The Growth and Non-Structural Carbohydrate Response Patterns of Siberian Elm (Ulmus pumila) under Salt Stress with Different Intensities and Durations
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Experimental Design
2.3. Measurement of Relative Growth Rate
2.4. Measurements of Leaf Mass Area, Photosynthetic-Related Traits and Midday Leaf Water Potential
2.5. Measurement of Root to Shoot Ratio
2.6. Measurement of Non-Structural Carbohydrates Concentration
2.7. Data Analysis
3. Results
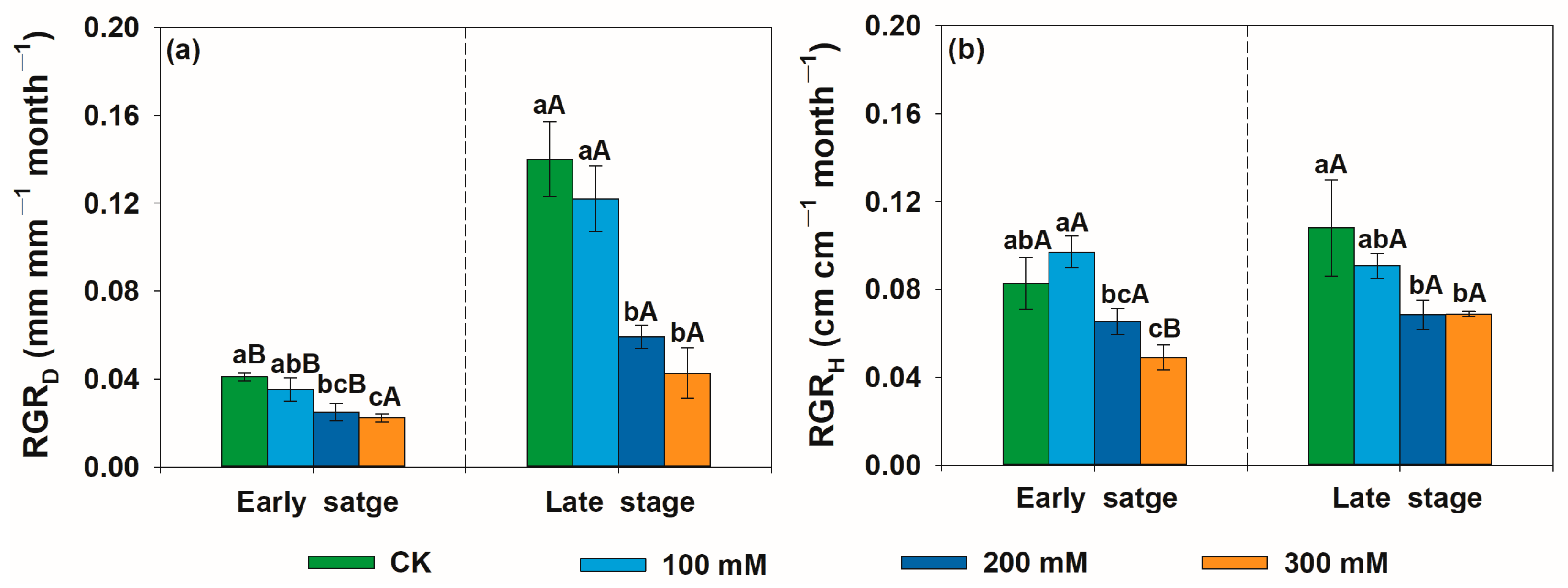
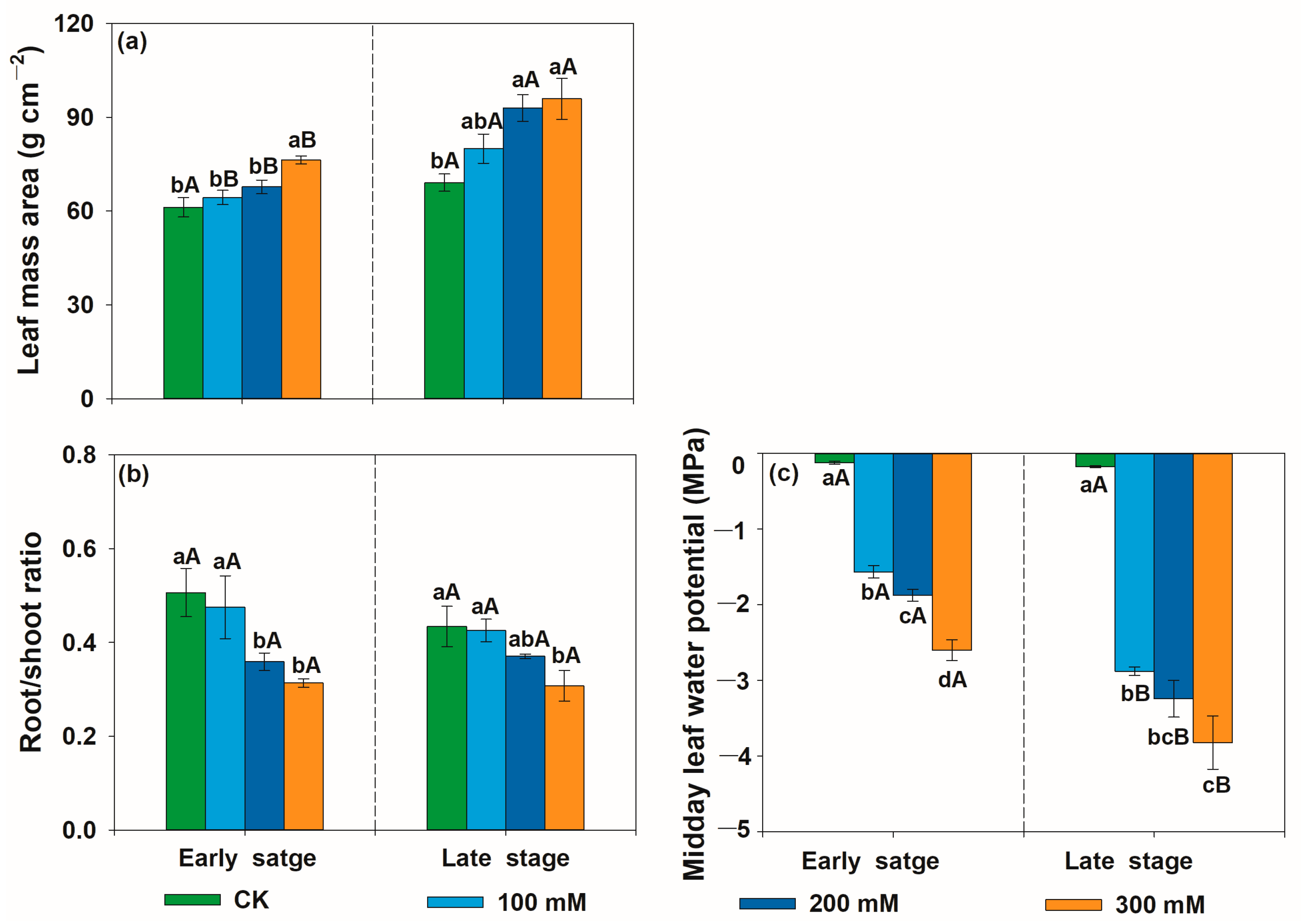
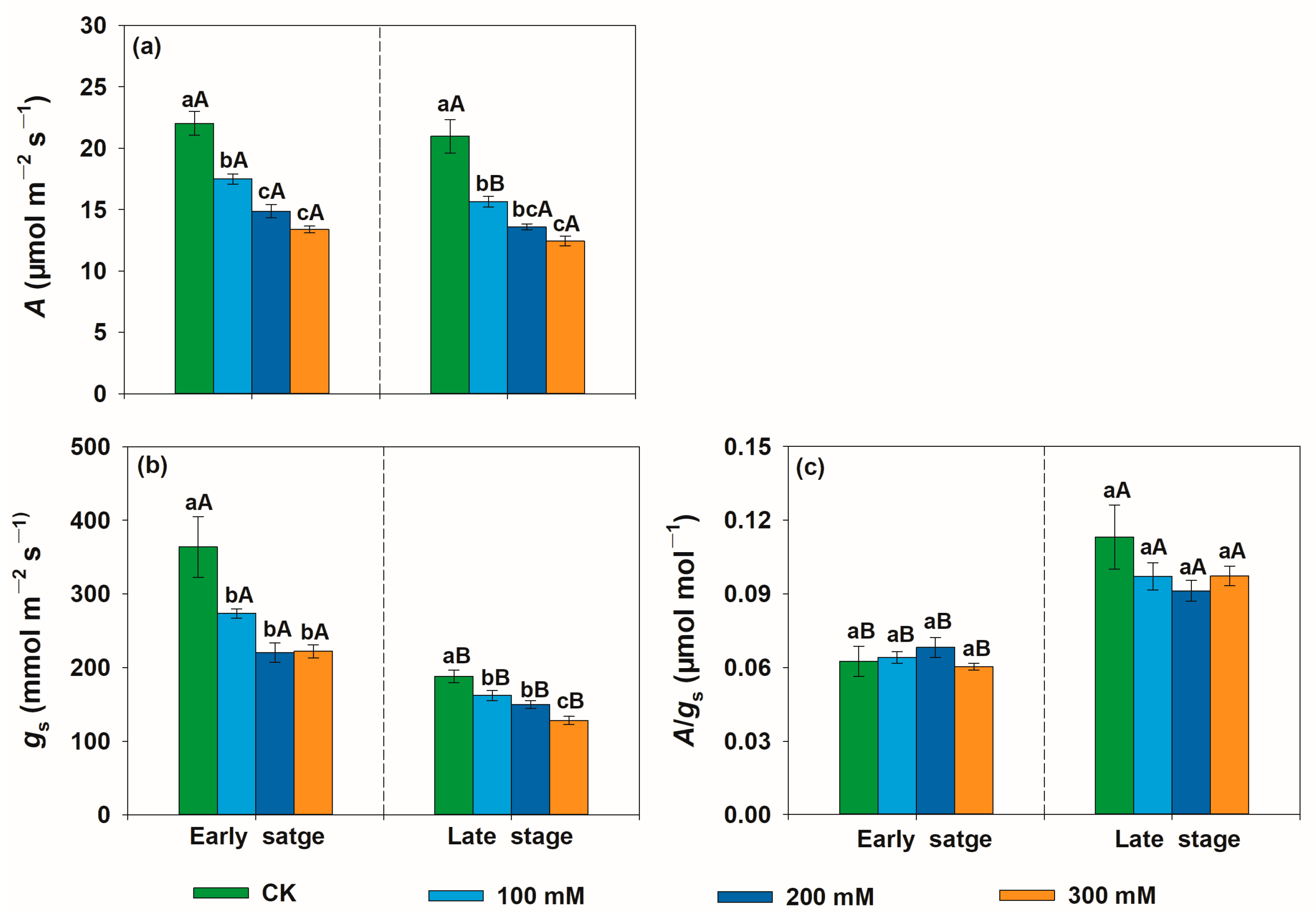
3.1. Effects of Salt Stress on Relative Growth Rate, Leaf Mass Area, Midday Leaf Water Potential, Root/Shoot Ratio, and Photosynthetic-Related Traits of U. pumila
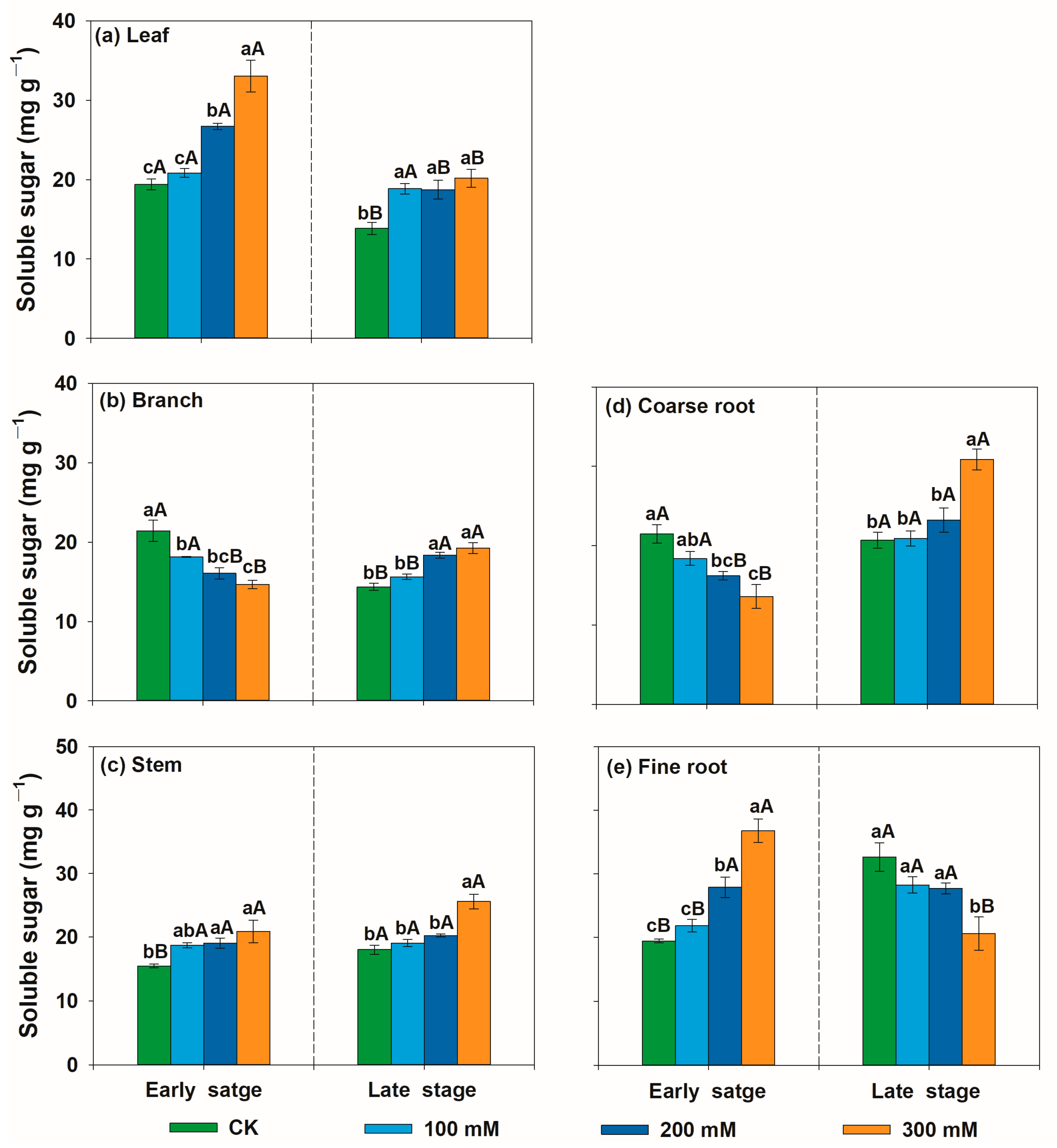
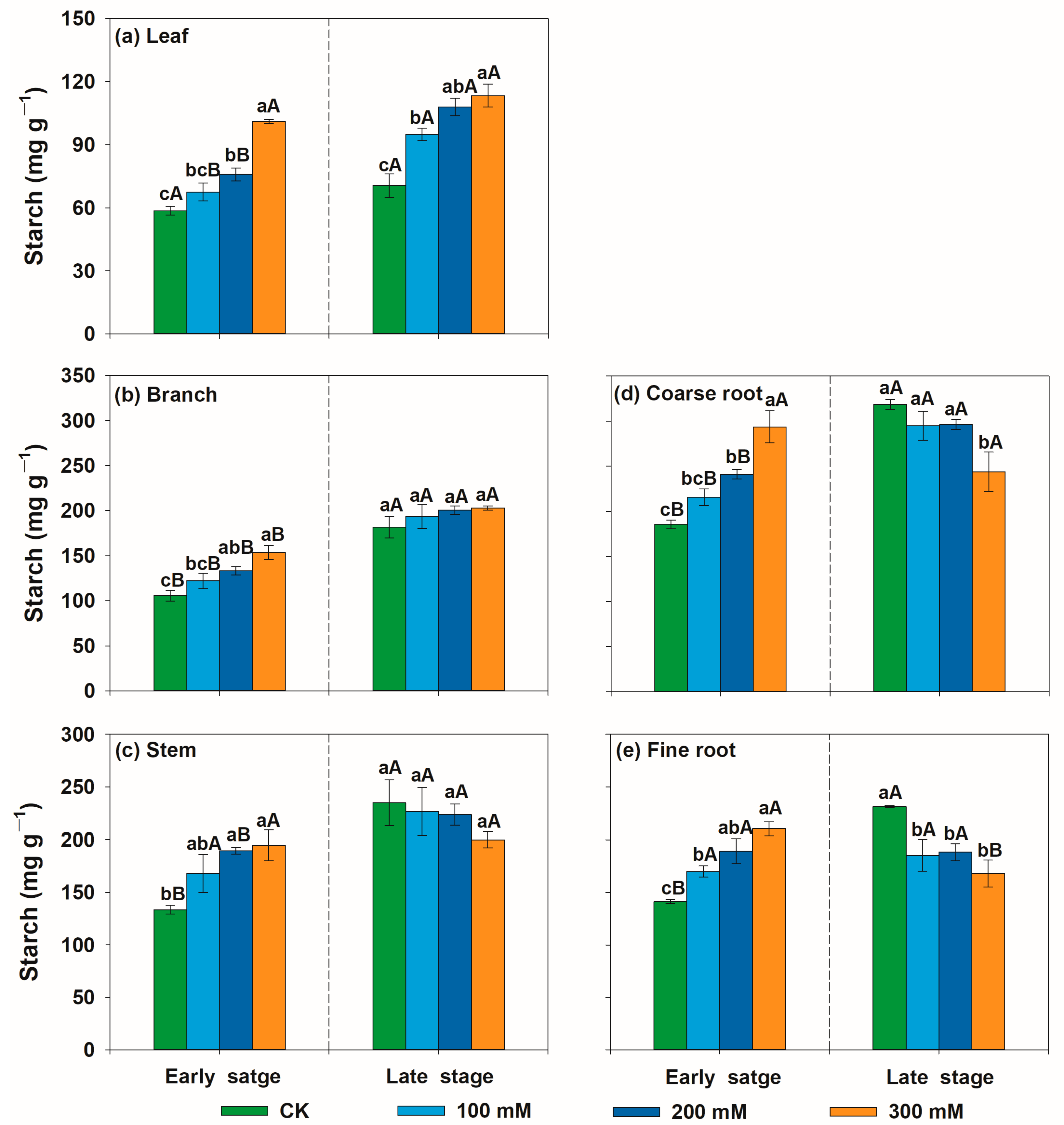
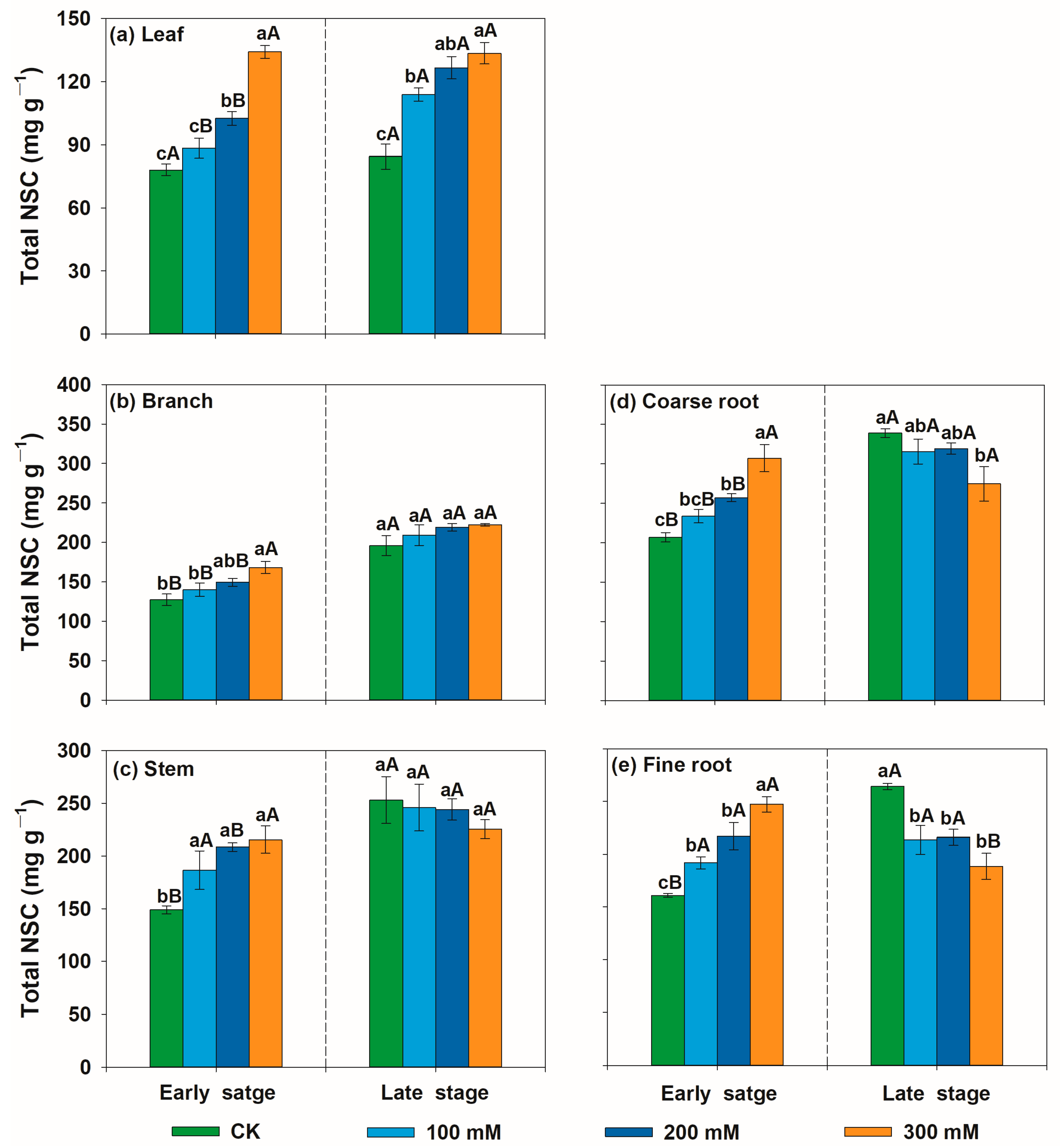
3.2. Variation Patterns of Non-Structural Carbohydrate Concentrations of U. pumila in Response to Salt Stress
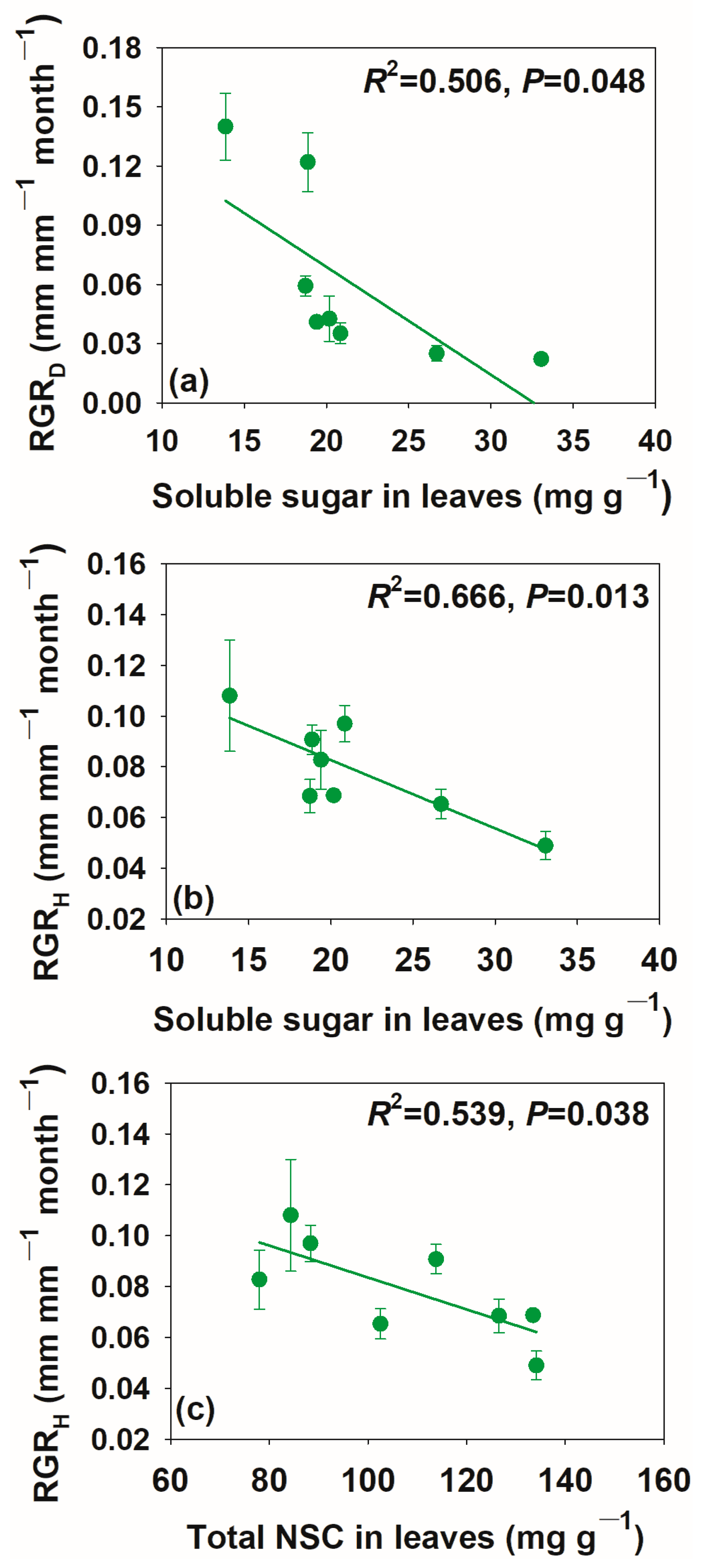
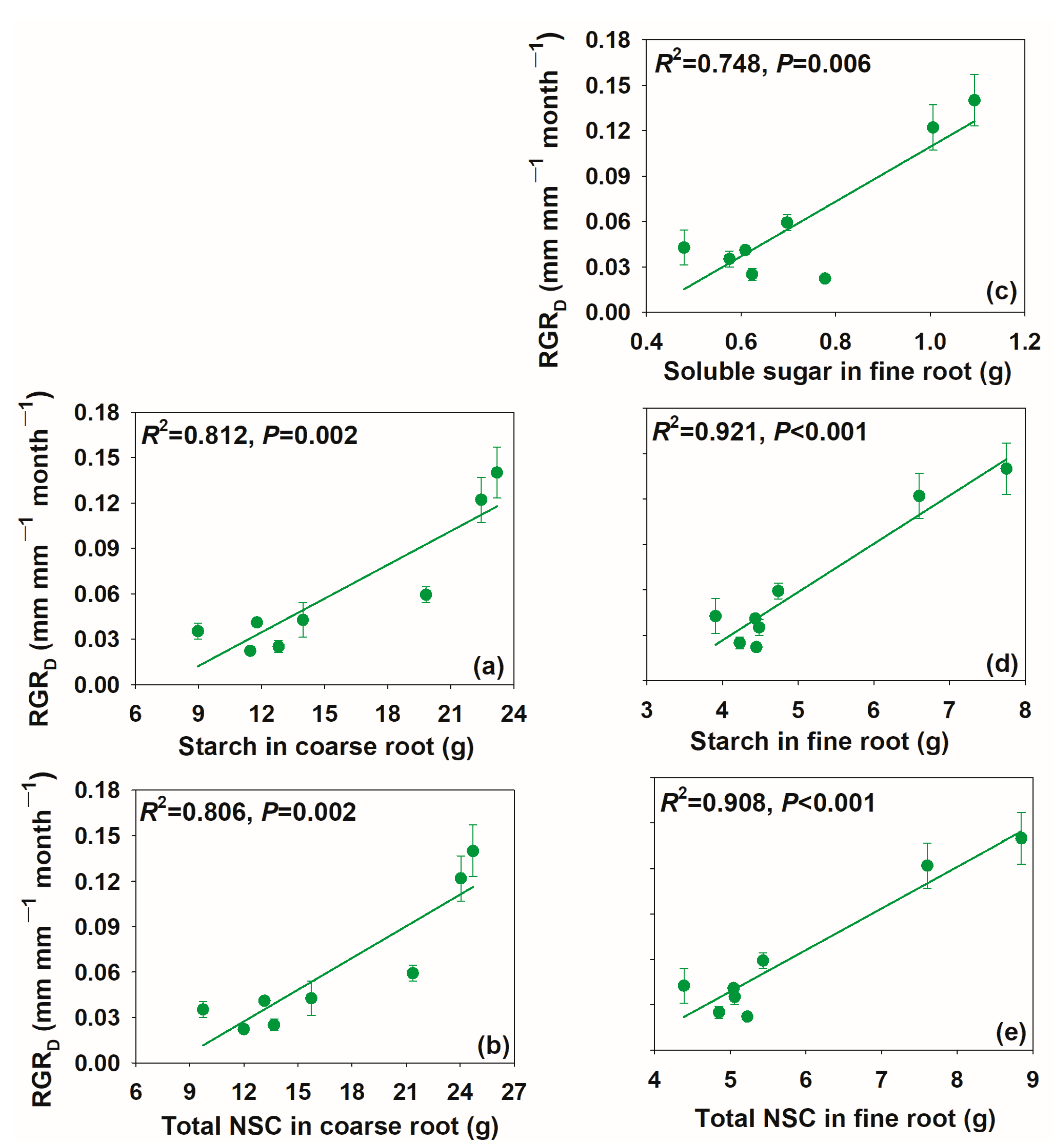
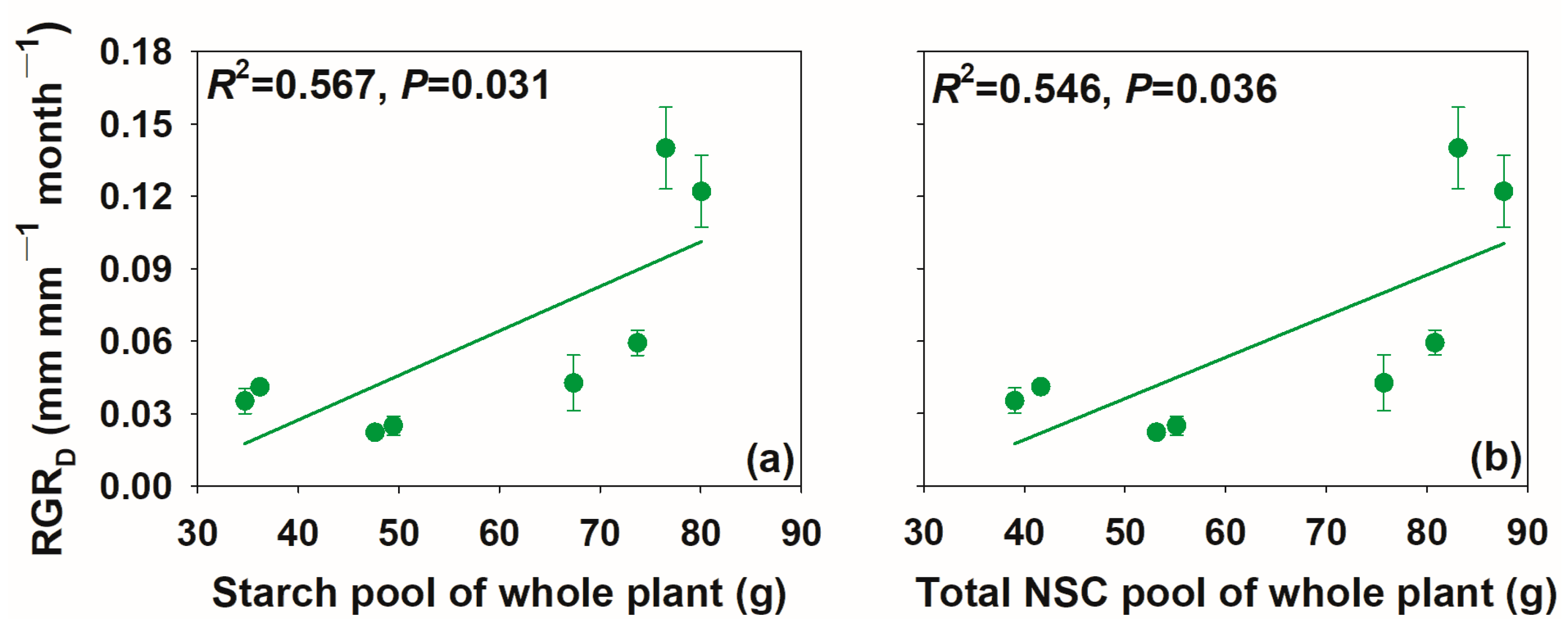
3.3. Relationships between Relative Growth Rate and Non-Structural Carbohydrate Concentrations and Pool Size
4. Discussion
4.1. Effects of Salt Stress on Relative Growth Rate, Leaf Mass Area, Midday Leaf Water Potential, Root-to-Shoot Ratio, and Photosynthetic-Related Traits of U. pumila
4.2. Variation Patterns of Non-Structural Carbohydrate Concentration Sof U. pumila in Response to Salt Stress
4.3. Relationships between Relative Growth Rate and Non-Structural Carbohydrate Concentrations and Pool Size
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Evelin, H.; Kapoor, R.; Giri, B. Arbuscular mycorrhizal fungi in alleviation of salt stress: A review. Ann. Bot. 2009, 104, 1263–1280. [Google Scholar] [CrossRef] [PubMed]
- Akramkhanov, A.; Martius, C.; Park, S.J.; Hendrickx, J.M.H. Environmental factors of spatial distribution of soil salinity on flat irrigated terrain. Geoderma 2011, 163, 55–62. [Google Scholar] [CrossRef]
- Cabot, C.; Sibole, J.V.; Barceló, J.; Poschenrieder, C. Lessons from crop plants struggling with salinity. Plant Sci. 2014, 226, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Munns, R. Comparative physiology of salt and water stress. Plant Cell Environ. 2002, 25, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Parida, A.K.; Das, A.B. Salt tolerance and salinity effects on plants: A review. Ecotoxicol. Environ. Safe 2005, 60, 324–349. [Google Scholar] [CrossRef] [PubMed]
- Polle, A.; Chen, S. On the salty side of life: Molecular, physiological and anatomical adaptation and acclimation of trees to extreme habitats. Plant Cell Environ. 2015, 38, 1794–1816. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhang, J.; Pei, D.; Yu, L. Combined effects of water stress and salinity on growth, physiological, and biochemical traits in two walnut genotypes. Physiol. Plant. 2021, 172, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Xia, J.; Shao, P.; Ma, J.; Gao, F.; Lang, Y.; Xing, X.; Dong, M.; Li, C. Response of the fine root morphological and chemical traits of Tamarix chinensis to water and salt changes in coastal wetlands of the Yellow River Delta. Front. Plant Sci. 2022, 13, 952830. [Google Scholar] [CrossRef]
