Eight-Year Survival and Growth of Sakhalin Fir (Abies sachalinensis) Seedlings with One Weeding Operation: Impact of Mechanical Site Preparation, Vegetation Release, Summer Planting, Stock Type, and Forwarder Trail
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site, Site Preparation, and Planting Design
2.2. Field Measurements
2.3. Statistical Analyses
2.3.1. Initial Seedling Distribution and Statistical Approach
2.3.2. Analyses of Differences between Stock Types and between Years
2.3.3. Analyses of Survival Rate and Height Growth Using GLMs and LMMs
2.3.4. Analyses of Survival Decline in the Sixth Year in the Forwarder Trail and Height Growth and Competition in the Fifth Year
3. Results
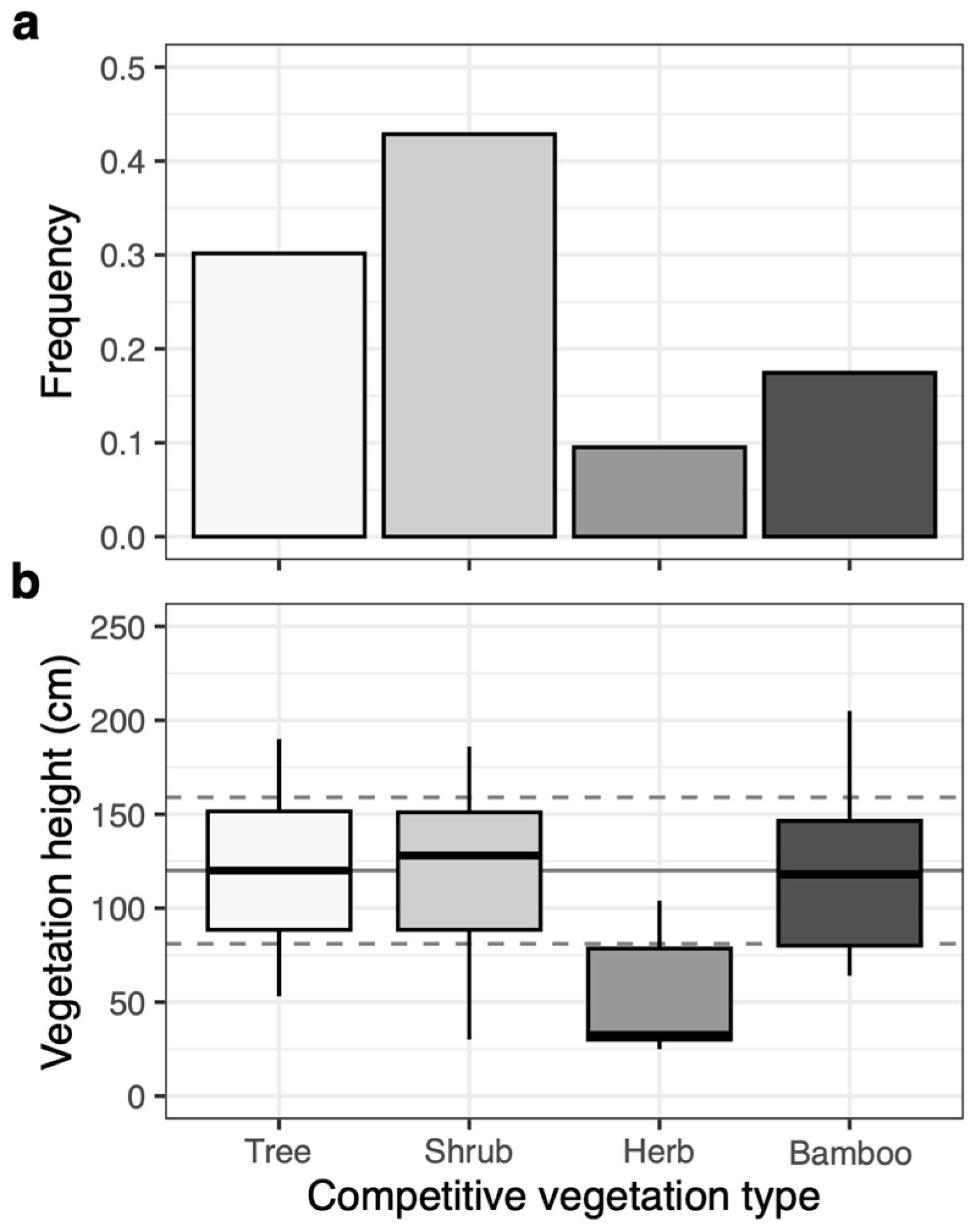
3.1. Competing Vegetation
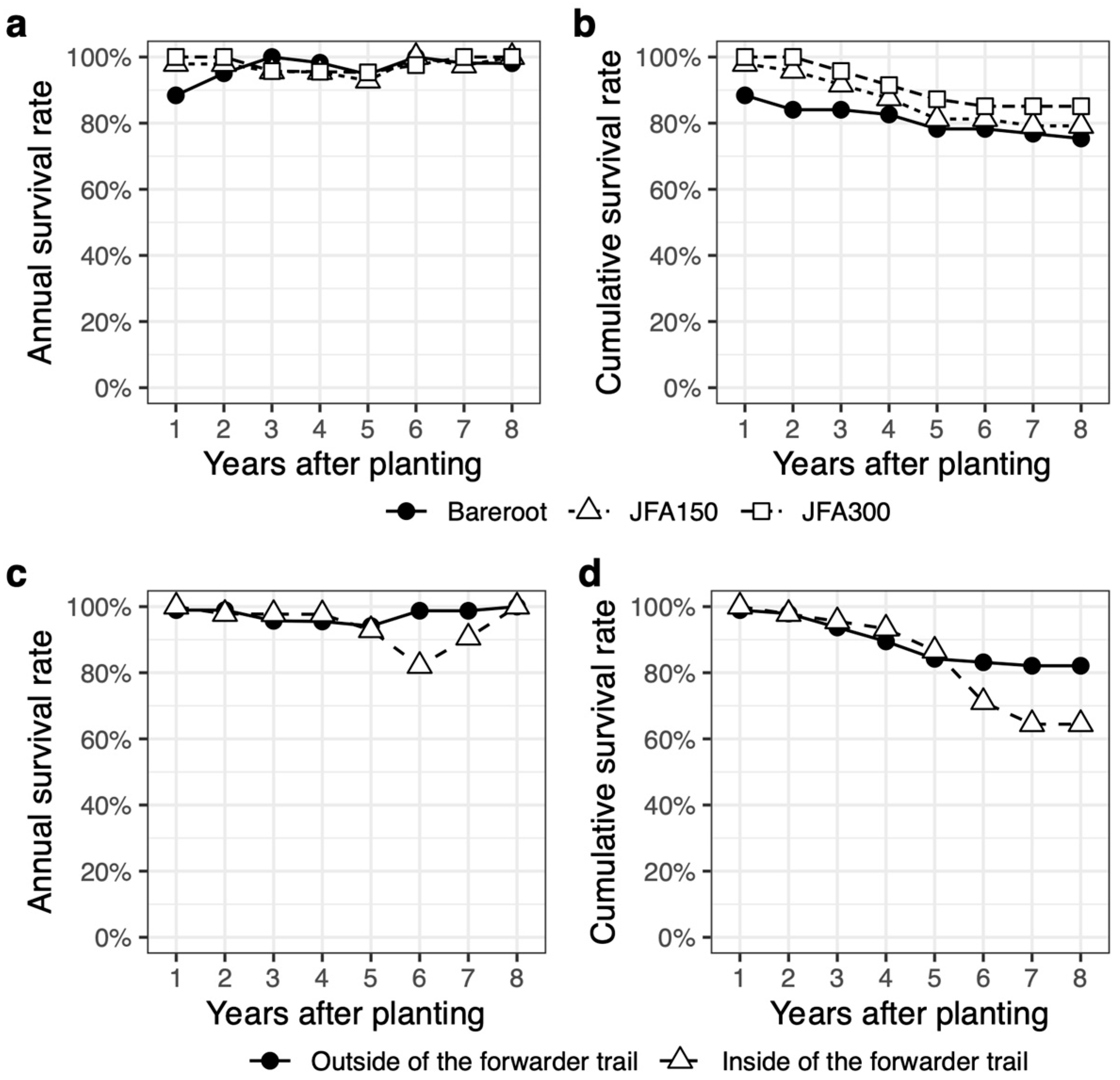
3.2. Survival Rate
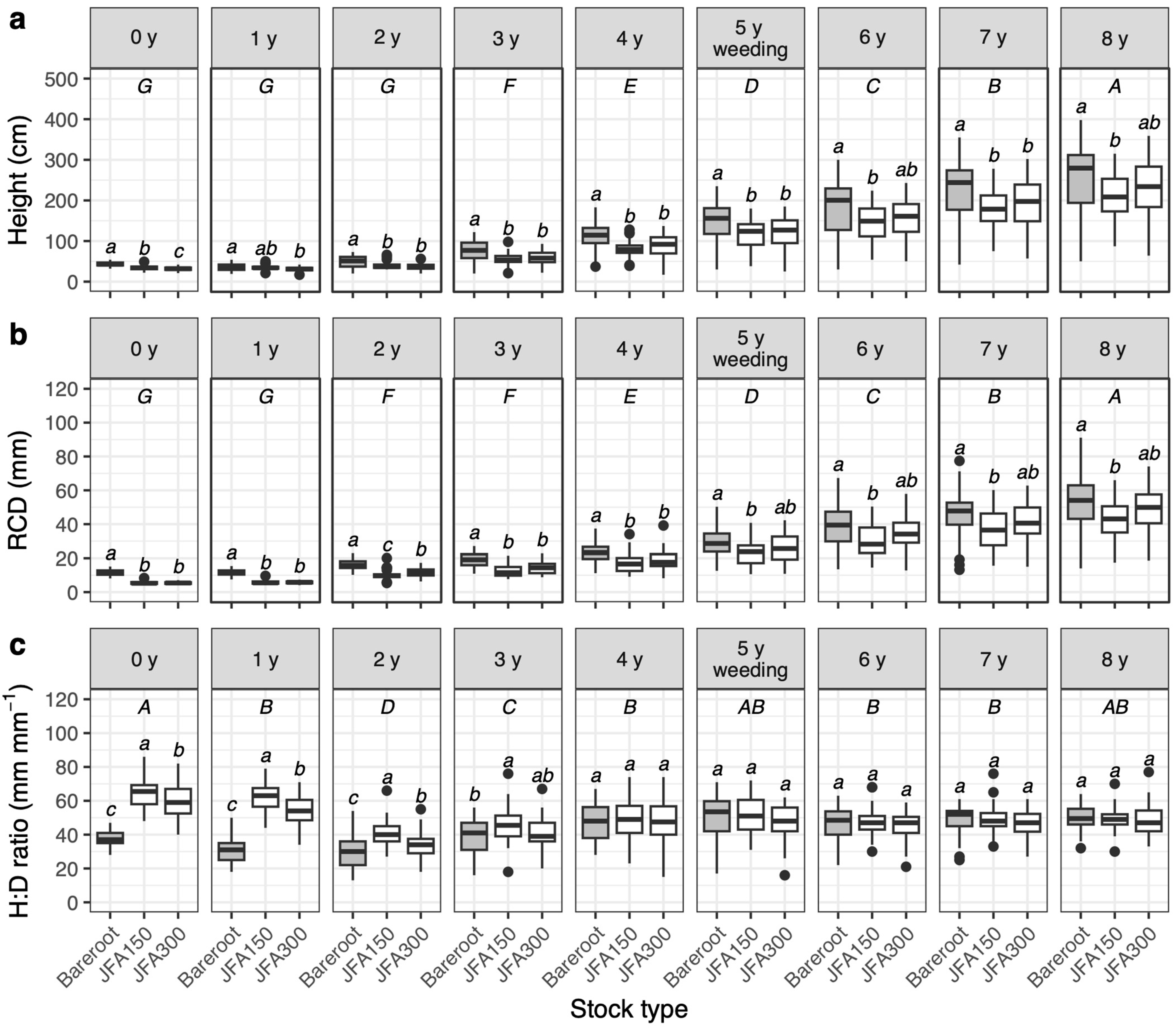
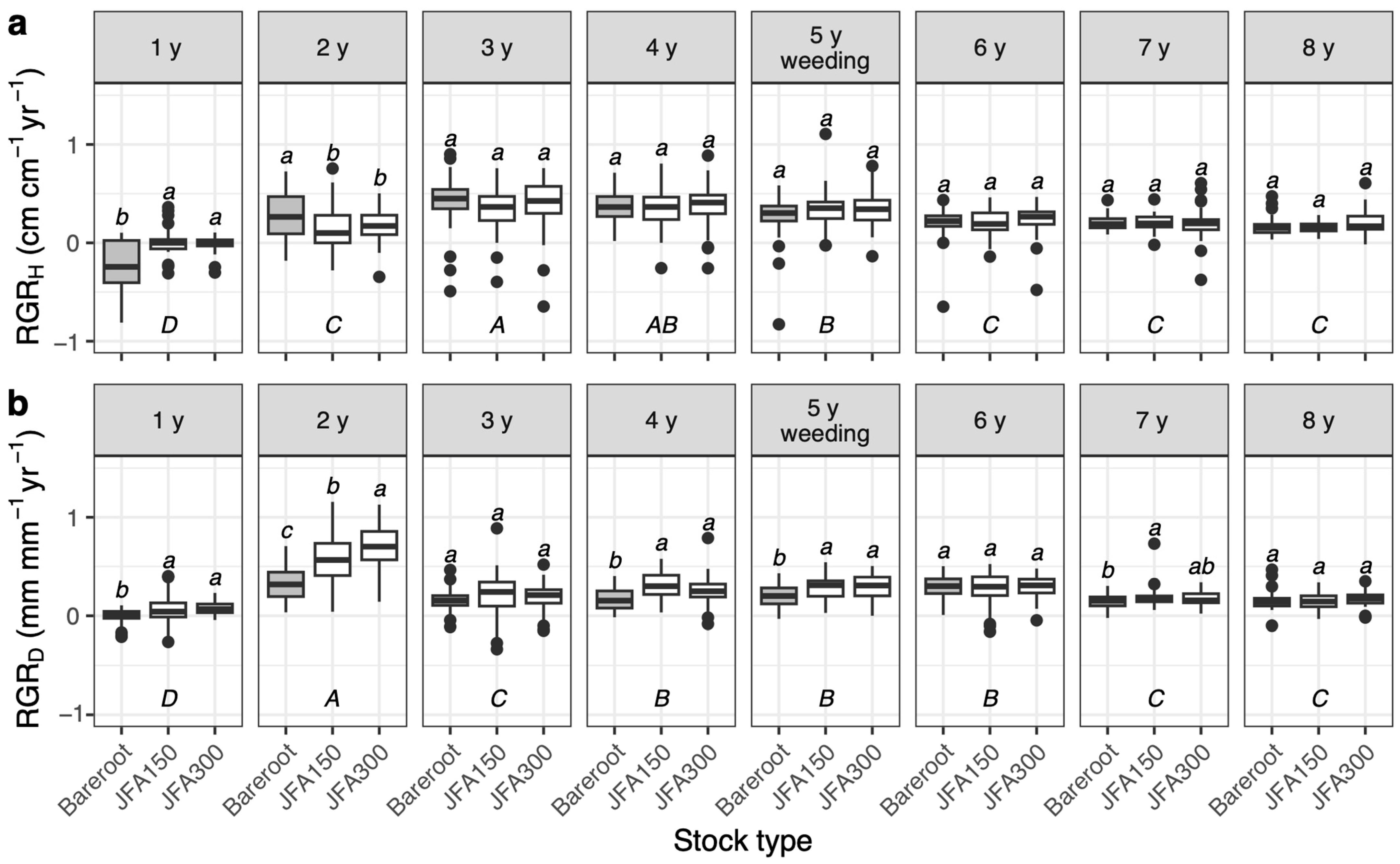
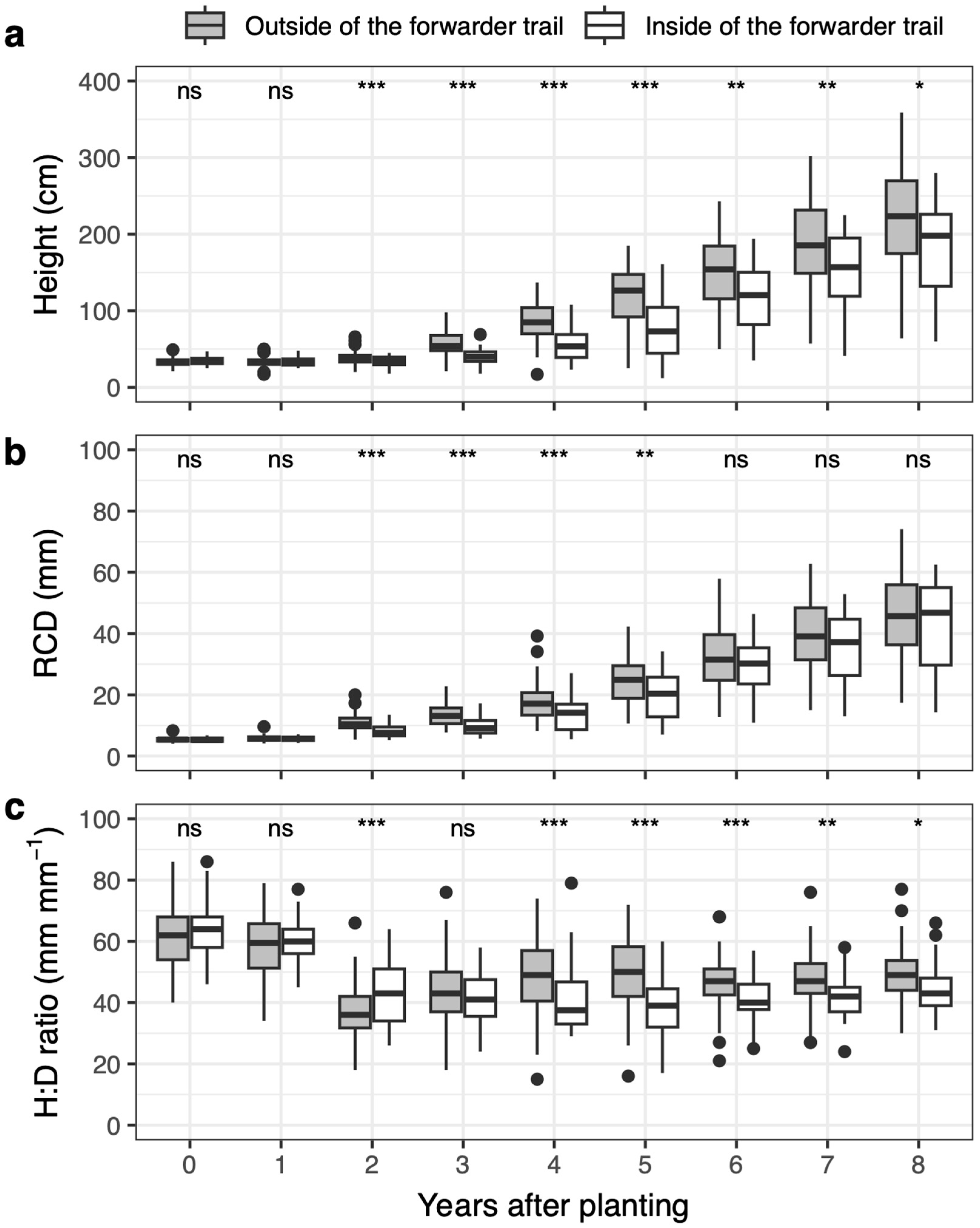
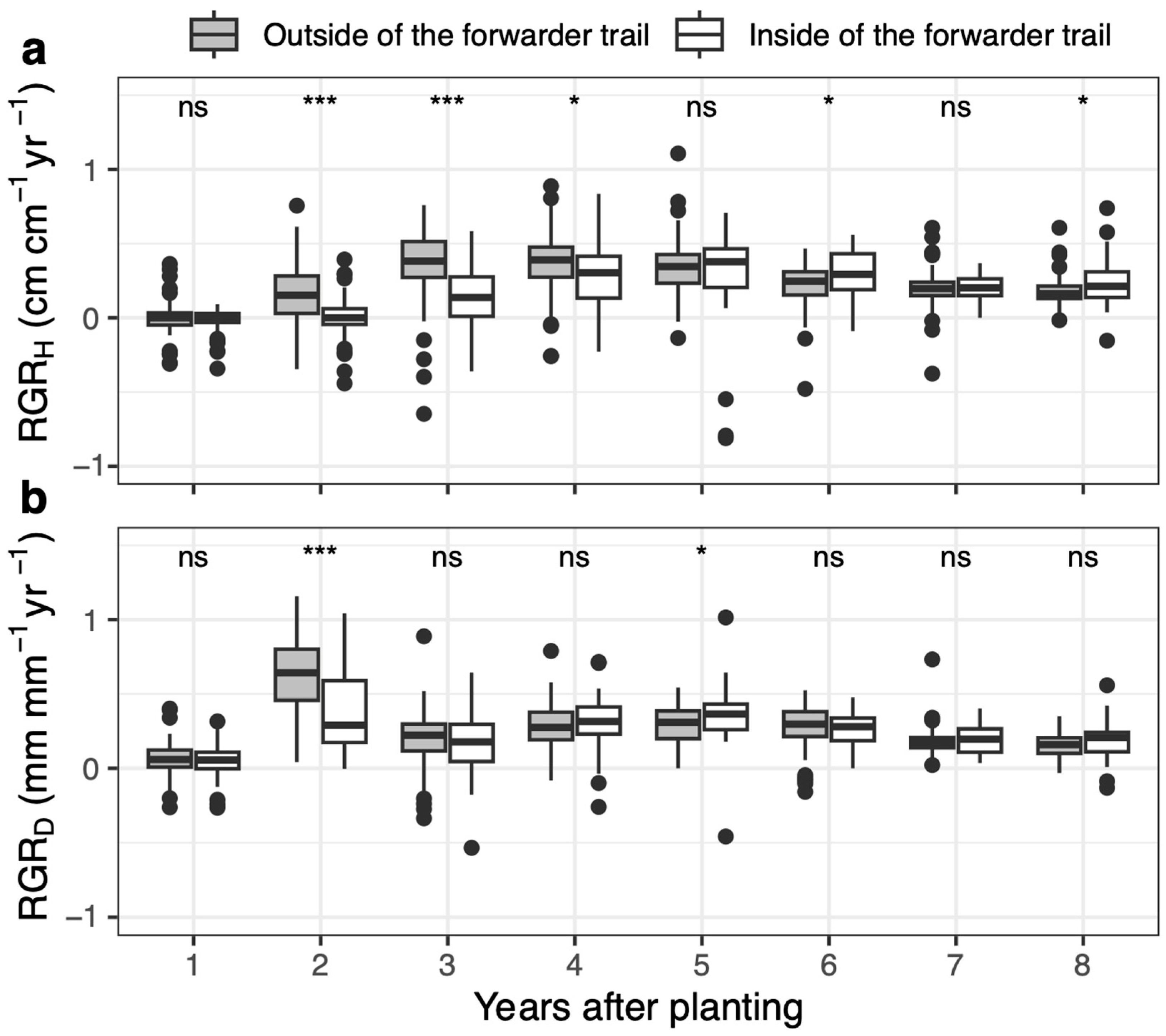
3.3. Seedling Growth
4. Discussion
4.1. Success in Sakhalin Fir Reforestation with One Weeding Operation in Eight Years
4.2. Negative Effects of Forwarder Trail
4.3. Effects of Stock Type and Summer Planting
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wagner, R.G.; Little, K.M.; Richardson, B.; McNabb, K. The role of vegetation management for enhancing productivity of the world’s forests. Forestry 2006, 79, 57–79. [Google Scholar] [CrossRef]
- Willoughby, I.; Balandier, P.; Bentsen, N.S.; McCarthy, N.; Claridge, J. Forest Vegetation Management in Europe: Current Practice and Future Requirements; COST Office: Brussels, Belgium, 2009; p. 156. [Google Scholar]
- McCarthy, N.; Bentsen, N.S.; Willoughby, I.; Balandier, P. The state of forest vegetation management in Europe in the 21st century. Eur. J. For. Res. 2011, 130, 7–16. [Google Scholar] [CrossRef]
- Richardson, B.; Vanner, A.; Ray, J.; Davenhill, N.; Coker, G. Mechanisms of Pinus radiata growth suppression by some common forest weed species. N. Z. J. For. Sci. 1996, 26, 421–437. [Google Scholar]
- Biring, B.S.; Comeau, P.G.; Fielder, P. Long-term effects of vegetation control treatments for release of Engelmann spruce from a mixed-shrub community in Southern British Columbia. Ann. For. Sci. 2003, 60, 681–690. [Google Scholar] [CrossRef]
- Davis, M.A.; Wrage, K.J.; Reich, P.B.; Tjoelker, M.G.; Schaeffer, T.; Muermann, C. Survival, growth, and photosynthesis of tree seedlings competing with herbaceous vegetation along a water-light-nitrogen gradient. Plant Ecol. 1999, 145, 341–350. [Google Scholar] [CrossRef]
- Oshima, Y. Ecological studies of Sasa communities. Bot. Mag. Tokyo 1961, 74, 199–210. [Google Scholar] [CrossRef]
- Nakashizuka, T.; Numata, M. Regeneration process of climax beech forests : I. Structure of a beech forest with the undergrowth of Sasa. Jpn. J. Ecol. 1982, 32, 57–67. [Google Scholar] [CrossRef]
