Differentiation in Leaf Functional Traits and Driving Factors of the Allopatric Distribution of Tetraploid and Octaploid Buddleja macrostachya in the Sino-Himalayan Region
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Study Sites
2.2. Leaf Morphological Traits
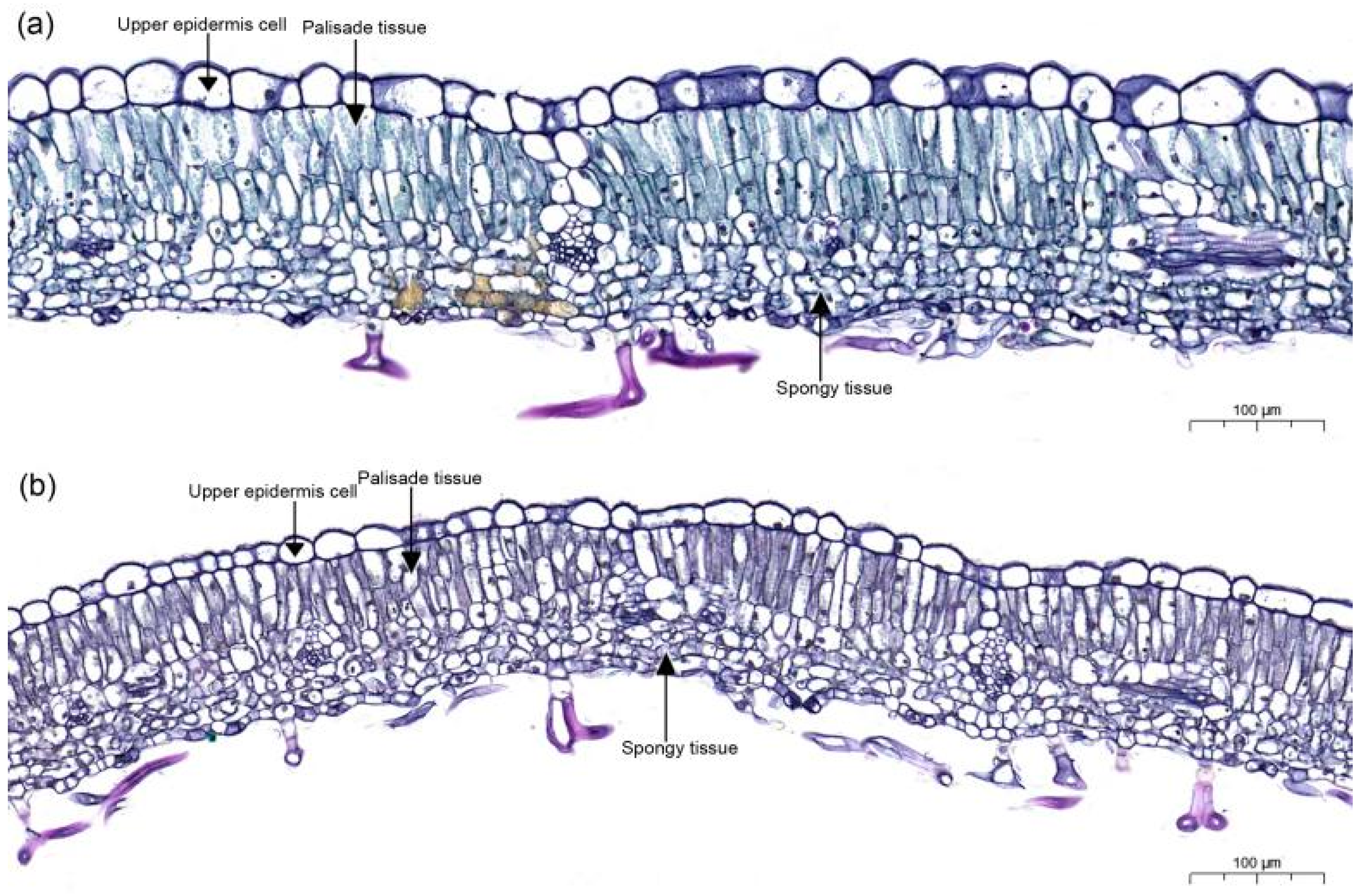
2.3. Leaf Anatomical Structure
2.4. Leaf Gas Exchange
2.5. Leaf Macroelements and Soil C:N:P Stoichiometry
2.6. Statistical Analysis
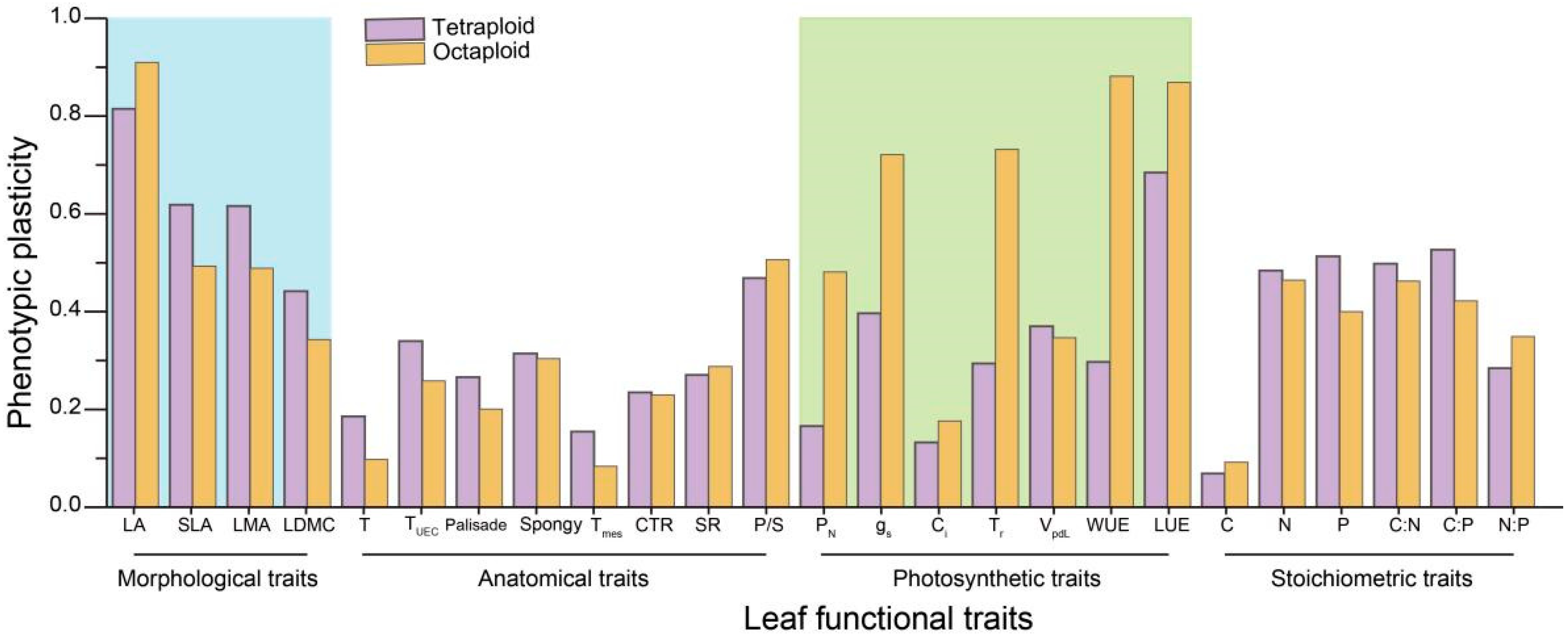
2.6.1. Phenotypic Plasticity between Tetraploid and Octaploid B. macrostachya
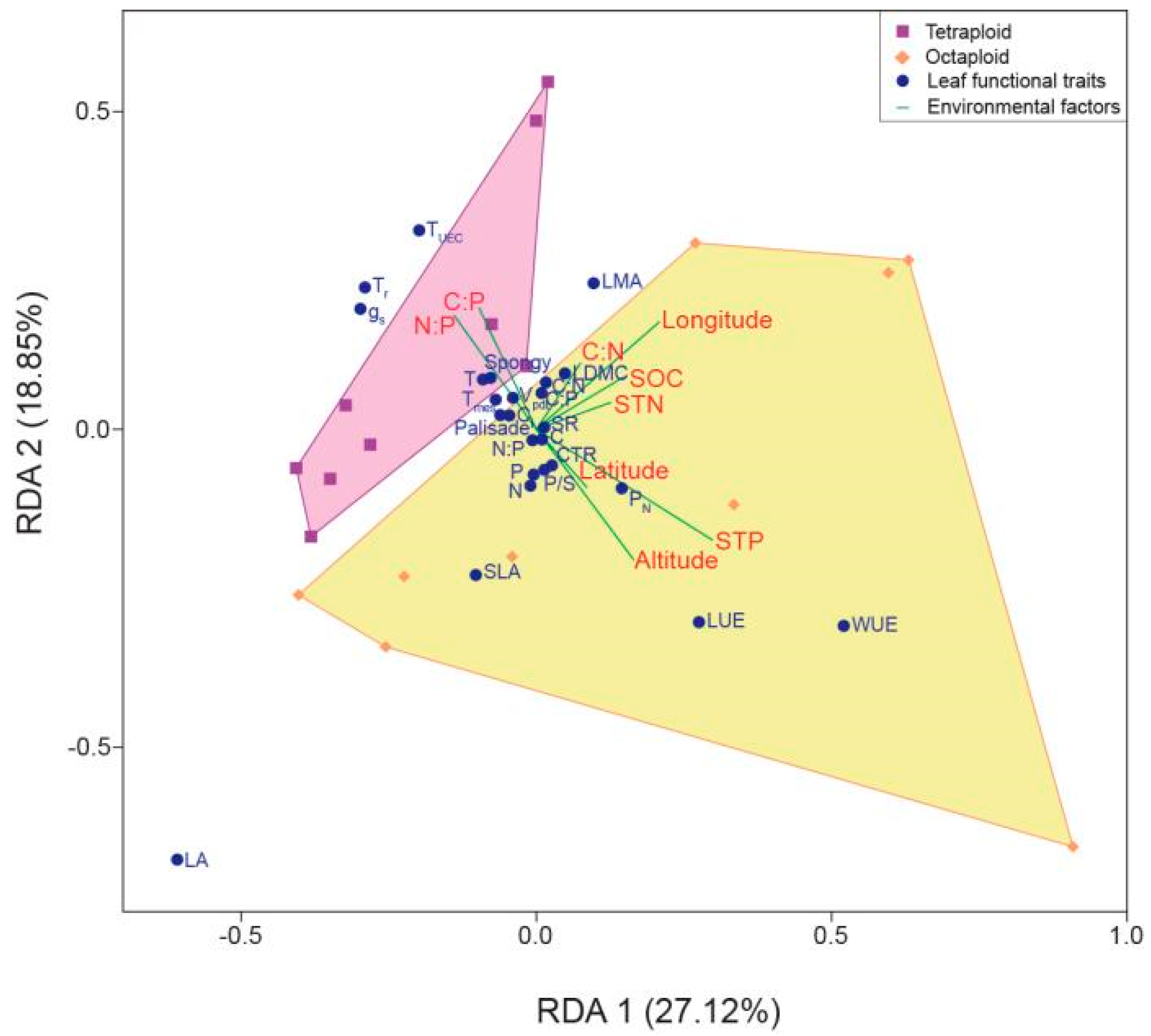
2.6.2. Correlation between Leaf Functional Traits and Environmental Factors within the B. macrostachya Complex
3. Results
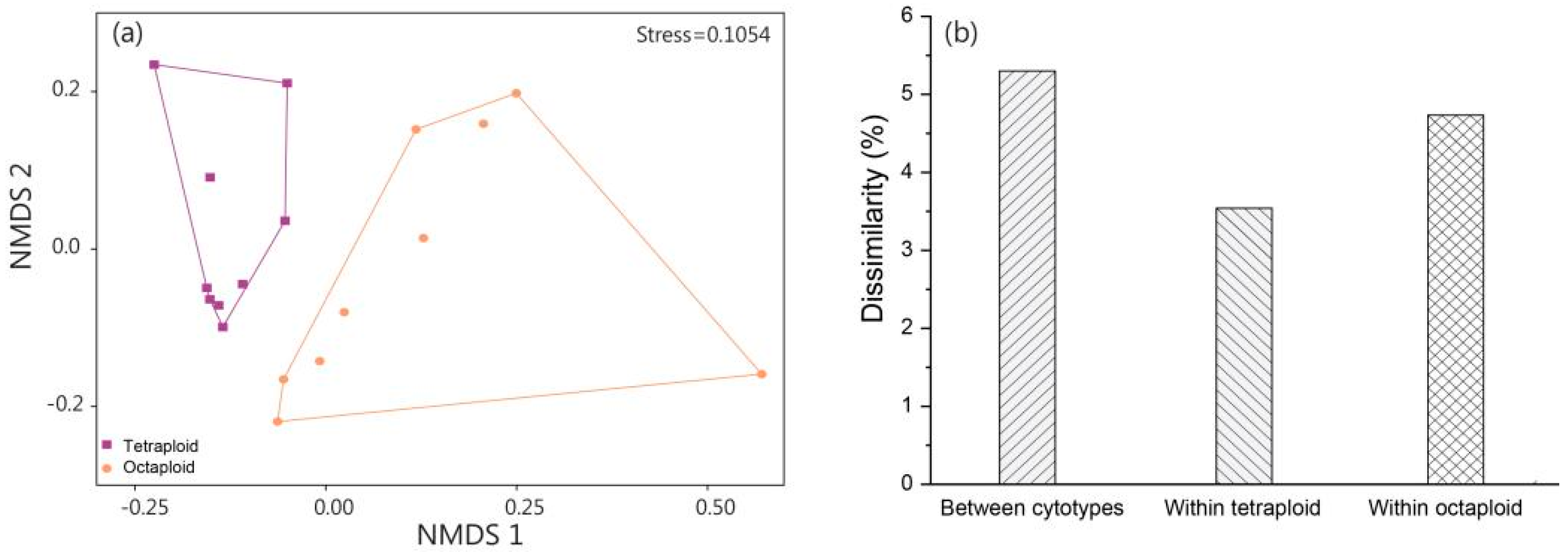
3.1. Comparison of Leaf Functional Traits between Tetraploid and Octaploid B. macrostachya
3.2. Comparison of Soil Stoichiometry in Tetraploid and Octaploid B. macrostachya
3.3. Relationship between Habitat Environmental Factors and Leaf Functional Traits within the B. macrostachya Complex
4. Discussion
4.1. Phenotypic Differentiation in Tetraploid and Octaploid B. macrostachya
4.2. Phenotypic Plasticity Enhances Species Adaptability within the B. macrostachya Complex
4.3. Abiotic Environmental Factors Influence Geographic Differentiation within the B. macrostachya Complex
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Cao, Q.; Zhang, X.; Gao, X.; Wang, L.; Jia, G. Effects of Ploidy Level on the Cellular, Photochemical and Photosynthetic Characteristics in Lilium FO Hybrids. Plant Physiol. Biochem. 2018, 133, 50–56. [Google Scholar] [CrossRef]
- Van de Peer, Y.; Mizrachi, E.; Marchal, K. The Evolutionary Significance of Polyploidy. Nat. Rev. Genet. 2017, 18, 411–424. [Google Scholar] [CrossRef]
- Palacio-López, K.; Beckage, B.; Scheiner, S.; Molofsky, J. The Ubiquity of Phenotypic Plasticity in Plants: A Synthesis. Ecol. Evol. 2015, 5, 3389–3400. [Google Scholar] [CrossRef]
- Guignard, M.S.; Nichols, R.A.; Knell, R.J.; Macdonald, A.; Romila, C.A.; Trimmer, M.; Leitch, I.J.; Leitch, A.R. Genome Size and Ploidy Influence Angiosperm Species’ Biomass Under Nitrogen and Phosphorus Limitation. New Phytol. 2016, 210, 1195–1206. [Google Scholar] [CrossRef]