- Yu, L.; Tang, S.; Guo, C.; Korpelainen, H.; Li, C. Differences in ecophysiological responses of Populus euphratica females and males exposed to salinity and alkali stress. Plant Physiol. Biochem. 2023, 198, 107707. [Google Scholar] [CrossRef]
- Dietze, M.C.; Sala, A.; Carbone, M.S.; Czimczik, C.I.; Mantooth, J.A.; Richardson, A.D.; Vargas, R. Nonstructural carbon in woody plants. Annu. Rev. Plant Biol. 2014, 65, 667–687. [Google Scholar] [CrossRef]
- Martínez-Vilalta, J.; Sala, A.; Asensio, M.D.; Galiano, L.; Hoch, G.; Palacio, S.; Piper, F.I.; Lloret, F. Dynamics of non-structural carbohydrates in terrestrial plants: A global synthesis. Ecol. Monogr. 2016, 86, 495–516. [Google Scholar] [CrossRef]
- Sala, A.; Piper, F.; Hoch, G. Physiological mechanisms of drought-induced tree mortality are far from being resolved. New Phytol. 2010, 186, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Hesse, B.D.; Goisser, M.; Hartmann, H.; Grams, T.E.E. Repeated summer drought delays sugar export from the leaf and impairs phloem transport in mature beech. Tree Physiol. 2019, 39, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Würth, M.K.R.; Peláez-Riedl, S.; Wright, S.J.; Körner, C.; 2005. Non-structural carbohydrate pools in a tropical forest. Oecologia 2005, 143, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Palacio, S.; Hoch, G.; Sala, A.; Korner, C.; Millard, P. Does carbon storage limit tree growth? New Phytol. 2014, 201, 1096–1100. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Lu, R.; Xia, J. Impacts of global environmental change drivers on non-structural carbohydrates in terrestrial plants. Funct. Ecol. 2020, 34, 1525–1536. [Google Scholar] [CrossRef]
- McDowell, N.G.; Pockman, W.T.; Allen, C.D.; Breshears, D.D.; Cobb, N.; Kolb, T.; Plaut, J.; Sperry, J.; West, A.; Williams, D.G.; et al. Mechanisms of plant survival and mortality during drought: Why do some plants survive while others succumb to drought? New Phytol. 2008, 178, 719–739. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, H.; Moura, C.F.; Anderegg, W.R.L.; Ruehr, N.K.; Salmon, Y.; Allen, C.D.; Arndt, S.K.; Breshears, D.D.; Davi, H.; Galbraith, D.; et al. Research frontiers for improving our understanding of drought-induced tree and forest mortality. New Phytol. 2018, 218, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Hoch, G.; Richter, A.; Korner, C. Non-structural carbon compounds in temperate forest trees. Plant Cell Environ. 2003, 26, 1067–1081. [Google Scholar] [CrossRef]
- Furze, M.E.; Wainwright, D.K.; Huggett, B.A.; Knipfer, T.; McElrone, A.J.; Brodersen, C.R. Ecologically driven selection of nonstructural carbohydrate storage in oak trees. New Phytol. 2021, 232, 567–578. [Google Scholar] [CrossRef]