- Noguchi, M.; Yoshida, T. Regeneration responses influenced by single-tree selection harvesting in a mixed-species tree community in northern Japan. Can. J. For. Res. 2007, 37, 1554–1562. [Google Scholar] [CrossRef]
- Nakagawa, M. A weeding-duration model for Abies sachalinensis plantations in Hokkaido, northern Japan. J. For. Res. 2013, 24, 131–136. [Google Scholar] [CrossRef]
- Ammer, C.; Balandier, P.; Bentsen, N.S.; Coll, L.; Löf, M. Forest vegetation management under debate: An introduction. Eur. J. For. Res. 2011, 130, 1–5. [Google Scholar] [CrossRef]
- Thiffault, N.; Roy, V. Living without herbicides in Québec (Canada): Historical context, current strategy, research and challenges in forest vegetation management. Eur. J. For. Res. 2011, 130, 117–133. [Google Scholar] [CrossRef]
- Ramantswana, M.; Guerra, S.P.S.; Ersson, B.T. Advances in the mechanization of regenerating plantation forests: A review. Curr. For. Rep. 2020, 6, 143–158. [Google Scholar] [CrossRef]
- Fortier, J.; Messier, C. Are chemical or mechanical treatments more sustainable for forest vegetation management in the context of the TRIAD? For. Chron. 2006, 82, 806–818. [Google Scholar] [CrossRef]
- Wyatt, S.; Rousseau, M.-H.; Nadeau, S.; Thiffault, N.; Guay, L. Social concerns, risk and the acceptability of forest vegetation management alternatives: Insights for managers. For. Chron. 2011, 87, 274–289. [Google Scholar] [CrossRef]
- Iddris, N.A.-A.; Formaglio, G.; Paul, C.; von Groß, V.; Chen, G.; Angulo-Rubiano, A.; Berkelmann, D.; Brambach, F.; Darras, K.F.A.; Krashevska, V.; et al. Mechanical weeding enhances ecosystem multifunctionality and profit in industrial oil palm. Nat. Sustain. 2023, 6, 683–695. [Google Scholar] [CrossRef]
- Mallik, A.; Bell, F.; Gong, Y. Effectiveness of delayed brush cutting and herbicide treatments for vegetation control in a seven-year-old jack pine plantation in northwestern Ontario, Canada. Silva Fenn. 2002, 36, 505–519. [Google Scholar] [CrossRef]
- Masaki, T.; Oguro, M.; Yamashita, N.; Otani, T.; Utsugi, H. Reforestation following harvesting of conifer plantations in Japan: Current issues from silvicultural and ecological perspectives. Reforesta 2017, 3, 125–141. [Google Scholar] [CrossRef]
- Ishizuka, W.; Goto, S. Modeling intraspecific adaptation of Abies sachalinensis to local altitude and responses to global warming, based on a 36-year reciprocal transplant experiment. Evol. Appl. 2012, 5, 229–244. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, M.; Kurahashi, A.; Kaji, M.; Hogetsu, T. The effects of selection cutting on regeneration of Picea jezoensis and Abies sachalinensis in the sub-boreal forests of Hokkaido, northern Japan. For. Ecol. Manag. 2001, 146, 15–23. [Google Scholar] [CrossRef]