- Wei, N.; Cronn, R.; Liston, A.; Ashman, T.L. Functional Trait Divergence and Trait Plasticity Confer Polyploid Advantage in Heterogeneous Environments. New Phytol. 2019, 221, 2286–2297. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, B.; Qi, S.; Dong, M.; Wang, Z.; Li, Y.; Chen, S.; Li, B.L.; Zhang, J. Ploidy and Hybridity Effects on Leaf Size, Cell Size and Related Genes Expression in Triploids, Diploids and Their Parents in Populus. Planta 2019, 249, 635–646. [Google Scholar] [CrossRef]
- Wang, L.J.; Cao, Q.Z.; Zhang, X.Q.; Jia, G. Effects of Polyploidization on Photosynthetic Characteristics in Three Lilium Species. Sci. Hortic. 2021, 2284, 110098. [Google Scholar] [CrossRef]
- López-Jurado, J.; Mateos-Naranjo, E.; Balao, F. Polyploidy Promotes Divergent Evolution Across the Leaf Economics Spectrum and Plant Edaphic Niche in the Dianthus broteri Complex. J. Ecol. 2022, 110, 605–618. [Google Scholar] [CrossRef]
- Alix, K.; Gérard, P.R.; Schwarzacher, T.; Heslop-Harrison, J.S. Polyploidy and Interspecific Hybridization: Partners for Adaptation, Speciation and Evolution in Plants. Ann. Bot. 2017, 120, 183–194. [Google Scholar] [CrossRef]
- Baker, R.L.; Yarkhunova, Y.; Vidal, K.; Ewers, B.E.; Weinig, C. Polyploidy and the Relationship between Leaf Structure and Function: Implications for Correlated Evolution of Anatomy, Morphology, and Physiology in Brassica. BMC Plant Biol. 2017, 17, 3. [Google Scholar] [CrossRef]
- Violle, C.; Navas, M.; Vile, D.; Kazakou, E.; Fortunel, C.; Hummel, I.; Garnier, E. Let the Concept of Trait be Functional! Oikos 2007, 116, 882–892. [Google Scholar] [CrossRef]
- Chen, G.; Sun, W.B.; Sun, H. Morphological Characteristics of Leaf Epidermis and Size Variation of Leaf, Flower and Fruit in Different Ploidy Levels in Buddleja macrostachya (Buddlejaceae). J. Syst. Evol. 2009, 47, 231–236. [Google Scholar] [CrossRef]
- Hulshof, C.M.; Swenson, N.G. Variation in Leaf Functional Trait Values within and Across Individuals and Species: An Example from a Costa Rican Dry Forest. Funct. Ecol. 2010, 24, 217–223. [Google Scholar] [CrossRef]
- Blonder, B.; Violle, C.; Enquist, B.J. Assessing the Causes and Scales of the Leaf Economics Spectrum Using Venation Networks in Populus tremuloides. J. Ecol. 2013, 101, 981–989. [Google Scholar] [CrossRef]