- Sala, A.; Mencuccini, M. Ecosystem science: Plump trees win under drought. Nat. Clim. Chang. 2014, 4, 666–667. [Google Scholar] [CrossRef]
- Hartmann, H.; Ziegler, W.; Trumbore, S. Lethal drought leads to reduction in nonstructural carbohydrates in Norway spruce tree roots but not in the canopy. Funct. Ecol. 2013, 27, 413–427. [Google Scholar] [CrossRef]
- Li, W.; Hartmann, H.; Adams, H.D.; Zhang, H.; Jin, C.; Zhao, C.; Guan, D.; Wang, A.; Yuan, F.; Wu, J. The sweet side of global change-dynamic responses of non-structural carbohydrates to drought, elevated CO2 and nitrogen fertilization in tree species. Tree Physiol. 2018, 38, 1706–1723. [Google Scholar] [CrossRef] [PubMed]
- Galvez, D.A.; Landhausser, S.M.; Tyree, M.T. Root carbon reserve dynamics in aspen seedlings: Does simulated drought induce reserve limitation? Tree Physiol. 2011, 31, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Hagedorn, F.; Joseph, J.; Peter, M.; Luster, J.; Pritsch, K.; Geppert, U.; Kerner, R.; Molinier, V.; Egli, S.; Schaub, M.; et al. Recovery of trees from drought depends on belowground sink control. Nat. Plants 2016, 2, 16111. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Cao, Y.; Chen, Y.; Liu, G. Non-structural carbohydrate dynamics in Robinia pseudoacacia saplings under three levels of continuous drought stress. Trees 2015, 29, 1837–1849. [Google Scholar] [CrossRef]
- Braga, N.D.; Vitória, A.P.; Souza, G.M.; Barros, C.F.; Freitas, L. Weak relationships between leaf phenology and isohydric and anisohydric behavior in lowland wet tropical forest trees. Biotropica 2016, 48, 453–464. [Google Scholar] [CrossRef]
- Körner, C. Carbon limitation in trees. J. Ecol. 2003, 91, 4–17. [Google Scholar] [CrossRef]
- McDowell, N.G.; Beerling, D.J.; Breshears, D.D.; Fisher, R.A.; Raffa, K.F.; Stitt, M. The interdependence of mechanisms underlying climate-driven vegetation mortality. Trends Ecol. Evol. 2011, 26, 523–532. [Google Scholar] [CrossRef]
- Hoch, G. Carbon reserves as indicators for carbon limitation in trees. In Progress in Botany Progress in Botany (Genetics—Physiology—Systematics—Ecology); Lüttge, U., Beyschlag, W., Eds.; Springer: Cham, Switzerland; Berlin, Germany, 2015; pp. 321–346. [Google Scholar]
- He, W.; Liu, H.; Qi, Y.; Liu, F.; Zhu, X. Patterns in nonstructural carbohydrate contents at the tree organ level in response to drought duration. Glob. Chang. Biol. 2020, 26, 3627–3638. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Ann. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [PubMed]
- Chaves, M.M.; Flexas, J.; Pinheiro, C. Photosynthesis under drought and salt stress: Regulation mechanisms from whole plant to cell. Ann. Bot. 2009, 103, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Amtmann, A.; Armengaud, P.; Volkov, V. Potassium Nutrition and Salt Stress; Blacdkwell Publishing: Oxford, UK, 2004. [Google Scholar]
