Abstract
Soil respiration (Rs) is a widely monitored parameter in global forest management that results in activities that contribute to ecosystem functions. Rs can vary depending on different disturbance levels and ecosystem types as a result of changes in forest management practices. Understanding the mechanisms through which different forest management practices affect Rs can provide a general reference for ecological management and restoration practices. However, the global drivers of Rs across different forest management practices have not been sufficiently studied in the literature. In this study, we investigated the changing trends in Rs based on the relationships evident between biomass and Rs across different forest management practices. We used simple linear models to explore the relationships between biomass (aboveground and belowground biomasses) and Rs at a global scale based on different types of forest management practices and biomes. We observed significant differences in the mean values of Rs among various forest management practices. Furthermore, significant positive relationships between forest biomass and Rs were evident globally. Soil temperature had a significant effect on Rs, but the influences of soil temperature and moisture on Rs changed with the variations in forest management practices. Biome type can regulate the relationships between forest biomass and Rs across different forest management practices. We observed that the relationships between forest biomass and Rs were the strongest for naturally regenerating forests, both with and without signs of management, in tropical and subtropical coniferous and temperate broadleaf and mixed forests. Forest plantations and agroforestry can favor the establishment of similar positive relationships in temperate forest biomes (i.e., temperate conifer forests and boreal forests/taiga). Our results show that aboveground and belowground biomasses can be applied as effective ecological indicators for monitoring Rs levels, depending on different forest management practices and biomes. In this study, we provide evidence for monitoring Rs levels under different forest management practices globally.
1. Introduction
Forest management practices are classified in the field as planning sustainable forest management, restoration, and conservation activities [1]. Understanding the role of forest management practices in terms of conducting ecological assessments can facilitate these decision-making processes [1,2]. In a previous study, Lesiv et al. (2022) [1] provided maps of global forest management classes that contributed to assessing ecosystem functions and services. The maintenance of soil functions is the goal of conserving and restoring ecosystem functions and services across a variety of forest management practices worldwide [3,4,5]. For example, it is necessary to assess soil health in agroforestry systems globally [6,7]. Soil health is a key ecological indicator of ecosystem functions and services [8,9]. With the rapid development of forest management practices, it is thus critical to assess soil health and soil functions so as to support the development of biodiverse organisms that sustain terrestrial life.
Soil respiration (Rs) is a critical indicator of soil health. Rs is a measure of biological activity and decomposition, and reflects the soil’s potential to encourage plant growth and produce soil microbes [9,10,11]. Carbon sequestration is a process mainly influenced by Rs because it is the largest outward flux of carbon (C) from terrestrial ecosystems [12]. A high Rs rate indicates high biological activity, which signifies a healthy soil that readily breaks down the organic residues and cycles the nutrients needed for tree growth in forests [12,13]. The soil microbial community and root biomass play vital roles in Rs activity in terrestrial ecosystems [11,13]. Furthermore, changes in soil moisture and temperature induced by land use can exert more significant effects on Rs levels [9,11,13]. Thus, biomass, in combination with the factors of soil moisture and temperature, greatly contributes to Rs activity, supporting the flux of carbon (C) from terrestrial ecosystems. Forest management practices can drive land use changes and biomass variation [12]. Understanding the potential impacts of forest management practices on the intensity and processes controlling Rs is crucial for accurately estimating the global carbon balance [12]. Thus, the appropriate selection of management methods can contribute to enhancing forest carbon sequestration on a global scale.
Ecological processes (e.g., biomass changes) vary depending on the changes occurring in forest management practices [1,12]. For example, there are significant differences in the aboveground and belowground biomasses between natural and planted forests. The increase in aboveground and belowground biomasses occurs as a result of forest development [14,15,16]. A high forest biomass value is closely linked to managed plantation forests. Previous studies have provided evidence for the relationship between biomass and Rs [17,18,19]. However, these studies focus on specific sites or cases of forest management practices. Our study presents a general analysis of the relationship between biomass and Rs across different forest management practices. Thus, it is possible to develop monitoring approaches for Rs by using biomass as an ecological indicator.
The effectiveness of forest management practices on ecosystem functions (e.g., Rs) depends on biome changes [20,21]. Therefore, it is necessary to implement different approaches to forest management for biomes on a global scale. The relationship between biomass and Rs can be generalized, and also guide the successful development of natural and planted forests [22,23,24]. For example, in temperate plantation forests, the effects of thinning on Rs and its components vary substantially over time [23,24,25]. Belowground biomass (i.e., fine root and microbial biomasses) should be incorporated into biogeochemical models to accurately predict the long-term effects of thinning on Rs in these biomes [26,27]. However, heavy thinning practices may not apply to tropical forests, and agroforestry can enhance direct forest conversions and the high biodiversity levels of tropical forests with high levels of carbon stocks. Biomass can differ when estimating Rs because of the changes in forest management practices [23,24,25]. Hence, in this study, we considered the effects of the biome on the relationships between biomass and Rs across different forest management practices.
Moreover, we investigated the effects of soil temperature and moisture on Rs rates under different forest management scenarios. The release of carbon dioxide from the soil through the process of respiration substantially contributes to fluxes occurring in the global carbon cycle [28,29]. Rs rates can also vary with soil temperature changes [30]. Respiration rates are likely to follow the current temperature response function, although higher latitudes are more responsive to warmer temperatures than lower latitudes [31]. Furthermore, soil moisture is a key index for understanding soil–plant–atmosphere interactions [32]. Linear relationship between Rs and water content levels, as a result of soil moisture, generally exists across different spatial scales [33,34]. However, the effects of soil temperature and moisture levels on Rs levels under different forest management scenarios, which can provide useful insights into the role of forest management concerning Rs from an environmental perspective, have rarely been investigated in the literature.
Thus, our study aims to explore the relationships between aboveground and belowground biomasses and Rs across different forest management practices at the global scale, which can ultimately provide a varied selection of appropriate management options in the future. Higher Rs rates signify highly active soils as well as a higher carbon efflux activity to the atmosphere [17,19,23,24]. Given that high Rs may not mean high biodiversity and soil health in some cases, we should favor management practices that support high Rs rates, which also imply a higher biomass accumulation rate. In this study, we propose five hypotheses: (1) positive relationships between biomass and Rs exist; (2) significant relationships between soil temperature and moisture and Rs exist; (3) the biomass–respiration relationships vary across different forest management practices; (4) the relationships of soil temperature and moisture with Rs depend on various forest management practices; and (5) the effects of forest management classes on biomass–respiration relationships depend on biome types.
2. Materials and Methods
2.1. Data on Forest Management Practices
Global forest management data were obtained from Lesiv et al. (2022) [1]. Forest management types were classified into six groups: (11) naturally regenerating forests without any signs of management, (20) naturally regenerating forests with signs of forest management, (31) planted forests (rotation > 15 years), (32) plantation forest (rotation ≤ 15 years), (40) oil palm plantations, and (53) agroforestry, similarly to the study of Lesiv et al. (2022) [1]. These forest management types were based on the human impact level and rotation time observed, distinguishable from visual interpretations of satellite imagery. The high soil respiration does not necessarily mean high biodiversity and soil health, but rather means a high rate of forest disturbances. To reduce such effects, the human impact level and rotation time observed from forest management practices are linked to the rate of forest disturbances. Based on the study conducted by Lesiv et al. (2022), we used 226,322 locations at a 100 m × 100 m resolution based on unique locations using Geo-Wiki (https://www.geo-wiki.org/), an online application used for crowdsourcing and the expert visual interpretation of satellite imagery [1]. The location dataset represents the status of forest ecosystems, and can be used to investigate the value of forests in terms of species, ecosystems, and their services [1].
We obtained a global map of the 14 biomes from Dinerstein (2017) [35]. Based on the studies performed by Olson et al. (2001) and Dinerstein et al. (2017) [35,36], we used a global map of 867 ecoregions belonging to 14 biome guide conservation actions and ecological processes. We used terrestrial biomes in our study; large land areas displayed characteristic geographically distinct assemblages of plant communities and environmental conditions [37,38]. The study area included six forest biomes: tropical and subtropical moist broadleaf forests, tropical and subtropical dry broadleaf forests, tropical and subtropical coniferous forests, temperate broadleaf and mixed forests, temperate conifer forests, and boreal forests/taiga.
In our study, however, we only considered temperate, tropical, and boreal forests, excluding forest masses in drylands, considering the importance of these ecosystems for carbon cycle assessment [29]. Tropical and subtropical dry broadleaf forests include the cases of forest management practices [1]. Hence, this biome was included in our study. The most efficient carbon capture systems are widely available in the research, and the carbon present in certain forests can be released into the atmosphere by the processes of respiration, decomposition, and combustion in a cyclical manner [39]. Hence, biomes are efficient for the activities of carbon sequestration and storage. Different forest management practices can help temperate, tropical, and boreal forests to capture higher levels of carbon by changing the factors of tree age structure and density in the stands.