- Onoda, Y.; Wright, I.J.; Evans, J.R.; Hikosaka, K.; Kitajima, K.; Niinemets, Ü.; Poorter, H.; Tosens, T.; Westoby, M. Physiological and Structural Tradeoffs Underlying the Leaf Economics Spectrum. New Phytol. 2017, 214, 1447–1463. [Google Scholar] [CrossRef]
- Liu, Y.; Dawson, W.; Prati, D.; Haeuser, E.; Feng, Y.; van Kleunen, M. Does Greater Specific Leaf Area Plasticity Help Plants to Maintain a High Performance When Shaded? Ann. Bot. 2016, 118, 1329–1336. [Google Scholar] [CrossRef]
- Yao, X.; Li, C.; Li, S.; Zhu, Q.; Zhang, H.; Wang, H.; Yu, C.; Martin, S.K.S.; Xie, F. Effect of Shade on Leaf Photosynthetic Capacity, Light-intercepting, Electron Transfer and Energy Distribution of Soybeans. Plant Growth Regul. 2017, 83, 409–416. [Google Scholar] [CrossRef]
- Scoffoni, C.; Rawls, M.; McKown, A.; Cochard, H.; Sack, L. Decline of Leaf Hydraulic Conductance with Dehydration: Relationship to Leaf Size and Venation Architecture. Plant Physiol. 2011, 156, 832–843. [Google Scholar] [CrossRef]
- Fan, Z.X.; Sterck, F.; Zhang, S.B.; Fu, P.L.; Hao, G.Y. Tradeoff between Stem Hydraulic Efficiency and Mechanical Strength Affects Leaf–stem Allometry in 28 Ficus Tree Species. Front. Plant Sci. 2017, 8, 1619. [Google Scholar] [CrossRef]
- Wright, I.J.; Reich, P.B.; Westoby, M.; Ackerly, D.D.; Baruch, Z.; Bongers, F.; Cavender-Bares, J.; Chapin, T.; Cornelissen, J.H.; Diemer, M.; et al. The Worldwide Leaf Economics Spectrum. Nature 2004, 428, 821–827. [Google Scholar] [CrossRef]
- Pritzkow, C.; Szota, C.; Williamson, V.G.; Arndt, S.K. Phenotypic Plasticity of Drought Tolerance Traits in a Widespread Eucalypt (Eucalyptus obliqua). Forests 2020, 11, 1371. [Google Scholar] [CrossRef]
- Grether, G.F. Environmental Change, Phenotypic Plasticity, and Genetic Compensation. Am. Nat. 2005, 166, E115–E123. [Google Scholar] [CrossRef]
- Reich, P.B. The World-wide ‘Fast–Slow’ Plant Economics Spectrum: A Traits Manifesto. J. Ecol. 2014, 102, 275–301. [Google Scholar] [CrossRef]
- Chen, G.; Sun, W.B.; Sun, H. Ploidy Variation in Buddleja L. (Buddlejaceae) in the Sino-Himalayan Region and Its Biogeographical Implications. Bot. J. Linn. Soc. 2007, 154, 305–312. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, S.; Meng, L.Z.; Wang, S.; Chen, Y.; Gong, W. Ploidy Differentiation and Floral Scent Divergence in Buddleja macrostachya (Scrophulariaceae) Complex. Biochem. Syst. Ecol. 2021, 96, 104271. [Google Scholar] [CrossRef]