- Sala, A.; Woodruff, D.R.; Meinzer, F.C. Carbon dynamics in trees: Feast or famine? Tree Physiol. 2012, 32, 764–775. [Google Scholar] [CrossRef] [PubMed]
- Piper, F.I.; Fajardo, A. Carbon dynamics of Acer pseudoplatanus seedlings under drought and complete darkness. Tree Physiol. 2016, 36, 1400–1408. [Google Scholar] [CrossRef] [PubMed]
- Piper, F.I.; Moreno-Meynard, P.; Fajardo, A. Nonstructural carbohydrates predict survival in saplings of temperate trees under carbon stress. Funct. Ecol. 2022, 36, 2806–2818. [Google Scholar] [CrossRef]
- McDowell, N.G. Mechanisms linking drought, hydraulics, carbon metabolism, and vegetation mortality. Plant Physiol. 2011, 155, 1051–1059. [Google Scholar] [CrossRef] [PubMed]
- Wiley, E.; Helliker, B. A re-evaluation of carbon storage in trees lends greater support for carbon limitation to growth. New Phytol. 2012, 195, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Bahamonde, C.; Piper, F.I.; Cavieres, L.A. Carbon allocation to growth and storage depends on elevation provenance in an herbaceous alpine plant of Mediterranean climate. Oecologia 2021, 195, 299–312. [Google Scholar] [CrossRef] [PubMed]
- Myers, J.A.; Kitajima, K. Carbohydrate storage enhances seedling shade and stress tolerance in a neotropical forest. J. Ecol. 2007, 95, 383–395. [Google Scholar] [CrossRef]
- Poorter, L.; Kitajima, K. Carbohydrate storage and light requirements of tropical moist and dry forest tree species. Ecology 2007, 88, 1000–1011. [Google Scholar] [CrossRef]
- Piper, F.I. Decoupling between growth rate and storage remobilization in broadleaf temperate tree species. Funct. Ecol. 2020, 34, 1180–1192. [Google Scholar] [CrossRef]
- Genet, H.; Breda, N.; Dufrene, E. Age-related variation in carbon allocation at tree and stand scales in beech (Fagus sylvatica L.) and sessile oak (Quercus petraea (Matt.) Liebl.) using a chronosequence approach. Tree Physiol. 2010, 30, 177–192. [Google Scholar] [CrossRef]
- Jiang, D.; Tang, Y.; Busso, C.A. Effects of vegetation cover on recruitment of Ulmus pumila L. in Horqin Sandy land, northeastern China. J. Arid Land 2014, 6, 343–351. [Google Scholar] [CrossRef]
- Amorós, M.D.; Mauri, P.V.; Curt, M.D. The influence of tree management practices on phenological growth stages of Ulmus pumila L. (Siberian elm). Ann. Appl. Biol. 2021, 179, 259–272. [Google Scholar] [CrossRef]
- Guo, J.; Xu, X.; Zhang, R.; Chen, X.; Xing, Y.; Li, B.; Liu, C.; Zhou, Y. Effect of short-term combined alkaline stress on antioxidant metabolism, photosynthesis, and leaf-air temperature difference in sorghum. Photosynthetica 2022, 60, 200–211. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, J.; Zhen, W.; Sun, T.; Hu, X. Abscisic acid alleviates harmful effect of saline-alkaline stress on tomato seedlings. Plant Physiol. Biochem. 2022, 175, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Zuo, L.; Zhang, S.; Zhang, J.; Liu, Y.; Yu, X.; Yang, M.; Wang, J. Primer development and functional classification of EST-SSR markers in Ulmus species. Tree Genet. Genomes 2020, 16, 74. [Google Scholar] [CrossRef]
