Phylogenetic Diversity, Host Specificity, and Distribution of the Wood-Decaying Fungus Phellinotus teixeirae in Western Colombia’s Seasonally Dry Tropical Forest
Abstract
:1. Introduction
2. Materials and Methods
2.1. Morphological Analyses
2.2. DNA Extraction, Amplification and Sequencing of Barcodes
2.3. Phylogenetic Analysis
2.4. Data Analysis
3. Results
3.1. Taxonomy–Record Description for Phellinotus Teixeirae Salvador-Montoya, Elias and Drechsler-Santos [MycoBank MB840997]
3.2. Phylogenetic Relationships
3.3. Patterns of Nucleotide Diversity
3.4. Analysis of Molecular Variance
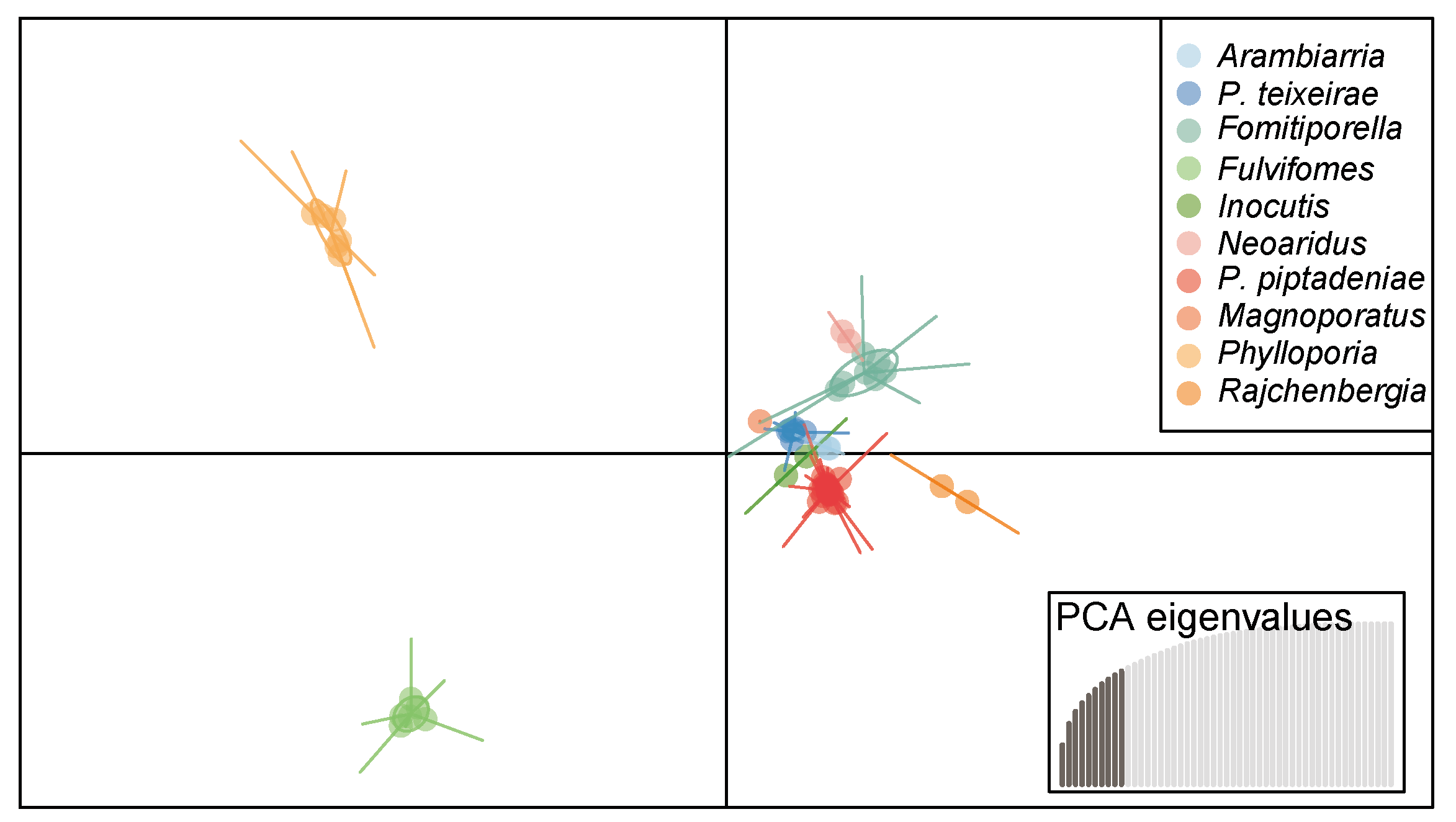
3.5. Discriminant Analysis of Principal Components
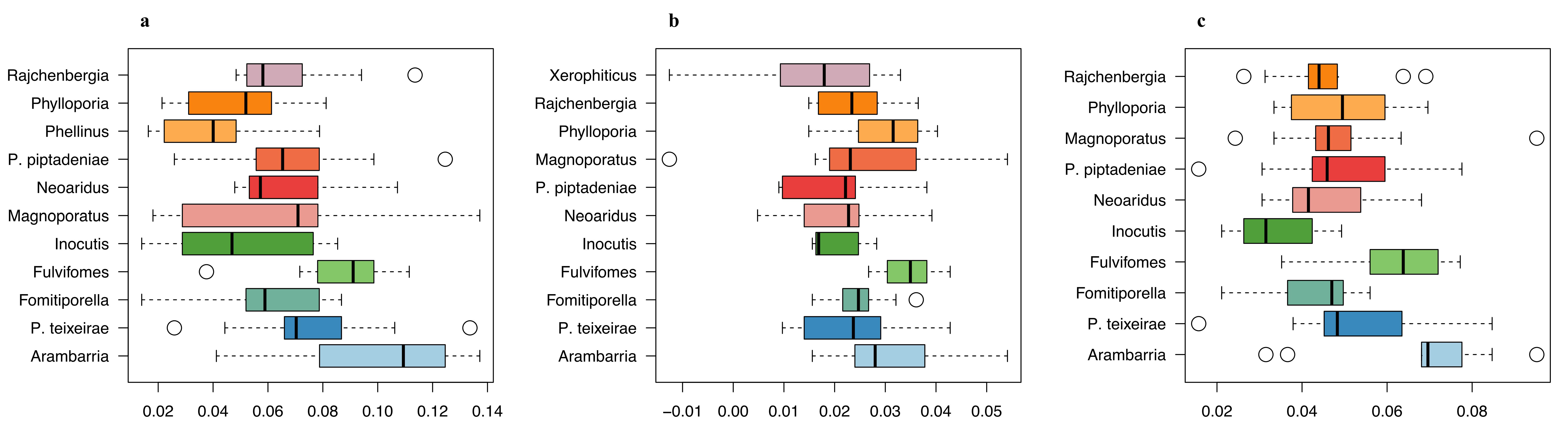
3.6. Patterns of SNP Allelic Distribution in Phellinotus Species
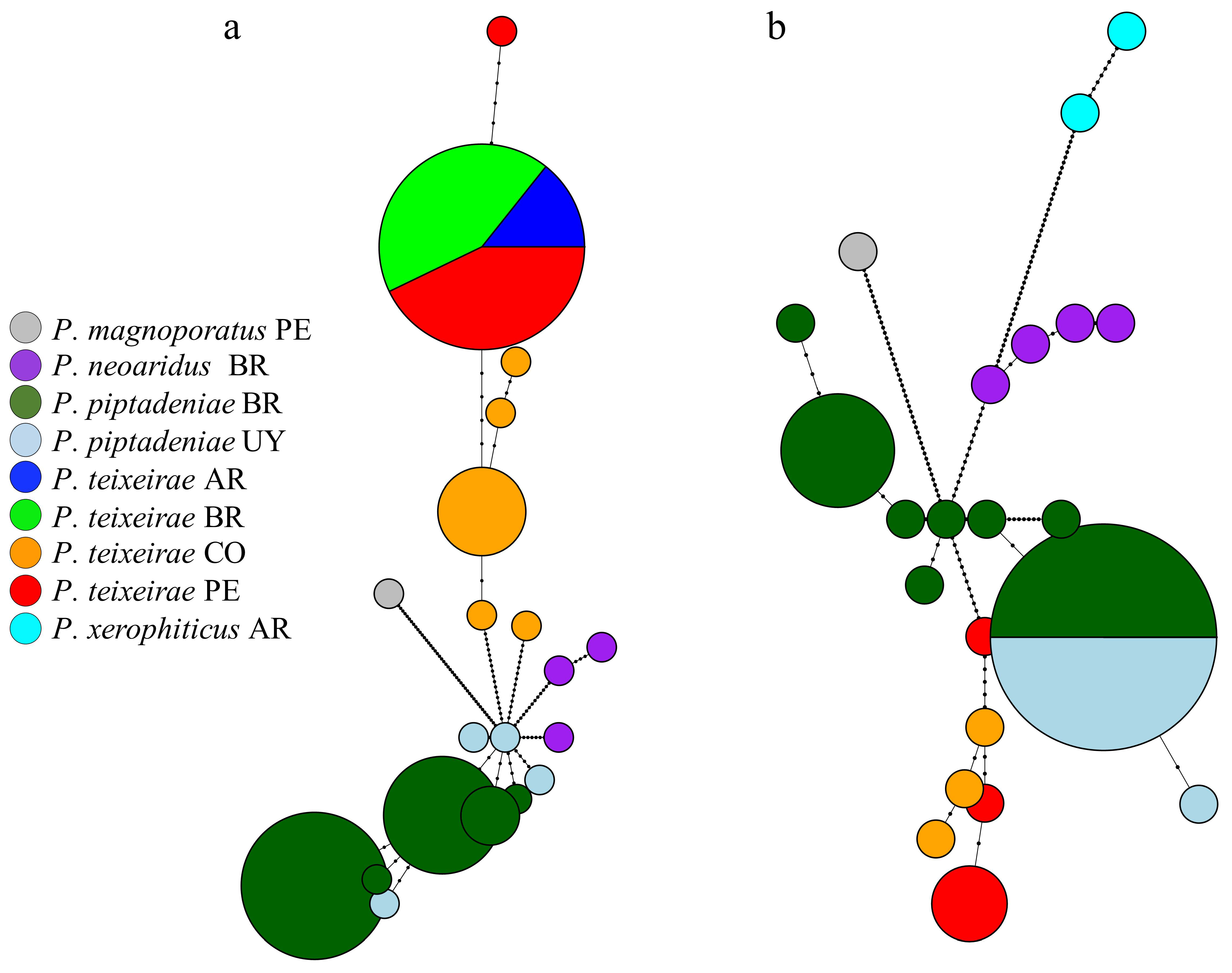
3.7. Network Analysis and Haplotype Distribution of Phellinotus Species
4. Discussion
4.1. Patterns and Processes of Genetic Diversity in Phellinotus Species
4.2. Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Elias, S.G.; Salvador-Montoya, C.A.; Costa-Rezende, D.H.; Guterres, D.C.; Fernandes, M.; Olkoski, D.; Klabunde, G.H.F.; Drechsler-Santos, E.R. Studies on the biogeography of Phellinotus piptadeniae (Hymenochaetales, Basidiomycota): Expanding the knowledge on its distribution and clarifying hosts relationships. Fungal Ecol. 2020, 45, 100912. [Google Scholar] [CrossRef]
- Salvador-Montoya, C.A.; Elias, S.G.; Popoff, O.F.; Robledo, G.L.; Urcelay, C.; Góes-Neto, A.; Martínez, S.; Drechsler-Santos, E.R. Neotropical Studies on Hymenochaetaceae: Unveiling the Diversity and Endemicity of Phellinotus. J. Fungi 2022, 8, 216. [Google Scholar] [CrossRef] [PubMed]
- Drechsler-Santos, E.R.; Robledo, G.L.; Limajúnior, N.C.; Malosso, E.; Reck, M.A.; Gibertoni, T.B.; De Queiroz Cavalcanti, M.A.; Rajchenberg, M. Phellinotus, a new neotropical genus in the Hymenochaetaceae (Basidiomycota, Hymenochaetales). Phytotaxa 2016, 261, 218–239. [Google Scholar] [CrossRef]
- Drechsler-Santos, E.R.; Santos, P.J.P.; Gibertoni, T.B.; Cavalcanti, M.A.Q. Ecological aspects of Hymenochaetaceae in an area of Caatinga (semi-arid) in Northeast Brazil. Fungal Divers. 2010, 42, 71–78. [Google Scholar] [CrossRef]
