Soil Carbon and Nitrogen Stocks and Their Influencing Factors in Different-Aged Stands of Sand-Fixing Caragana korshinskii in the Mu Us Desert of Northwest China
Abstract
:1. Introduction
2. Materials and Methods
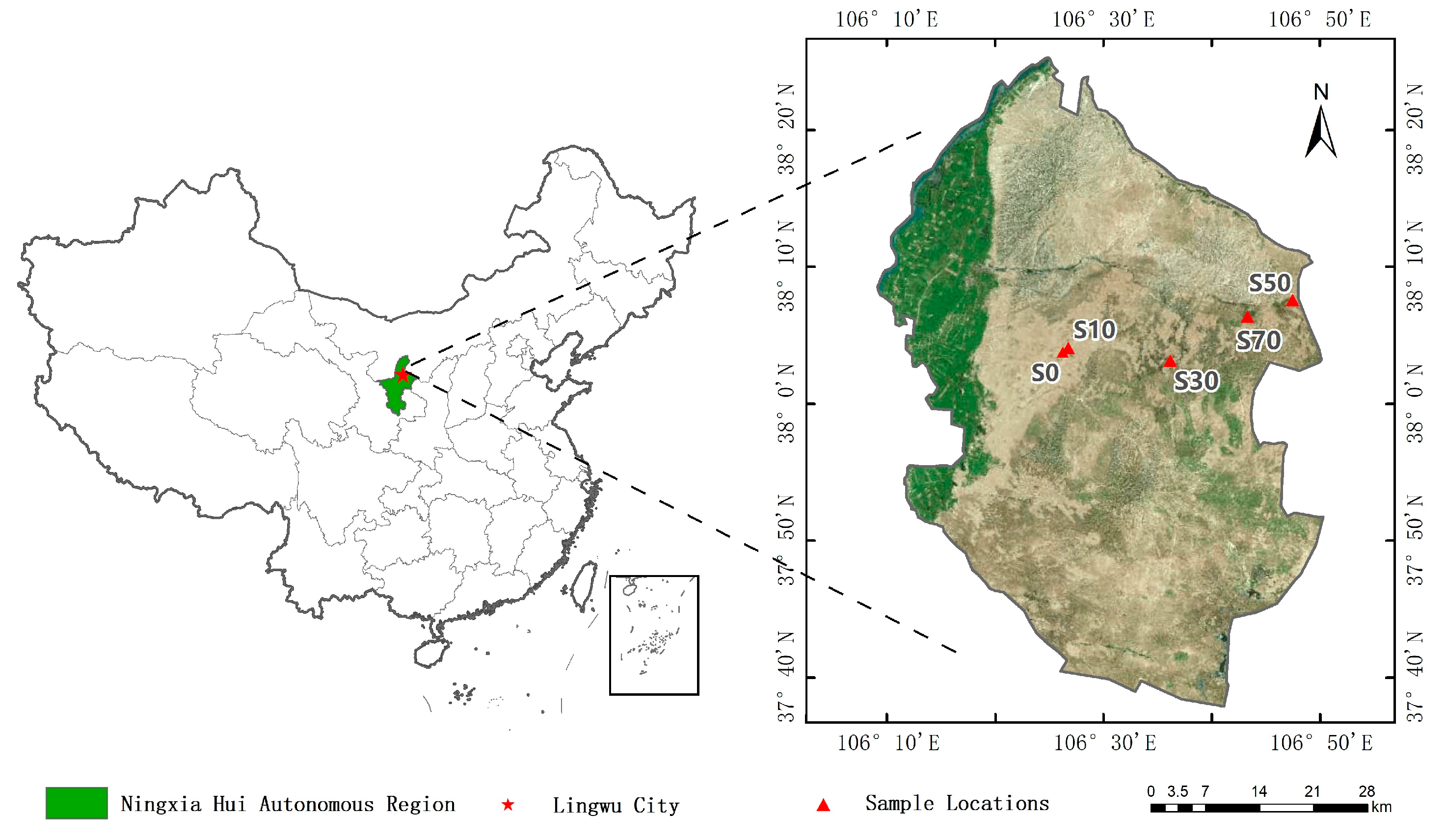
2.1. Study Area
2.2. Experimental Design
2.3. Plant Sampling and Collection
2.4. Soil Sampling and Measurements
2.5. Calculation of Soil C and N Stocks
2.6. Statistical Analysis
3. Results
3.1. Changes in Aboveground Plants after C. korshinskii Afforestation
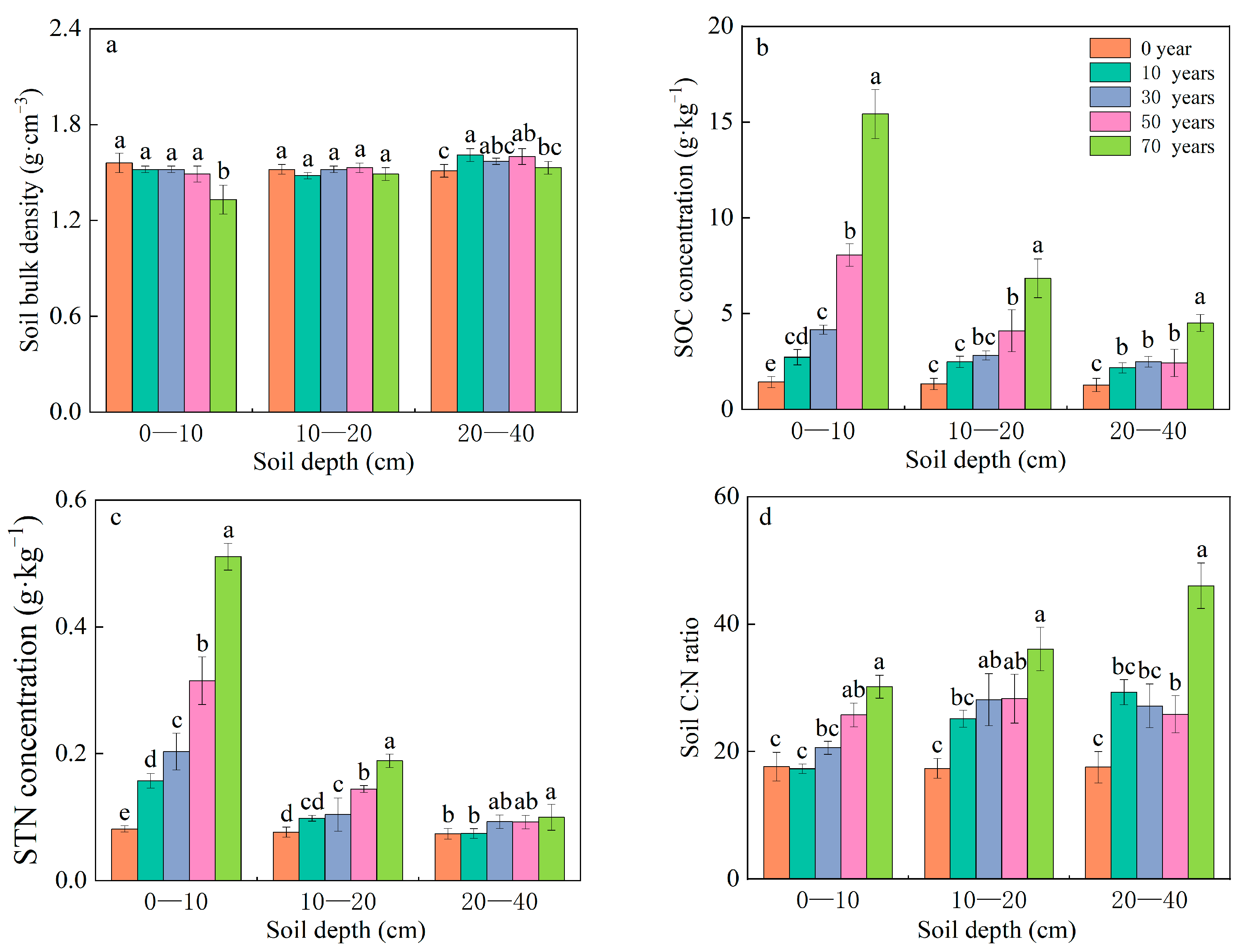
3.2. Effects of Years of C. korshinskii Afforestation on SBD and the SOC and STN Contents
3.3. Distribution Characteristics of SOC and STN Stocks in Response to C. korshinskii Afforestation
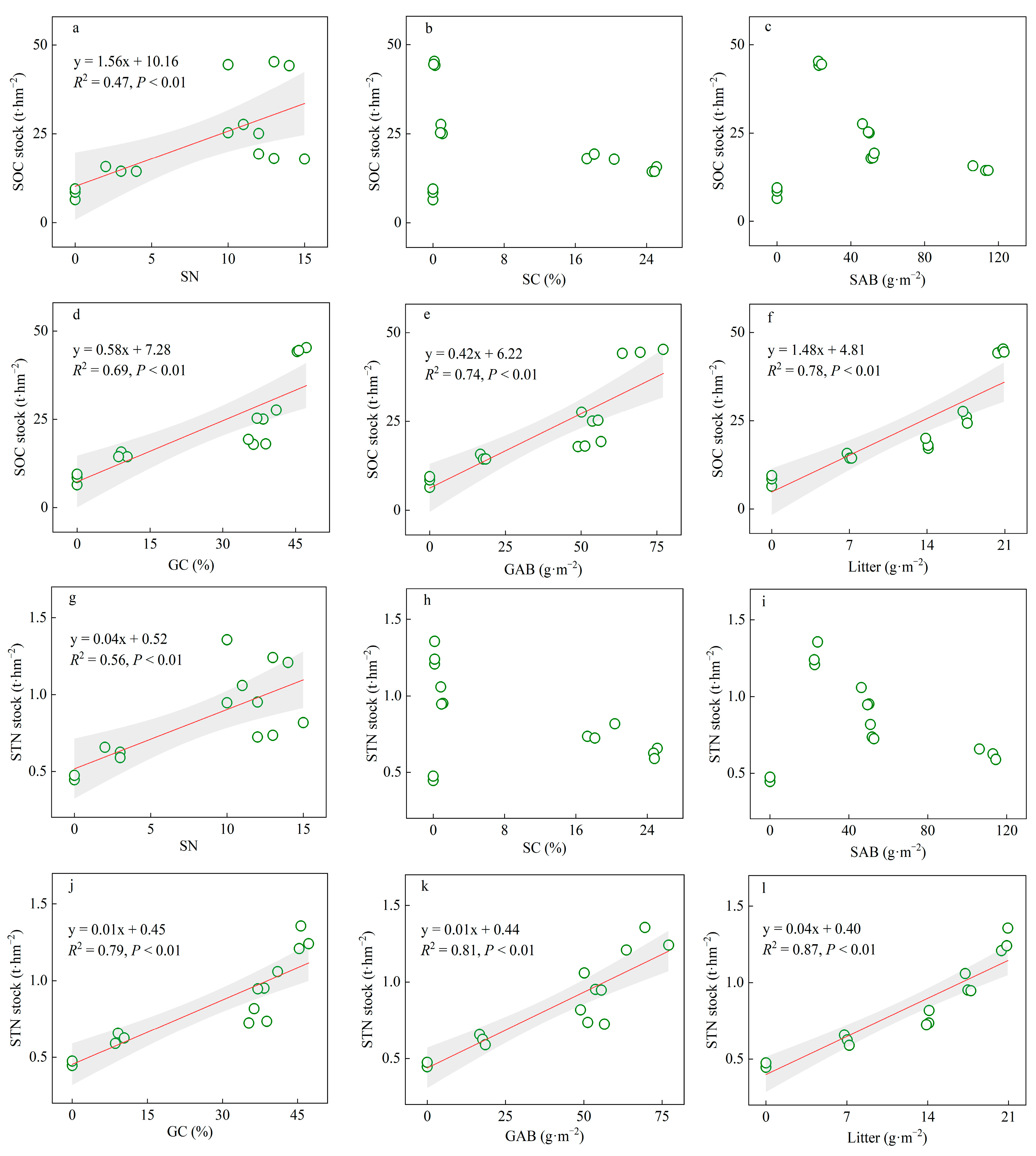
3.4. Correlations of SOC and STN Storage with Aboveground Plant Characteristics
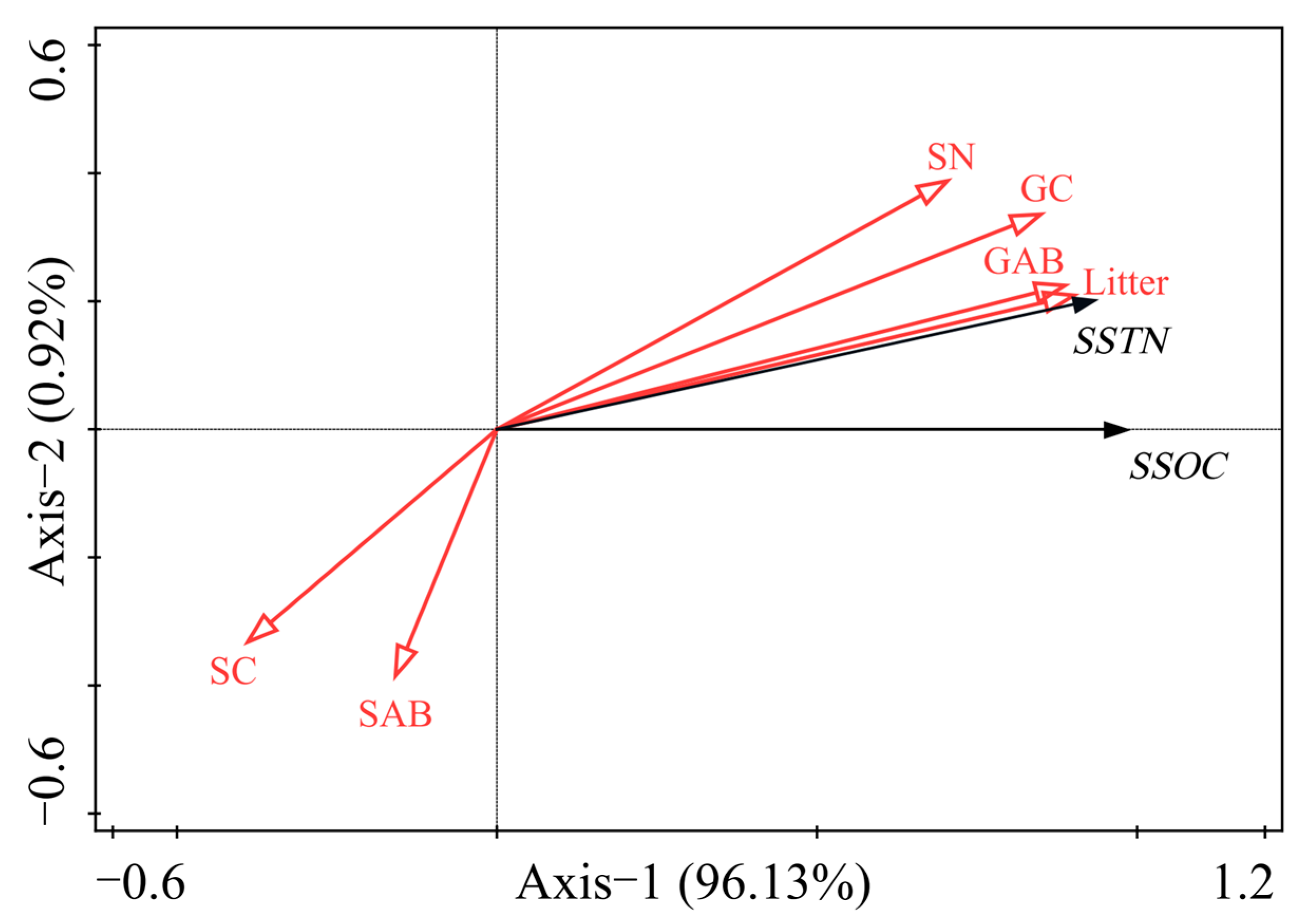
3.5. Relation of SOC and STN Stocks to Vegetation Characteristics
4. Discussion
4.1. Effects of C. korshinskii Afforestation on SOC and STN
4.2. Effects of C. korshinskii Afforestation on Stocks of SOC and STN
4.3. Correlations of Soil C and N during Afforestation
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Becerril-Piña, R.; Mastachi-Loza, C.A.; González-Sosa, E. Assessing desertification risk in the semi-arid highlands of central Mexico. J. Arid Environ. 2015, 120, 4–13. [Google Scholar] [CrossRef]
- Torres, L.; Abraham, E.M.; Rubio, C. Desertification research in Argentina. Land Degrad. Dev. 2015, 26, 433–440. [Google Scholar] [CrossRef]
- Lal, R. Potential of desertification control to sequester carbon and mitigate the greenhouse effect. Clim. Chang. 2001, 51, 35–72. [Google Scholar] [CrossRef]
- Reynolds, J.F.; Smith, D.M.S.; Lambin, E.F.; Turner, B.L.; Mortimore, M.; Batterbury, S.P.J.; Downing, T.E.; Dowlatabadi, H.; Fernandez, R.J.; Herrick, J.E. Global desertification: Building a science for dryland development. Science 2007, 316, 847–851. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Baquerizo, M.; Maestre, F.T.; Gallardo, A.; Bowker, M.A. Decoupling of soil nutrient cycles as a function of aridity in global drylands. Nature 2013, 502, 672–676. [Google Scholar] [CrossRef]
