Intraspecific Trait Variation Regulates Biodiversity and Community Productivity of Shrublands in Drylands
Abstract
:1. Introduction
2. Materials and Methods
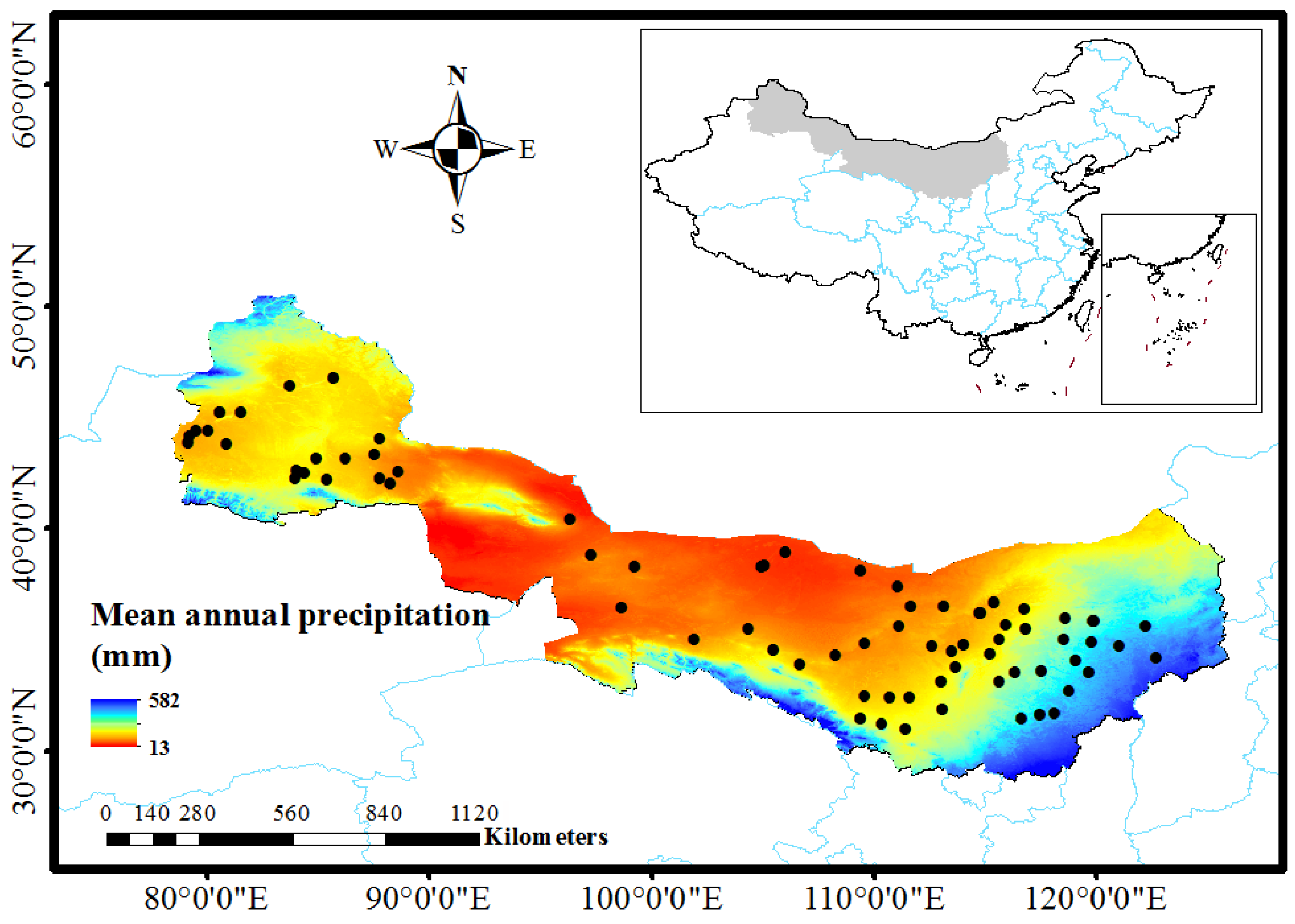
2.1. Study Site and Field Sampling
2.2. Quantification the Effect of External and Internal Filters on Plant Functional Traits
2.3. Calculating Functional Redundancy of Plant Functional Traits
2.4. Classification the Plant Functional Types of Shrub Plants
2.5. Environmental Predictors
2.6. Statistical Analysis
3. Results
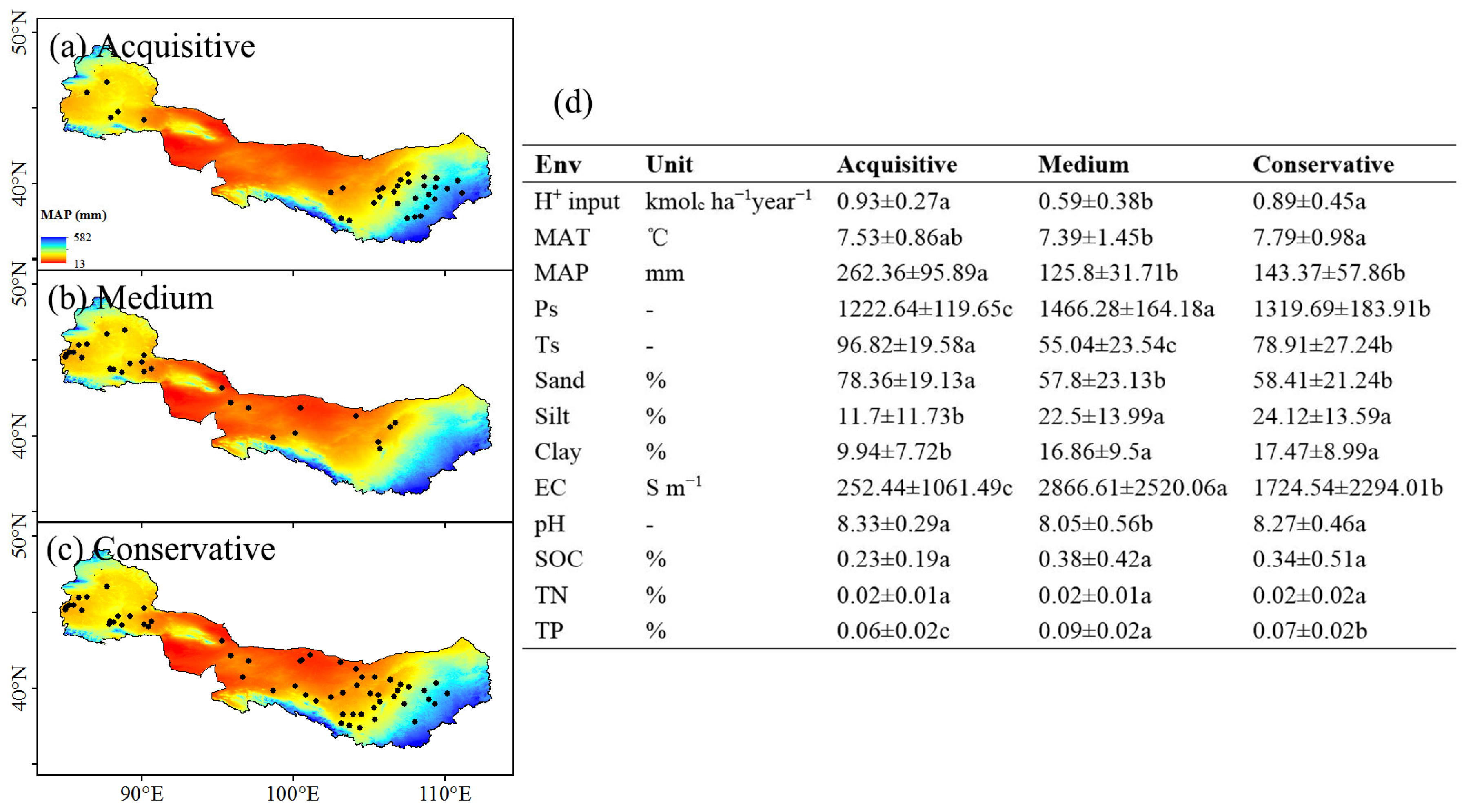
3.1. The Classification of Resource-Use Strategy for Shrublands in Northwest China
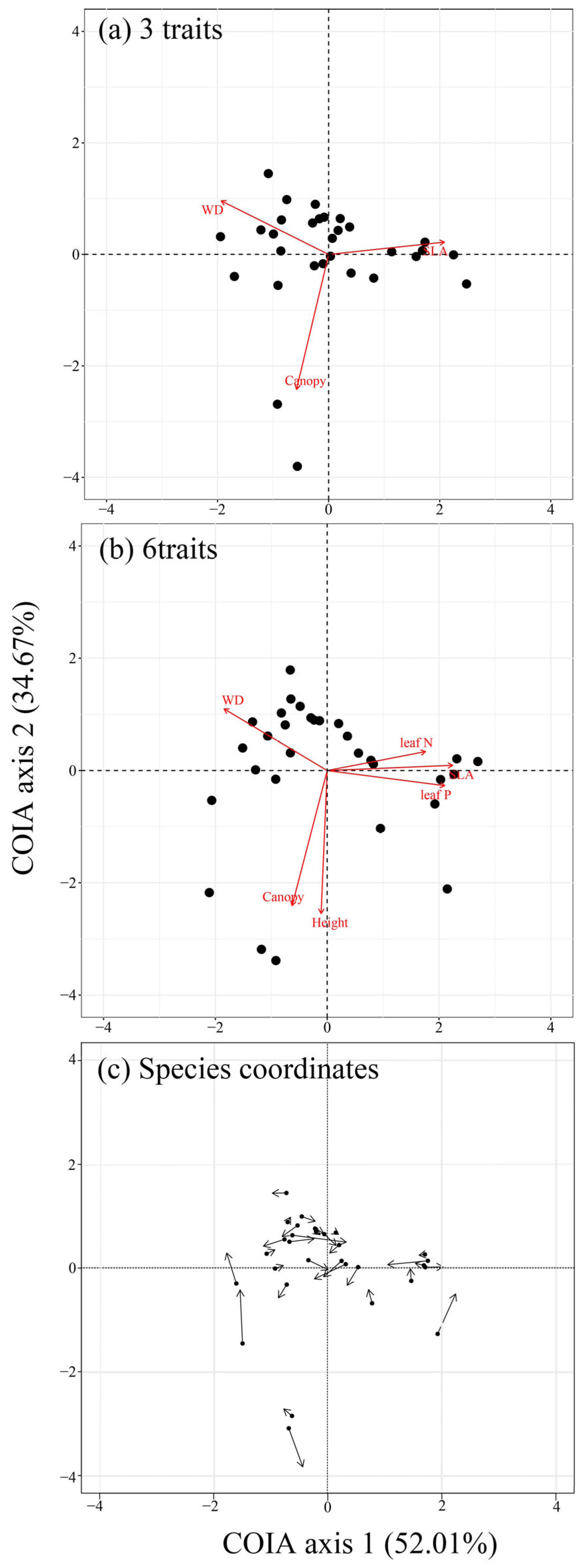
3.2. The Results of Co-Inertia Analysis
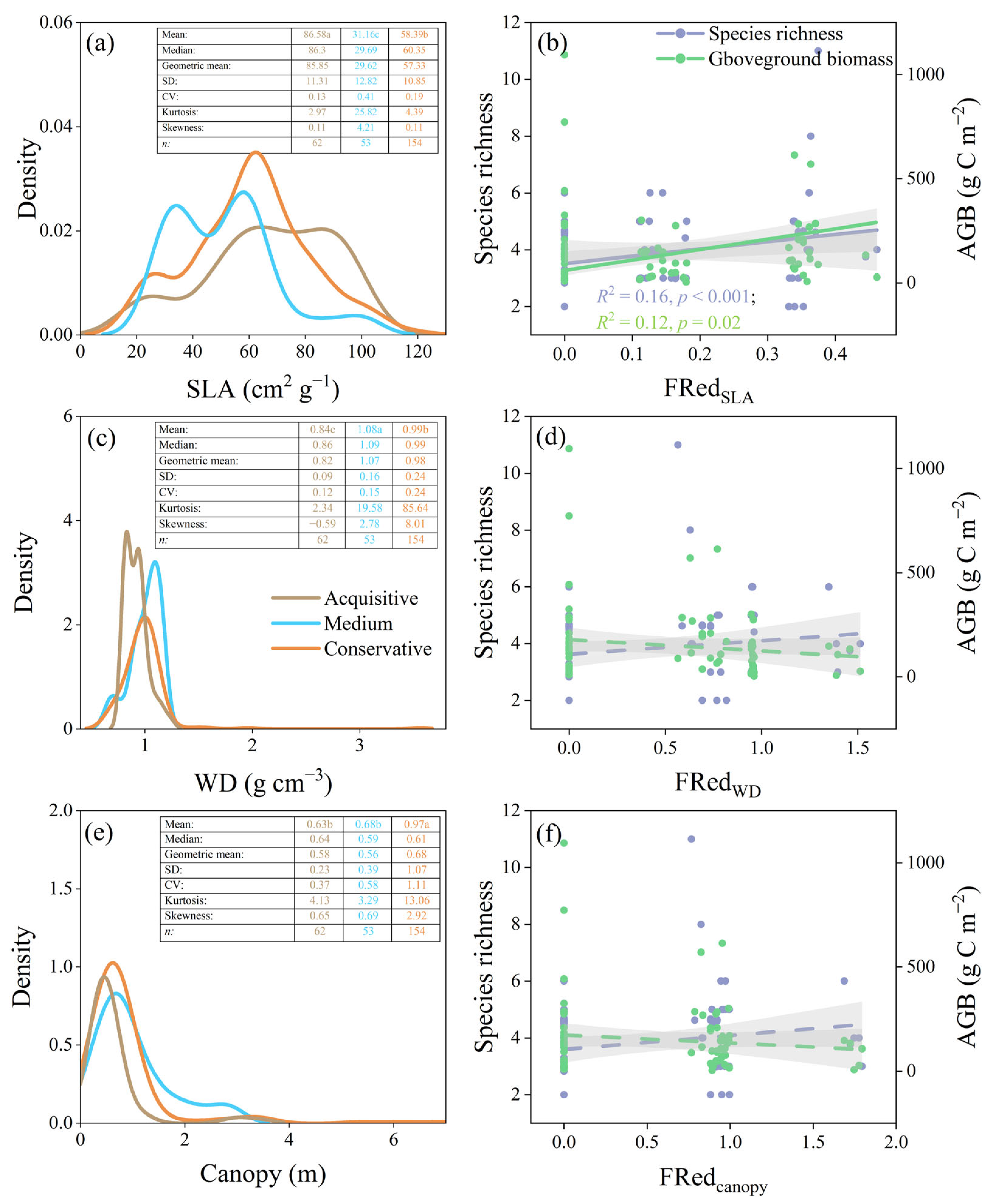
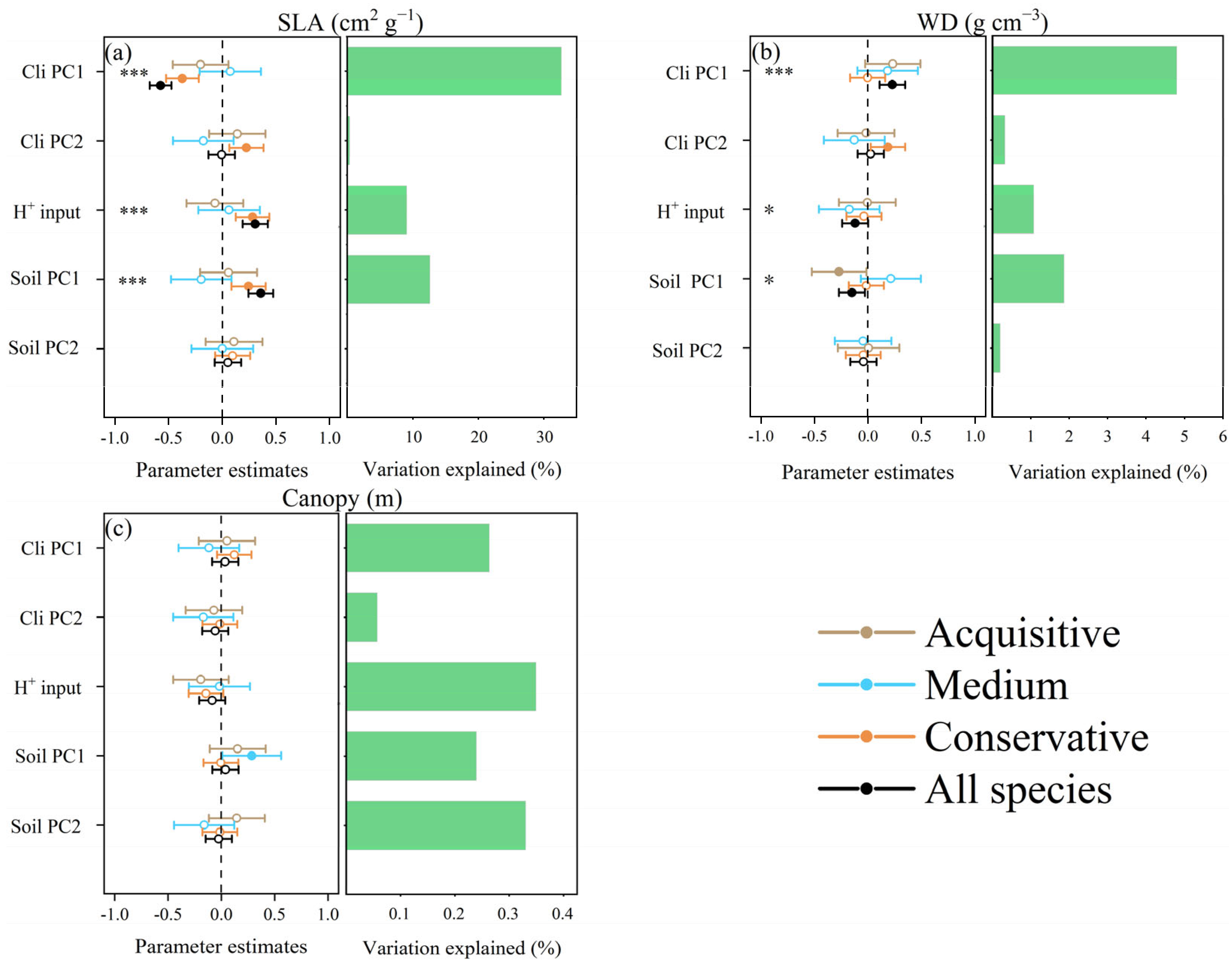