- McKown, A.D.; Guy, R.D.; Klápště, J.; Geraldes, A.; Friedmann, M.; Cronk, Q.C.; El-Kassaby, Y.A.; Mansfield, S.D.; Douglas, C.J. Geographical and Environmental Gradients shape Phenotypic Trait Variation and Genetic Structure in Populus trichocarpa. New Phytol. 2014, 201, 1263–1276. [Google Scholar] [CrossRef]
- Hu, Y.K.; Zhang, Y.L.; Liu, G.F.; Pan, X.; Yang, X.; Li, W.B.; Dai, W.H.; Tang, S.L.; Tao, X.; Chen, L.Y.; et al. Intraspecific N and P Stoichiometry of Phragmites australis: Geographic Patterns and Variation Among Climatic Regions. Sci. Rep. 2017, 7, 43018. [Google Scholar] [CrossRef]
- Osnas, J.L.; Katabuchi, M.; Kitajima, K.; Wright, S.J.; Reich, P.B.; Van Bael, S.A.; Kraft, N.J.; Samaniego, M.J.; Pacala, S.W.; Lichstein, J.W. Divergent Drivers of Leaf Trait Variation within Species, Among Species, and Among Functional Groups. Proc. Natl. Acad. Sci. USA 2018, 115, 5480–5485. [Google Scholar] [CrossRef]
- Midolo, G.; De Frenne, P.; Hölzel, N.; Wellstein, C. Global Patterns of Intraspecific Leaf Trait Responses to Elevation. Global Chang. Biol. 2019, 25, 2485–2498. [Google Scholar] [CrossRef]
- Akram, M.A.; Wang, X.; Shrestha, N.; Zhang, Y.; Sun, Y.; Yao, S.; Li, J.; Hou, Q.; Hu, W.; Ran, J.; et al. Variations and Driving Factors of Leaf Functional Traits in the Dominant Desert Plant Species Along an Environmental Gradient in the Drylands of China. Sci. Total Environ. 2023, 897, 165394. [Google Scholar] [CrossRef]
- Liu, W.S.; Li, Z.; Danhui, Q. Variation in Leaf Traits at Different Altitudes Reflects the Adaptive Strategy of Plants to Environmental Changes. Ecol. Evol. 2020, 10, 8166–8175. [Google Scholar] [CrossRef]
- Smart, S.M.; Glanville, H.C.; Blanes, M.C.; Mercado, L.M.; Emmett, B.A.; Jones, D.L.; Cosby, B.; Marrs, R.H.; Butler, A.B.; Marshall, M.R.; et al. Leaf Dry Matter Content is Better at Predicting Above-ground Net Primary Production Than Specific Leaf Area. Funct. Ecol. 2017, 31, 1336–1344. [Google Scholar] [CrossRef]
- Liu, X.; Chen, H.; Sun, T.; Li, D.; Wang, X.; Mo, W.; Wang, R.; Zhang, S. Variation in Woody Leaf Anatomical Traits Along the Altitudinal Gradient in Taibai Mountain, China. Glob. Ecol. Conserv. 2021, 26, e01523. [Google Scholar] [CrossRef]