- Akashi, N.; Nitta, N.; Ohno, Y. Effect of forest management on understory vascular plants in planted Abies sachalinensis forests. For. Ecol. Manag. 2021, 497, 119521. [Google Scholar] [CrossRef]
- Nakagawa, M.; Kanno, M.; Yasaka, M. A weeding-duration model for Larix kaempferi plantations in Hokkaido, northern Japan. J. For. Res. 2017, 16, 319–324. [Google Scholar] [CrossRef]
- Takiya, M. Silvicultural method of Larix kaempferi and Abies sachalinensis to the low-cost forestry in Hokkaido. Boreal For. Res. 2014, 62, 3–6. (In Japanese) [Google Scholar]
- Kayama, M.; Koike, T. Growth characteristics of dwarf bamboo distributed in the northern part of Japan. In Bamboo—Current and Future Prospects; Khalil, H.A., Ed.; IntechOpen: London, UK, 2018; pp. 185–199. [Google Scholar] [CrossRef]
- Lei, T.T.; Koike, T. Functional leaf phenotypes for shaded and open environments of a dominant dwarf bamboo (Sasa senanensis) in Northern Japan. Int. J. Plant Sci. 1998, 159, 812–820. [Google Scholar] [CrossRef]
- Tobita, H.; Utsugi, H.; Kitao, M.; Kayama, M.; Uemura, A.; Kitaoka, S.; Maruyama, Y. Variation in photoinhibition among Sasa senanensis, Quercus mongolica, and Acer mono in the understory of a deciduous broad-leaved forest exposed to canopy gaps caused by typhoons. Trees 2010, 24, 307–319. [Google Scholar] [CrossRef]
- Löf, M.; Dey, D.C.; Navarro, R.M.; Jacobs, D.F. Mechanical site preparation for forest restoration. New For. 2012, 43, 825–848. [Google Scholar] [CrossRef]
- Fargione, J.; Haase, D.L.; Burney, O.T.; Kildisheva, O.A.; Edge, G.; Cook-Patton, S.C.; Chapman, T.; Rempel, A.; Hurteau, M.D.; Davis, K.T.; et al. Challenges to the reforestation pipeline in the United States. Front. For. Glob. Chang. 2021, 4, 629198. [Google Scholar] [CrossRef]
- Oya, S.; Kuramoto, S.; Koyama, Y.; Nakazawa, M.; Taki, S.; Utsugi, H. Effects of mechanical site preparation on controlling competing vegetation and weeding reduction. J. Jpn For. Eng. Soc. 2021, 36, 99–110, (In Japanese with English Summary). [Google Scholar] [CrossRef]
- Harayama, H.; Tsuyama, I.; Kitao, M.; Yamada, T.; Furuya, N.; Utsugi, H.; Sasaki, S. Effects of seedling size, stock type, and mechanical site preparation method on initial survival and growth of Japanese larch (Larix kaempferi) seedlings. Forests 2023, 14, 784. [Google Scholar] [CrossRef]
- Yamazaki, H.; Yoshida, T. Various scarification treatments produce different regeneration potentials for trees and forbs through changing soil properties. J. For. Res. 2020, 25, 41–50. [Google Scholar] [CrossRef]
- Harayama, H.; Tsuyama, I.; Uemura, A.; Kitao, M.; Han, Q.; Kuramoto, S.; Utsugi, H. Growth and survival of hybrid larch F1 (Larix gmelinii var. japonica × L. kaempferi) and Japanese larch under various intensities of competition. New For. 2023, 54, 945–961. [Google Scholar] [CrossRef]
