns | 1.53 ns | 0.37 ns | 0.31 ns |
| Morphology indexes | |||||
| Ground diameter (GD) | 30.84 *** | 1.04 ns | 3.38 * | 1.22 ns | 3.06 * |
| Height (H) | 4.65 ** | 2.90 * | 1.46 ns | 1.31 ns | 2.80 * |
| Net height increment (NH) | 2.92 * | 3.35 * | 1.38 ns | 1.45 ns | 0.72 ns |
| Leaf count (LC) | 4.42 ** | 5.82 ** | 1.98 ns | 4.64 ** | 4.00 * |
| Biomass indexes | |||||
| Root biomass (RB) | 25.33 *** | 2.15 † | 11.02 *** | 6.95 *** | 5.90 ** |
| Stem biomass (SB) | 22.24 *** | 3.66 * | 1.156 ns | 1.11 ns | 1.64 ns |
| Leaf biomass (LB) | 4.05 * | 2.93 * | 1.53 ns | 3.05 * | 3.22 * |
| Root–shoot ratio | 21.84 *** | 3.97 * | 16.16 *** | 9.52 *** | 18.17 *** |
| Total biomass (TB) | 22.59 *** | 2.41 * | 8.08 ** | 5.61 ** | 4.48 ** |
| Response Variable | Level of Treatment | Aboveground Variables of P. amabilis | |||||
|---|---|---|---|---|---|---|---|
| Ground Diameter (GD, mm) | Height (H, cm) | Net Height Increment (NH, cm) | Stem Biomass (SB, g) | Leaf Count (LC) | Leaf Biomass (LB, g) | ||
| Light intensity (%) | 8.5 | 1.7 ± 0.1 c | 17.2 ± 0.3 c | 7.7 ± 0.4 b | 0.2 ± 0.0 c | 110.6 ± 10.6 b | 0.4 ± 0 b |
| 15 | 1.8 ± 0.1 c | 16.8 ± 0.4 c | 8.1 ± 0.8 b | 0.3 ± 0.0 c | 97.7 ± 8.4 b | 0.4 ± 0 b | |
| 28 | 2.4 ± 0.1 b | 19.8 ± 0.5 ab | 9 ± 0.7 ab | 0.6 ± 0.0 b | 145.7 ± 14.1 a | 0.6 ± 0.1 a | |
| 75 | 2.3 ± 0.1 b | 17.6 ± 0.7 bc | 9.6 ± 1.3 ab | 0.5 ± 0.1 b | 110 ± 12.5 b | 0.4 ± 0.1 b | |
| 100 (control) | 2.8 ± 0.1 a | 20.8 ± 0.6 a | 10.7 ± 0.8 a | 0.9 ± 0.1 a | 156.7 ± 25.1 a | 0.6 ± 0.1 a | |
| Water addition (mm) | 800 | 2.3 ± 0.2 a | 19.9 ± 0.8 a | 9.7 ± 0.7 a | 0.5 ± 0.1 b | 95.8 ± 13.1 c | 0.4 ± 0.1 c |
| 1200 | 2.2 ± 0.2 a | 17.7 ± 1.5 ab | 8.7 ± 1.2 ab | 0.6 ± 0.1 a | 158.7 ± 26.1 a | 0.6 ± 0.1 a | |
| 1600 | 2.2 ± 0.1 a | 19.5 ± 0.7 a | 10.6 ± 0.8 a | 0.5 ± 0.1 ab | 120.8 ± 11.7 bc | 0.5 ± 0.1 abc | |
| 2000 | 2.1 ± 0.2 a | 18.3 ± 1.2 ab | 9.1 ± 0.7 ab | 0.5 ± 0.1 ab | 140.2 ± 7.1 ab | 0.5 ± 0.1 ab | |
| 2400 | 2.2 ± 0.1 a | 16.7 ± 0.6 b | 7.1 ± 0.5 b | 0.4 ± 0.0 b | 105.1 ± 11.1 c | 0.4 ± 0.1 bc | |
| Groundwater level (cm) | 0 (control) | 2.3 ± 0.2 a | 19.4 ± 1.1 a | 9.9 ± 1.1 a | 0.5 ± 0.1 a | 136.3 ± 12.9 a | 0.6 ± 0.1 a |
| 7 | 2.4 ± 0.1 a | 19.7 ± 1.1 a | 9.9 ± 1.0 a | 0.6 ± 0.1 a | 129.7 ± 13.0 a | 0.5 ± 0.1 a | |