- Qi, X.; Chen, L.; Hu, Z.; Shen, W.; Xu, H.; Ma, L.; Wang, G.; Jing, Y.; Wang, X.; Zhang, B.; et al. Cytology, transcriptomics, and mass spectrometry imaging reveal changes in late-maturation elm (Ulmus pumila) seeds. J. Plant Physiol. 2022, 271, 153639. [Google Scholar] [CrossRef]
- Zhang, L.; Xie, S.; Yang, C.; Cao, D.; Fan, S.; Zhang, X. Comparative transcriptome analysis reveals candidate genes and pathways for potential branch growth in elm (Ulmus pumila) cultivars. Biology 2022, 11, 711. [Google Scholar] [CrossRef]
- Carlson, C.A.; Burkhart, H.E.; Allen, H.L.; Fox, T.R. Absolute and relative changes in tree growth rates and changes to the stand diameter distribution of Pinus taeda as a result of midrotation fertilizer applications. Can. J. For. Res. 2008, 38, 2063–2071. [Google Scholar] [CrossRef]
- Seibt, U.; Rajabi, A.; Griffiths, H.; Berry, J.A. Carbon isotopes and water use efficiency: Sense and sensitivity. Oecologia 2008, 155, 441–454. [Google Scholar] [CrossRef]
- Furze, M.E.; Huggett, B.A.; Aubrecht, D.M.; Stolz, C.D.; Carbone, M.S.; Richardson, A.D. Whole-tree nonstructural carbohydrate storage and seasonal dynamics in five temperate species. New Phytol. 2019, 221, 1466–1477. [Google Scholar] [CrossRef] [PubMed]
- Ladjal, M.; Huc, R.; Ducrey, M. Drought effects on hydraulic conductivity and xylem vulnerability to embolism in diverse species and provenances of Mediterranean cedars. Tree Physiol. 2005, 25, 1109–1117. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nardini, A.; Battistuzzo, M.; Savi, T. Shoot desiccation and hydraulic failure in temperate woody angiosperms during an extreme summer drought. New Phytol. 2013, 200, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Wright, I.J.; Reich, P.B.; Cornelissen, J.H.C.; Falster, D.S.; Groom, P.K.; Hikosaka, K.; Lee, W.; Lusk, C.H.; Niinemets, U.; Oleksyn, J. Modulation of leaf economic traits and trait relationships by climate. Glob. Ecol. Biogeogr. 2005, 14, 411–421. [Google Scholar] [CrossRef]
- Markesteijn, L.; Poorter, L.; Paz, H.; Sack, L.; Paz, H.; Bongers, L. Ecological differentiation in xylem cavitation resistance is associated with stem and leaf structural traits. Plant Cell Environ. 2011, 34, 137–148. [Google Scholar] [CrossRef]
- Reich, P.B. The world-wide ‘fast-slow’ plant economics spectrum: A traits manifesto. J. Ecol. 2014, 102, 275–301. [Google Scholar] [CrossRef]
- Niinemets, Ü. Does the touch of cold make evergreen leaves tougher? Tree Physiol. 2016, 36, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Sancho-Knapik, D.; Escudero, A.; Mediavilla, S.; Scoffoni, C.; Zailaa, J.; Cavender-Bares, J.; Alvarez-Arenas, T.G.; Molins, A.; Alonso-Forn, D.; Ferrio, J.P.; et al. Deciduous and evergreen oaks show contrasting adaptive responses in leaf mass per area across environments. New Phytol. 2021, 230, 521–534. [Google Scholar] [CrossRef]