- Zhang, F.; Lu, K.; Gu, Y.; Zhang, L.; Li, W.; Li, Z. Effects of Low-temperature Stress and Brassinolide Application on the Photosynthesis and Leaf Structure of TungTree Seedlings. Front. Plant Sci. 2020, 10, 1767. [Google Scholar] [CrossRef]
- Zou, Z.; Zeng, F.; Zeng, Z.; Du, H.; Tang, H.; Zhang, H. The Variation in the Stoichiometric Characteristics of the Leaves and Roots of Karst Shrubs. Forests 2021, 12, 852. [Google Scholar] [CrossRef]
- Bao, S.D. Soil and Agricultural Chemistry Analysis, 3rd ed.; China Agriculture Press: Beijing, China, 2000. [Google Scholar]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 83–93. [Google Scholar]
- Brooker, R.; Brown, L.K.; George, T.S.; Pakeman, R.J.; Palmer, S.; Ramsay, L.; Schöb, C.; Schurch, N.; Wilkinson, M.J. Active and Adaptive Plasticity in a Changing Climate. Trends Plant Sci. 2022, 27, 717–728. [Google Scholar] [CrossRef]
- Cai, J.; Pan, X.; Xiao, Y.; Wang, Y.; Li, G.; Wang, Y.; Zhang, M.; Wang, L. Below-ground Root Nutrient-acquisition Strategies are More Sensitive to Long-term Grazing Than Above-ground Leaf Traits Across a Soil Nutrient Gradient. Funct. Ecol. 2024, 1–11. [Google Scholar] [CrossRef]
- Everingham, S.E.; Offord, C.A.; Sabot, M.E.; Moles, A.T. Leaf Morphological Traits Show Greater Responses to Changes in Climate Than Leaf Physiological Traits and Gas Exchange Variables. Ecol. Evol. 2024, 14, e10941. [Google Scholar] [CrossRef]
- Deguchi, R.; Koyama, K. Photosynthetic and Morphological Acclimation to High and Low Light Environments in Petasites japonicus subsp. giganteus. Forests 2020, 11, 1365. [Google Scholar] [CrossRef]
- Amoako, F.K.; Sagervanshi, A.; Hussain, M.A.; Pitann, B.; Mühling, K.H. Transcriptional and Physiological Analyses Uncover the Mineralization and Uptake Mechanisms of Phytic acid in Symbiotically Grown Vicia faba plants. Plant Physiol. Bioch. 2024, 211, 108723. [Google Scholar] [CrossRef]
- Zhang, J.; He, N.; Liu, C.; Xu, L.; Yu, Q.; Yu, G. Allocation Strategies for Nitrogen and Phosphorus in Forest Plants. Oikos 2018, 127, 1506–1514. [Google Scholar] [CrossRef]
- Cao, J.; Hong, P.; Zhan, C.; He, S. Dynamics in Stoichiometric Traits and Carbon, Nitrogen, and Phosphorus Pools Across Three Different-aged Picea asperata Mast. Plantations on the Eastern Tibet Plateau. Forests 2020, 11, 1346. [Google Scholar] [CrossRef]