- Salvador-Montoya, C.A.; Robledo, G.L.; Cardoso, D.; Borba-Silva, M.A.; Fernandes, M.; Drechsler-Santos, E.R. Phellinus piptadeniae (Hymenochaetales: Hymenochaetaceae): Taxonomy and host range of a species with disjunct distribution in South American seasonally dry forests. Plant Syst. Evol. 2015, 301, 1887–1896. [Google Scholar] [CrossRef]
- Pildain, M.B.; Cendoya, R.R.; Ortiz-Santana, B.; Becerra, J.; Rajchenberg, M. A discussion on the genus Fomitiporella (Hymenochaetaceae, hymenochaetales) and first record of F. americana from southern South America. MycoKeys 2018, 38, 77–91. [Google Scholar] [CrossRef] [PubMed]
- Salvador-Montoya, C.A.; Popoff, O.F.; Góes-Neto, A.; Drechsler-Santos, E.R. Global phylogenetic and morphological reassessment of Fomitiporella s.l. (Hymenochaetales, Basidiomycota): Taxonomic delimitation of Fomitiporella s.s. and segregation of Rajchenbergia, gen. nov. Plant Syst. Evol. 2020, 306, 1–27. [Google Scholar] [CrossRef]
- Salvador-Montoya, C.A.; Martínez, M.; Drechsler-Santos, E.R. Taxonomic update of species closely related to Fulvifomes robiniae in America. Mycol. Prog. 2022, 21, 95. [Google Scholar] [CrossRef]
- Floudas, D.; Binder, M.; Riley, R.; Barry, K.; Blanchette, R.A.; Henrissat, B.; Martínez, A.T.; Otillar, R.; Spatafora, J.W.; Yadav, J.S.; et al. The paleozoic origin of enzymatic lignin decomposition reconstructed from 31 fungal genomes. Science 2012, 336, 1715–1719. [Google Scholar] [CrossRef]
- Larsson, K.H.; Parmasto, E.; Fischer, M.; Langer, E.; Nakasone, K.K.; Redhead, S.A. Hymenochaetales: A molecular phylogeny for the hymenochaetoid clade. Mycologia 2006, 98, 926–936. [Google Scholar] [CrossRef]
- Zhou, L.W.; Dai, Y.C. Phylogeny and taxonomy of Phylloporia (Hymenochaetales): New species and a worldwide key to the genus. Mycologia 2012, 104, 211–222. [Google Scholar] [CrossRef] [PubMed]
- He, S.H.; Dai, Y.C. Taxonomy and phylogeny of Hymenochaete and allied genera of Hymenochaetaceae (Basidiomycota) in China. Fungal Divers. 2012, 56, 77–93. [Google Scholar] [CrossRef]
- Amalfi, M.; Decock, C. Fomitiporia castilloi sp. nov. and multiple clades around F. apiahyna and F. texana in meso-and South America evidenced by multiloci phylogenetic inferences. Mycologia 2013, 105, 873–887. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.M.; Yu, H.Y.; Zhou, L.W.; Decock, C.; Vlasák, J.; Dai, Y.C. Phylogeny and taxonomy of the Inonotus linteus complex. Fungal Divers. 2013, 58, 159–169. [Google Scholar] [CrossRef]
