Characteristics and Driving Mechanisms of Understory Vegetation Diversity Patterns in Central and Southern China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Sources
2.2. Detection and Comparison of Vegetation Diversity Patterns
2.3. Exploring Mechanisms of Community Assembly
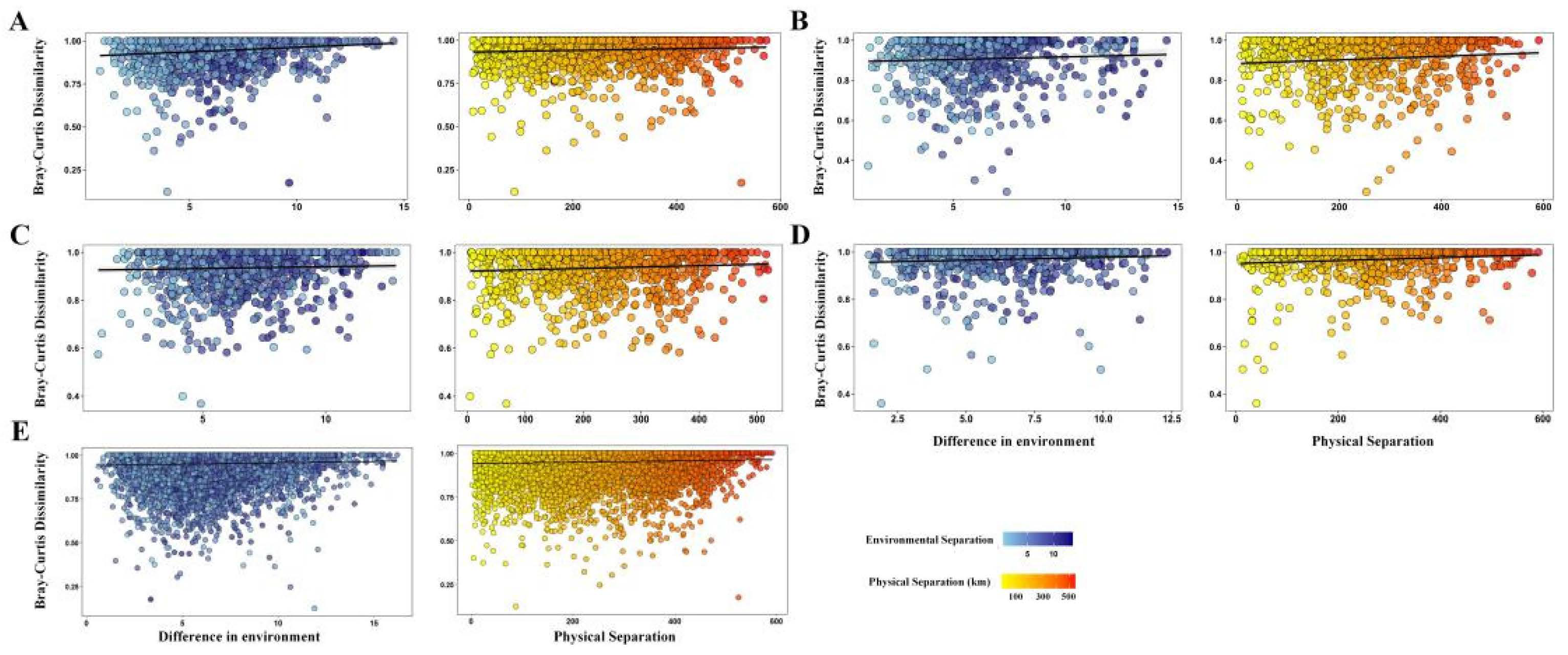
2.4. Driving Factors of Community Assembly
3. Results
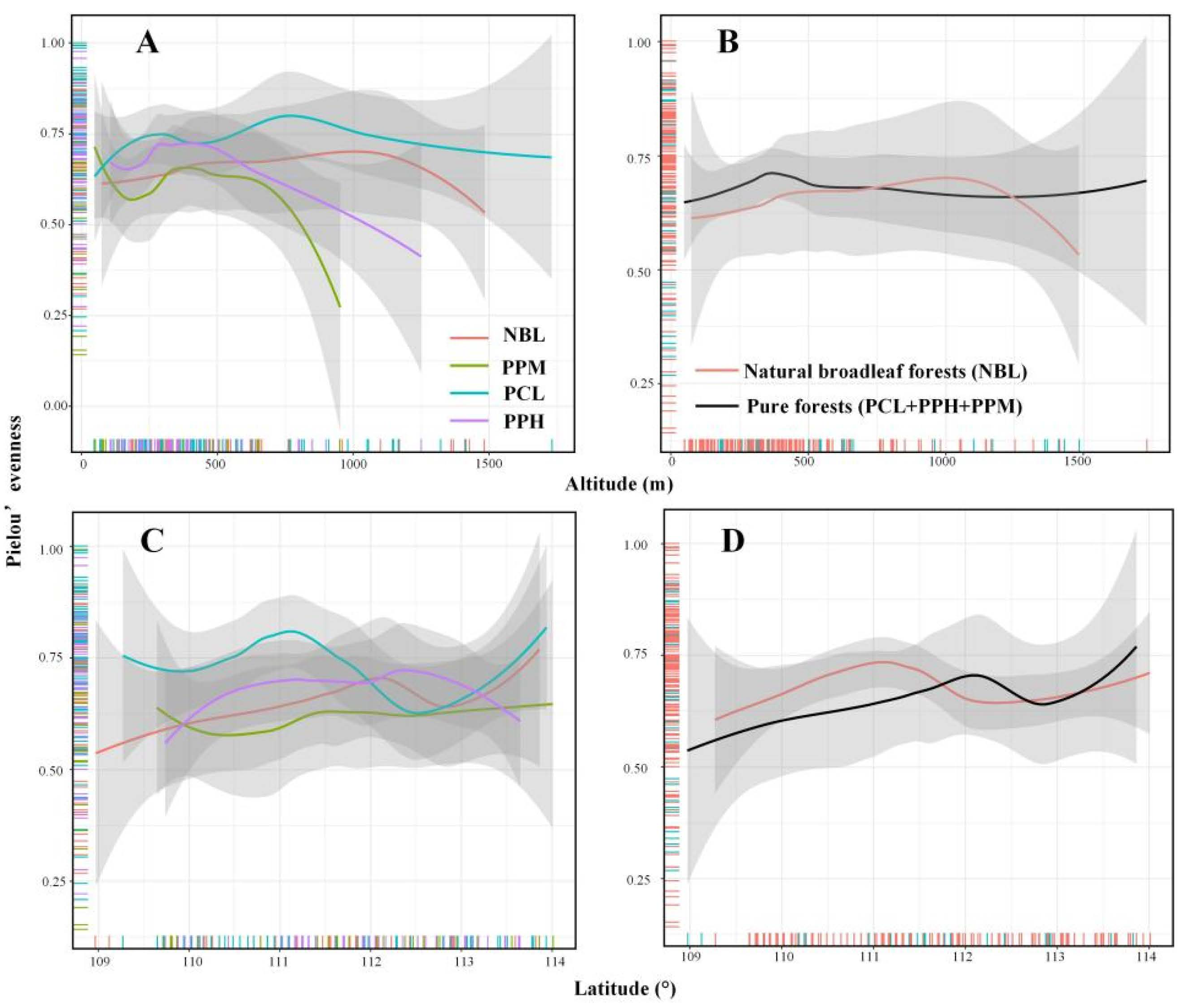
3.1. Vertical/Horizontal Diversity Distribution Pattern and Species Pool
3.2. Comparison of Multiple Diversity Patterns Based on SAD
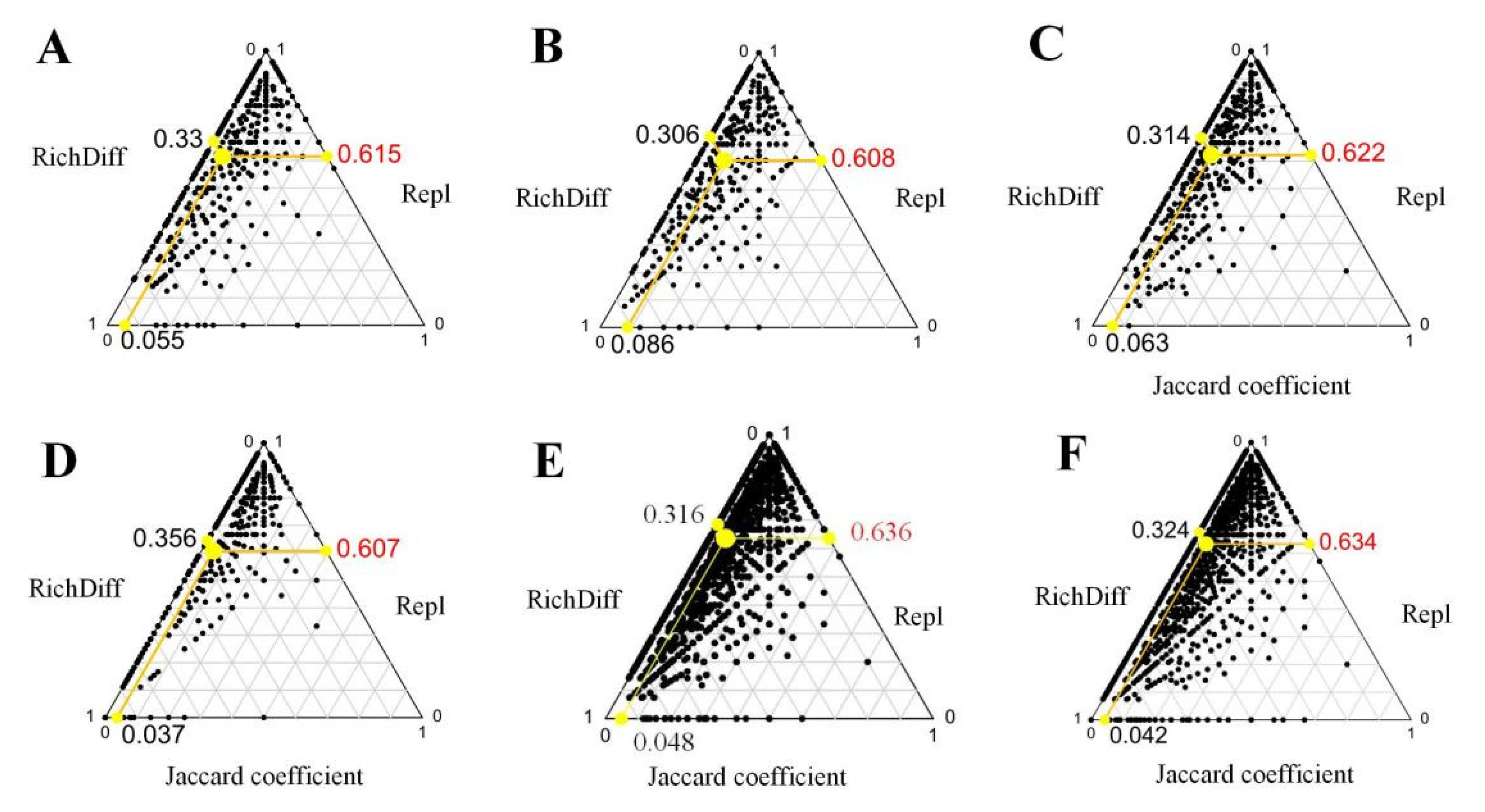
3.3. Mechanisms of Community Assembly
3.4. Driving Factors of Diversity Construction
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reese, G.C.; Wilson, K.R.; Flather, C.H. Performance of species richness estimators across assemblage types and survey parameters. Global Ecol. Biogeogr. 2014, 23, 585–594. [Google Scholar] [CrossRef]
- Lewis, R.J.; de Bello, F.; Bennett, J.A.; Fibich, P.; Finerty, G.E.; Götzenberger, L.; Hiiesalu, I.; Kasari, L.; Lepš, J.; Májeková, M.; et al. Applying the dark diversity concept to nature conservation. Conserv. Biol. 2016, 31, 40–47. [Google Scholar] [CrossRef]
- Hill, M.J.; Biggs, J.; Thornhill, I.; Briers, R.A.; Ledger, M.; Gledhill, D.G.; Wood, P.J.; Hassall, C. Community heterogeneity of aquatic macroinvertebrates in urban ponds at a multi-city scale. Landscape Ecol. 2018, 33, 389–405. [Google Scholar] [CrossRef]
- Wang, X.Z.; Ellwood, M.D.F.; Ai, D.; Zhang, R.; Wang, G. Species abundance distributions as a proxy for the niche-neutrality continuum. J. Plant Ecol. 2018, 11, 445–452. [Google Scholar] [CrossRef]
- Poot, P.; Lambers, H. Are Trade-offs in allocation pattern and root morphology related to species abundance? A congeneric comparison between rare and common species in the south-western Australian flora. J. Ecol. 2003, 91, 58–67. [Google Scholar] [CrossRef]
- Sugihara, G.; Bersier, L.F.; Southwood, T.R.E.; Pimm, S.L.; May, R.M. Predicted correspondence between species abundances and dendrograms of niche similarities. Proc. Natl. Acad. Sci. USA 2003, 100, 5246–5251. [Google Scholar] [CrossRef]
- Chase, J.M. Towards a really unified theory for metacommunities. Funct. Ecol. 2005, 19, 182–186. [Google Scholar] [CrossRef]
- Ugland, K.I.; Lambshead, P.J.D.; McGill, B.; Gary, J.S.; O’Dea, N.; Ladle, R.J.; Whittaker, R.J. Modelling dimensionality in species abundance distributions: Description and evaluation of the Gambin model. Evol. Ecol. Res. 2007, 9, 313–324. [Google Scholar]