- Wang, Y.L.; Li, L.; Paudel, B.R.; Zhao, J.L. Genomic Insights into High-Altitude Adaptation: A Comparative Analysis of Roscoea alpina and R. purpurea in the Himalayas. Int. J. Mol. Sci. 2024, 25, 2265. [Google Scholar] [CrossRef]
- Van Laere, K.; Van Huylenbroeck, J.; Van Bockstaele, E. Introgression of Yellow Flower Colour in Buddleja davidii by Means of Polyploidisation and Interspecific Hybridisation. Hortic. Sci. 2011, 38, 96–103. [Google Scholar] [CrossRef]
- Pérez-Ramos, I.M.; Matías, L.; Gómez-Aparicio, L.; Godoy, Ó. Functional Traits and Phenotypic Plasticity Modulate Species Coexistence Across Contrasting Climatic Conditions. Nat. Commun. 2019, 10, 2555. [Google Scholar] [CrossRef]
- Aycan, M.; Nahar, L.; Baslam, M.; Mitsui, T. Transgenerational Salinity Tolerance Plasticity in Rice: Unraveling Nongenetic Phenotypic Modifications and Environmental Influences. J. Exp. Bot. 2024, erae211. [Google Scholar] [CrossRef]
- Uyeda, J.C.; Hansen, T.F.; Arnold, S.J.; Pienaar, J. The Millionyear Wait for Macroevolutionary Bursts. Proc. Natl. Acad. Sci. USA 2011, 108, 15908–15913. [Google Scholar] [CrossRef]
- Henn, J.J.; Buzzard, V.; Enquist, B.J.; Halbritter, A.H.; Klanderud, K.; Maitner, B.S.; Michaletz, S.T.; Pötsch, C.; Seltzer, L.; Telford, R.J.; et al. Intraspecific Trait Variation and Phenotypic Plasticity Mediate Alpine Plant Species Response to Climate Change. Front. Plant Sci. 2018, 9, 408249. [Google Scholar] [CrossRef]
- Love, J.M.; Ferris, K.G. Local Adaptation to an Altitudinal Gradient: The Interplay Between Mean Phenotypic Trait Variation and Phenotypic Plasticity in Mimulus laciniatus. Perspect. Plant Ecol. 2024, 63, 125795. [Google Scholar] [CrossRef]
- De Storme, N.; Mason, A. Plant Speciation Through Chromosome Instability and Ploidy Change: Cellular Mechanisms, Molecular Factors and Evolutionary Relevance. Curr. Plant Biol. 2014, 1, 10–33. [Google Scholar] [CrossRef]
- Seguí, J.; Lázaro, A.; Traveset, A.; Salgado-Luarte, C.; Gianoli, E. Phenotypic and Reproductive Responses of an Andean Violet to Environmental Variation Across an Elevational Gradient. Alp. Bot. 2018, 128, 59–69. [Google Scholar] [CrossRef]


| Populations | Abbreviation | Ploidy | Altitude (m) | Latitude | Longitude | Average Annual Air Temperature (°C) | Average Annual Precipitation (mm) |