- Zhou, L.W.; Qin, W.M. Phylogeny and taxonomy of the recently proposed genus phellinopsis (Hymenochaetales, Basidiomycota). Mycologia 2013, 105, 689–696. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.-W. Fulvifomes hainanensis sp. nov. and F. indicus comb. nov. (Hymenochaetales, Basidiomycota) evidenced by a combination of morphology and phylogeny. Mycoscience 2014, 55, 70–77. [Google Scholar] [CrossRef]
- Zhu, L.; Song, J.; Zhou, J.L.; Si, J.; Cui, B.K. Species diversity, phylogeny, divergence time, and biogeography of the genus sanghuangporus (Basidiomycota). Front. Microbiol. 2019, 10, 812. [Google Scholar] [CrossRef] [PubMed]
- Neves, M.A. Guide to the common fungi of the semiarid region of Brazil. In Common Fungi of the Semiarid Region of Brazil; Neves, M.A., Baseia, I., Dreschler-Santos, E., Góes-Neto, A., Eds.; TECC Editora: Florianópolis, Brazil, 2013; p. 131. [Google Scholar]
- Riggio, J.; Baillie, J.E.M.; Brumby, S.; Ellis, E.; Kennedy, C.M.; Oakleaf, J.R.; Tepe, T.; Theobald, D.M.; Venter, O.; Watson, J.E.M.; et al. Global human influence maps reveal clear opportunities in conserving Earth’s remaining intact terrestrial ecosystems. Glob. Chang. Biol. 2020, 26, 4344–4356. [Google Scholar] [CrossRef]
- Ocón, J.P.; Ibanez, T.; Franklin, J.; Pau, S.; Keppel, G.; Rivas-Torres, G.; Shin, M.E.; Gillespie, T.W. Global tropical dry forest extent and cover: A comparative study of bioclimatic definitions using two climatic data sets. PLoS ONE 2021, 16, 0252063. [Google Scholar] [CrossRef]
- Etter, A.; Andrade, A.; Saavedra, K.; Cortés, J. Actualización de la Lista Roja de los Ecosistemas Terrestres de Colombia: Conocimiento del riesgo de ecosistemas como herramienta para la gestión. In Estado y Tendencias de la Biodiversidad Continental de Colombia; Moreno, L.A., Rueda, C., Andrade, G.I., Eds.; Instituto de Investigación de Recursos Biológicos Alexander von Humboldt: Bogotá, DC, USA, 2017. [Google Scholar]
- Gómez-Montoya, N.; Ríos Sarmiento, C.; Zora-Vergara, B.; Benjumea-Aristizabal, C.; Santa-Santa, D.J.; Zuluaga-Moreno, M.; Franco-Molano, A.E. Diversidad de macrohongos (Basidiomycota) de Colombia: Listado de especies. Actual. Biológicas 2022, 44, 1–94. [Google Scholar] [CrossRef]
- ColFungi. Useful Fungi of Colombia. Facil by R Bot Gard Kew Publ Internet. Available online: https://colfungi.org/2023 (accessed on 10 July 2023).