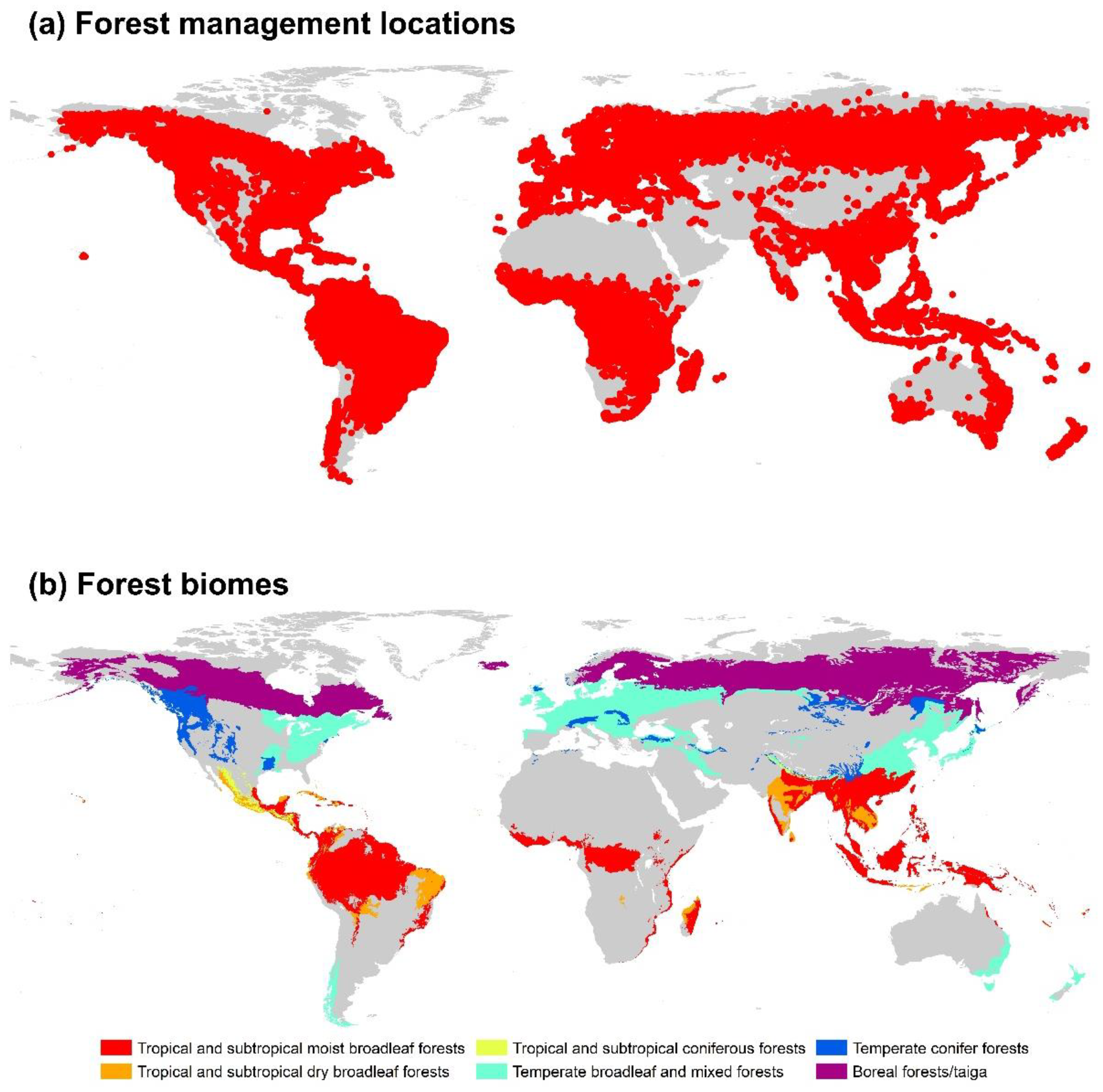
To improve the accuracy of the different forest management practices, we overlapped 226,322 locations with the above-mentioned six forest biomes, excluding global dry zones [39]. The soil in dry regions is sandy with extremely low organic matter, low nutrient levels, and low microbial activity, which results in considerable uncertainties concerning the biomass–Rs relationship. However, in our study, we only used locations presenting the six forest biomes relevant to our study. Finally, 159,835 locations in the six forest biomes were used for further analysis (Figure 1).
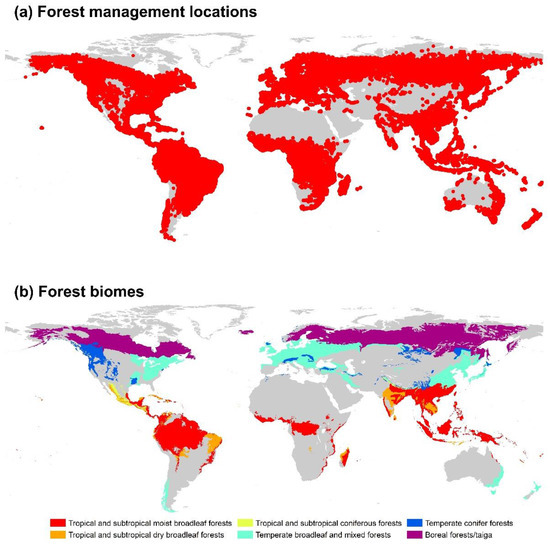
Figure 1.
Locations of forest management practices (a) and biomes (b) in our study.
2.2. Data on Soil Respiration
Soil respiration data obtained from 2000 to 2014 were obtained from Huang et al. (2020) [40], which were based on comprehensive literature checks of field Rs measurements. This dataset followed the selection criteria of the records available in the literature: (1) the dataset strictly focused on annual (year-round) Rs measurements; (2) the studies that applied an infrared gas analyzer or gas chromatograph were selected for both autotrophic and heterotrophic respiration processes; and (3) in cases where more than one set of year-round Rs measurements were completed at one site and for one year, the data were averaged to estimate the mean annual Rs rates for the site and year [30]. The final dataset used in this study contained 1292 annual Rs measurements collected from 701 sites between 2000 and 2014 [40].
Based on the abovementioned dataset, the product of the annual global Rs rates collected from 2000 to 2014 was generated at a 1 km × 1 km spatial resolution for each grid cell, using the available MODIS data and statistical models based on biome types. Rs rates varied among different biomes due to the global relationships between the factors of climate and vegetation. In their study, Huang et al. (2020) established biome-scale models for improving the estimation performance of global Rs based on field measurements and satellite remote sensing technology. Here, Rs is considered as the annual soil respiration rate (g C m−2 year−1) [40]. Under a 10-fold cross-validation method, this database was used to perform model accuracy estimations using four statistical models (multiple nonlinear regression (MNLR), random forest regression (RFR), support vector regression (SVR), and artificial neural network (ANN)) by comparing the root mean square error (RMSE) and coefficient of determination (R2) [40]. These models could account for more spatiotemporal variabilities in the global Rs rates than machine learning algorithm models. The model parameters of different numbers or types were adjusted and tested to ensure that the Rs results were accurate at a 1 km × 1 km spatial resolution for each grid cell. This database is relatively robust when measuring Rs rates [40]. To conduct a spatial analysis at the global scale, a 1 km by 1 km spatial resolution dataset was created from the original remote sensing data products based on the nearest neighbor resampling method based on 701 sites studied between 2000 and 2014. We averaged the annual global Rs rates for each cell grid from 2000 to 2014 [40]. Bare lands, water bodies, urban lands, and built-up lands were excluded from our study area. The Rs indices could be represented at a spatial scale.
2.3. Data on Forest Biomass
In this study, we classified forest biomass into aboveground and belowground biomasses. The original aboveground biomass data were generated at a 1 km × 1 km spatial resolution using a global dataset of aboveground live biomass (i.e., dry mass) stored in forests with a spatial resolution of 1 ha, based on the study conducted by Santoro et al. (2021) [41]. We obtained the data for belowground biomass (i.e., root biomass) from the study conducted by Huang et al. (2020) [40] based on 10,307 field measurements of forest root biomasses worldwide, including global observations of forest structure, climatic conditions, topography, land management, and soil characteristics. This is a spatially explicit global high-resolution (~1 km) root biomass dataset that includes the assessment of fine and coarse roots [40].
2.4. Data on Soil Temperature and Moisture
Global maps indicating the annual soil temperature data, including mean, minimum, and maximum temperatures, were used to calculate nine bioclimatic variables used in our study: (1) AMT—annual mean temperature; (2) MDTR—mean diurnal temperature range (mean of monthly temperature (max temp–min temp)); (3) isothermality (×100); (4) TS—temperature seasonality (standard deviation × 100); (5) MTWM—maximum temperature of the warmest month; (6) MTCM—minimum temperature of the coldest month; (7) ATR—annual temperature range; (8) MTWQ—mean temperature of the wettest quarter; and (9) MTDQ—mean temperature of the driest quarter. The data were obtained from a previous study (https://zenodo.org/records/4558663, accessed on 27 August 2020) [42]. The corresponding temperature values based on ERA5L were calculated using the means of the average temperature for each month over the 1981–2016 period and averaging the 12 monthly values from January to December to produce one annual value. This procedure was repeated for a soil depth in the range of 0–5 cm, as well as concerning the offsets in the mean, minimum, and maximum temperatures [42]. All variable map layers were projected onto a unified pixel grid in EPSG:4326 (WGS84) at a 30 arc-sec resolution (≈1 × 1 km at the equator). Soil temperature data indicate that seasonal variations and climatic conditions control Rs rates for particular types of vegetation and forest management practices [42].
We downloaded a map from the study of Guevara et al. (2021) [43], which indicated soil moisture levels. The dataset comprised gap-free global mean annual soil moisture predictions for 28 years (1991–2018) across a 15 km grid at a soil depth of 0–5 cm. This was a new soil moisture dataset that had high granularity with validation methods and a modeling approach that could be applied on a global scale. Subsequently, we computed the average and standard deviation values for soil moisture over the 28-year period (1991–2018) for further analysis [43].
2.5. Analyses
To explore the relationships of aboveground and belowground biomass, as well as soil temperature and moisture, with Rs, we used a general linear mixed model on a global scale [44]. In our study, the random factor was forest management practices, the response variable was Rs, and the explanatory variables were biomass (aboveground and belowground biomasses), soil temperature, and moisture (mean and standard deviation values obtained from 1991 to 2018). We used marginal R2 (R2m) and conditional R2 (R2c) values to quantify the observed relationships under a general linear mixed model. The R2m value of the model was reported using just fixed effects, while the R2c value of the full model was employed considering the random factors. The predictors are organized in a matrix where the rows are the study locations and the columns are the aboveground and belowground biomass, soil temperature and moisture, and Rs. The matrix was used as the input in a general linear mixed model. We compared the results of the gap between the R2m and R2c to test whether the changes in forest management classes (i.e., random factors) affect the relationships of biomass, soil temperature, and moisture with Rs. The relative importance of the above-mentioned explanatory variables was quantified using the general linear mixed model [44]. We then used the Tukey post hoc test to compare the average Rs values for each forest management/biome type. To explore the dependence of different forest management approaches and biomes, we used simple linear models to determine the relationships evident between biomass and Rs, and between soil temperature and moisture and Rs, based on the biome types, as well as different forest management practices. All analyses were conducted using the glmm. hp and lme4 packages using R v4.2.3 (https://www.r-project.org/).