- Kitao, M.; Harayama, H.; Han, Q.; Agathokleous, E.; Uemura, A.; Furuya, N.; Ishibashi, S. Springtime photoinhibition constrains regeneration of forest floor seedlings of Abies sachalinensis after a removal of canopy trees during winter. Sci. Rep. 2018, 8, 6310. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Noguchi, M.; Akibayashi, Y.; Noda, M.; Kadomatsu, M.; Sasa, K. Twenty years of community dynamics in a mixed conifer broad-leaved forest under a selection system in northern Japan. Can. J. For. Res. 2006, 36, 1363–1375. [Google Scholar] [CrossRef]
- Li, J.; Morimoto, J.; Hotta, W.; Suzuki, S.N.; Owari, T.; Toyoshima, M.; Nakamura, F. The 30-year impact of post-windthrow management on the forest regeneration process in northern Japan. Landsc. Ecol. Eng. 2023, 19, 227–242. [Google Scholar] [CrossRef]
- Claveau, Y.; Messier, C.; Comeau, P.G.; Coates, K.D. Growth and crown morphological responses of boreal conifer seedlings and saplings with contrasting shade tolerance to a gradient of light and height. Can. J. For. Res. 2002, 32, 458–468. [Google Scholar] [CrossRef]
- Dobrowolska, D.; Bončina, A.; Klumpp, R. Ecology and silviculture of silver fir (Abies alba Mill.): A review. J. For. Res. 2017, 22, 326–335. [Google Scholar] [CrossRef]
- Grossnickle, S.C.; El-Kassaby, Y.A. Bareroot versus container stocktypes: A performance comparison. New For. 2016, 47, 1–51. [Google Scholar] [CrossRef]
- Cambi, M.; Certini, G.; Neri, F.; Marchi, E. The impact of heavy traffic on forest soils: A review. For. Ecol. Manag. 2015, 338, 124–138. [Google Scholar] [CrossRef]
- Labelle, E.R.; Hansson, L.; Högbom, L.; Jourgholami, M.; Laschi, A. Strategies to mitigate the effects of soil physical disturbances caused by forest machinery: A comprehensive review. Curr. For. Rep. 2022, 8, 20–37. [Google Scholar] [CrossRef]
- Harayama, H.; Tobita, H.; Kitao, M.; Kon, H.; Ishizuka, W.; Kuromaru, M.; Kita, K. Enhanced summer planting survival of Japanese larch container-grown seedlings. Forests 2021, 12, 1115. [Google Scholar] [CrossRef]
- Sasaki, S. Some considerations of tree planting layouts for future full-mechanized harvesting for gentle terrain forests in Hokkaido, Japan. Boreal For. Res. 2014, 62, 7–10. (In Japanese) [Google Scholar] [CrossRef]
- Ampoorter, E.; De Frenne, P.; Hermy, M.; Verheyen, K. Effects of soil compaction on growth and survival of tree saplings: A meta-analysis. Basic and Appl. Ecol. 2011, 12, 394–402. [Google Scholar] [CrossRef]
- Sugai, T.; Yokoyama, S.; Tamai, Y.; Mori, H.; Marchi, E.; Watanabe, T.; Satoh, F.; Koike, T. Evaluating soil–root interaction of hybrid larch seedlings planted under soil compaction and nitrogen loading. Forests 2020, 11, 947. [Google Scholar] [CrossRef]