- Liu, H.; Wu, X.; Yu, P.; Xu, C.; Allen, C.D.; Hartmann, H.; Wei, X.; Dan, Y. Nature-based framework for sustainable afforestation in global drylands under changing climate. Glob. Chang. Biol. 2022, 28, 2202–2220. [Google Scholar] [CrossRef] [PubMed]
- Conant, R.T.; Klopatek, J.M.; Klopatek, C.C. Environmental factors controlling soil respiration in three semiarid ecosystems. Soil Sci. Soc. Am. J. 2000, 64, 383–390. [Google Scholar] [CrossRef]
- Wohlfahrt, G.; Fenstermaker, L.F.; Arnone, J.A. Large annual net ecosystem CO2 uptake of a Mojave Desert ecosystem. Glob. Chang. Biol. 2008, 14, 1475–1487. [Google Scholar] [CrossRef]
- Xu, A.T.; Wu, B.; Fang, B.; Gao, Y.; Li, E.L.; Cao, Y.L.; Qi, L.; Gao, J.L.; Zhi, M.; Liu, M.H. Different responses of growing season ecosystem CO2 fluxes to rain addition in a desert ecosystem. Plants 2023, 12, 1158. [Google Scholar] [CrossRef]
- Mcgonigle, T.P.; Turner, W.G. Grasslands and Croplands Have Different Microbial Biomass Carbon Levels per Unit of Soil Organic Carbon. Agriculture 2017, 7, 57. [Google Scholar] [CrossRef]
- Batjes, N.H. Total carbon and nitrogen in the soils of the world. Eur. J. Soil Sci. 2014, 65, 10–21. [Google Scholar] [CrossRef]
- Esser, G.; Kattge, J.; Sakalli, A. Feedback of carbon and nitrogen cycles enhances carbon sequestration in the terrestrial biosphere. Glob. Chang. Biol. 2011, 17, 819–842. [Google Scholar] [CrossRef]
- Liu, X.; Yang, T.; Wang, Q.; Huang, F.; Li, L.H. Dynamics of soil carbon and nitrogen stocks after afforestation in arid and semi-arid regions: A meta-analysis. Sci. Total Environ. 2018, 618, 1658–1664. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.Q.; Field, C.B.; Jackson, R.B. Does nitrogen constrain carbon cycling, or does carbon input stimulate nitrogen cycling? Ecology 2006, 87, 3–4. [Google Scholar] [CrossRef]
- Li, X.J.; Li, X.R.; Wang, X.P.; Yang, H.T. Changes in soil organic carbon fractions after afforestation with xerophytic shrubs in the Tengger Desert, northern China. Eur. J. Soil Sci. 2016, 67, 184–195. [Google Scholar] [CrossRef]