3. Results
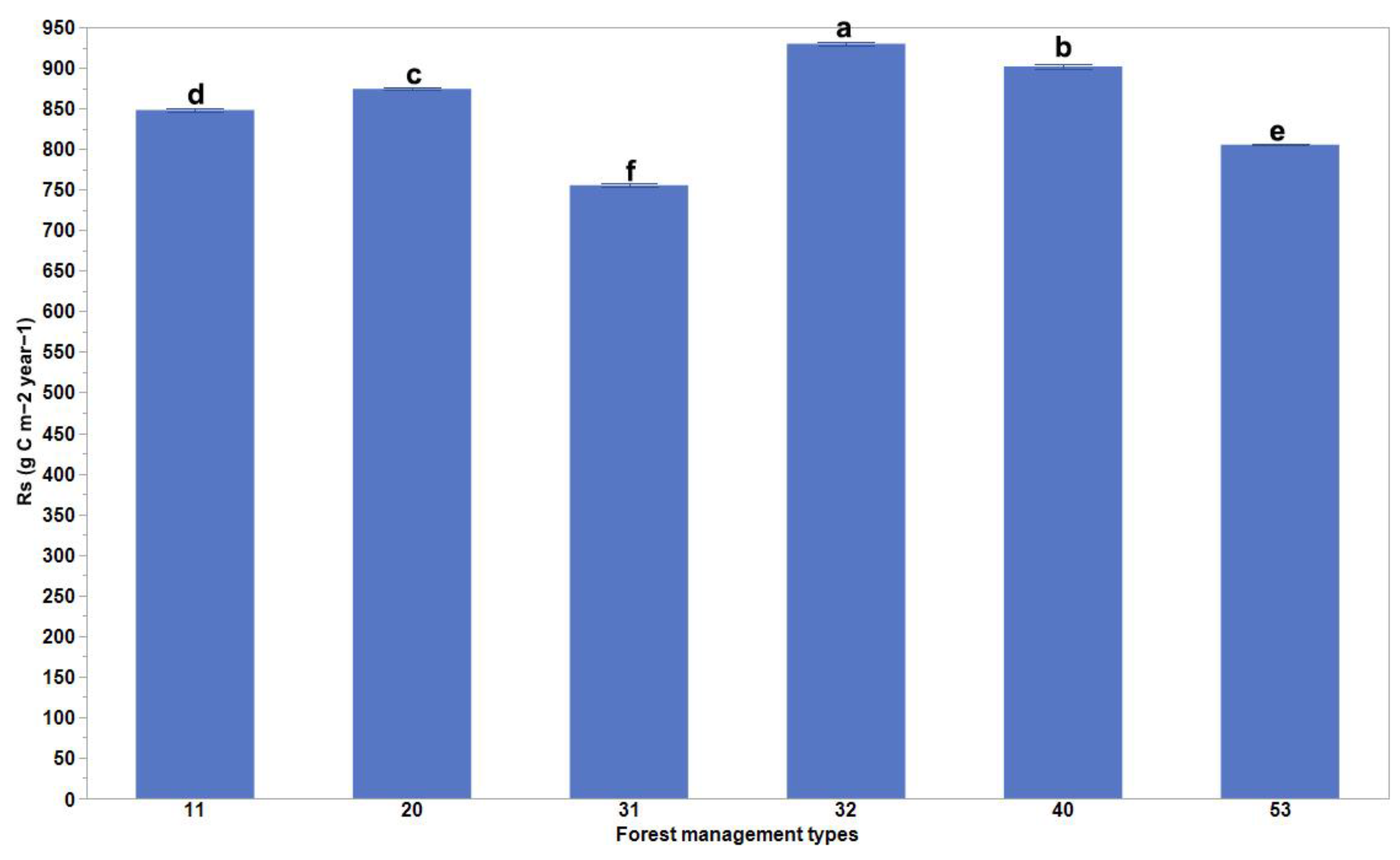
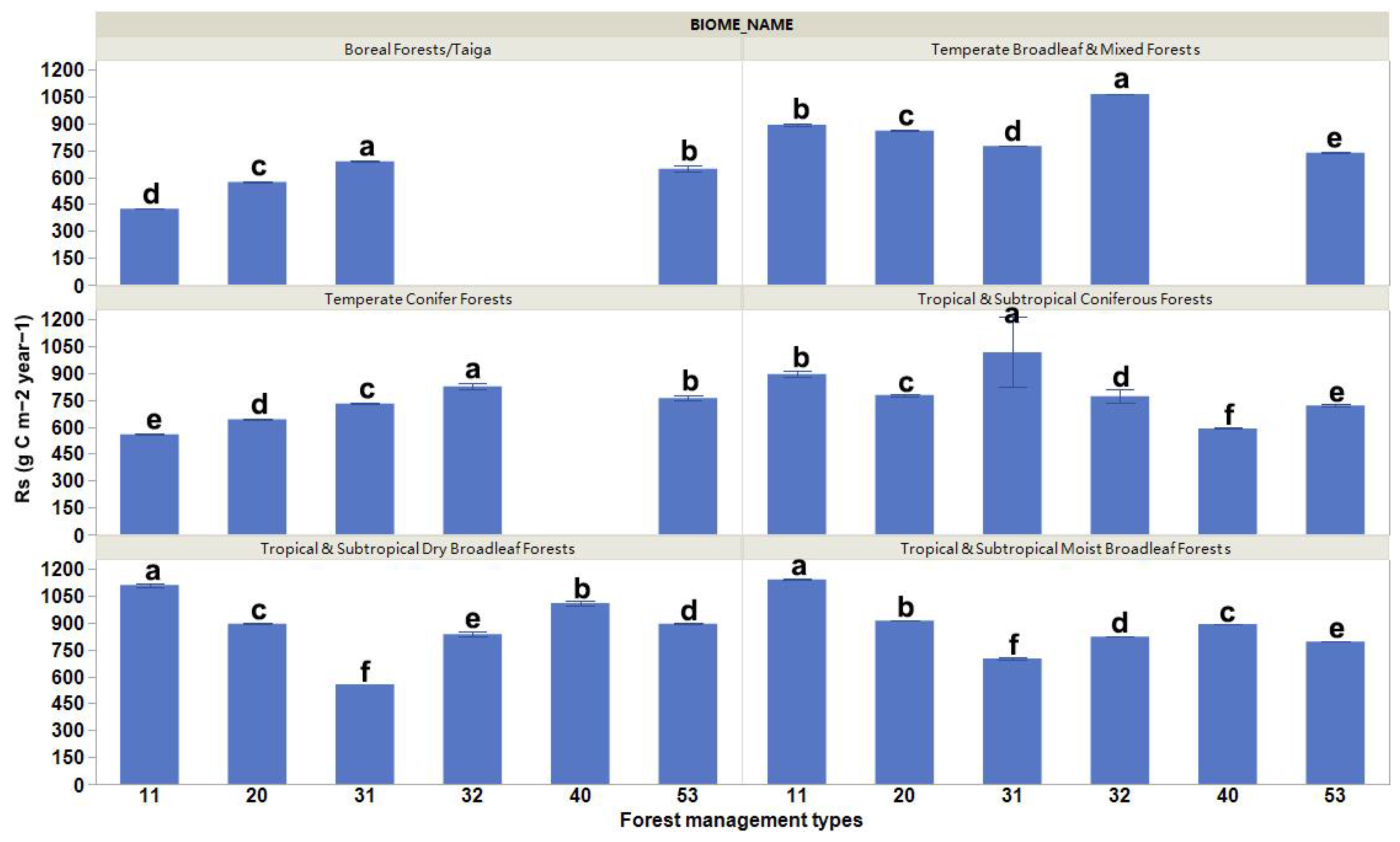
Our results present significant differences in the mean values of Rs for the different forest management practices (Figure 2). Globally, the Rs rates were the highest for plantation forests (rotation ≤ 15 years) and oil palm plantations, and the lowest for planted forests (rotation > 15 years) (Figure 2). These differences varied depending on the biome changes that occurred (Figure 3). The highest Rs rates were evident for planted forests (rotation > 15 years) in boreal forests/taiga and tropical and subtropical coniferous forest areas, plantation forests (rotation ≤ 15 years) in temperate broadleaf, mixed forest and temperate conifer forest areas, and naturally regenerating forests without any signs of management located in primary forests in tropical and subtropical coniferous forest and tropical and subtropical moist broadleaf forest areas (Figure 3).
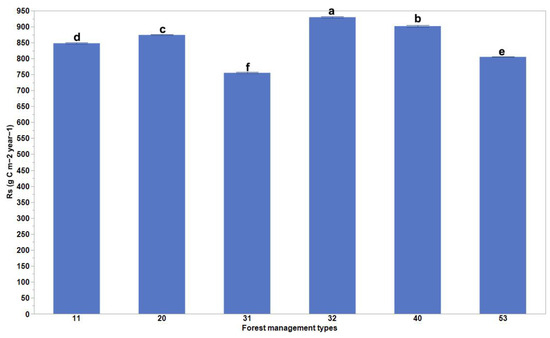
Figure 2.
Comparison of soil respiration rates as a result of different forest management practices. Columns coupled with bars represent mean ± standard error values. The six forest management types are classified as (11) naturally regenerating forests without any signs of management, (20) naturally regenerating forests with signs of forest management, (31) planted forests (rotation > 15 years), (32) plantation forest (rotation ≤ 15 years), (40) oil palm plantations, and (53) agroforestry [1]. Multiple comparisons using Tukey post hoc test and the compact letter display.