- Castellani, A. Maintenance and cultivation of common pathogenic fungi in man in sterile distilled water: Further resaerches. J. Trop. Med. Hyg. 1967, 70, 181–184. [Google Scholar]
- Miettinen, O.; Niemelä, T.; Spirin, W. Northern Antrodiella species: The identity of A. semisupina, and type studies of related taxa. Mycotaxon 2006, 96, 211–239. [Google Scholar]
- Miettinen, O.; Vlasák, J.; Rovoire, B.; Spirin, V. Fungal Systematics and Evolution VOLUME. Fungal Syst. Evol. 2018, 1, 169–215. [Google Scholar]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and Direct Sequencing of Fungal Ribosomal Rna Genes for Phylogenetics. In PCR Protocols; Innis, M.D.H.G., Sninsky, J., White, T., Eds.; Academic Press: Cambridge, MA, USA, 1990; pp. 315–322. [Google Scholar] [CrossRef]
- Haynes, K.A.; Westerneng, T.J.; Fell, J.W.; Moens, W. Rapid detection and identification of pathogenic fungi by polymerase chain reaction amplification of large subunit ribosomal DNA. Med. Mycol. 1995, 33, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Vilgalys, R.; Hester, M. Rapid Genetic Identification and Mapping of Enzymatically Amplified Ribosomal DNA from SeveralCryptococcus Species. J. Bacteriol. 1990, 172, 4238–4246. [Google Scholar] [CrossRef] [PubMed]
- Brandon Matheny, P.; Wang, Z.; Binder, M.; Curtis, J.M.; Lim, Y.W.; Henrik Nilsson, R.; Hofstetter, V.; Ammirati, J.F.; Schoch, C.L.; Langer, E.; et al. Contributions of rpb2 and tef1 to the phylogeny of mushrooms and allies (Basidiomycota, Fungi). Mol. Phylogenet. Evol. 2007, 43, 430–451. [Google Scholar] [CrossRef] [PubMed]
- Oghenekaro, A.O.; Miettinen, O.; Omorusi, V.I.; Evueh, G.A.; Farid, M.A.; Gazis, R.; Asiegbu, F.O. Molecular phylogeny of Rigidoporus microporus isolates associated with white rot disease of rubber trees (Hevea brasiliensis). Fungal Biol. 2014, 118, 495–506. [Google Scholar] [CrossRef] [PubMed]
- Ewing, B.; Green, P. Base-calling of automated sequencer traces using phred. II. Error probabilities. Genome Res. 1998, 8, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Gordon, D.; Abajian, C.; Green, P. Consed: A Graphical Tool for Sequence Finishing. Genome Res. 1998, 8, 195–202. [Google Scholar] [CrossRef]
- Gordon, D.; Green, P. Consed: A graphical editor for next-generation sequencing. Bioinformatics 2013, 29, 2936–2937. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Larsson, A. AliView: A fast and lightweight alignment viewer and editor for large datasets. Bioinformatics 2014, 30, 3276–3278. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.T.; Schmidt, H.A.; Von Haeseler, A.; Minh, B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Kalyaanamoorthy, S.; Minh, B.Q.; Wong, T.K.F.; Von Haeseler, A.; Jermiin, L.S. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods 2017, 14, 587–589. [Google Scholar] [CrossRef] [PubMed]
- Hoang, D.T.; Chernomor, O.; von Haeseler, A.; Minh, B.Q.; Vinh, L.S. UFBoot2: Improving the Ultrafast Bootstrap Approximation. Mol. Biol. Evol. 2018, 35, 518–522. [Google Scholar] [CrossRef] [PubMed]
- Guindon, S.; Dufayard, J.F.; Lefort, V.; Anisimova, M.; Hordijk, W.; Gascuel, O. New algorithms and methods to estimate maximum-likelihood phylogenies: Assessing the performance of PhyML 3.0. Syst. Biol. 2010, 59, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Hall, T.A. BioEdit: A user friendly biological seque. Nucleid Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Jombart, T.; Devillard, S.; Balloux, F. Discriminant analysis of principal components: A new method for the analysis of genetically structured populations. BMC Genet. 2010, 11, 94. [Google Scholar] [CrossRef] [PubMed]
- Heberle, H.; Meirelles, V.G.; da Silva, F.R.; Telles, G.P.; Minghim, R. InteractiVenn: A web-based tool for the analysis of sets through Venn diagrams. BMC Bioinform. 2015, 16, 169. [Google Scholar] [CrossRef]
- Forero, E.; Romero, C. Estudios de Leguminosas en Colombia; Academia Colombiana de Ciencias Exactas, Físicas y Naturales: Bogotá, Colombia, 2005; Volume 25. [Google Scholar]
- Duke, J.A.; Wain, K.K. Medicinal plants of the world. Computer index with more than 85000 entries. In Handbook of Medicinal Herbs; Longman gr.: Boca Raton, FL, USA, 1981. [Google Scholar]
- Manimaran, P.; Sanjay, M.R.; Senthamaraikannan, P.; Yogesha, B.; Barile, C.; Siengchin, S. A new study on characterization of Pithecellobium dulce fiber as composite reinforcement for light-weight applications. J. Nat. Fibers 2020, 17, 359–370. [Google Scholar] [CrossRef]
- Misra, G.; Nigam, S.K. Pithecolobium duke: A tree of commercial importance. Pharm. Biol. 1978, 16, 158–162. [Google Scholar] [CrossRef]
- Preethi, S.; Marysaral, A. GC-MS Analysis of Microwave Assisted Ethanolic Extract of Pithecellobium dulce. Malaya J. Biosci. 2014, 1, 242–247. [Google Scholar]