- Li, X.J.; Li, Y.F.; Xie, T.; Zhang, Z.Q.; Li, X.R. Recovery of soil carbon and nitrogen stocks following afforestation with xerophytic shrubs in the Tengger Desert, North China. Catena 2022, 214, 106277. [Google Scholar] [CrossRef]
- Grünzweig, J.M.; Gelfand, I.; Fried, Y.; Yakir, D. Biogeochemical factors contributing to enhanced carbon storage following afforestation of a semi-arid shrubland. Biogeosciences 2007, 4, 891–904. [Google Scholar] [CrossRef]
- Xiang, Y.X.; Chen, S.K.; Pan, P.; Ouyang, X.; Ning, J.; Qi, L.; University, J.A. Stoichiometric traits of C, N and P of leaf-litter-soil system of Pinus massoniana forest. J. For. Environ. 2019, 39, 120. [Google Scholar]
- Chai, Q.L.; Ma, Z.Y.; An, Q.Q.; Wu, G.L.; Chang, X.F.; Zheng, J.Y.; Wang, G.J. Does Caragana korshinskii plantation increase soil carbon continuously in a water limited landscape on the Loess Plateau, China? Land Degrad. Dev. 2019, 30, 1691–1698. [Google Scholar] [CrossRef]
- Li, Y.Q.; Chen, Y.P.; Wang, X.Y.; Niu, Y.Y.; Jie, L. Improvements in soil carbon and nitrogen capacities after shrub planting to stabilize sand dunes in China’ s Horqin Sandy Land. Sustainability 2017, 9, 662. [Google Scholar] [CrossRef]
- Tong, X.F.; Yang, H.L.; Ning, Z.Y.; Zhang, Z.Q.; Li, Y.L. Biomass estimation models for dominant sand-fixing shrubs in Horqin sand land. J. Desert Res. 2018, 38, 553–559. [Google Scholar]
- Liu, Y.; Ren, Y.; Gao, G.L.; Ding, G.D.; Zhang, Y.; Wang, J.Y.; Guo, Y. Distribution characteristics of soil carbon, nitrogen and phosphorus storage in Pinus sylvestris var. mongolica plantations. Sci. Soil Water Conserv. 2021, 19, 27–34. [Google Scholar]
- Zhao, P.B.; He, Y.; Huang, Y.Y.; Li, Y.Y.; Qiu, K.Y.; Xie, Y.Z.; Zhao, X.J.; Chen, l.; Liu, W.S.; Li, H.Q. Spatial heterogeneity of soil nutrients under different stand-fixing shrubs in southern Mu Us sandy land, China. Acta Agrestia Sin. 2021, 29, 2040–2048. [Google Scholar]
- He, L.W. Stoichiometric Characteristics of Caragana korshinskii Leaves of Different Planting Years in Ningxia baijitan; Ningxia University: Yinchuan, China, 2021. [Google Scholar]
- Yu, S.; Li, X.W.; Wang, R.X.; Yang, J.L.; Ma, L. Succession mechanism and dynamics in artificial Caragana korshinskii sand-fixing forests of different ages in Baijitan of Lingwu. Acta Prataculturae Sin. 2024, 33, 13–23. [Google Scholar]