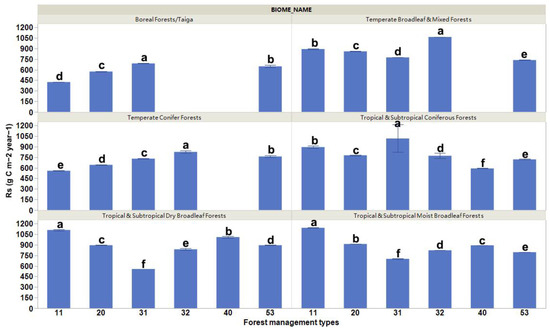
Figure 3.
Comparison of soil respiration rates as a result of different forest management practices across six forest biomes. Columns coupled with bars represent mean ± standard error values. The six forest management types are classified as (11) naturally regenerating forests without any signs of management, (20) naturally regenerating forests with signs of forest management, (31) planted forests (rotation > 15 years), (32) plantation forest (rotation ≤ 15 years), (40) oil palm plantations, and (53) agroforestry [1]. Multiple comparisons using Tukey post hoc test and the compact letter display.
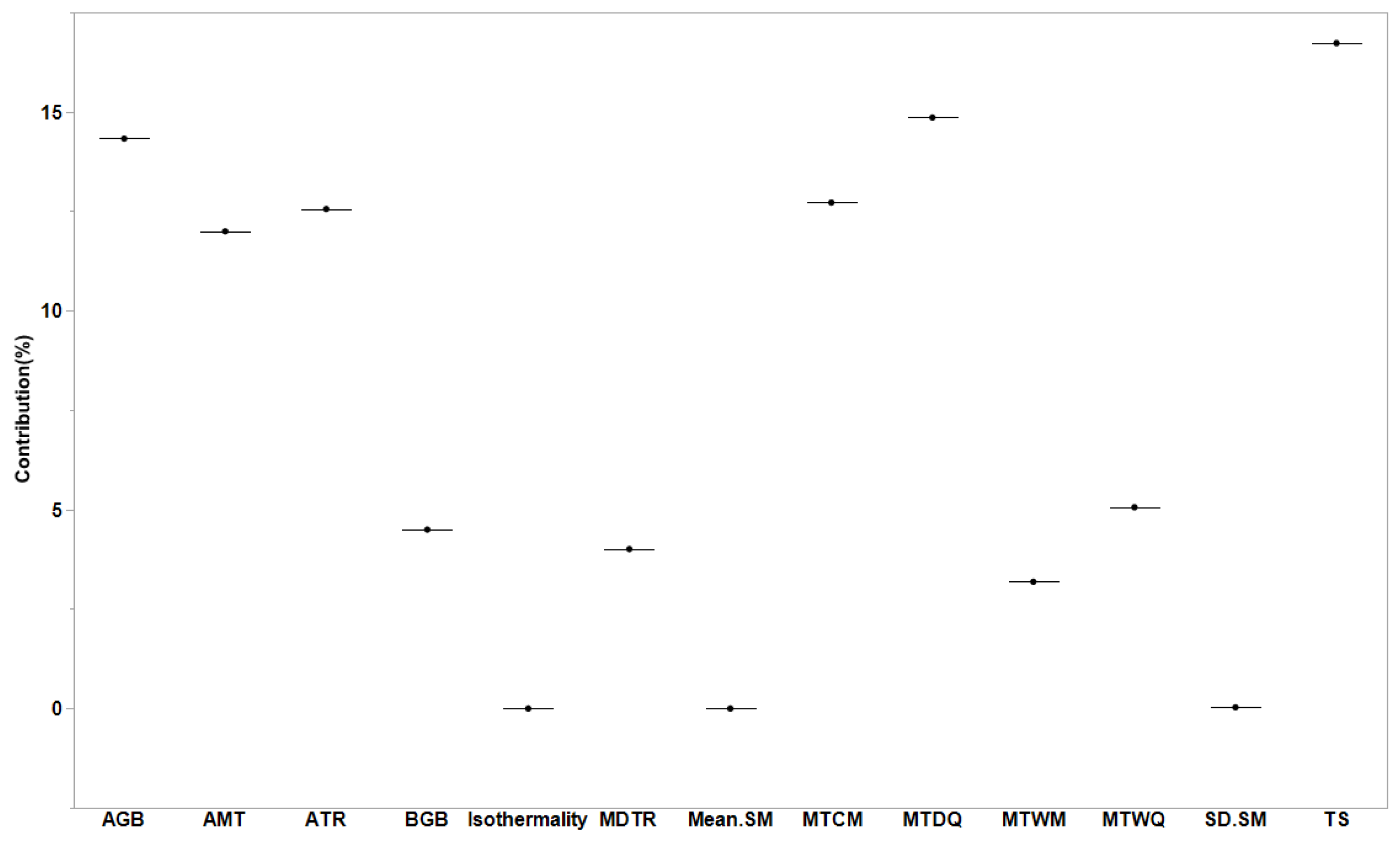
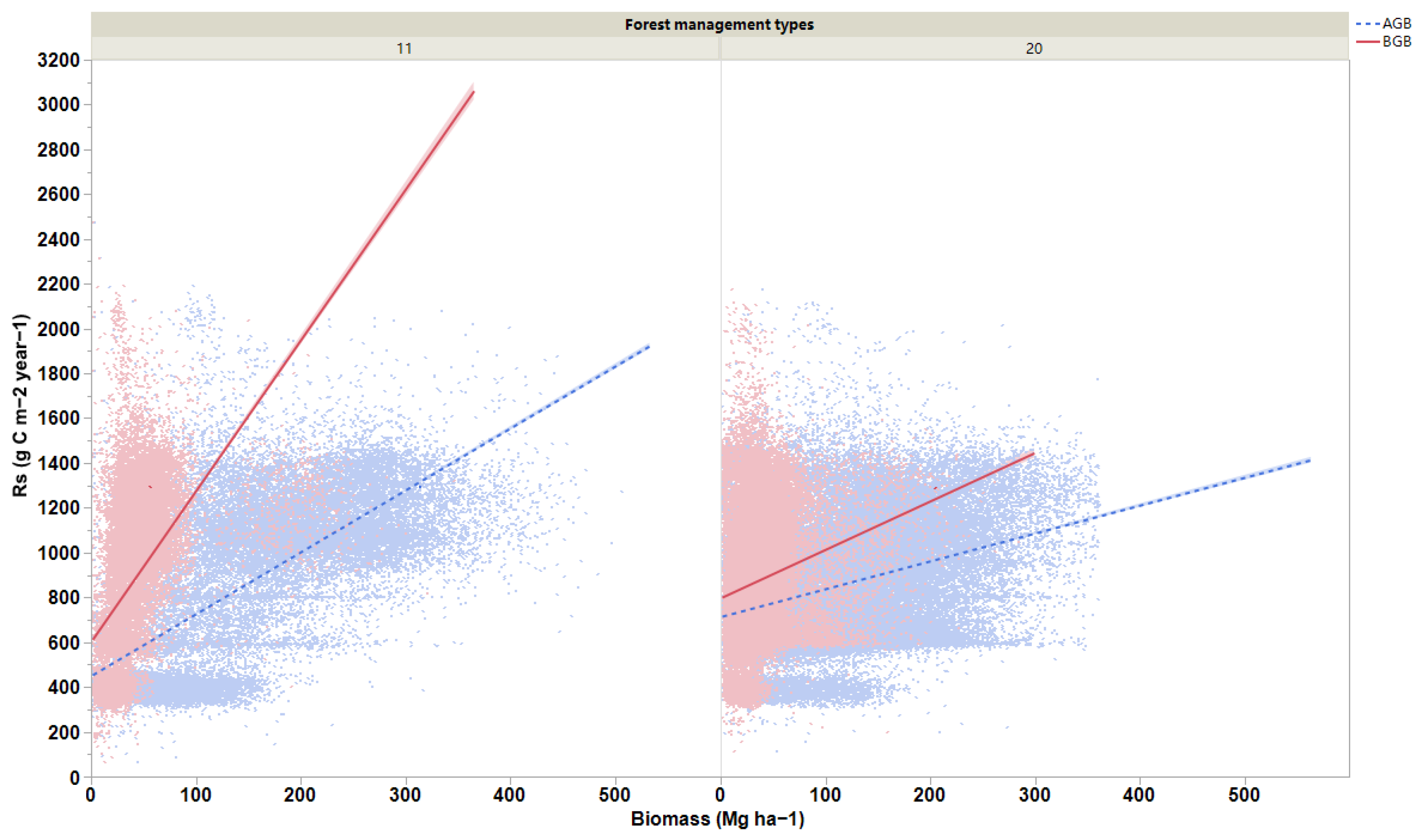
We observed that forest biomass and soil conditions had significant effects on Rs rates globally (R2m = 0.30 and R2c = 0.33). The factors of AMT, TS, MTCM, MTWQ, MTDQ, and aboveground biomass had substantial effects on Rs; however, soil moisture had the least effect on Rs (Figure 4). The gap between R2m and R2c values indicates that these effects varied depending on the changes in forest management classes. We also observed that the strongest positive relationships between biomass and Rs existed for naturally regenerating forests without signs of management (R2 = 0.49 for aboveground and R2 = 0.28 for belowground; p < 0.0001; Figure 5) and for naturally regenerating forests with signs of forest management (R2 = 0.11 for aboveground; p < 0.0001; Figure 5). Other forest management classes can lead to the establishment of weak relationships between forest biomass and Rs globally. Based on the slope results we obtained in our study, the effects of aboveground biomass on Rs rates were evidently lower than those of the belowground biomass at the global scale.
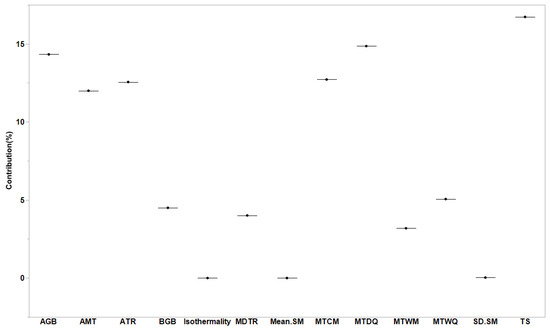
Figure 4.