- Ulrich, W.; Soliveres, S.; Maestre, F.T.; Gotelli, N.J.; Quero, J.L.; Delgado-Baquerizo, M.; Bowker, M.A.; Eldridge, D.J.; Ochoa, V.; Gozalo, B.; et al. Climate and soil attributes determine plant species turnover in global drylands. J. Biogeogr. 2014, 41, 2307–2319. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tang, Z.; Fang, J. Contribution of environmental filtering and dispersal limitation to species turnover of temperate deciduous broad-leaved forests in China. Appl. Veg. Sci. 2015, 18, 34–42. [Google Scholar] [CrossRef]
- Myers, J.A.; LaManna, J. The promise and pitfalls of beta diversity in ecology and conservation. J. Veg. Sci. 2016, 27, 1081–1083. [Google Scholar] [CrossRef]
- Zhang, C.; He, F.; Zhang, Z.; Zhao, X.; Godaw, K.V. Latitudinal gradients and ecological drivers of β-diversity vary across spatial scales in a temperate forest region. Glob. Ecol. Biogeogr. 2020, 29, 1257–1264. [Google Scholar] [CrossRef]
- Tan, L.; Fan, C.; Zhang, C.; Gadow, K.V.; Fan, X. How beta diversity and the underlying causes vary with sampling scales in the Changbai mountain forests. Ecol. Evol. 2017, 7, 10116–10123. [Google Scholar] [CrossRef] [PubMed]
- Legendre, P. Interpreting the replacement and richness difference components of beta diversity. Global Ecol. Biogeogr. 2014, 23, 1324–1334. [Google Scholar] [CrossRef]
- Leprieur, F.; Tedesco, P.; Hugueny, B.; Beauchard, O.; Dürr, H.; Brosse, S.; Oberdorff, T. Partitioning global patterns of freshwater fish beta diversity reveals contrasting signatures of past climate changes. Ecol. Lett. 2011, 14, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Si, X.; Baselga, A.; Ding, P.; Machado, R.B. Revealing betadiversity patterns of breeding bird and lizard communities on inundated land-bridge islands by separating the turnover and nestedness components. PLoS ONE 2015, 10, e0127692. [Google Scholar] [CrossRef] [PubMed]
- Laganière, J.; Angers, D.A.; Paré, D. Carbon accumulation in agricultural soils after afforestation: A meta-analysis. Global Change Biol. 2010, 16, 439–453. [Google Scholar] [CrossRef]
- Feng, J.; Xu, X.; Wu, J.; Zhang, Q.; Zhang, D.; Li, Q.; Long, C.; Chen, Q.; Cheng, X. Inhibited enzyme activities in soil macroaggregates contribute to enhanced soil carbon sequestration under afforestation in central China. Sci. Total Environ. 2018, 640–641, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Cai, L.; He, Z.; Wang, R.; Wu, P.; Ma, X. Thinning increases understory diversity and biomass, and improves soil properties without decreasing growth of Chinese fir in southern China. Environ. Sci. Pollut. Res. Int. 2016, 23, 24135–24150. [Google Scholar] [CrossRef] [PubMed]
- Janusz, C.; Radosaw, G.; Kamil, M.; Magdalena, J.; Jan, T.; Łukaszb, S. The influence of the forest management in the Biaowiea forest on the species structure of the forest community. Forest Ecol. Manag. 2021, 496, 119363. [Google Scholar] [CrossRef]