|---|---|---|---|---|---|---|---|
| Daxueshan, Lincang | DXS | Tetraploids | 2012 | N 24°06′50.18″ | E 99°56′15.32″ | 23.3 | 1032 |
| Fengchunling, Yuangyang | FCL | Tetraploids | 1562 | N 23°00′12.22″ | E 103°01′58.35″ | 17.9 | 1562.3 |
| Baohua, Honghe | BH | Tetraploids | 1205 | N 23°12′44.35″ | E 102°12′44.35″ | 24.5 | 1150 |
| Shuangjiang, Lincang | SJ | Tetraploids | 1983 | N 23°38′32.83″ | E 100°01′58.32″ | 19.5 | 995.3 |
| Hepingzi, Yuanjiang | HPZ | Tetraploids | 2117 | N 23°40′00.70″ | E 101°45′51.46″ | 17 | 946 |
| Daweishan, Pingbian | DWS | Tetraploids | 2100 | N 22°54′28.09″ | E 103°41′56.12″ | 16 | 2012 |
| Adebo, Jinping | ADB | Tetraploids | 2115 | N 22°54′03.05″ | E 103°12′07.68″ | 21 | 1500 |
| Xiaoheishan, Longling | XHS | Tetraploids | 2487 | N 24°29′49.30″ | E 98°51′24.88″ | 16.4 | 1699 |
| Meidong, Lvcun | MD | Tetraploids | 1600 | N 23°0′12.99″ | E 102°31′2.45″ | 16.6 | 2400 |
| Laozhai, Mengzi | LZ | Octoploids | 2566 | N 23°23′36.53″ | E 103°48′29.86″ | 14 | 1273.4 |
| Jinxingshan, Longling | JXS | Octoploids | 2164 | N 24°35′03.59″ | E 98°54′23.44″ | 14.9 | 1837.7 |
| Yongcui, Nanjian | YC | Octoploids | 2413 | N 24°56′08.28″ | E 100°23′30.10″ | 27 | 867.6 |
| Gaoligongshan, Lushui | GLGS | Octoploids | 3105 | N 25°58′21.21″ | E 98°41′1.87″ | 11 | 1667 |
| Cangshan, Dali | CS | Octoploids | 1561 | N 23°00′14.93″ | E 100°06′06.83″ | 15.7 | 1078 |
| Xima, Dehong | XM | Octoploids | 1951 | N 24°42′53.85″ | E 97°44′32.23″ | 16 | 1552 |
| Bozhushan, Wenshan | BZS | Octoploids | 2201 | N 23°21′40.96″ | E 103°54′22.13″ | 22.6 | 1054 |
| Baicaoling, Dayao | BCL | Octoploids | 3000 | N 26°05′9.84″ | E 101°09′7.06″ | 17.2 | 793 |
| Wujiexiang, Chuxiong | WJX | Octoploids | 2487 | N 25°04′10.68″ | E 101°0′53.32″ | 15.5 | 893.8 |
| Leaf Functional Traits | Tetraploid (Mean ± SE) | Octaploid (Mean ± SE) | Significance |
|---|---|---|---|
| Morphological traits (n = 45) | |||
| LA (cm2) | 165.62 ± 26.69 | 137.5 ± 34.45 | * |
| SLA (m2 kg−1) | 111.00 ± 9.57 | 134.45 ± 8.90 | * |
| LMA (g m2) | 101.65 ± 12.48 | 81.01 ± 5.38 | * |
| LDMC (g g−1) | 0.23 ± 0.02 | 0.21 ± 0.01 | * |
| Anatomical traits (n = 45) | |||
| T (μm) | 126.64 ± 2.41 | 97.75 ± 1.42 | * |
| TUEC (μm) | 18.30 ± 0.80 | 9.08 ± 0.28 | * |