- Bradbury, P.J.; Zhang, Z.; Kroon, D.E.; Casstevens, T.M.; Ramdoss, Y.; Buckler, E.S. TASSEL: Software for association mapping of complex traits in diverse samples. Bioinformatics 2007, 23, 2633–2635. [Google Scholar] [CrossRef] [PubMed]
- Pennington, R.; Prado, D.; Pendry, C. Neotropical seasonally dry forests and quaternary vegetation changes. J. Biogeogr. 2000, 27, 261–273. [Google Scholar]
- Pinto-Ruiz, R.; Hernández, D.; Gómez, H.; Cobos, M.; Quiroga, R.; Pezo, D. Árboles forrajeros de tres regiones ganaderas de Chiapas, México: Usos y características nutricionales. Univ. Cienc. 2010, 26, 19–31. [Google Scholar]
- De la Puente, J.; Villa, A.; Astie, W. Inventario Forestal del Gobierno de Jalisco; Secretaría de Agricultura y Ganadería, Subsecretaría Forestal y de la Fauna, Dirección general del Inventario Nacional Forestal: Mexico City, Mexico, 1970; pp. 1–64. [Google Scholar]
- Carabias, J.; Arizpe, L. El deterioro ambiental: Cambios nacionales, cambios globales. In Desarrollo Sustentable: Hacia una Política Ambiental; Azuela, A., Carabias, J., Provencio, E., Quadri, G., Eds.; Universidad Nacional Autónoma de México, Coordinación de Humanidades: Ciudad de México, Mexico, 1993; pp. 43–59. [Google Scholar]
- Nogueira-Melo, G.S.; Santos, P.J.P.; Gibertoni, T.B. Host-exclusivity and host-recurrence by wood decay fungi (Basidiomycota-Agaricomycetes) in Brazilian mangroves. Acta Bot. Bras. 2017, 31, 566–570. [Google Scholar] [CrossRef]
- He, M.Q.; Zhao, R.L.; Hyde, K.D.; Begerow, D.; Kemler, M.; Yurkov, A.; McKenzie, E.H.C.; Raspé, O.; Kakishima, M.; Sánchez-Ramírez, S.; et al. Notes, outline and divergence times of Basidiomycota. Fungal Divers. 2019, 99, 105–367. [Google Scholar] [CrossRef]
- Wakeley, J. Coalescent Theory: An Introduction. In Greenwood Village and Colo, 1st ed.; Roberts & Co. Publishers: Greenwood Village, CO, USA, 2009; pp. 1–326. [Google Scholar]
- Iwanaga, T.; Anzawa, K.; Mochizuki, T. Variations in ribosomal DNA copy numbers in a genome of Trichophyton interdigitale. Mycoses 2020, 63, 1133–1140. [Google Scholar] [CrossRef] [PubMed]
- Paloi, S.; Luangsa-ard, J.J.; Mhuantong, W.; Stadler, M.; Kobmoo, N. Intragenomic variation in nuclear ribosomal markers and its implication in species delimitation, identification and barcoding in fungi. Fungal Biol. Rev. 2022, 42, 1–33. [Google Scholar] [CrossRef]
- Averbeck, K.T.; Eickbush, T.H. Monitoring the mode and tempo of concerted evolution in the Drosophila melanogaster rDNA locus. Genetics 2005, 171, 1837–1846. [Google Scholar] [CrossRef]
- Ganley, A.R.D.; Kobayashi, T. Highly efficient concerted evolution in the ribosomal DNA repeats: Total rDNA repeat variation revealed by whole-genome shotgun sequence data. Genome Res. 2007, 17, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Keller, I.; Chintauan-Marquier, I.C.; Veltsos, P.; Nichols, R.A. Ribosomal DNA in the grasshopper Podisma pedestris: Escape from concerted evolution. Genetics 2006, 174, 863–874. [Google Scholar] [CrossRef] [PubMed]
- McTaggart, S.J.; Dudycha, J.L.; Omilian, A.; Crease, T.J. Rates of recombination in the ribosomal DNA of apomictically propagated Daphnia obtusa lines. Genetics 2007, 175, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Londoño-Caicedo, J.M.; Uribe-Londoño, M.; Buitrago-Bitar, M.A.; Cortés, A.J.; Muñoz-Flórez, J.E. Molecular Identification and Phylogenetic Diversity of Native Entomopathogenic Nematodes, and Their Bacterial Endosymbionts, Isolated from Banana and Plantain Crops in Western Colombia. Agronomy 2023, 13, 1373. [Google Scholar] [CrossRef]
- Lavrinienko, A.; Jernfors, T.; Koskimäki, J.J.; Pirttilä, A.M.; Watts, P.C. Does Intraspecific Variation in rDNA Copy Number Affect Analysis of Microbial Communities? Trends Microbiol. 2021, 29, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Simon, U.K.; Weiß, M. Intragenomic variation of fungal ribosomal genes is higher than previously thought. Mol. Biol. Evol. 2008, 25, 2251–2254. [Google Scholar] [CrossRef] [PubMed]
- Long, E.; Dawid, B. Drosophila of Ribosomal melanogaster DNA Insertions in Drosohpila melanogaster. Cell 1979, 18, 1185–1196. [Google Scholar] [CrossRef] [PubMed]
- Eickbush, T.H.; Eickbush, D.G. Finely orchestrated movements: Evolution of the ribosomal RNA genes. Genetics 2007, 175, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Cortés, A.J.; López-Hernández, F.; Osorio-Rodriguez, D. Predicting Thermal Adaptation by Looking Into Populations’ Genomic Past. Front. Genet. 2020, 11, 1–14. [Google Scholar] [CrossRef]