- Yu, L.; Huang, Z.; Li, Z.; Korpelainen, H.; Li, C. Sex-specific strategies of nutrient resorption associated with leaf economics in Populus euphratica. J. Ecol. 2022, 110, 2062–2073. [Google Scholar] [CrossRef]
- Moser, B.; Kipfer, T.; Richter, S.; Egli, S.; Wohlgemuth, T. Drought resistance of Pinus sylvestris seedlings conferred by plastic root architecture rather than ectomycorrhizal colonization. Ann. For. Sci. 2015, 72, 303–309. [Google Scholar] [CrossRef]
- Zhou, G.; Zhou, X.; He, Y.; Shao, J.; Hu, Z.; Liu, R.; Zhou, H.; Hosseinibai, S. Grazing intensity significantly affects belowground carbon and nitrogen cycling in grassland ecosystems: A meta-analysis. Glob. Chang. Biol. 2017, 23, 1167–1179. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Zhou, X.; Nie, Y.; Bai, S.; Zhou, L.; Shao, J.; Cheng, W.; Wang, J.; Hu, F.; Fu, Y. Drought-induced changes in root biomass largely result from altered root morphological traits: Evidence from a synthesis of global field trials. Plant Cell Environ. 2018, 41, 2589–2599. [Google Scholar] [CrossRef] [PubMed]
- Cimato, A.; Castelli, S.; Tattini, M.; Traversi, M.L. An ecophysiological analysis of salinity tolerance in olive. Environ. Exp. Bot. 2010, 68, 214–221. [Google Scholar] [CrossRef]
- Singh, J.; Sastry, E.V.D.; Singh, V. Effect of salinity on tomato (Lycopersicon esculentum Mill.) during seed germination stage. Physiol. Mol. Biol. Pla. 2012, 18, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, H.; Yang, M.; Du, L. No evidence for local adaptation to salt stress in the existing populations of invasive Solidago canadensis in China. PLoS ONE 2012, 12, e0175252. [Google Scholar] [CrossRef] [PubMed]
- Dasgan, H.Y.; Aktas, H.; Abak, K.; Cakmak, I. Determination of screening techniques to salinity tolerance in tomatoes and investigation of genotype responses. Plant Sci. 2002, 163, 695–703. [Google Scholar] [CrossRef]
- Li, X.; Wan, S.; Kang, Y.; Chen, X.; Chu, L. Chinese rose (Rosa chinensis) growth and ion accumulation under irrigation with waters of different salt contents. Agric. Water Manag. 2016, 163, 180–189. [Google Scholar] [CrossRef]
- Koyro, H.W. Effect of salinity on growth, photosynthesis, water relations and solute composition of the potential cash crop halophyte Plantago coronopus L. Environ. Exp. Bot. 2006, 56, 136–146. [Google Scholar] [CrossRef]
- Jacqueline, S.; Kay, M.; Paul, C.E.; Boon, P.I. Interactive effects of salinity and water depth on the growth of Melaleuca ericifolia Sm. (Swamp paperbark) seedlings. Aquat. Bot. 2006, 86, 213–222. [Google Scholar] [CrossRef]
- Ma, Y.; Su, B.; Han, Y.; Wu, X.; Zhou, W.; Wang, Q.; Zhou, L.; Yu, D. Response of photosynthetic characteristics and non-structural carbohydrate accumulation of Betula ermanii seedlings to drought stress. Chin. J. Appl. Ecol. 2021, 32, 513–520, (In Chinese with English Abstract). [Google Scholar] [CrossRef]
- Hartmann, H.; Ziegler, W.; Kolle, O.; Trumbore, S. Thirst beats hunger-declining hydration during drought prevents carbon starvation in Norway spruce saplings. New Phytol. 2013, 200, 340–349. [Google Scholar] [CrossRef] [PubMed]