- Mariotti, B.; Hoshika, Y.; Cambi, M.; Marra, E.; Feng, Z.; Paoletti, E.; Marchi, E. Vehicle-induced compaction of forest soil affects plant morphological and physiological attributes: A meta-analysis. For. Ecol. Manag. 2020, 462, 118004. [Google Scholar] [CrossRef]
- Hunt, R. Plant Growth Curves. The Functional Approach to Plant Growth Analysis; Edward Arnold Ltd.: London, UK, 1982; p. 248. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous inference in general parametric models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef] [PubMed]
- Firth, D. Bias reduction of maximum likelihood estimates. Biometrika 1993, 80, 27–38. [Google Scholar] [CrossRef]
- Kassambara, A. ggpubr: ‘ggplot2’ Based Publication Ready Plots. R Package Version 0.6.0. 2023. Available online: https://CRAN.R-project.org/package=ggpubr (accessed on 1 May 2024).
- Fox, J.; Weisberg, S. An {R} Companion to Applied Regression, 3rd ed.; Sage Publications: Thousand Oaks, CA, USA, 2019; p. 608. [Google Scholar]
- Bartoń, K. MuMIn: Multi-Model Inference. R Package Version 1.47.5. 2023. Available online: https://CRAN.R-project.org/package=MuMIn (accessed on 1 May 2024).
- Funakoshi, S. Shoot growth and winter bud formation in Abies sachalinensis Mast. Res. Bull. Coll. Exp. For. Hokkaido Univ. 1985, 42, 785–808, (In Japanese with English Summary). [Google Scholar]
- Nakagawa, M.; Kurahashi, A. Factors affecting soil-based natural regeneration of Abies sachalinensis following timber harvesting in a sub-boreal forest in Japan. New For. 2005, 29, 199–205. [Google Scholar] [CrossRef]
- Yoshida, T.; Iga, Y.; Ozawa, M.; Noguchi, M.; Shibata, H. Factors influencing early vegetation establishment following soil scarification in a mixed forest in northern Japan. Can. J. For. Res. 2005, 35, 175–188. [Google Scholar] [CrossRef]
- Yoshida, T.; Yamazaki, H.; Miyamoto, T. Scarification with surface soil replacement can promote understory reinitiation as well as the growth of a secondary birch stand. J. For. Res. 2022, 28, 51–56. [Google Scholar] [CrossRef]
- Iijima, H.; Shibuya, M.; Saito, H. Examination of the coexistence mechanism of two major conifers in Hokkaido, northern Japan, based on differences in suitable germination conditions and shade tolerance. Écoscience 2015, 16, 352–360. [Google Scholar] [CrossRef]
- Wright, E.F.; Canham, C.D.; Coates, K.D. Effects of suppression and release on sapling growth for 11 tree species of northern, interior British Columbia. Can. J. For. Res. 2000, 30, 1571–1580. [Google Scholar] [CrossRef]
- Hansen, J.; Beck, E. The fate and path of assimilation products in the stem of 8-year-old Scots pine (Pinus sylvestris L.) trees. Trees 1990, 4, 16–21. [Google Scholar] [CrossRef]
- Hansen, J.; Beck, E. Seasonal changes in the utilization and turnover of assimilation products in 8-year-old Scots pine (Pinus sylvestris L.) trees. Trees 1994, 8, 172–182. [Google Scholar] [CrossRef]