- Jü, L.; Xu, H.; Jin, X.J.; Wang, B.; Wang, X.H.; Ma, J.G. Establishment of the biomass models of Caragana korshinskii based on site factors and their interaction. J. Northwest For. Univ. 2023, 38, 1–7. [Google Scholar]
- Gul, S.; Whalen, J.K.; Thomas, B.W.; Sachdeva, V.; Deng, H. Physico-chemical properties and microbial responses in biochar-amended soils: Mechanisms and future directions. Agric. Ecosyst. Environ. 2015, 206, 46–59. [Google Scholar] [CrossRef]
- Wang, D.D.; Chakraborty, S.; Weindorf, D.C.; Li, B.; Sharma, A.; Paul, S.; Ali, M.N. Synthesized use of VisNIR DRS and PXRF for soil characterization: Total carbon and total nitrogen. Geoderma 2015, 243–244, 157–167. [Google Scholar] [CrossRef]
- Dong, Z.L. The Study of Artificial Haloxylon Forest Distribution of Soil Carbon and Nitrogen Storage and Its Influence Factors in Arid Desert Area; Chinese Academy of Forestry: Beijing, China, 2014. [Google Scholar]
- An, F.J.; Su, Y.Z.; Niu, Z.R.; Liu, T.N. Effects of fine particulate matter input on soil carbon and nitrogen accumulation after establishment of Haloxylon ammodendron plantations on shifting sand dunes in arid area. J. Desert Res. 2021, 41, 147–156. [Google Scholar]
- Post, W.M.; Kwon, K.C. Soil carbon sequestration and land-use change: Processes and potential. Glob. Chang. Biol. 2000, 6, 317–328. [Google Scholar] [CrossRef]
- Feng, Q.; Endo, K.N.; Cheng, G.D. Soil carbon in desertified land in relation to site characteristics. Geoderma 2002, 106, 21–43. [Google Scholar] [CrossRef]
- Qi, Y.B.; Chang, Q.R.; Liu, M.Y.; Liu, J.; Chen, T. Characteristics of desertified soil in desertification reversing process by artificial vegetation. Agric. Res. Arid Areas 2011, 29, 180–185. [Google Scholar]
- Li, C.J.; Zeng, F.J.; Guo, J.H.; Refkat; Liu, B. Soil properties of different sandy lands under different vegetation recovering levels: A case in Southern Taklimakan Desert. Arid Zone Res. 2015, 32, 1061–1067. [Google Scholar]