- Vasco-Palacios, A.; Lücking, R.; Moncada, B.; Palacio, M.; Motato-Vásquez, V. A Critical Assessment of Biogeographic Distribution Patterns of Colombian Fungi. In Ctalogue Fungi Colombia, 1st ed.; De Almedia, R., Lüking, R., Vasco-Palacios, A., Gaya, E., Diazgranados, M., Eds.; Kew Royal Botanical Garden: Richmond, UK, 2022; pp. 120–137. [Google Scholar]
- Vesth, T.C.; Nybo, J.L.; Theobald, S.; Frisvad, J.C.; Larsen, T.O.; Nielsen, K.F.; Hoof, J.B.; Brandl, J.; Salamov, A.; Riley, R.; et al. Investigation of inter- and intraspecies variation through genome sequencing of Aspergillus section Nigri. Nat. Genet. 2018, 50, 1688–1695. [Google Scholar] [CrossRef]
- Cortés, A.J.; Restrepo-Montoya, M.; Bedoya-Canas, L.E. Modern Strategies to Assess and Breed Forest Tree Adaptation to Changing Climate. Front. Plant Sci. 2020, 11, 583323. [Google Scholar] [CrossRef] [PubMed]
- Guevara-Escudero, M.; Osorio, A.N.; Cortés, A.J. Integrative pre-breeding for biotic resistance in forest trees. Plants 2021, 10, 2022. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, Z.; Gao, M.; Li, T.; Cui, X.; Zu, J.; Sang, S.; Fan, W.; Zhang, H. Intraspecific Comparative Analysis Reveals Genomic Variation of Didymella arachidicola and Pathogenicity Factors Potentially Related to Lesion Phenotype. Biology 2023, 12, 476. [Google Scholar] [CrossRef] [PubMed]
- Shnyreva, A.; Badalyan, S.; Shnyreva, A. Analysis of Intraspecies Genetic Variability among Collections of Medicinal Red Belt Conk Mushroom, Fomitopsis pinicola (Agaricomycetes). Int. J. Med. Mushrooms 2019, 21, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, R.H.; Kristiansson, E.; Ryberg, M.; Hallenberg, N.; Larsson, K.H. Intraspecific ITS variability in the Kingdom Fungi as expressed in the international sequence databases and its implications for molecular species identification. Evol. Bioinform. 2008, 4, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Bolaños, A.C.; Ramos, V.L.; Londoño, J.M.; Castillo, A.; Vitali, V.M.; Mello, A.; Muñoz, J.E. Detecting Manganese Peroxidase (MnP) Gene in Ganoderma Species Detecting Manganese Peroxidase (MnP) gene in Ganoderma species. Cryptogamie 2018, 39, 325–340. [Google Scholar] [CrossRef]
- Arenas, S.; Cortés, A.J.; Mastretta, A.; Jaramillo, J.P. Evaluating the accuracy of genomic prediction for the management and conservation of relictual natural tree populations. Tree Genet. Genomes 2021, 17, 12. [Google Scholar] [CrossRef]
- Cortés, A.J. On the origin of the common bean. Am. J. Plant Sci. 2013, 4, 1998–2000. [Google Scholar] [CrossRef]




| Passport Data | GenBank Accession Number | |||||||
|---|---|---|---|---|---|---|---|---|
| Genera | Fungi Species | Country (ISO) | Reference Collection | Tree Host | ITS | nLSU | tef1 | rpb2 |
| Arambarria Rajchenb. and Pildain | A. destruens Rajchenb. and Pildain * | AR | CIEFAPcc 347 | Diostea juncea | KP347538 | KP347523 | KY907666 | – |
| A. cognata (Bres.) Rajchnb. Pildain | UY | CGP473 | Dodonaea viscosa | KY907683 | KY907687 | KY907675 | – | |
| A. cognata | UY | CGP474 | Dodonaea viscosa | KY907682 | KY907692 | KY907676 | – | |
| Fomitiporella Murrill | F. austroasiana Y.C. Dai, X.H. Ji and J. Vlasák (T) | CN | Dai 16244 | On fallen angiosperm trunk | MG657328 | MG657320 | – | – |
| F. caryophylli (Racib.) T. Wagner and M. Fisch. | IN | CBS 448.76 | Shorea robusta | AY558611 | AY059021 | – | – | |
| F. chinensis (Pilát) Y.C. Dai, X.H. Ji and J. Vlasák | CN | Cui 11230 | Quercus sp. | KX181309 | KY693759 | KY693958 | – | |
| F. inermis (Ellis & Everh.) Murrill | US | JV 1009/56 | Ilex mucronata | KX181306 | KX181347 | – | – | |
| F. subinermis Y.C. Dai, X.H. Ji and Vlasák | CN | Dai 15114 | On root of angispoerm tree | KX181308 | KX181344 | – | – | |
| F. umbrinella (Bres.) Murrill * | US | JV 0509/114 | Unknown | KX181314 | KX181336 | – | – | |
| F. umbrinella (Bres.) Murrill | BR | FLOR 51648 | MK802943 | MK802941 | – | – | ||
| Fulvifomes Murrill | F. elaeodendri Tchotet, M.P.A. Coetzee, Rajchenb. and Jol. Roux | ZA | CMW 47825 | Elaeodendron croceum | MH599094 | MH599134 | MT108964 | – |
| F. centroamericanus Y.C. Dai, X.H. Ji and J. Vlasák (T) | GT | JV 0611_III | On living angiosperm tree | KX960763 | KX960764 | – | – | |
| F. fastuosus (Lév.) Bondartseva and S. Herrera | PH | CBS 213.36 | Gliricidia sepium | AY558615 | AY059057 | – | – | |
| F. nilgheriensis (Mont.) Bondartseva and S. Herrera | US | CBS 209.36 | On dead deciduous wood | AY558633 | AY059023 | – | – | |
| F. robiniae (Murrill) Murrill | US | CBS 211.36 | Robinia pseudoacacia | AY558646 | AY059038 | – | – | |
| F. squamosus Salvador-Montoya and Drechsler-Santos | PE | USM258361 | Acacia macracantha | MF479267 | MF479266 | – | – | |
| Inocutis Fiasson and Niemelä | I. dryophilus (Berk.) Fiasson and Niemelä | US | DLL 2012-001 | Quercus alba | KU139186 | KU139255 | – | KU139317 |