- Wyka, T.P.; Żytkowiak, R.; Oleksyn, J. Seasonal dynamics of nitrogen level and gas exchange in different cohorts of Scots pine needles: A conflict between nitrogen mobilization and photosynthesis? Eur. J. For. Res. 2016, 135, 483–493. [Google Scholar] [CrossRef]
- Rab, M.A. Recovery of soil physical properties from compaction and soil profile disturbance caused by logging of native forest in Victorian Central Highlands, Australia. For. Ecol. Manag. 2004, 191, 329–340. [Google Scholar] [CrossRef]
- Sohrabi, H.; Jourgholami, M.; Tavankar, F.; Venanzi, R.; Picchio, R. Post-harvest evaluation of soil physical properties and natural regeneration growth in steep-slope terrains. Forests 2019, 10, 1034. [Google Scholar] [CrossRef]
- Kozlowski, T.T. Soil compaction and growth of woody plants. Scand. J. For. Res. 1999, 14, 596–619. [Google Scholar] [CrossRef]
- Startsev, A.D.; McNabb, D.H. Effects of skidding on forest soil infiltration in west-central Alberta. Can. J. Soil Sci. 2000, 80, 617–624. [Google Scholar] [CrossRef]
- Sutherland, B.J. Preventing Soil Compaction and Rutting in the Boreal Forest of Western Canada: A Practical Guide to Operating Timber-Harvesting Equipment; FERIC: Pointe-Claire, QC, Canada, 2003; p. 52. [Google Scholar]
- Picchio, R.; Mederski, P.S.; Tavankar, F. How and how much, do harvesting activities affect forest soil, regeneration and stands? Curr. For. Rep. 2020, 6, 115–128. [Google Scholar] [CrossRef]
- DeArmond, D.; Ferraz, J.B.S.; Higuchi, N. Natural recovery of skid trails: A review. Can. J. For. Res. 2021, 51, 948–961. [Google Scholar] [CrossRef]
- Zenner, E.K.; Fauskee, J.T.; Berger, A.L.; Puettmann, K.J. Impacts of skidding traffic intensity on soil disturbance, soil recovery, and aspen regeneration in north central Minnesota. North. J. A. For. 2007, 24, 177–183. [Google Scholar] [CrossRef]
- Goutal, N.; Boivin, P.; Ranger, J. Assessment of the natural recovery rate of soil specific volume following forest soil compaction. Soil Sci. Soc. Am. J. 2012, 76, 1426–1435. [Google Scholar] [CrossRef]
- Picchio, R.; Tavankar, F.; Nikooy, M.; Pignatti, G.; Venanzi, R.; Lo Monaco, A. Morphology, growth and architecture response of beech (Fagus orientalis lipsky) and maple tree (Acer velutinum boiss.) seedlings to soil compaction stress caused by mechanized logging operations. Forests 2019, 10, 771. [Google Scholar] [CrossRef]
- Naghdi, R.; Solgi, A.; Labelle, E.R.; Zenner, E.K. Influence of ground-based skidding on physical and chemical properties of forest soils and their effects on maple seedling growth. Eur. J. For. Res. 2016, 135, 949–962. [Google Scholar] [CrossRef]