- Cao, C.Y.; Jiang, D.M.; Teng, X.H.; Jiang, Y.; Liang, W.J.; Cui, Z.B. Soil chemical and microbiological properties along a chronosequence of Caragana microphylla Lam. plantations in the Horqin sandy land of northeast China. Appl. Soil Ecol. 2008, 40, 78–85. [Google Scholar] [CrossRef]
- Poeplau, C.; Don, A.; Vesterdal, L.; Leifeld, J.; Wesemael, B.V.; Schumacher, J.; Gensior, A. Temporal dynamics of soil organic carbon after land-use change in the temperate zone- carbon response functions as a model approach. Glob. Chang. Biol. 2011, 17, 2415–2427. [Google Scholar] [CrossRef]
- Guo, J.; Wang, B.; Wang, G.B.; Wu, Y.Q.; Cao, F.L. Afforestation and agroforestry enhance soil nutrient status and carbon sequestration capacity in eastern China. Land Degrad. Dev. 2019, 31, 392–403. [Google Scholar] [CrossRef]
- Cao, Y.F.; Li, Y.; Li, C.H.; Huang, G.; Lü, G.H. Relationship between presence of the desert shrub Haloxylon ammodendron and microbial communities in two soils with contrasting textures. Appl. Soil Ecol. 2016, 103, 93–100. [Google Scholar] [CrossRef]
- Chen, Y.L.; Zhang, Z.S.; Zhao, Y.; Hu, Y.G.; Zhang, D.H. Soil carbon storage along a 46-year revegetation chronosequence in a desert area of northern China. Geoderma Int. J. Soil Sci. 2018, 325, 28–36. [Google Scholar] [CrossRef]
- Niu, X.W.; Zhang, Q.; Yang, Z.P.; Chen, B.; Shi, Q.L.; Liu, P.; Lei, L. Research on change of soil properities of Caragana plantation in North-west of Shanxi Province. Acta Bot. Boreali-Occident. Sin. 2003, 4, 628–632. [Google Scholar]
- Yang, M.H.; Zhu, Z.M.; Cao, M.M.; Wang, C.J.; Xie, Y. Study on the correlation of soil vegetation in different desertification stages on the southeastern edge of Mu Us Sandy Land. J. Northwest A&F Univ. (Nat. Sci. Ed.) 2010, 38, 181–187. [Google Scholar]
- Berg, B. Decomposing litter; limit values; humus accumulation, locally and regionally. Appl. Soil Ecol. 2018, 123, 494–508. [Google Scholar] [CrossRef]
- Lange, M.; Eisenhauer, N.; Sierra, C.A.; Bessler, H.; Engels, C.; Griffiths, R.I.; Mellado-Vázquez, P.G.; Malik, A.A.; Roy, J.; Scheu, S. Plant diversity increases soil microbial activity and soil carbon storage. Nat. Commun. 2015, 6, 6707. [Google Scholar] [CrossRef]