| Palisade (μm) | 51.53 ± 1.81 | 44.72 ± 1.35 | * |
| Spongy (μm) | 41.36 ± 1.73 | 30.92 ± 1.27 | * |
| Tmes (μm) | 92.89 ± 1.65 | 75.64 ± 0.89 | * |
| CTR (%) | 40.91 ± 1.44 | 45.87 ± 1.34 | * |
| SR (%) | 32.69 ± 1.26 | 31.6 ± 1.20 | NS |
| P/S | 1.33 ± 0.11 | 1.56 ± 0.14 | NS |
| Photosynthetic traits (n = 45) | |||
| PN (μmol CO2 m−2 s−1) | 20.85 ± 0.48 | 23.97 ± 1.67 | NS |
| gs (mol H2O m−2 s−1) | 0.37 ± 0.02 | 0.30 ± 0.03 | * |
| Ci (μmol μmol−1) | 314.79 ± 4.83 | 305.21 ± 5.15 | * |
| Tr (mmol H2O m−2 s−1) | 6.97 ± 0.27 | 5.37 ± 0.48 | * |
| VpdL (KPa) | 1.91 ± 0.10 | 1.74 ± 0.08 | * |
| WUE (μmol mmol) | 3.08 ± 0.13 | 6.90 ± 2.14 | * |
| LUE (mol mol | 0.03 ± 0 | 0.04 ± 0.01 | * |
| Stoichiometric characteristics (n = 27) | |||
| C (mg g−1) | 441.47 ± 2.14 | 444.02 ± 2.52 | NS |
| N (mg g−1) | 19.04 ± 0.61 | 25.06 ± 0.66 | * |
| P (mg g−1) | 1.71 ± 0.07 | 2.25 ± 0.06 | * |
| C:N Ratio | 23.77 ± 0.82 | 18.10 ± 0.56 | * |
| C:P Ratio | 269.53 ± 12.30 | 202.28 ± 7.01 | * |
| N:P Ratio | 11.29 ± 0.24 | 11.25 ± 0.28 | NS |
| Cytotype | SOC | STN | STP | C:N | C:P | N:P |
|---|---|---|---|---|---|---|
| (mg g−1) | (mg g−1) | (mg g−1) | ||||
| Tetraploid | 22.87 ± 2.27 | 0.99 ± 0.10 | 0.50 ± 0.05 | 23.53 ± 1.17 | 53.47 ± 7.33 | 2.19 ± 0.22 |
| Octaploid | 33.20 ± 3.09 | 1.53 ± 0.14 | 0.93 ± 0.12 | 21.67 ± 0.99 | 48.35 ± 6.43 | 2.12 ± 0.22 |
| Significance | * | * | * | NS | NS | NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, W.; Li, H.; Fu, H.; Wang, C. Differentiation in Leaf Functional Traits and Driving Factors of the Allopatric Distribution of Tetraploid and Octaploid Buddleja macrostachya in the Sino-Himalayan Region. Forests 2024, 15, 1007. https://doi.org/10.3390/f15061007
Gong W, Li H, Fu H, Wang C. Differentiation in Leaf Functional Traits and Driving Factors of the Allopatric Distribution of Tetraploid and Octaploid Buddleja macrostachya in the Sino-Himalayan Region. Forests. 2024; 15(6):1007. https://doi.org/10.3390/f15061007
Chicago/Turabian StyleGong, Weichang, He Li, Hongbo Fu, and Chuanming Wang. 2024. "Differentiation in Leaf Functional Traits and Driving Factors of the Allopatric Distribution of Tetraploid and Octaploid Buddleja macrostachya in the Sino-Himalayan Region" Forests 15, no. 6: 1007. https://doi.org/10.3390/f15061007