- Lamb, D.; Erskine, P.D.; Parrotta, J.A. restoration of degraded tropicalForest landscapes. Science 2005, 310, 1628–1632. [Google Scholar] [CrossRef] [PubMed]
- Suding, K.; Higgs, E.S.; Palmer, M.; Callicott, J.B.; Anderson, C.B.; Baker, M.E.; Gutrich, J.J.; Hondula, K.L.; LaFevor, M.C.; Larson, B.M.H.; et al. Committing to ecological restoration. Science 2015, 348, 638–640. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Fu, B.; Stafford-Smith, M.; Wang, S.; Zhao, W. Improve forest restoration initiatives to meet Sustainable Development Goal 15. Nat. Ecol. Evol. 2020, 5, 10–13. [Google Scholar] [CrossRef] [PubMed]
- Gamfeldt, L.; Snall, T.; Bagchi, R.; Jonsson, M.; Gustafsson, L.; Kjellander, P.; Ruiz-Jaen, M.C.; Froberg, M.; Stendahl, J.; Philipson, C.D.; et al. Higher levels of multiple ecosystem services are found in forests with more tree species. Nat. Commun. 2013, 4, 1340. [Google Scholar] [CrossRef] [PubMed]
- Ares, A.; Neill, A.R.; Puettmann, K.J. Understory abundance, species diversity and functional attribute response to thinning in coniferous stands. Forest Ecol. Manag. 2010, 260, 1104–1113. [Google Scholar] [CrossRef]
- Barbier, S.; Gosselin, F.; Balandier, P. Influence of tree species on understory vegetation diversity and mechanisms involved-A critical review for temperate and boreal forests. Forest Ecol. Manag. 2008, 254, 1–15. [Google Scholar] [CrossRef]
- Seiwa, K.; Eto, Y.; Hishita, M.; Masaka, K. Effects of thinning intensity on species diversity and timber production in a conifer (Cryptomeria japonica) plantation in Japan. J. Forest Res. 2012, 17, 468–478. [Google Scholar] [CrossRef]
- Preston, F.W. The commonness and rarity of species. Ecology 1984, 29, 254–283. [Google Scholar] [CrossRef]
- De Bello, F.; Fibich, P.; Zelený, D.; Kopecký, M.; Mudrák, O.R.; Chytrý, M.; Pyšek, P.; Wild, J.; Michalcová, D.; Sádlo, J.; et al. Measuring size and composition of species pools: A comparison of dark diversity estimates. Ecol. Evol. 2016, 6, 4088–4101. [Google Scholar] [CrossRef] [PubMed]
- Pärtel, M.; Szava-Kovats, R.; Zobel, M. Dark diversity: Shedding light on absent species. Trends Ecol. Evol. 2011, 26, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.H.; Wang, Y.T.; Walther, B.A.; Chao, A. An improved nonparametric lower bound of species richness via a modified Good-Turing frequency formula. Biometrics 2014, 70, 671–682. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.P.; Belle, G.V. Nonparametric estimation of species richness. Biometrics 1984, 40, 119–129. [Google Scholar] [CrossRef]
- Whittaker, R.H. Dominance and diversity in land plant communities. Science 1965, 147, 250–260. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.B. Methods for fitting dominance/diversity curves. J. Veg. Sci. 1991, 2, 35–46. [Google Scholar] [CrossRef]