- Xu, X.H.; Zhang, W.H.; Zhang, H.; Zhen, H.W.; Wang, Z.H.; Zheng, J.W. Study on the species diversity dyna mics of Sophora davidii community in Loess hilly region. J. Agric. Cult. Univ. Hebei 2006, 29, 1–5. [Google Scholar]
- Bowker, M.A.; Maestre, F.T.; Escolar, C. Biological crusts as a model system for examining the biodiversity-ecosystem function relationship in soils. Soil Biol. Biochem. 2009, 42, 405–417. [Google Scholar] [CrossRef]
- Housman, D.C.; Powers, H.H.; Collins, A.D.; Belnap, J. Carbon and nitrogen fixation differ between successional stages of biological soil crusts in the Colorado Plateau and Chihuahuan desert. J. Arid Environ. 2006, 66, 620–634. [Google Scholar] [CrossRef]
- Martens, D.A.; Reedy, T.E.; Lewis, D. Soil carbon content and composition of 130-year crop, pasture and forest land-use managements. Glob. Chang. Biol. 2003, 10, 65–78. [Google Scholar] [CrossRef]
- Li, X.J.; Yang, H.T.; Shi, W.L.; Li, Y.F.; Guo, Q. Afforestation with xerophytic shrubs accelerates soil net nitrogen nitrification and mineralization in the Tengger Desert, Northern China. Catena 2018, 169, 11–20. [Google Scholar] [CrossRef]
- Hoogmoed, M.; Cunningham, S.C.; Baker, P.J.; Beringer, J.; Cavagnaro, R.T. Is there more soil carbon under nitrogen-fixing trees than under non-nitrogen-fixing trees in mixed-species restoration plantings? Agric. Ecosyst. Environ. 2014, 188, 80–84. [Google Scholar] [CrossRef]
- Morris, S.J.; Bohm, S.; Haile-Mariam, S.; Paul, E.A. Evaluation of carbon accrual in afforested agricultural soils. Glob. Chang. Biol. 2007, 13, 1145–1156. [Google Scholar] [CrossRef]
- Guo, Y.J.; Ma, X.J.; Yu, S.; Xu, D.M. Effects of reseeding on soil organic carbon content and its distribution in degrade desert steppes. Acta Agrestia Sin. 2019, 27, 315–319. [Google Scholar]
- Weintraub, S.R.; Wieder, W.R.; Cleveland, C.C.; Townsend, A.R. Organic matter inputs shift soil enzyme activity and allocation patterns in a wet tropical forest. Biogeochemistry 2013, 114, 313–326. [Google Scholar] [CrossRef]
- Tian, H.Q.; Chen, G.H.; Zhang, C.; Melillo, J.M.; Hall, C.A.S. Pattern and variation of C:N:P ratios in China’s soils: A synthesis of observational data. Biogeochemistry 2010, 98, 139–151. [Google Scholar] [CrossRef]