- Man, R.; Greenway, K.J. Effects of soil moisture and species composition on growth and productivity of trembling aspen and white spruce in planted mixtures: 5-year results. New For. 2011, 44, 23–38. [Google Scholar] [CrossRef]
- Pinto, J.R.; Marshall, J.D.; Dumroese, R.K.; Davis, A.S.; Cobos, D.R. Establishment and growth of container seedlings for reforestation: A function of stocktype and edaphic conditions. For. Ecol. Manag. 2011, 261, 1876–1884. [Google Scholar] [CrossRef]
- Collet, C.; Chenost, C. Using competition and light estimates to predict diameter and height growth of naturally regenerated beech seedlings growing under changing canopy conditions. Forestry 2006, 79, 489–502. [Google Scholar] [CrossRef]
- Craine, J.M.; Dybzinski, R.; Robinson, D. Mechanisms of plant competition for nutrients, water and light. Funct. Ecol. 2013, 27, 833–840. [Google Scholar] [CrossRef]
- Brais, S. Persistence of soil compaction and effects on seedling growth in northwestern Quebec. Soil Sci. Soc. Am. J. 2001, 65, 1263–1271. [Google Scholar] [CrossRef]
- Harayama, H.; Tsuyama, I. Performance of container seedlings after outplanting analyzed from planting database in Hokkaido. For. Tree Breed. Hokkaido 2020, 63, 10–14. (In Japanese) [Google Scholar]
- Rose, R.; Scott Ketchum, J. Interaction of initial seedling diameter, fertilization and weed control on Douglas-fir growth over the first four years after planting. Ann. For. Sci. 2003, 60, 625–635. [Google Scholar] [CrossRef]
- Thiffault, N.; Jobidon, R.; Munson, A.D. Comparing large containerized and bareroot conifer stock on sites of contrasting vegetation composition in a non-herbicide scenario. New For. 2014, 45, 875–891. [Google Scholar] [CrossRef]
- Grossnickle, S.C.; MacDonald, J.E. Why seedlings grow: Influence of plant attributes. New For. 2018, 49, 1–34. [Google Scholar] [CrossRef]
- Morrissey, R.C.; Jacobs, D.F.; Davis, A.S.; Rathfon, R.A. Survival and competitiveness of Quercus rubra regeneration associated with planting stocktype and harvest opening intensity. New For. 2010, 40, 273–287. [Google Scholar] [CrossRef]
- Ivetic, V.; Grossnickle, S.; Skoric, M. Forecasting the field performance of Austrian pine seedlings using morphological attributes. iForest 2017, 10, 99–107. [Google Scholar] [CrossRef]
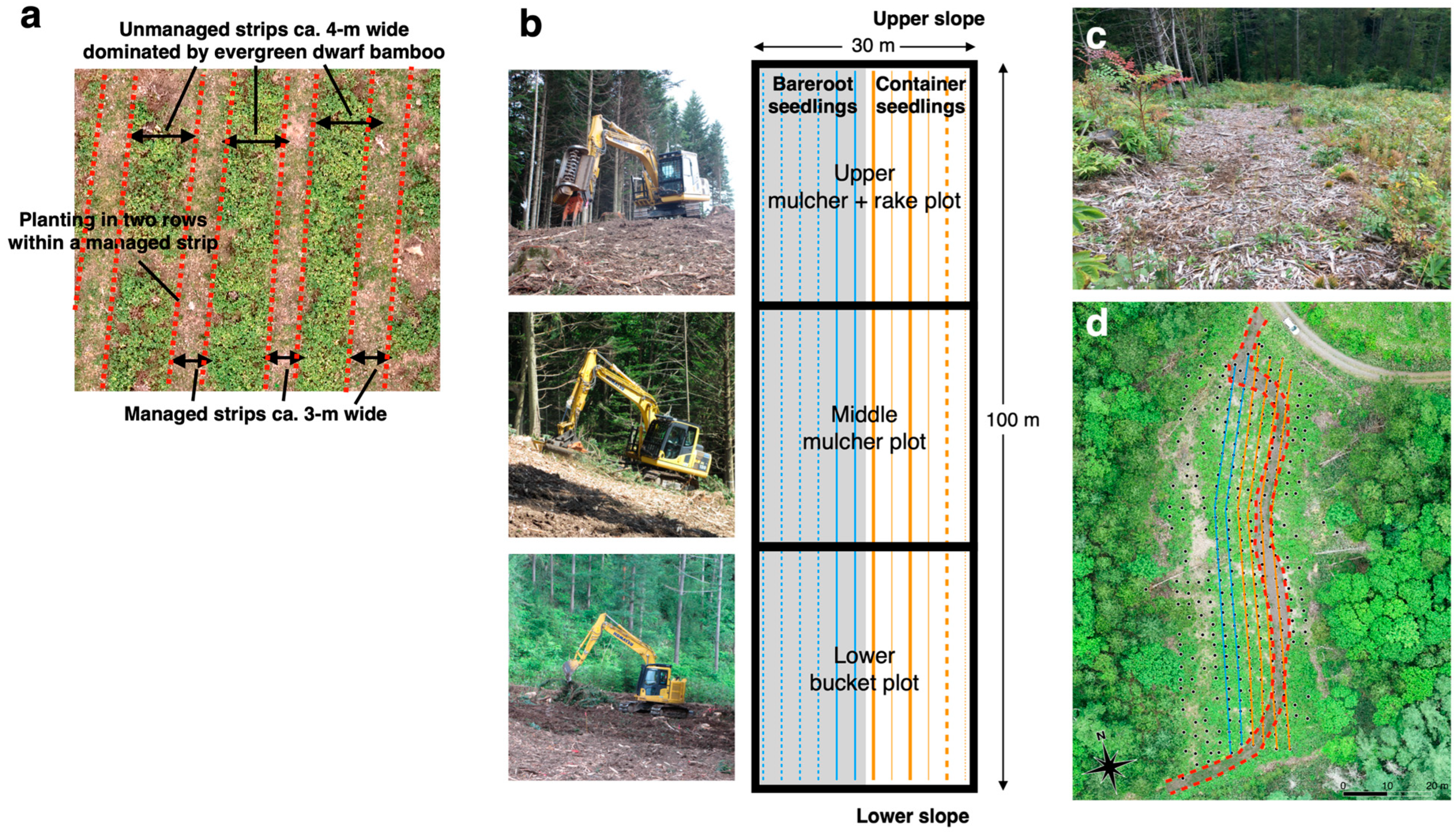

| Plot Name | Lower-Bucket | Middle-Mulcher | Upper-Mulcher + Rake | Total | |||
|---|---|---|---|---|---|---|---|
| MSP | Bucket | Mulcher | Mulcher + Rake | ||||
| Slope position | Lower | Middle | Upper | ||||
| Forwarder trail | Outside | Inside | Outside | Inside | Outside | Inside | |
| Bareroot | 21 | 0 | 26 | 0 | 22 | 2 | 71 |
| Container-JFA150 | 14 | 6 | 17 | 9 | 17 | 7 | 70 |
| Container-JFA300 | 14 | 6 | 17 | 9 | 16 | 8 | 70 |
| Subtotal | 49 | 12 | 60 | 18 | 55 | 17 | |
| Total | 61 | 78 | 72 | 211 | |||
| Height (cm) | RCD (mm) | n | |