- Matthews, T.J.; Sadler, J.P.; Kubota, Y.; Woodall, C.W.; Pugh, T.A.M. Systematic variation in North American tree species abundance distributions along macroecological climatic gradients. Global Ecol. Biogeogr. 2019, 28, 601–611. [Google Scholar] [CrossRef]
- Ulrich, W.; Kusumoto, B.; Shiono, T.; Kubota, Y. Climatic and geographic correlates of global forest tree species–abundance distributions and community evenness. J. Veg. Sci. 2015, 27, 295–305. [Google Scholar] [CrossRef]
- Boulangeat, I.; Lavergne, S.; Van Es, J.; Garraud, L.; Thuiller, W. Niche breadth, rarity and ecological characteristics within a regional flora spanning large environmental gradients. J. Biogeogr. 2012, 39, 204–214. [Google Scholar] [CrossRef]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer-Verlag: New York, NY, USA, 2016; ISBN 978-3-319-24277-4. [Google Scholar]
- Dray, S.; Pélissier, R.; Pierre, C.; Fortin, M.; Legendre, P.; Peres-Neto, P.; Bellier, E.; Bivand, R.; Blanchet, F.G.; Ceres, M.; et al. Community ecology in the age of multivariate multiscale spatial analysis. Ecol. Monogr. 2012, 82, 257–275. [Google Scholar] [CrossRef]
- Chen, S.; Jiang, G.; Ouyang, Z.; Xu, W.; Xiao, Y. Relative importance of water, energy, and heterogeneity in determining regional pteridophyte and seed plant richness in China. J. Syst. Evol. 2011, 49, 95–107. [Google Scholar] [CrossRef]
- Gu, Y.; Han, S.; Zhang, J.H.; Chen, Z.J.; Wang, W.J.; Feng, Y.; Jiang, Y.J.; Geng, S.C. Temperature-dominated driving mechanisms of the plant diversity in temperate forests, Northeast China. Forests. 2020, 11, 227. [Google Scholar] [CrossRef]
- Lewis, S.L.; Wheeler, C.E.; Mitchard, E.T.A.; Koch, A. Restoring natural forests is the best way to remove atmospheric carbon. Nature 2019, 568, 25–28. [Google Scholar] [CrossRef] [PubMed]
- Alexander, S.; Nelson, C.R.; Aronson, J.; Lamb, D.; Cliquet, A.; Erwin, K.L.; Finlayson, C.M.; Groot, R.S.; Harris, J.A.; Higgs, E.S.; et al. Opportunities and Challenges for Ecological Restoration within REDD+. Restor. Ecol. 2011, 19, 683–689. [Google Scholar] [CrossRef]
- Volkov, I.; Banavar, J.R.; Hubbell, S.P.; Maritan, A. Neutral theory and relative species abundance in ecology. Nature 2003, 424, 1035–1037. [Google Scholar] [CrossRef] [PubMed]
- Barange, M.; Campos, B. Models of species abundance: A critique of and an alternative to the dynamics model. Mar. Ecol. Prog. Ser. 1991, 69, 293–298. [Google Scholar] [CrossRef]
- Weiher, E.; Freund, D.; Bunton, T.; Stefanski, A.; Lee, T.; Bentivenga, S. Advances, challenges and a developing synthesis of ecological community assembly theory. Philos. T. R. Soc. B. 2011, 366, 2403–2413. [Google Scholar] [CrossRef] [PubMed]
- Gratani, L.; Crescente, M.F.; Varone, L.; Fabrini, G.; Digiulio, E. Growth pattern and photosynthetic activity of different bamboo species growing in the Botanical Garden of Rome. Flora 2008, 203, 77–84. [Google Scholar] [CrossRef]