| I. jamaicensis (Murrill) A.M. Gottlieb, J.E. Wright and Moncalvo | US | RLG 15819 | Quercus arizonica | KY907684 | KY907703 | – | – | |
| I. rheades (Pers.) Fiasson and Niemelä * | RU | CBS | – | – | MH866581 | – | – | |
| Inonotus P. Karst. | I. cuticularis (Bull.) P. Karst. * | – | JV 1109/89-J | – | KF446595 | – | KF446610 | – |
| I. griseus L.W. Zhou | CN | Dai 13436 | – | KX674583 | KX832925 | KY693959 | KX364919 | |
| I. vitis A.A. Brown, D.P, Lawr. And K. Baumgartner (T) | US | OC1/CBS 1453555 | Vitis vinifera | MN108118 | MN113944 | MN114509 | MN104164 | |
| Phellinotus Drechsler-Santos, Robledo and Rajchenb. | P. magnoporatus Salvador-Mont. And Drechsler-Santos | PE | USM 250523 | Ocotea aurantiodora | MZ954859 | MZ964981 | OK000625 | – |
| P. neoaridus Drechsler-Santos and Robledo * (T) | BR | URM 80362 | Caesalpinia sp. | KM211294 | KM211286 | – | – | |
| P. neoaridus | BR | URM77673 | – | MZ954857 | – | – | – | |
| P. neoaridus | BR | URM83203 | – | MZ954858 | MZ964977 | – | – | |
| P. neoaridus | BR | HUEFS 122186 | Cenostigma pyramidale | – | MZ964976 | – | – | |
| P. neoaridus | BR | URM 80579 | Caesalpinia sp. | – | MZ964978 | – | – | |
| P.piptadeniae (Teixeira) Drechsler-Santos and Robledo | BR | URM 80345 | Senegalia sp. | KM211291 | KM211283 | – | – | |
| P. piptadeniae | BR | URM 80322 | Mimosa sp. | KM211290 | KM211282 | – | – | |
| P. piptadeniae (T) | BR | URM 80361 | Mimosa sp. | KM211288 | KM211280 | – | – | |
| P. piptadeniae | BR | URM 80768 | Piptadenia stipulaceae | KM211289 | KM211281 | – | – | |
| P. piptadeniae | BR | URM 80766 | Mimosa sp. | KM211293 | KM211285 | – | – | |
| P. piptadeniae | BR | URM 80360 | Mimosa sp. | KM211292 | KM211284 | – | – | |
| P. piptadeniae | BR | FLOR 51451 | Piptadenia gonoacantha | MZ954839 | MZ964964 | – | – | |
| P. piptadeniae | BR | FLOR 63105 | Piptadenia gonoacantha | MZ954847 | MZ964971 | OK000617 | – | |
| P. piptadeniae | BR | FLOR 63111 | Piptadenia gonoacantha | MZ954845 | MZ964969 | OK000618 | – | |
| P. piptadeniae | BR | FLOR 62129 | – | MZ954840 | MZ964965 | – | – | |
| P. piptadeniae | BR | FLOR 62132 | – | MZ954841 | MZ964966 | – | – | |
| P. piptadeniae | BR | FLOR 63627 | Piptadenia gonoacantha | KP412305 | KP412282 | – | – | |
| P. piptadeniae | BR | FLOR 63101 | Piptadenia gonoacantha | MZ954846 | MZ964970 | OK000619 | – | |
| P. piptadeniae | UY | MVHC 5756 | Calliandra tweediei | MZ954842 | MZ964968 | – | – | |
| P. piptadeniae | UY | MVHC 5754 | Calliandra tweediei | MZ954843 | MZ964967 | – | – | |
| P. piptadeniae | UY | MVHC 5561 | Calliandra tweediei | MZ954844 | KT266877 | – | – | |
| P. piptadeniae | UY | MVHC 5562 | Calliandra tweediei | KT266876 | KT266878 | – | – | |
| P. piptadeniae | BR | FLOR 39572 | Piptadenia gonoacantha | MZ954848 | – | – | – | |
| P. teixeirae Salvador-Mont., Elias and Drechsler-Santos (T: corresponds to type specimens) | PE | USM 250528 | Pithecellobium excelsum | MZ954853 | MZ964972 | – | – | |
| Phellinotus Drechsler-Santos, Robledo and Rajchenb. | P. teixeirae | PE | USM 258362 | Libidibia glabrata | MZ954854 | MZ964975 | OK000621 | OK000626 |
| P. teixeirae | PE | USM 278225 | Libidibia glabrata | MZ954855 | MZ964974 | OK000622 | OK000627 | |
| P. teixeirae | BR | URM 80889 | Pytyrocarpa moniliformis | MZ954852 | – | – | – | |
| P. teixeirae | BR | URM 80403 | Piptadenia sp. | MZ054849 | – | – | – | |
| P. teixeirae | BR | URM 80636 | Pytyrocarpa moniliformis | MZ954850 | – | – | – | |
| P. teixeirae | PE | USM 258366 | Libidibia glabrata | MZ954856 | MZ964973 | – | – | |
| P. teixeirae | AR | CTES 515266 | – | MZ954851 | – | – | – | |
| P. teixeirae | CO | ACB 431 | Pithecellobium dulce | OR205894 | OR186199 | OR209163 | – | |
| P. teixeirae | CO | ACB 432 | Pithecellobium dulce | OR205895 | OR186200 | OR209164 | OR204685 | |
| P. teixeirae | CO | ACB 433 | Pithecellobium dulce | OR205896 | – | OR209165 | OR204686 | |
| P. teixeirae | CO | ACB 460 | Pithecellobium dulce | OR205897 | – | OR209166 | OR204687 | |
| P. teixeirae | CO | ACB 463 | Pithecellobium dulce | OR205898 | – | OR209167 | OR204688 | |
| P. teixeirae | CO | ACB 553 | Pithecellobium dulce | OR205899 | OR186201 | OR209168 | – | |
| P. teixeirae | CO | ACB 848 | Pithecellobium dulce | OR205900 | – | – | – | |
| P. xerophyticus Robledo, Urcelay and Drechsler-Santos (T) | AR | CORD 3551 | Prosopis sp. | – | MZ964979 | OK000624 | OK000629 | |
| P. xerophyticus | AR | CORD 3552 | Prosopis sp. | – | MZ964980 | OK000623 | OK000628 | |
| Phylloporia Murrill | P. crataegi L.W. Zhou and Y.C. Dai | CN | Dai 18133 | Crataegus spp. | MH151191 | MH165865 | MH167431 | MH161224 |
| P. elegans Ferreira-Lopes, Robledo and Drechsler-Santos | BR | FLOR 51178 | – | KJ639049 | KJ631408 | – | – | |