|---|---|---|---|
| Surviving | 87 ± 6 | 22.1 ± 1.4 | 32 |
| Dead | 39 ± 9 | 11.7 ± 1.5 | 7 |
| p-value | 0.0016 ** | 0.0016 ** |
| Two-Year Survival | Eight-Year Survival | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Fixed Effects | Category | Estimate | SE | z-Value | p-Value | Estimate | SE | z-Value | p-Value |
| Intercept | 1.61 | 0.53 | 3.00 | 0.002 | −0.05 | 0.47 | −0.12 | 0.908 | |
| Stock type (vs. bareroot) | Container | 2.07 | 0.56 | 3.69 | <0.001 | 0.54 | 0.37 | 1.45 | 0.143 |
| Forwarder trail (vs. outside trail) | Inside trail | Not selected | −1.08 | 0.39 | 2.74 | 0.006 | |||
| Initial height | Not selected | Not selected | |||||||
| Initial H:D ratio | Not selected | Not selected | |||||||
| Plot | Middle-mulcher | 0.25 | 0.71 | 0.35 | 0.728 | Not selected | |||
| (vs. lower-bucket) | Upper- mulcher + rake | −0.87 | 0.62 | −1.40 | 0.163 | ||||
| Height after Eight Years | RCD after Eight Years | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Fixed Effects | Category | Estimate | SE | t-Value | p-Value | Estimate | SE | t-Value | p-Value |
| Intercept | 131.9 | 49.3 | 2.68 | 0.008 | 37.5 | 10.36 | 25.76 | <0.001 | |
| Initial height | 2.9 | 1.1 | 2.63 | 0.009 | 0.34 | 0.23 | 1.47 | 0.145 | |
| Initial H:D ratio | Not selected | Not selected | |||||||
| Stock type (vs. bareroot) | Container | −8.7 | 16.8 | −0.52 | 0.605 | −3.03 | 3.52 | −0.86 | 0.392 |
| Forwarder trail (vs. outside trail) | Inside | −40.1 | 15.2 | −2.64 | 0.009 | −3.33 | 3.19 | −1.05 | 0.298 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harayama, H.; Tsuyama, I.; Yamada, T.; Kitao, M.; Furuya, N.; Yazaki, K.; Sugai, T.; Uemura, A.; Sasaki, S.; Utsugi, H. Eight-Year Survival and Growth of Sakhalin Fir (Abies sachalinensis) Seedlings with One Weeding Operation: Impact of Mechanical Site Preparation, Vegetation Release, Summer Planting, Stock Type, and Forwarder Trail. Forests 2024, 15, 1012. https://doi.org/10.3390/f15061012
Harayama H, Tsuyama I, Yamada T, Kitao M, Furuya N, Yazaki K, Sugai T, Uemura A, Sasaki S, Utsugi H. Eight-Year Survival and Growth of Sakhalin Fir (Abies sachalinensis) Seedlings with One Weeding Operation: Impact of Mechanical Site Preparation, Vegetation Release, Summer Planting, Stock Type, and Forwarder Trail. Forests. 2024; 15(6):1012. https://doi.org/10.3390/f15061012
Chicago/Turabian StyleHarayama, Hisanori, Ikutaro Tsuyama, Takeshi Yamada, Mitsutoshi Kitao, Naoyuki Furuya, Kenichi Yazaki, Tetsuto Sugai, Akira Uemura, Shozo Sasaki, and Hajime Utsugi. 2024. "Eight-Year Survival and Growth of Sakhalin Fir (Abies sachalinensis) Seedlings with One Weeding Operation: Impact of Mechanical Site Preparation, Vegetation Release, Summer Planting, Stock Type, and Forwarder Trail" Forests 15, no. 6: 1012. https://doi.org/10.3390/f15061012