- Xu, Q.; Liang, C.F.; Chen, J.H.; Li, Y.C.; Qin, H.; Fuhrmann, J. Rapid bamboo invasion (expansion) and its effects on biodiversity and soil processes. Glob. Ecol. Conserv. 2019, 21, e00787. [Google Scholar] [CrossRef]
- Rahbek, C. The role of spatial scale and the perception of large-scale speciesrichness patterns. Ecol. Lett. 2005, 8, 224–239. [Google Scholar] [CrossRef]
- Mayor, S.J.; Cahill, J.F., Jr.; He, F.; Solymos, P.; Boutin, S. Regional boreal biodiversity peaks at intermediate human disturbance. Nat. Commun. 2012, 3, 1142. [Google Scholar] [CrossRef] [PubMed]
- Grime, J. Competitive exclusion in herbaceous vegetation. Nature 1973, 242, 344–347. [Google Scholar] [CrossRef]
- Connell, J. Diversity in tropical rain forests and coral reefs-High diversity of trees and corals is maintained only in a non-equilibrium state. Science 1978, 199, 1302–1310. [Google Scholar] [CrossRef] [PubMed]
- Oommen, M.A.; Shanker, K. Elevational species richness patterns emerge from multiple local mechanisms in Himalayan woody plants. Ecology 2005, 86, 3039–3047. [Google Scholar] [CrossRef]
- Lu, Z.X.; Yang, M.; Li, B.; Hu, J.J.; Yu, H.B. Elevational patterns of seed plants and the driving mechanisms in the Himalaya. Ying Yong Sheng Tai Xue Bao 2023, 34, 1787–1796. [Google Scholar] [PubMed]
- Zhou, Y.; Ochola, A.C.; Njogu, A.W.; Boru, B.H.; Mwachala, G.; Hu, G.; Xin, H.; Wang, Q. The species richness pattern of vascular plants along a tropical elevational gradient and the test of elevational Rapoport’s rule depend on different life-forms and phytogeographic affinities. Ecol. Evol. 2019, 9, 4495–4503. [Google Scholar] [CrossRef]
- Gao, D.; Fu, L.; Sun, J.; Li, Y.; Cao, Z.; Liu, Y.; Xu, P.; Zhao, J. The mid-domain effect and habitat complexity applied to elevational gradients: Moss species richness in a temperate semihumid monsoon climate mountain of China. Ecol. Evol. 2021, 11, 7448–7460. [Google Scholar] [CrossRef] [PubMed]
- Zu, K.; Wang, Z.; Zhu, X.; Lenoir, J.; Shrestha, N.; Lyu, T.; Luo, A.; Li, Y.; Ji, C.; Peng, S.; et al. Upward shift and elevational range contractions of subtropical mountain plants in response to climate change. Sci. Total Environ. 2021, 783, 146896. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, D.M. The disturbing history of intermediate disturbance. Oikos 1999, 84, 145–147. [Google Scholar] [CrossRef]
- Catford, J.A.; Daehler, C.C.; Murphy, H.T.; Sheppard, A.W.; Hardesty, B.D.; Westcott, D.A.; Rejmánek, M.; Bellingham, P.J. Theintermediate disturbance hypothesis and plant invasions: Implications for species richness and management. Perspectives in Plant Ecology. Evol. Syst. 2012, 14, 231–241. [Google Scholar]