- Sala, A.; Hoch, G. Height-related growth declines in ponderosa pine are not due to carbon limitation. Plant Cell Environ. 2009, 32, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Fajardo, A.; Piper, F.I. An experimental approach to explain the southern Andes elevational treeline. Am. J. Bot. 2014, 101, 788–795. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Shi, H.; He, R.; Liu, H.; Zhu, W.; Yu, D.; Zhang, Q.; Dang, H. Prioritized carbon allocation to storage of different functional types of species at the upper range limits is driven by different environmental drivers. Sci. Total Environ. 2021, 773, 145581. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, H.; Trumbore, S. Understanding the roles of nonstructural carbohydrates in forest trees-from what we can measure to what we want to know. New Phytol. 2016, 211, 386–403. [Google Scholar] [CrossRef]
- Guo, Q.; Wu, X.; Korpelainen, H.; Li, C. Stronger intraspecific competition aggravates negative effects of drought on the growth of Cunninghamia lanceolata. Environ. Exp. Bot. 2020, 175, 104042. [Google Scholar] [CrossRef]
- O’Brien, M.J.; Leuzinger, S.; Philipson, C.D.; Tay, J.; Hector, A. Drought survival of tropical tree seedlings enhanced by nonstructural carbohydrate levels. Nat. Clim. Chang. 2014, 4, 710–714. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, C. Mechanisms of carbon source sink limitations to tree growth. Chin. J. Plant Ecol. 2019, 43, 1036–1047, (In Chinese with English Abstract). [Google Scholar] [CrossRef]
- Lin, X.; Liu, L.; Dong, T.; Fang, Q.; Guo, Q. Effects of non-structural carbohydrate and nitrogen allocation on the ability of Populus deltoides and P. cathayana to resist soil salinity stress. Chin. J. Plant Ecol. 2021, 45, 961–971, (In Chinese with English Abstract). [Google Scholar] [CrossRef]
- Silva, E.N.; Ferrira-Silva, S.L.; Viegas, R.A.; Viegas, R.A.; Silveira, J.A.G. The role of organic and inorganic solutes in the osmotic adjustment of drought-stressed Jatropha curcas plants. Environ. Exp. Bot. 2010, 69, 279–285. [Google Scholar] [CrossRef]
- Brunner, I.; Herzog, C.; Dawes, M.A.; Arend, M.; Sperisen, C. How tree roots respond to drought. Front. Plant Sci. 2015, 6, 547. [Google Scholar] [CrossRef]
- Smeekens, S. Sugar-induced signal transduction in plants. Annu. Rev. Plant Biol. 2000, 51, 49–81. [Google Scholar] [CrossRef] [PubMed]
- Nardini, A.; Casolo, V.; Dal Borgo, A.; Savi, T.; Stenni, B.; Bertoncin, P.; Zini, L.; McDowell, N.G. Rooting depth, water relations and non-structural carbohydrate dynamics in three woody angiosperms differentially affected by an extreme summer drought. Plant Cell Environ. 2015, 39, 618–627. [Google Scholar] [CrossRef] [PubMed]
- Trifilò, P.; Kiorapostolou, N.; Petruzzellis, F.; Vitti, S.; Petit, G.; Lo Gullo, M.A.; Nardini, A.; Casolo, V. Hydraulic recovery from xylem embolism in excised branches of twelve woody species: Relationships with parenchyma cells and non-structural carbohydrates. Plant Physiol. Bioch. 2019, 139, 513–520. [Google Scholar] [CrossRef]