| P. gabonensis Decock and Yombiy. | GA | MUCL 55571 | Dichostema glaucescens | KU198355 | KU198353 | – | – | |
| P. nodostipitata Ferreira-Lopes, Robledo and Drechsler-Santos | BR | FLOR 51153 | On living roots | KJ639057 | KJ631414 | – | – | |
| P. parasitica Murrill * | AR | Leif Ryvarden 19843 | – | KU198361 | – | – | – | |
| P. pectinata (Klotzsch) Ryvarden | AU | Voucher 113 | – | MH151181 | MH165867 | MH167421 | MH161213 | |
| P. pseudopectinata Yuan Y. Chen and B.K. Cui | CN | Cui 13749 | Angiosperm | MF410323 | KX242356 | MH167429 | MH161222 | |
| Rajchenbergia Salvador-Montoya, Popoff & Drechsler-Santos | R. mangrovei (Y.C. Dai, X.H. Ji and J. Vlasák) Salvador-Mont. Drechsler-Santos and Popoff | FR | JV1612/25-J | Conocarpus erectus | MG657325 | MG657331 | – | – |
| R. pertenuis (Xavier de Lima and Oliveira-Filho) Salvador-Mont. Popoff and Drechsler-Santos | BR | PPT111 | On dead wood | MG806100 | MG806101 | – | – | |
| R. tenuissima (H.Y. Yu, C.L. Zhao and Y.C. Dai) Salvador-Mont. Drechsler-Santos and Popoff | CN | Dai12245 | Angiosperm | KC999902 | KC456242 | – | – | |
| Sanghuangporus Sheng H. Wu, L.W. Zhou and Y.C. Dai | S. baumii (Pilát) L.W. Zhou and Y.C. Dai | CN | Dai 16900 | – | MF772785 | MF772802 | MF977782 | MF973476 |
| S. sanghuang (Sheng H. Wu, T. Hatt and Y.C. Dai) Sheng H. Wu, L.W. Zhouand Y.C. Dai * | CN | Cui 14419 | – | MF772789 | MF772810 | MF977790 | MF973483 | |
| S. vaninii (Ljub) L.W. Zhou and Y.C. Dai | US | DMR-95-1-T | Populus tremuloides | KU139198 | KU139258 | KU139380 | KU139318 | |
| S. zonatus (Y.C. Dai and X.M. Tian) L.W. Zhou and Y.C. Dai | CN | Dai 10841 | Living angiosperm tree | JQ860306 | KP030775 | MF977797 | MF973490 | |
| Tropicoporus L.W. Zhou, Y.C. Dai and Sheng H. Wu | T. dependens (Murrill) L.W. Zhou, Y.C. Dai and Vlasák | US | JV 0409/12-J | Angiosperm wood | KC778777 | MF772818 | MF977799 | MF973492 |
| T. drechsleri Salvador-Mont. and Popoff | AR | CTES 570140 | Patagonula americana | MG242439 | MG242444 | – | – | |
| T. excentrodendri L.W. Zhou and Y.C. Dai * (T) | CN | Yuan 6232 | Excentrodendron tonkinense | KP030790 | – | – | – | |
| T. texanus A.A. Brown, D.P. Lawr. and K. Baumgartner (T) | US | TX9-CBS 145.357 | Vitis vinifera | MN108124 | MN113950 | MN114515 | MN104178 | |
| Root | P. igniarius (L.) Quél. * | CZ | JV 9411/5 | – | KR013061 | – | KR013092 | – |
| Gene Name | Taxa | Length | S | π | θW | Tajima’s D |
|---|---|---|---|---|---|---|
| ITS | All | 737 | 246 | 0.032 | 59.32 | −2.935 ** |
| ITS | P. piptadeniae | 457 | 38 | 0.002 | 11.24 | −3.663 *** |
| ITS | P. teixeirae | 628 | 13 | 0.005 | 4.08 | −0.561 |
| LSU | All | 992 | 170 | 0.028 | 42.9 | −2.424 * |
| LSU | P. neoaridus | 746 | 4 | 0.0013 | 2.181 | −5.07 *** |
| LSU | P. piptadeniae | 557 | 44 | 0.0018 | 13.26 | −3.87 *** |
| LSU | P. teixeirae | 726 | 16 | 0.002 | 6.53 | −3.049 *** |
| RBP2 | All | 1247 | 61 | 0.022 | 23.52 | 0.289 |
| RBP2 | P. teixeirae | 1201 | 5 | 0.002 | 2.189 | 0.196 |
| TEF | All | 504 | 117 | 0.06 | 38.74 | −1.134 |
| Species/Locality Level | Df | Sum Sq | Mean Sq | Sigma | % | p-Value |
|---|---|---|---|---|---|---|
| Between species | 3 | 1812.04 | 604.01 | 79.28 | 70.09 | 0.0019 |
| Between samples within spp. | 4 | 148.81 | 37.20 | 1.11 | 0.98 | 0.117 |
| Between localities | 28 | 916.14 | 32.72 | 32.72 | 28.93 | 0.0009 |
| Total | 35 | 2877 | 82.20 | 113.11 | 100 | – |
| Species level | ||||||
| Between species | 3 | 1812.04 | 604.01 | 79.84 | 70.58 | 0.0009 |
| Between samples | 32 | 1064.96 | 33.23 | 33.23 | 29.42 | – |
| Total | 35 | 2877 | 82.20 | 113.12 | 100 | – |
| Locality level | ||||||
| Between localities | 3 | 410.58 | 136.87 | 9.64 | 11.11 | 0.044 |
| Between samples | 32 | 2466.42 | 77.08 | 77.076 | 88.89 | – |
| Total | 35 | 2877 | 82.20 | 86.71 | 100 | – |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bolaños-Rojas, A.C.; Londoño-Caicedo, J.M.; Cortés, A.J.; Motato-Vásquez, V. Phylogenetic Diversity, Host Specificity, and Distribution of the Wood-Decaying Fungus Phellinotus teixeirae in Western Colombia’s Seasonally Dry Tropical Forest. Forests 2024, 15, 1008. https://doi.org/10.3390/f15061008
Bolaños-Rojas AC, Londoño-Caicedo JM, Cortés AJ, Motato-Vásquez V. Phylogenetic Diversity, Host Specificity, and Distribution of the Wood-Decaying Fungus Phellinotus teixeirae in Western Colombia’s Seasonally Dry Tropical Forest. Forests. 2024; 15(6):1008. https://doi.org/10.3390/f15061008
Chicago/Turabian StyleBolaños-Rojas, Ana C., Jorge M. Londoño-Caicedo, Andrés J. Cortés, and Viviana Motato-Vásquez. 2024. "Phylogenetic Diversity, Host Specificity, and Distribution of the Wood-Decaying Fungus Phellinotus teixeirae in Western Colombia’s Seasonally Dry Tropical Forest" Forests 15, no. 6: 1008. https://doi.org/10.3390/f15061008