- Fox, J.W. The intermediate disturbance hypothesis should be abandoned. Trends Ecol. Evol. 2013, 28, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Sheil, D.; Burslem, D.F.R.P. Defining and defending Connell’s intermediate disturbance hypothesis: A response to Fox. Trends Ecol. Evol. 2013, 28, 571–572. [Google Scholar] [CrossRef]
- Legendre, P.; Gallagher, E.D. Ecologically meaningful transformations for ordination of species data. Oecologia 2001, 129, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Tuomisto, H.; Ruokolainen, K.; Yli-Halla, M. Dispersal, environment, and floristic variation of western Amazonian forests. Science 2003, 299, 241–244. [Google Scholar] [CrossRef] [PubMed]
- Tilman, D. Niche tradeoffs, neutrality, and community structure: A stochastic theory of resource competition, invasion, and community assembly. Proc. Natl. Acad. Sci. USA 2004, 101, 10854–10861. [Google Scholar] [CrossRef] [PubMed]
- Chave, J. Neutral theory and community ecology. Ecol. Lett. 2004, 7, 241–253. [Google Scholar] [CrossRef]
- Jiang, L.; Lv, G.; Gong, Y.; Li, Y.; Wang, H.; Wu, D. Characteristics and driving mechanisms of species beta diversity in desert plant communities. PLoS ONE 2021, 16, e0245249. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.M.; Tuomisto, H.; Borcard, D.; Legendre, P.; Clark, D.B.; Olivas, P.C. Explaining variation in tropical plant community composition: Influence of environmental and spatial data quality. Oecologia 2008, 155, 593–604. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Cánovas, C.; Millán, A.; Velasco, J.; Vaughan, I.P.; Ormerod, S.J. Contrasting effects of natural and anthropogenic stressors on beta diversity in river organisms. Global Ecol. Biogeogr. 2013, 22, 796–805. [Google Scholar] [CrossRef]
- Yang, J.; Su, P.; Zhou, Z.; Shi, R.; Ding, X. Environmental filtering rather than dispersal limitation dominated plant community assembly in the Zoige Plateau. Ecol. Evol. 2022, 12, e9117. [Google Scholar] [CrossRef] [PubMed]
- Myers, J.A.; Chase, J.M.; Jiménez, I.; Jørgensen, P.M.; Araujo-Murakami, A.; Paniagua-Zambrana, N.; Seidel, R. Beta-diversity in temperate and tropical forests reflects dissimilar mechanisms of community assembly. Ecol. Lett. 2013, 16, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Nishizawa, K.; Shinohara, N.; Cadotte, M.W.; Mori, A.S. The latitudinal gradient in plant community assembly processes: A meta-analysis. Ecol. Lett. 2022, 25, 1711–1724. [Google Scholar] [CrossRef]
- Qian, H.; Chen, S.B.; Mao, L.F.; Ouyang, Z.Y. Drivers of β-diversity along latitudinal gradients revisited. Global Ecol. Biogeogr. 2013, 22, 659–670. [Google Scholar] [CrossRef]