| 14 | 2.3 ± 0.2 a | 18.1 ± 1.1 a | 9.2 ± 0.9 a | 0.6 ± 0.1 a | 135.1 ± 26.9 a | 0.5 ± 0.1 a | |
| 21 | 2.1 ± 0.1 ab | 17.9 ± 1.2 a | 8.3 ± 0.6 a | 0.5 ± 0.1 a | 105.4 ± 9.7 a | 0.4 ± 0.0 a | |
| 28 | 2 ± 0.2 b | 17.1 ± 0.7 a | 7.8 ± 0.7 a | 0.4 ± 0.1 a | 114.2 ± 15.0 a | 0.4 ± 0.1 a | |
| Fertilizer type | River sand (control) | 2.3 ± 0.2 a | 18.2 ± 0.9 a | 8.1 ± 0.5 a | 0.5 ± 0.1 a | 141.9 ± 11.8 a | 0.5 ± 0.0 a |
| Urea | 2.1 ± 0.1 a | 19.3 ± 1.2 a | 10.2 ± 1.0 a | 0.4 ± 0.1 a | 83.8 ± 9.7 b | 0.3 ± 0.1 b | |
| Compound fertilizer | 2.2 ± 0.2 a | 18.5 ± 0.8 a | 9.3 ± 1.0 a | 0.4 ± 0.1 a | 122.5 ± 12.8 ab | 0.5 ± 0.1 ab | |
| Cow manure | 2.2 ± 0.2 a | 17.0 ± 1.3 a | 8.3 ± 0.7 a | 0.5 ± 0.1 a | 128.1 ± 13.0 a | 0.5 ± 0.1 a | |
| Rapeseed cake | 2.3 ± 0.2 a | 19.3 ± 1.0 a | 9.3 ± 1.0 a | 0.6 ± 0.1 a | 144.4 ± 25.2 a | 0.6 ± 0.1 a | |
| Rhizosphere soil type | River sand (control) | 2.4 ± 0.2 a | 19.0 ± 1.0 a | 8.6 ± 0.7 a | 0.5 ± 0.1 ab | 126.3 ± 8.8 ab | 0.5 ± 0.0 ab |
| P. massoniana | 2.0 ± 0.1 b | 16.8 ± 1.0 b | 8.1 ± 0.7 a | 0.5 ± 0.0 ab | 104.3 ± 11.9 b | 0.4 ± 0.1 b | |
| C. camphora | 2.2 ± 0.2 ab | 19.1 ± 1.1 ab | 9.4 ± 1.1 a | 0.5 ± 0.1 ab | 135.5 ± 18.5 ab | 0.6 ± 0.1 a | |
| Mixed forest | 2.2 ± 0.2 ab | 17.5 ± 1.0 ab | 9.1 ± 0.7 a | 0.4 ± 0.1 b | 104.5 ± 10.1 b | 0.4 ± 0.0 b | |
| P. armandii | 2.2 ± 0.2 ab | 19.7 ± 1.1 ab | 9.9 ± 1.1 a | 0.6 ± 0.1 a | 150.0 ± 24.9 a | 0.6 ± 0.1 a | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tong, J.; Ouyang, D.; Wang, J.; Yan, X.; Fu, R.; Chen, F.; Fang, X.; Bu, W.; Lin, X.; Li, J. Light Intensity: A Key Ecological Factor in Determining the Growth of Pseudolarix amabilis Seedlings. Forests 2024, 15, 684. https://doi.org/10.3390/f15040684
Tong J, Ouyang D, Wang J, Yan X, Fu R, Chen F, Fang X, Bu W, Lin X, Li J. Light Intensity: A Key Ecological Factor in Determining the Growth of Pseudolarix amabilis Seedlings. Forests. 2024; 15(4):684. https://doi.org/10.3390/f15040684
Chicago/Turabian StyleTong, Jie, Dawei Ouyang, Ji Wang, Xueqin Yan, Rurao Fu, Fusheng Chen, Xiangmin Fang, Wensheng Bu, Xiaofan Lin, and Jianjun Li. 2024. "Light Intensity: A Key Ecological Factor in Determining the Growth of Pseudolarix amabilis Seedlings" Forests 15, no. 4: 684. https://doi.org/10.3390/f15040684