Importance of biomass (aboveground and belowground biomasses), soil temperature, and moisture (mean and standard error values obtained from 1991 to 2018) for soil respiration based on the general linear mixed model. Nine soil temperature variables are presented in Section 2. SM represents soil moisture.
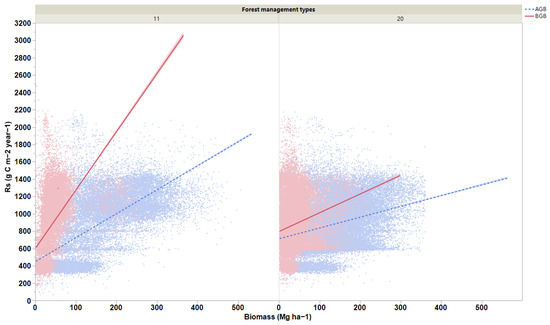
Figure 5.
Regression lines for relationships of aboveground (AGB) and belowground (BGB) biomasses with soil respiration rates for (11) naturally regenerating forests without any signs of management and (20) naturally regenerating forests with signs of forest management. All the relationships are significant (p < 0.05).
We can observe that the effects of soil temperature and moisture on Rs rates depend on different forest management practices (Table 1). The strongest relationships between soil temperature and moisture (i.e., AMT, MDTR, TS, MTWM, MTCM, ATR, MTWQ, MTDQ, and mean SM) and Rs can be observed in naturally regenerating forests without any signs of management (Table 1). In addition, AMT, MTWM, MTCM, MTWQ, and MTDQ present strong effects on the Rs rates in plantation forests (rotation ≤ 15 years; Table 1). AMT has positive effects on Rs in naturally regenerating forests without any signs of management, but has negative effects on Rs in plantation forests (rotation ≤ 15 years; Table 1). In naturally regenerating forests with signs of forest management, only TS has an effect on Rs (Table 1). Based on the slope results, it can be observed that the soil moisture level is enhanced in naturally regenerating forests without any signs of management, planted forests (rotation > 15 years), and oil palm plantations. MDTR had negative effects on Rs rates in naturally regenerating forests without any signs of management, naturally regenerating forests with signs of forest management, and planted forests (rotation > 15 years).

Table 1.
Relationships of soil temperature and moisture with soil respiration rates based on simple linear models.
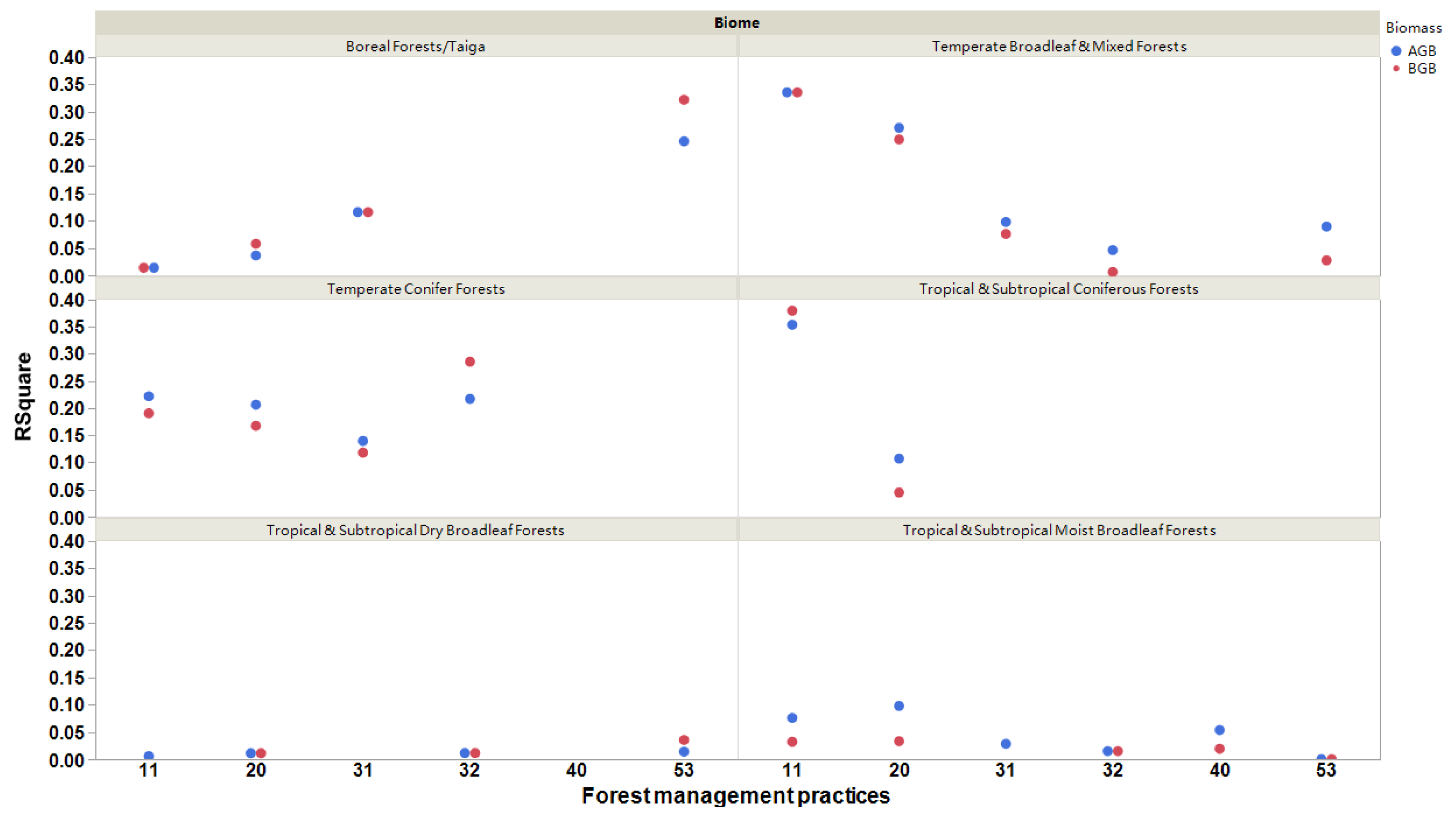
Interestingly, we observed that biome types could regulate the relationships between forest biomass and Rs across different forest management practices (Figure 6). All the relationships were positive. The relationships between forest biomass and Rs rates were the strongest for naturally regenerating forests, both with and without signs of management, in tropical and subtropical coniferous forests and temperate broadleaf and mixed forests. When analyzing planted forests (rotation > 15 years), we observed that the relationships between forest biomass and Rs rates were the strongest in temperate conifer forests, boreal forests, and taiga (R2 > 0.12; p < 0.0001; Figure 6). For agroforestry, we determined that boreal forests/taiga had the strongest relationships, but the R2 values of belowground biomass were higher than those of aboveground biomass (Figure 6). In other biomes, the relationships between forest biomass and Rs rates were weak due to R2 < 0.1, except for aboveground biomass vs. Rs in tropical and subtropical coniferous forests in naturally regenerating forests with signs of forest management (Figure 6).
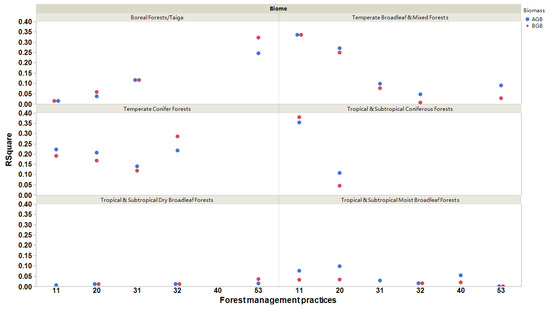
Figure 6.
The significant relationships (R2) of aboveground (AGB) and belowground (BGB) biomasses with soil respiration rates for different forest management practices and biome types. All the relationships are significant (p < 0.05). The six forest management types classified as (11) naturally regenerating forests without any signs of management, (20) naturally regenerating forests with signs of forest management, (31) planted forests (rotation >15 years), (32) plantation forest (rotation ≤15 years), (40) oil palm plantations, and (53) agroforestry [1].
4. Discussion
4.1. Relationships between Biomass and Soil Respiration
Globally, different forest management types result in different Rs levels. The highest Rs rate was evident in plantation forests (rotation ≤ 15 years), and the lowest Rs rate occurred in planted forests (rotation > 15 years), indicating the presence of high Rs rates in the early stages of planted forests. Rs rates are highly dependent on soil temperature and moisture conditions [45,46]. We observed that, in the early stages of plantation forests, the soil temperature levels were relatively high, and the soil moisture level was relatively low because of the decreasing stand density and leaf area [47,48]. Rs rates increase with increasing temperature, and it has also been established that Rs decreases when the soil is either very dry or wet [49]. Hence, it is possible that the effects of different forest management types on Rs rates occur due to the changes in soil temperature and moisture levels.
Furthermore, we determined the relationships between aboveground and belowground biomasses and Rs at the global scale. Rs rates on a large spatial scale are determined by shifts in aboveground and belowground biomass allocations [50,51]. Litter decomposition, which is associated with microbial respiration, is likely to be a major component of Rs [52,53]. Aboveground biomass is frequently removed and transformed into litter with a short lifespan [23,53]. Litter decomposition plays a critical role in regulating soil carbon and nitrogen cycling between plants and soils in forest ecosystems, and the litter layer mediates the soil microclimate by buffering the soil surface and atmosphere [53,54]. Microbial respiration in the surface layer is associated with a high rate of litter component decomposition, which can promote Rs [55]. High belowground biomass, including plant roots and closely associated microbial organisms, significantly contribute to Rs [26].