- Sanitha, K.S.; Dharmendra, P.S.; Rajeev, S. Functional and taxonomic (α and β) diversity patterns of macrobenthic communities along a depth gradient (19-2639 m): A case study from the southern Indian continental margin. Deep. Sea Res. Part I Oceanogr. Res. Pap. 2020, 159, 103250. [Google Scholar]
- Li, X.; Li, Y.; Zhang, J.; Peng, S.; Chen, Y.; Cao, Y. The effects of forest thinning on understory diversity in China: A meta-analysis. Land. Degrad. Dev. 2020, 31, 1225–1240. [Google Scholar] [CrossRef]
- Zilliox, C.; Gosselin, F. Tree species diversity and abundance as indicators of understory diversity in French mountain forests: Variations of the relationship in geographical and ecological space. For. Ecol. Manag. 2014, 321, 105–116. [Google Scholar] [CrossRef]
- Zellweger, F.; Braunisch, V.; Morsdorf, F.; Baltensweiler, A.; Abegg, M.; Roth, T.; Bugmann, H.; Bollmann, K. Disentangling the effects of climate, topography, soil and vegetation on stand-scale species richness in temperate forests. For. Ecol. Manag. 2015, 349, 36–44. [Google Scholar] [CrossRef]
- Jocque, M.; Field, R.; Brendonck, L.; De Meester, L. Climatic control of dispersal-ecological specialization trade-offs: A metacommunity process at the heart of the latitudinal diversity gradient? Glob. Ecol. Biogeogr. 2010, 19, 244–252. [Google Scholar] [CrossRef]




| Code | Description |
|---|---|
| Bio1 | Annual mean temperature |
| Bio2 | Mean diurnal range [mean of monthly (max temp.–min temp.)] |
| Bio3 | Isothermality (Bio2/Bio7) (×100) |
| Bio4 | Temperature seasonality (standard deviation × 100) |
| Bio5 | Max temperature of the warmest month |
| Bio6 | Min temperature of the coldest month |
| Bio7 | Temperature annual range (Bio5-Bio6) |
| Bio8 | Mean temperature of the wettest quarter |
| Bio9 | Mean temperature of the driest quarter |
| Bio10 | Mean temperature of the warmest quarter |
| Bio11 | Mean temperature of the coldest quarter |
| Bio12 | Annual precipitation |
| Bio13 | Precipitation of the wettest month |
| Bio14 | Precipitation of the driest month |
| Bio15 | Precipitation seasonality (coefficient of variation) |
| Bio16 | Precipitation of the wettest quarter |
| Bio17 | Precipitation of the driest quarter |
| Bio18 | Precipitation of the warmest quarter |
| Bio19 | Precipitation of the coldest quarter |
| Model | PCL | PPM | PPH | NBL | ||||
|---|---|---|---|---|---|---|---|---|
| Value | Variance | Value | Variance | Value | Variance | Value | Variance | |
| Observed species | 192 | / | 162 | / | 196 | / | 206 | / |
| Chao model | 452 | 69.17 | 456 | 89.84 | 375 | 46.01 | 528 | 81.12 |
| Jackknife model | 314 | 23.66 | 266 | 23.75 | 312 | 23.94 | 346 | 29.12 |
| Bootstrap model | 242 | 11.49 | 204 | 11.24 | 245 | 11.97 | 263 | 13.29 |
| Type | PCL: Cunninghamia lanceolata | ||||
| Distribution model | M1 | M2 | M3 | M4 | M5 |