- Tomasella, M.; Petrussa, E.; Petruzzellis, F.; Nardini, A.; Casolo, V. The possible role of non-structural carbohydrates in the regulation of tree hydraulics. Int. J. Mol. Sci. 2020, 21, 144. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Calcerrada, J.; Li, M.; López, R.; Cano, F.J.; Oleksyn, J.; Atkin, O.K.; Pita, P.; Aranda, I.; Gil, L. Drought-induced shoot dieback starts with massive root xylem embolism and variable depletion of nonstructural carbohydrates in seedlings of two tree species. New Phytol. 2017, 213, 597–610. [Google Scholar] [CrossRef] [PubMed]
- Shibata, R.; Kurokawa, H.; Shibata, M.; Tanaka, H.; Iida, S.; Masaki, T.; Nakashizuka, T. Relationships between resprouting ability, species traits and resource allocation patterns in woody species in a temperate forest. Funct. Ecol. 2016, 30, 1205–1215. [Google Scholar] [CrossRef]
- Santos, M.; Barros, V.; Lima, L.; Frosi, G.; Santos, M.G. Whole plant water status and non-structural carbohydrates under progressive drought in a Caatinga deciduous woody species. Trees 2021, 35, 1257–1266. [Google Scholar] [CrossRef]
- Imaji, A.; Seiwa, K. Carbon allocation to defense, storage, and growth in seedlings of two temperate broad-leaved tree species. Oecologia 2010, 162, 273–281. [Google Scholar] [CrossRef]
- Wyka, T.P.; Karolewski, P.; Żytkowiak, R.; Chmielarz, P.; Oleksyn, J. Whole-plant allocation to storage and defense in juveniles of related evergreen and deciduous shrub species. Tree Physiol. 2016, 36, 536–547. [Google Scholar] [CrossRef] [PubMed]
- Chapin, F.S., III; Schulze, E.; Mooney, H.A. The ecology and economics of storage in plants. Annu. Rev. Ecol. Syst. 1990, 21, 423–447. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, P.; Yang, C.; Zhang, X.; Tong, B.; Xie, X.; Li, X.; Fan, S. The Growth and Non-Structural Carbohydrate Response Patterns of Siberian Elm (Ulmus pumila) under Salt Stress with Different Intensities and Durations. Forests 2024, 15, 1004. https://doi.org/10.3390/f15061004
Jiang P, Yang C, Zhang X, Tong B, Xie X, Li X, Fan S. The Growth and Non-Structural Carbohydrate Response Patterns of Siberian Elm (Ulmus pumila) under Salt Stress with Different Intensities and Durations. Forests. 2024; 15(6):1004. https://doi.org/10.3390/f15061004
Chicago/Turabian StyleJiang, Peipei, Cheng Yang, Xuejie Zhang, Boqiang Tong, Xiaoman Xie, Xianzhong Li, and Shoujin Fan. 2024. "The Growth and Non-Structural Carbohydrate Response Patterns of Siberian Elm (Ulmus pumila) under Salt Stress with Different Intensities and Durations" Forests 15, no. 6: 1004. https://doi.org/10.3390/f15061004
APA StyleJiang, P., Yang, C., Zhang, X., Tong, B., Xie, X., Li, X., & Fan, S. (2024). The Growth and Non-Structural Carbohydrate Response Patterns of Siberian Elm (Ulmus pumila) under Salt Stress with Different Intensities and Durations. Forests, 15(6), 1004. https://doi.org/10.3390/f15061004