Forest plantations and agroforestry have developed rapidly worldwide, contributing to Rs and playing an important role in regulating pools of soil carbon and carbon cycling in terrestrial ecosystems, which directly affect the atmospheric concentration of CO2 [17,24,56,57]. Numerous studies have conducted field and experimental work to explore the effects of forest thinning on Rs rates [24,25,58,59,60]. Forest thinning has been widely used to enhance tree growth, optimize stand structure, promote biodiversity, reduce the numbers of tree pests and diseases, decrease wildfire risks, and maintain a healthy ecosystem function in experimental and field studies [23,57,58,59]. Based on the results obtained from field and experimental studies, it is evident that thinning and understory plant removal activities inevitably affect Rs and its components by altering the environment of plantation forests [24,25,57,58]. Previous studies have shown that thinning can directly inhibit Rs by reducing aboveground and belowground biomasses [24,25,60,61].
Based on the slope results we obtained in our study, it is evident that the effects of aboveground biomass on Rs rates are lower than those of the belowground biomass at the global scale, indicating that microbial respiration coupled with plant roots plays a more critical role in Rs activity than aboveground litter decomposition. However, the influence of aboveground and belowground biomasses on Rs rates depends on the changes in forest management practices. The effects of biomass on Rs rates are evident in naturally regenerating forests globally, but are less obvious in other forest management classes. Naturally regenerating forests have relatively high photosynthetic assimilation rates, soil microbial activity, root density, and biomass values, in comparison to plantation forests [62,63]. Hence, naturally regenerating forests present stronger effects on Rs than plantation forests. Our study provided a general mechanism for assessing Rs variations in forest management types, ranging from natural to plantation forests, from the perspective of aboveground and belowground biomasses.
4.2. Relationships between Soil Temperature and Moisture and Soil Respiration
In our study, soil temperature presented a significant relationship with Rs, but a weak relationship between soil moisture and Rs at a global scale was also evident. However, we observed that the effects of soil temperature and moisture on Rs rates depended on forest management types. High temperatures were associated with low Rs values in boreal forests. Our study has shown that forest management practices could regulate the effects of soil temperature and moisture on Rs rates. Specifically, annual and extreme temperatures had substantial effects on Rs rates in naturally regenerating and plantation forests worldwide without displaying any signs of management (rotation ≤ 15 years). Although the soil temperature and moisture dependence of Rs were thoroughly examined in our study, our results show that such effects depend on different forest management practices.
Previous field experiments have demonstrated that Rs was more sensitive to temperature changes as the soil moisture level increased. The effects of changes in soil temperature and moisture caused by aridity on carbon cycle variability under different forest management practices should also be considered in the research. The changes in forest management types on soil conditions can be attributed to the response of Rs to drought [64]. Drought can lead to a significant reduction in vegetation productivity by decreasing the water availability for plant tolerance, inhibiting extracellular enzyme diffusion in the context of plant water stress, and in turn inducing a substantial reduction in Rs activity [65,66]. Furthermore, different disturbance levels can alter the relationships between soil temperature and Rs rates [67,68]. Forest management practices are applied based on the degree of land disturbance [1]. Our results indicate that the annual mean temperature has a positive effect on Rs rates in naturally regenerating forests without any signs of management, but a negative effect on Rs in plantation forests (rotation ≤ 15 years). Temperature seasonality can also influence Rs rates during different forest management practices in the form of environmental constraints, such as great temperature fluctuations and severe water scarcity [69,70,71]. Soil moisture was also significantly associated with Rs, only in naturally regenerating forests without any signs of management. Relatively low soil moisture levels and warm temperatures can change the Rs rates in natural vegetation [56]. Hence, we should pay more attention to soil conditions (e.g., temperature and moisture) for the purpose of monitoring Rs rates across different forest management practices.
4.3. Biome Effects
Our results show that the effects of aboveground and belowground biomasses on Rs rates depend on biome changes. Our results also show that positive relationships between biomass and Rs exist widely in naturally regenerating forests across all 14 biomes surveyed in our study. Based on the analysis we performed on the biome effects, we determined that planted forests favored similar positive relationships in temperate forests. Rs can be enhanced for planted forests (rotation > 15 years), plantation forests (rotation ≤ 15 years), and agroforestry in temperate conifer and boreal forests/taiga by increasing aboveground and belowground biomasses. Furthermore, the Rs rate was the highest in planted forests in these two biomes.
As a crucial indicator of forest growth and quality, estimating the aboveground biomass plays a key role in monitoring the global carbon cycle and performing forest health assessments, which present significant results concerning Rs–biomass relationships in temperate conifer forests [70,72,73]. At smaller spatial scales (e.g., within forests), stand biomass is not strongly associated with Rs in temperate conifer forests [72]. Larger biomasses do not necessarily imply higher metabolic rates, even if the trees being studied are old, or have a higher proportion of dead wood due to old age, compared to younger, more metabolically active trees that account for relatively little in terms of biomass [66,74,75]. Thus, an increase in biomass can result in greater increases in Rs rates in different forest areas, as biomass is only an indirect proxy of autotrophic and, even more so, heterotrophic metabolic activity.
Furthermore, in our study, we observed that boreal forests/taiga presented the strongest relationship between biomass and Rs in the agroforestry category, indicating that biomass should be used to estimate Rs rates for agroforestry in boreal forests/taiga. Agroforestry is the system of growing trees, other woody perennials, crops, or pastures on the same land [72,73]. Agroforestry practices have recently received considerable attention in the research as a useful strategy to increase the number of carbon sinks in soils [74,76,77]. The selection of proper tree species and management techniques to rapidly increase boreal forests/taiga biomasses when introducing agroforestry systems has also been suggested in the research [76,77,78]. However, our study did not provide robust evidence for the existence of positive relationships between biomass and Rs rates in tropical and subtropical biomes (e.g., tropical and subtropical coniferous forests). Our results can be applied to forest management practices for naturally regenerating forests.
5. Conclusions
We can conclude that global positive relationships exist between aboveground and belowground biomasses and Rs in naturally regenerating forests, particularly in tropical and subtropical coniferous forests. Hence, aboveground and belowground biomasses could be used as effective ecological indicators for monitoring Rs activity, depending on the different forest management practices and biomes employed. In our results, a general reference for ecosystem functions related to Rs under different forest management practices globally is successfully provided.
Author Contributions
Conceptualization, C.H. and J.-P.L.; methodology, C.H. and J.-P.L.; software, C.H. and J.-P.L.; validation, C.H. and J.-P.L.; formal analysis, C.H. and J.-P.L.; investigation, C.H. and J.-P.L.; resources, C.H. and J.-P.L.; data curation, C.H. and J.-P.L.; writing—original draft preparation, C.H. and J.-P.L.; writing—review and editing, J.-Z.W.; visualization, J.-P.L.; supervision, J.-P.L.; project administration, J.-P.L.; funding acquisition, J.-P.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Key Research and Development Program of China (No. 2023YFD220040504).
Data Availability Statement
All relevant data are within the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Lesiv, M.; Schepaschenko, D.; Buchhorn, M.; See, L.; Dürauer, M.; Georgieva, I.; Jung, M.; Hofhansl, F.; Schulze, K.; Bilous, A.; et al. Global forest management data for 2015 at a 100 m resolution. Sci. Data 2022, 9, 199. [Google Scholar] [CrossRef] [PubMed]