| Parameter 1 (c) | / | 0.019063 | 0.32079 | 0.10158 | 0.10158 |
| Parameter 2 (γ) | / | / | 1.0937 | −0.80123 | −0.80123 |
| Parameter 3 (β) | / | / | / | / | 2.08 × 10−5 |
| Deviance | 197.348 | 225.301 | 84.686 | 10.123 | 10.123 |
| BIC | 660.96 | 694.17 | 558.81 | 484.25 | 489.51 |
| Type | PPM: Pinus massoniana | ||||
| Distribution model | M1 | M2 | M3 | M4 | M5 |
| Parameter 1 (c) | / | 0.020138 | 0.40219 | 0.083082 | 0.14684 |
| Parameter 2 (γ) | / | / | 0.93738 | −0.7145 | −0.85093 |
| Parameter 3 (β) | / | / | / | / | 1.674 |
| Deviance | 105.222 | 98.301 | 45.2 | 14.952 | 11.101 |
| BIC | 496.59 | 494.76 | 446.74 | 416.49 | 417.73 |
| Type | PPH: Phyllostachys heterocycla | ||||
| Distribution model | M1 | M2 | M3 | M4 | M5 |
| Parameter 1 (c) | / | 0.016446 | 0.42198 | 0.07339 | 0.1477 |
| Parameter 2 (γ) | / | / | 0.92587 | −0.69644 | −0.85728 |
| Parameter 3 (β) | / | / | / | / | 2.352 |
| Deviance | 125.6891 | 118.5444 | 53.436 | 17.9576 | 9.6419 |
| BIC | 603.15 | 601.29 | 541.46 | 505.98 | 502.94 |
| Type | NBL: natural broad-leaved | ||||
| Distribution model | M1 | M2 | M3 | M4 | M5 |
| Parameter 1 (c) | / | 0.011986 | 0.30838 | 0.052334 | 0.053539 |
| Parameter 2 (γ) | / | / | 0.7301 | −0.60032 | −0.60554 |
| Parameter 3 (β) | / | / | / | 0.07024 | |
| Deviance | 131.6266 | 90.1187 | 45.9177 | 8.7498 | 8.7383 |
| BIC | 605.83 | 569.65 | 530.78 | 493.61 | 498.93 |
| Type | M1 | M2 | M3 | M4 | |
|---|---|---|---|---|---|
| NBL | Statistic r | 0.1325 | 0.04006 | 0.1005 | 0.09754 |
| Significance | 0.0173 * | 0.184 | 0.0217 * | 0.011 * | |
| PPM | Statistic r | 0.08493 | 0.001371 | 0.1467 | 0.08907 |
| Significance | 0.1243 | 0.481 | 0.0038 * | 0.059 | |
| PCL | Statistic r | 0.1578 | 0.09842 | 0.1172 | 0.008024 |
| Significance | 0.0023 * | 0.0072 * | 0.009 * | 0.385 | |
| PPH | Statistic r | −0.02315 | 0.009001 | 0.0611 | 0.06377 |
| Significance | 0.6429 | 0.433 | 0.1232 | 0.109 | |
| Pure forest | Statistic r | 0.04333 | 0.04238 | 0.0746 | 0.05378 |
| Significance | 0.0748 | 0.0618 | 4.00 × 10−4 * | 0.0031 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, Y.; Tian, Y.; Song, Q.; Deng, N. Characteristics and Driving Mechanisms of Understory Vegetation Diversity Patterns in Central and Southern China. Forests 2024, 15, 1056. https://doi.org/10.3390/f15061056
Xiao Y, Tian Y, Song Q, Deng N. Characteristics and Driving Mechanisms of Understory Vegetation Diversity Patterns in Central and Southern China. Forests. 2024; 15(6):1056. https://doi.org/10.3390/f15061056
Chicago/Turabian StyleXiao, Yaqin, Yuxin Tian, Qingan Song, and Nan Deng. 2024. "Characteristics and Driving Mechanisms of Understory Vegetation Diversity Patterns in Central and Southern China" Forests 15, no. 6: 1056. https://doi.org/10.3390/f15061056