- Herbert, D.A.; Williams, M.; Rastetter, E.B. A model analysis of N and P limitation on carbon accumulation in Amazonian secondary forest after alternate land-use abandonment. Biogeochemistry 2003, 65, 121–150. [Google Scholar] [CrossRef]
- LeBaue, D.S.; Treseder, K.K. Nitrogen limitation of net primary productivity in terrestrial ecosystems is globally distributed. Ecology 2008, 89, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Schulte-Uebbing, L.; De Vries, W. Global-scale impacts of nitrogen deposition on tree carbon sequestration in tropical, and boreal forests: A meta-analysis. Glob. Chang. Biol. 2018, 24, e416–e431. [Google Scholar] [CrossRef]
- Han, X.Y.; Gao, G.Y.; Li, Z.S.; Chang, R.Y.; Jiao, L.; Fu, B.J. Effects of plantation age and precipitation gradient on soil carbon and nitrogen changes following afforestation in the Chinese Loess Plateau. Land Degrad. Dev. 2019, 30, 2298–2310. [Google Scholar] [CrossRef]



| Stand Age (years) | Dominant Species | Species Number | Shrub Coverage (%) | Shrub Aboveground Biomass (g·m−2) | Grass Coverage (%) | Grass Aboveground Biomass (g·m−2) | Total Litter (g·m−2) |
|---|---|---|---|---|---|---|---|
| 0 | — | — | — | — | — | — | — |
| 10 | Caragana korshinskii, Corispermum mongolicum | 2.8 ± 0.8 | 24.9 ± 0.6 | 111.2 ± 3.2 | 9.3 ± 0.8 | 17.7 ± 0.7 | 7.0 ± 0.4 |
| 30 | C. korshinskii, Chenopodium album, Heteropappus altaicus, C. mongolicum, Setaria viridis | 13.4 ± 1.1 | 18.6 ± 1.3 | 51.9 ± 0.7 | 36.8 ± 1.5 | 52.3 ± 1.5 | 14.0 ± 0.2 |
| 50 | C. korshinskii, Artemisia scoparia, Bassia dasyphylla, Eragrostis pilosa, S. viridis | 11.2 ± 0.8 | 0.9 ± 0.1 | 48.6 ± 1.4 | 38.8 ± 1.6 | 53.2 ± 0.9 | 17.5 ± 0.2 |
| 70 | C. korshinskii, C. album, A. scoparia, Enneapogon desvauxii, Asparagus cochinchinensis | 12.5 ± 1.5 | 0.2 ± 0.0 | 23.1 ± 0.7 | 46.1 ± 0.8 | 70.1 ± 1.6 | 20.7 ± 0.2 |
| F | — | 35.6 | 726.7 | 662.2 | 331.5 | 83.5 | 2129.9 |
| p | — | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Stand Age (years) | Soil Bulk Density (g·cm−3) | SOC Concentration (g·kg−1) | STN Concentration (g·kg−1) | Soil C:N Ratio | SOC Stock (t·hm−2) | STN Stock (t·hm−2) |
|---|---|---|---|---|---|---|