- Keenan, R.J. Climate change impacts and adaptation in forest management: A review. Ann. For. Sci. 2015, 72, 145–167. [Google Scholar] [CrossRef]
- Peng, C. Understanding the role of forest simulation models in sustainable forest management. Environ. Impact Assess. Rev. 2000, 20, 481–501. [Google Scholar] [CrossRef]
- Varma, V.K.; Ferguson, I.; Wild, I. Decision support system for the sustainable forest management. For. Ecol. Manag. 2000, 128, 49–55. [Google Scholar] [CrossRef]
- Colombo, F.; Macdonald, C.A.; Jeffries, T.C.; Powell, J.R.; Singh, B.K. Impact of forest management practices on soil bacterial diversity and consequences for soil processes. Soil Biol. Biochem. 2016, 94, 200–210. [Google Scholar] [CrossRef]
- Muchane, M.N.; Sileshi, G.W.; Gripenberg, S.; Jonsson, M.; Pumariño, L.; Barrios, E. Agroforestry boosts soil health in the humid and sub-humid tropics: A meta-analysis. Agric. Ecosyst. Environ. 2020, 295, 106899. [Google Scholar] [CrossRef]
- Fahad, S.; Chavan, S.B.; Chichaghare, A.R.; Uthappa, A.R.; Kumar, M.; Kakade, V.; Pradhan, A.; Jinger, D.; Rawale, G.; Yadav, D.K.; et al. Agroforestry systems for soil health improvement and maintenance. Sustainability 2022, 14, 14877. [Google Scholar] [CrossRef]
- Lehmann, J.; Bossio, D.A.; Kögel-Knabner, I.; Rillig, M.C. The concept and future prospects of soil health. Nat. Rev. Earth Environ. 2020, 1, 544–553. [Google Scholar] [CrossRef] [PubMed]
- Jian, J.; Du, X.; Stewart, R.D. A database for global soil health assessment. Sci. Data 2020, 7, 16. [Google Scholar] [CrossRef]
- Carbone, M.S.; Still, C.J.; Ambrose, A.R.; Dawson, T.E.; Williams, A.P.; Boot, C.M.; Schaeffer, S.M.; Schimel, J.P. Seasonal and episodic moisture controls on plant and microbial contributions to soil respiration. Oecologia 2011, 167, 265–278. [Google Scholar] [CrossRef]
- Phillips, C.L.; Bond-Lamberty, B.; Desai, A.R.; Lavoie, M.; Risk, D.; Tang, J.; Todd-Brown, K.; Vargas, R. The value of soil respiration measurements for interpreting and modeling terrestrial carbon cycling. Plant Soil 2017, 413, 1–25. [Google Scholar] [CrossRef]
- Peng, Y.; Thomas, S.C.; Tian, D. Forest management and soil respiration: Implications for carbon sequestration. Environ. Rev. 2008, 16, 93–111. [Google Scholar] [CrossRef]
- Tufekcioglu, A.; Raich, J.W.; Isenhart, T.M.; Schultz, R.C. Soil respiration within riparian buffers and adjacent crop fields. Plant Soil 2001, 229, 117–124. [Google Scholar] [CrossRef]
- Hynynen, J.; Salminen, H.; Ahtikoski, A.; Huuskonen, S.; Ojansuu, R.; Siipilehto, J.; Lehtonen, M.; Eerikäinen, K. Long-term impacts of forest management on biomass supply and forest resource development: A scenario analysis for Finland. Eur. J. For. Res. 2015, 134, 415–431. [Google Scholar] [CrossRef]
- Erb, K.-H.; Kastner, T.; Plutzar, C.; Bais, A.L.S.; Carvalhais, N.; Fetzel, T.; Gingrich, S.; Haberl, H.; Lauk, C.; Niedertscheider, M.; et al. Unexpectedly large impact of forest management and grazing on global vegetation biomass. Nature 2018, 553, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Verkerk, P.J.; Fitzgerald, J.B.; Datta, P.; Dees, M.; Hengeveld, G.M.; Lindner, M.; Zudin, S. Spatial distribution of the potential forest biomass availability in Europe. For. Ecosyst. 2019, 6, 5. [Google Scholar] [CrossRef]
- Dornbush, M.E.; Raich, J.W. Soil temperature, not aboveground plant productivity, best predicts intra-annual variations of soil respiration in central Iowa grasslands. Ecosystems 2006, 9, 909–920. [Google Scholar] [CrossRef]
- Metcalfe, D.B.; Fisher, R.A.; Wardle, D.A. Plant communities as drivers of soil respiration: Pathways, mechanisms, and significance for global change. Biogeosciences 2011, 8, 2047–2061. [Google Scholar] [CrossRef]
- Chen, X.; Chen, H.Y. Plant diversity loss reduces soil respiration across terrestrial ecosystems. Glob. Chang. Biol. 2019, 25, 1482–1492. [Google Scholar] [CrossRef]
- Jandl, R.; Lindner, M.; Vesterdal, L.; Bauwens, B.; Baritz, R.; Hagedorn, F.; Johnson, D.W.; Minkkinen, K.; Byrne, K.A. How strongly can forest management influence soil carbon sequestration? Geoderma 2007, 137, 253–268. [Google Scholar] [CrossRef]
- Liu, L.; Wang, X.; Lajeunesse, M.J.; Miao, G.; Piao, S.; Wan, S.; Wu, Y.; Wang, Z.; Yang, S.; Li, P.; et al. A cross-biome synthesis of soil respiration and its determinants under simulated precipitation changes. Glob. Chang. Biol. 2016, 22, 1394–1405. [Google Scholar] [CrossRef] [PubMed]
- Major, J.; Lehmann, J.; Rondon, M.; Goodale, C. Fate of soil-applied black carbon: Downward migration, leaching and soil respiration. Glob. Chang. Biol. 2010, 16, 1366–1379. [Google Scholar] [CrossRef]
- Zhang, H.; Ying, B.; Hu, Y.; Wang, Y.; Yu, X.; Tang, C. Response of soil respiration to thinning is altered by thinning residue treatment in Cunninghamia lanceolata plantations. Agric. For. Meteorol. 2022, 324, 109089. [Google Scholar] [CrossRef]
- Zhao, B.; Ballantyne, A.P.; Meng, S.; Zhao, G.; Zheng, Z.; Zhu, J. Understory plant removal counteracts tree thinning effect on soil respiration in a temperate forest. Glob. Chang. Biol. 2022, 28, 6102–6113. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Han, H.; Kang, F.; Liu, K.; Song, Y.; Zhou, B.; Li, Y. Short-term effects of thinning on soil respiration in a pine (Pinus tabulaeformis) plantation. Biol. Fertil. Soils 2014, 50, 357–367. [Google Scholar] [CrossRef]
- McCormack, M.L.; Dickie, I.A.; Eissenstat, D.M.; Fahey, T.J.; Fernandez, C.W.; Guo, D.; Helmisaari, H.; Hobbie, E.A.; Iversen, C.M.; Jackson, R.B.; et al. Redefining fine roots improves understanding of below-ground contributions to terrestrial biosphere processes. New Phytol. 2015, 207, 505–518. [Google Scholar] [CrossRef]
- Ren, C.; Chen, J.; Deng, J.; Zhao, F.; Han, X.; Yang, G.; Tong, X.; Feng, Y.; Shelton, S.; Ren, G. Response of microbial diversity to C: N: P stoichiometry in fine root and microbial biomass following afforestation. Biol. Fertil. Soils 2017, 53, 457–468. [Google Scholar] [CrossRef]
- Schlesinger, W.H.; Andrews, J.A. Soil respiration and the global carbon cycle. Biogeochemistry 2000, 48, 7–20. [Google Scholar] [CrossRef]
- Poulter, B.; Frank, D.; Ciais, P.; Myneni, R.B.; Andela, N.; Bi, J.; Broquet, G.; Canadell, J.G.; Chevallier, F.; Liu, Y.Y.; et al. Contribution of semi-arid ecosystems to interannual variability of the global carbon cycle. Nature 2014, 509, 600–603. [Google Scholar] [CrossRef]
- Lavigne, M.B.; Foster, R.J.; Goodine, G. Seasonal and annual changes in soil respiration in relation to soil temperature, water potential and trenching. Tree Physiol. 2004, 24, 415–424. [Google Scholar] [CrossRef]
- Crous, K.Y.; Uddling, J.; De Kauwe, M.G. Temperature responses of photosynthesis and respiration in evergreen trees from boreal to tropical latitudes. New Phytol. 2022, 234, 353–374. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Fu, B.; Zhang, L.; Xu, Z. Soil moisture–plant interactions: An ecohydrological review. J. Soils Sediments 2019, 19, 1–9. [Google Scholar] [CrossRef]
- Lobell, D.B.; Asner, G.P. Moisture effects on soil reflectance. Soil Sci. Soc. Am. J. 2002, 66, 722–727. [Google Scholar] [CrossRef]
- Ainiwaer, M.; Ding, J.; Kasim, N.; Wang, J.; Wang, J. Regional scale soil moisture content estimation based on multi-source remote sensing parameters. Int. J. Remote Sens. 2020, 41, 3346–3367. [Google Scholar] [CrossRef]
- Dinerstein, E.; Olson, D.; Joshi, A.; Vynne, C.; Burgess, N.D.; Wikramanayake, E.; Hahn, N.; Palminteri, S.; Hedao, P.; Noss, R.; et al. An ecoregion-based approach to protecting half the terrestrial realm. BioScience 2017, 67, 534–545. [Google Scholar] [CrossRef] [PubMed]
- Olson, D.M.; Dinerstein, E.; Wikramanayake, E.D.; Burgess, N.D.; Powell, G.V.; Underwood, E.C.; D’Amico, J.A.; Itoua, I.; Strand, H.E.; Morrison, J.C.; et al. Terrestrial Ecoregions of the World: A New Map of Life on Earth: A new global map of terrestrial ecoregions provides an innovative tool for conserving biodiversity. BioScience 2001, 51, 933–938. [Google Scholar] [CrossRef]
- Wan, J.Z.; Wang, C.J.; Marquet, P.A. Environmental heterogeneity as a driver of terrestrial biodiversity on a global scale. Prog. Phys. Geogr. 2023, 47, 912–930. [Google Scholar] [CrossRef]
- Conradi, T.; Slingsby, J.A.; Midgley, G.F.; Nottebrock, H.; Schweiger, A.H.; Higgins, S.I. An operational definition of the biome for global change research. New Phytol. 2020, 227, 1294–1306. [Google Scholar] [CrossRef] [PubMed]
- Ai, J.; Qi, X.; Zhang, R.; He, M.; Li, J.; Xu, R.; Li, Y.; Sarmah, S.; Wang, H.; Zhao, J. A Novel Approach for Ecosystem Respiration Simulation in Drylands. Front. Ecol. Evol. 2023, 11, 1186272. [Google Scholar] [CrossRef]
- Huang, N.; Wang, L.; Song, X.P.; Black, T.A.; Jassal, R.S.; Myneni, R.B.; Wu, C.; Song, W.; Ji, D.; Yu, S.; et al. Spatial and temporal variations in global soil respiration and their relationships with climate and land cover. Sci. Adv. 2020, 6, eabb8508. [Google Scholar] [CrossRef]
- Santoro, M.; Cartus, O.; Carvalhais, N.; Rozendaal, D.; Avitabilie, V.; Araza, A.; de Bruin, S.; Herold, M.; Quegan, S.; Rodríguez-Veiga, P.; et al. The global forest above-ground biomass pool for 2010 estimated from high-resolution satellite observations. Earth Syst. Sci. Data Discuss. 2020, 13, 3927–3950. [Google Scholar] [CrossRef]