| 0 | 1.5 ± 0.5 | 1.4 ± 0.4 | 0.1 ± 0.1 | 17.5 ± 1.9 | 8.15 ± 0.2 | 0.5 ± 0.5 |
| 10 | 1.5 ± 0.0 | 2.5 ± 0.1 | 0.1 ± 0.0 | 22.4 ± 0.3 | 14.8 ± 0.4 | 0.6 ± 0.0 |
| 30 | 1.5 ± 0.0 | 3.24 ± 0.1 | 0.1 ± 0.0 | 23.7 ± 1.0 | 18.4 ± 0.4 | 0.8 ± 0.1 |
| 50 | 1.5 ± 0.1 | 4.9 ± 0.2 | 0.2 ± 0.0 | 26.4 ± 0.7 | 26.0 ± 0.8 | 1.0 ± 0.1 |
| 70 | 1.5 ± 0.1 | 8.9 ± 0.5 | 0.3 ± 0.0 | 33.5 ± 1.1 | 44.6 ± 0.3 | 1.3 ± 0.1 |
| F | 4.6 | 291.5 | 293.5 | 28.7 | 499.9 | 105.8 |
| p | <0.05 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Vegetation Properties | Explained (%) | Contribution (%) | F | p |
|---|---|---|---|---|
| Litter (g·m−2) | 79.7 | 83.9 | 39.3 | 0.002 ** |
| Number of species | 9.4 | 9.9 | 7.7 | 0.018 * |
| Shrub coverage (%) | 3.1 | 3.2 | 3.1 | 0.118 |
| Grass aboveground biomass (g·m−2) | 1.5 | 1.6 | 1.6 | 0.256 |
| Shrub aboveground biomass (g·m−2) | 0.8 | 0.9 | 0.8 | 0.416 |
| Grass coverage (%) | 0.5 | 0.5 | 0.5 | 0.520 |
| Characteristic parameter | RDA1 | RDA2 | RDA3 | RDA4 |
| Eigenvalue | 0.9613 | 0.0092 | 0.0294 | 0.0001 |
| Explained variation | 94.04 | 94.96 | 99.44 | 100.00 |
| Correlation | 0.9786 | 0.7152 | 0.0000 | 0.0000 |
| Test of significance of all canonical axes | 0.016 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, S.; Yang, J.; Norghauer, J.M.; Yang, J.; Yang, B.; Zhang, H.; Li, X. Soil Carbon and Nitrogen Stocks and Their Influencing Factors in Different-Aged Stands of Sand-Fixing Caragana korshinskii in the Mu Us Desert of Northwest China. Forests 2024, 15, 1018. https://doi.org/10.3390/f15061018
Yu S, Yang J, Norghauer JM, Yang J, Yang B, Zhang H, Li X. Soil Carbon and Nitrogen Stocks and Their Influencing Factors in Different-Aged Stands of Sand-Fixing Caragana korshinskii in the Mu Us Desert of Northwest China. Forests. 2024; 15(6):1018. https://doi.org/10.3390/f15061018
Chicago/Turabian StyleYu, Shuang, Junlong Yang, Julian M. Norghauer, Jun Yang, Bo Yang, Hongmei Zhang, and Xiaowei Li. 2024. "Soil Carbon and Nitrogen Stocks and Their Influencing Factors in Different-Aged Stands of Sand-Fixing Caragana korshinskii in the Mu Us Desert of Northwest China" Forests 15, no. 6: 1018. https://doi.org/10.3390/f15061018