- Lembrechts, J.J.; Hoogen, J.v.D.; Aalto, J.; Ashcroft, M.B.; De Frenne, P.; Kemppinen, J.; Kopecký, M.; Luoto, M.; Maclean, I.M.D.; Crowther, T.W.; et al. Global maps of soil temperature. Glob. Chang. Biol. 2021, 28, 3110–3144. [Google Scholar] [CrossRef]
- Guevara, M.; Taufer, M.; Vargas, R. Gap-free global annual soil moisture: 15 km grids for 1991–2018. Earth Syst. Sci. Data 2021, 13, 1711–1735. [Google Scholar] [CrossRef]
- Lai, J.; Zou, Y.; Zhang, S.; Zhang, X.; Mao, L. glmm. hp: An R package for computing individual effect of predictors in generalized linear mixed models. J. Plant Ecol. 2022, 15, 1302–1307. [Google Scholar] [CrossRef]
- Akinremi, O.O.; McGinn, S.M.; McLean, H.D.J. Effects of soil temperature and moisture on soil respiration in barley and fallow plots. Can. J. Soil Sci. 1999, 79, 5–13. [Google Scholar] [CrossRef]
- Curiel Yuste, J.; Baldocchi, D.D.; Gershenson, A.; Goldstein, A.; Misson, L.; Wong, S. Microbial soil respiration and its dependency on carbon inputs, soil temperature and moisture. Glob. Chang. Biol. 2007, 13, 2018–2035. [Google Scholar] [CrossRef]
- Tang, X.L.; Zhou, G.Y.; Liu, S.G.; Zhang, D.Q.; Liu, S.Z.; Li, J.; Zhou, C.Y. Dependence of soil respiration on soil temperature and soil moisture in successional forests in southern China. J. Integr. Plant Biol. 2006, 48, 654–663. [Google Scholar] [CrossRef]
- Von Arx, G.; Graf Pannatier, E.; Thimonier, A.; Rebetez, M. Microclimate in forests with varying leaf area index and soil moisture: Potential implications for seedling establishment in a changing climate. J. Ecol. 2013, 101, 1201–1213. [Google Scholar] [CrossRef]
- Davidson, E.A.; Belk, E.; Boone, R.D. Soil water content and temperature as independent or confounded factors controlling soil respiration in a temperate mixed hardwood forest. Glob. Chang. Biol. 1998, 4, 217–227. [Google Scholar] [CrossRef]
- Yan, Y.; Quan, Q.; Meng, C.; Wang, J.; Tian, D.; Wang, B.; Zhang, R.; Niu, S. Varying soil respiration under long-term warming and clipping due to shifting carbon allocation toward below-ground. Agric. For. Meteorol. 2021, 304, 108408. [Google Scholar] [CrossRef]
- Heinen, R.; Thakur, M.P.; Hiddes De Fries, J.R.; Steinauer, K.; Vandenbrande, S.; Jongen, R.; Bezemer, T.M. Foliar herbivory on plants creates soil legacy effects that impact future insect herbivore growth via changes in plant community biomass allocation. Funct. Ecol. 2022, 36, 1047–1062. [Google Scholar] [CrossRef]
- Cisneros-Dozal, L.M.; Trumbore, S.E.; Hanson, P.J. Effect of moisture on leaf litter decomposition and its contribution to soil respiration in a temperate forest. J. Geophys. Res. 2007, 112, G01013. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, J.; Zhao, R.; Guo, Y.; Hao, L. Aboveground net primary productivity and soil respiration display different responses to precipitation changes in desert grassland. J. Plant Ecol. 2021, 15, 57–70. [Google Scholar] [CrossRef]
- Valentini, C.M.A.; Sanches, L.; de Paula, S.R.; Vourlitis, G.L.; de Souza Nogueira, J.; Pinto, O.B., Jr.; de Almeida Lobo, F. Soil respiration and aboveground litter dynamics of a tropical transitional forest in northwest Mato Grosso, Brazil. J. Geophys. Res. 2008, 113, G00B10. [Google Scholar] [CrossRef]
- Fierer, N.; Strickland, M.S.; Liptzin, D.; Bradford, M.A.; Cleveland, C.C. Global patterns in belowground communities. Ecol. Lett. 2009, 12, 1238–1249. [Google Scholar] [CrossRef] [PubMed]
- Dacal, M.; García-Palacios, P.; Asensio, S.; Cano-Díaz, C.; Gozalo, B.; Ochoa, V.; Maestre, F.T. Contrasting mechanisms underlie short-and longer-term soil respiration responses to experimental warming in a dryland ecosystem. Glob. Chang. Biol. 2020, 26, 5254–5266. [Google Scholar] [CrossRef]
- Bae, K.; Lee, D.K.; Fahey, T.J.; Woo, S.Y.; Quaye, A.K.; Lee, Y.K. Seasonal variation of soil respiration rates in a secondary forest and agroforestry systems. Agrofor. Syst. 2013, 87, 131–139. [Google Scholar] [CrossRef]
- Pang, X.; Bao, W.; Zhu, B.; Cheng, W. Responses of soil respiration and its temperature sensitivity to thinning in a pine plantation. Agric. For. Meteorol. 2013, 171, 57–64. [Google Scholar] [CrossRef]
- Wang, C.-J.; Wang, R.; Yu, C.-M.; Pubu, Y.; Sun, W.-G.; Dang, X.-F.; Li, Q.-F.; Wan, J.-Z. Determinants of species assemblages of insect pests in alpine forest ecosystems of western China. For. Ecosyst. 2021, 8, 71. [Google Scholar] [CrossRef]
- Epron, D.; Nouvellon, Y.; Roupsard, O.; Mouvondy, W.; Mabiala, A.; Saint-André, L.; Joffre, R.; Jourdan, C.; Bonnefond, J.-M.; Berbigier, P.; et al. Spatial and temporal variations of soil respiration in a Eucalyptus plantation in Congo. For. Ecol. Manag. 2004, 202, 149–160. [Google Scholar] [CrossRef]
- Yang, L.; Qin, J.; Geng, Y.; Zhang, C.; Pan, J.; Niu, S.; Tian, D.; Zhao, X.; Wang, J. Long-term effects of forest thinning on soil respiration and its components in a pine plantation. For. Ecol. Manag. 2022, 513, 120189. [Google Scholar] [CrossRef]
- Hua, F.; Bruijnzeel, L.A.; Meli, P.; Martin, P.A.; Zhang, J.; Nakagawa, S.; Miao, X.; Wang, W.; McEvoy, C.; Peña-Arancibia, J.L.; et al. The biodiversity and ecosystem service contributions and trade-offs of forest restoration approaches. Science 2022, 376, 839–844. [Google Scholar] [CrossRef] [PubMed]
- Messier, C.; Bauhus, J.; Sousa-Silva, R.; Auge, H.; Baeten, L.; Barsoum, N.; Bruelheide, H.; Caldwell, B.; Cavender-Bares, J.; Dhiedt, E.; et al. For the sake of resilience and multifunctionality, let’s diversify planted forests! Conserv. Lett. 2022, 15, e12829. [Google Scholar] [CrossRef]
- Ahlström, A.; Raupach, M.R.; Schurgers, G.; Smith, B.; Arneth, A.; Jung, M.; Reichstein, M.; Canadell, J.G.; Friedlingstein, P.; Jain, A.K.; et al. The dominant role of semi-arid ecosystems in the trend and variability of the land CO2 sink. Science 2015, 348, 895–899. [Google Scholar] [CrossRef] [PubMed]
- Manzoni, S.; Schaeffer, S.M.; Katul, G.; Porporato, A.; Schimel, J.P. A theoretical analysis of microbial eco-physiological and diffusion limitations to carbon cycling in drying soils. Soil Biol. Biochem. 2014, 73, 69–83. [Google Scholar] [CrossRef]
- Misiak, M.; Goodall-Copestake, W.P.; Sparks, T.H.; Worland, M.R.; Boddy, L.; Magan, N.; Convey, P.; Hopkins, D.W.; Newsham, K.K. Inhibitory effects of climate change on the growth and extracellular enzyme activities of a widespread Antarctic soil fungus. Glob. Chang. Biol. 2021, 27, 1111–1125. [Google Scholar] [CrossRef] [PubMed]
- Concilio, A.; Ma, S.; Ryu, S.R.; North, M.; Chen, J. Soil respiration response to experimental disturbances over 3 years. For. Ecol. Manag. 2006, 228, 82–90. [Google Scholar] [CrossRef]
- Hagemann, U.; Moroni, M.T.; Gleißner, J.; Makeschin, F. Disturbance history influences downed woody debris and soil respiration. For. Ecol. Manag. 2010, 260, 1762–1772. [Google Scholar] [CrossRef]
- Lloyd, J.; Taylor, J.A. On the temperature dependence of soil respiration. Funct. Ecol. 1994, 8, 315–323. [Google Scholar] [CrossRef]
- Jia, X.; Mu, Y.; Zha, T.; Wang, B.; Qin, S.; Tian, Y. Seasonal and interannual variations in ecosystem respiration in relation to temperature, moisture, and productivity in a temperate semi-arid shrubland. Sci. Total Environ. 2020, 709, 136210. [Google Scholar] [CrossRef]
- Jian, J.; Bahn, M.; Wang, C.; Bailey, V.L.; Bond-Lamberty, B. Prediction of annual soil respiration from its flux at mean annual temperature. Agric. For. Meteorol. 2020, 287, 107961. [Google Scholar] [CrossRef]
- Tang, J.; Qi, Y.; Xu, M.; Misson, L.; Goldstein, A.H. Forest thinning and soil respiration in a ponderosa pine plantation in the Sierra Nevada. Tree Physiol. 2005, 25, 57–66. [Google Scholar] [CrossRef]
- Wang, Z.; Ji, L.; Hou, X.; Schellenberg, M.P. Soil respiration in semiarid temperate grasslands under various land management. PLoS ONE 2016, 11, e0147987. [Google Scholar]
- Jose, S. Agroforestry for ecosystem services and environmental benefits: An overview. Agrofor. Syst. 2009, 76, 1–10. [Google Scholar] [CrossRef]
- Rodríguez-Calcerrada, J.; Salomón, R.; Barba, J.; Gordaliza, G.G.; Curiel Yuste, J.; Magro, C.; Gil, L. Regeneration in the understory of declining overstory trees contributes to soil respiration homeostasis along succession in a sub-Mediterranean beech forest. Forests 2019, 10, 727. [Google Scholar] [CrossRef]
- Jose, S.; Bardhan, S. Agroforestry for biomass production and carbon sequestration: An overview. Agrofor. Syst. 2012, 86, 105–111. [Google Scholar] [CrossRef]
- Kukumägi, M.; Ostonen, I.; Kupper, P.; Truu, M.; Tulva, I.; Varik, M.; Aosaar, J.; Sõber, J.; Lõhmus, K. The effects of elevated atmospheric humidity on soil respiration components in a young silver birch forest. Agric. For. Meteorol. 2014, 194, 167–174. [Google Scholar] [CrossRef]
- Ma, Y.Q.; Wang, C.J.; Chen, Z.; Yu, F.H.; Wan, J.Z. Linking Forest Management Practices to the Functional Composition of Plant Communities. Forests 2023, 14, 1939. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).