3.3. Relationships between Environment Pressure, Assembly Processes, and Ecosystem Functions
4. Discussion
4.1. Precipitation Regimes Shaping Resource-Use Strategy Division of Shrub Community in Northwest China
4.2. Intraspecific Trait Variation Mediates Assembly Processes and Ecosystem Functions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, S.Y.; Miao, L.J.; Jiang, Z.H.; Wang, G.J.; Gnyawali, K.R.; Zhang, J.; Zhang, H.; Fang, K.; He, Y.; Li, C. Projected drought conditions in Northwest China with CMIP6 models under combined SSPs and RCPs for 2015–2099. Adv. Clim. Chang Res. 2020, 11, 210–217. [Google Scholar] [CrossRef]
- Miao, L.; Li, S.; Zhang, F.; Chen, T.; Shan, Y.; Zhang, Y. Future Drought in the Dry Lands of Asia Under the 1.5 and 2.0 °C Warming Scenarios. Earths Future 2020, 8, e2019EF001337. [Google Scholar] [CrossRef]
- Zhu, S.; Li, C.; Shao, H.; Ju, W.; Lv, N. The response of carbon stocks of drylands in Central Asia to changes of CO2 and climate during past 35 years. Sci. Total Environ. 2019, 687, 330–340. [Google Scholar] [CrossRef] [PubMed]
- Deng, M.; Liu, W.; Li, P.; Jiang, L.; Li, S.; Jia, Z.; Yang, S.; Guo, L.; Wang, Z.; Liu, L. Intraspecific trait variation drives grassland species richness and productivity under changing precipitation. Ecosphere 2021, 12, e03707. [Google Scholar] [CrossRef]
- Li, K.; Liu, X.; Song, L.; Gong, Y.; Lu, C.; Yue, P.; Tian, C.; Zhang, F. Response of alpine grassland to elevated nitrogen deposition and water supply in China. Oecologia 2015, 177, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Diao, H.; Chen, X.; Zhao, X.; Dong, K.; Wang, C. Effects of nitrogen addition and precipitation alteration on soil respiration and its components in a saline-alkaline grassland. Geoderma 2022, 406, 115541. [Google Scholar] [CrossRef]
- Gong, H.; Han, Y.; Li, J.; Liu, Z.; Hou, R.; Zhang, Y.; Dou, W.; Wang, B.; Ouyang, Z. Responses of Phragmites australis to Nitrogen Addition along Salinity Gradients in Coastal Saline-Alkali Soil. Land 2022, 11, 2320. [Google Scholar] [CrossRef]
- Delgado-Baquerizo, M.; Maestre, F.T.; Reich, P.B.; Jeffries, T.C.; Gaitan, J.J.; Encinar, D.; Berdugo, M.; Campbell, C.D.; Singh, B.K. Microbial diversity drives multifunctionality in terrestrial ecosystems. Nat. Commun. 2016, 7, 10541. [Google Scholar] [CrossRef] [PubMed]
- Wagg, C.; Bender, S.F.; Widmer, F.; van der Heijden, M.G.A. Soil biodiversity and soil community composition determine ecosystem multifunctionality. Proc. Natl. Acad. Sci. USA 2014, 111, 5266–5270. [Google Scholar] [CrossRef]
- Grace, J.B.; Anderson, T.M.; Seabloom, E.W.; Borer, E.T.; Adler, P.B.; Harpole, W.S.; Hautier, Y.; Hillebrand, H.; Lind, E.M.; Paertel, M.; et al. Integrative modelling reveals mechanisms linking productivity and plant species richness. Nature 2016, 529, 390–393. [Google Scholar] [CrossRef]
- Walde, M.; Allan, E.; Cappelli, S.L.; Didion-Gency, M.; Gessler, A.; Lehmann, M.M.; Pichon, N.A.; Grossiord, C. Both diversity and functional composition affect productivity and water use efficiency in experimental temperate grasslands. J. Ecol. 2021, 109, 3877–3891. [Google Scholar] [CrossRef]
- Chatanga, P.; Sieben, E.J.J. Variation in Plant Functional Composition of the Afromontane Palustrine Wetlands Along an Altitudinal Gradient in Lesotho. Wetlands 2020, 40, 2539–2552. [Google Scholar] [CrossRef]
- Thomas, H.J.D.; Myers-Smith, I.H.; Bjorkman, A.D.; Elmendorf, S.C.; Blok, D.; Cornelissen, J.H.C.; Forbes, B.C.; Hollister, R.D.; Normand, S.; Prevey, J.S.; et al. Traditional plant functional groups explain variation in economic but not size-related traits across the tundra biome. Glob. Ecol. Biogeogr. 2019, 28, 78–95. [Google Scholar] [CrossRef] [PubMed]
- Thakur, D.; Hadincova, V.; Schnablova, R.; Synkova, H.; Haisel, D.; Wilhelmova, N.; Dostalek, T.; Munzbergova, Z. Differential effect of climate of origin and cultivation climate on structural and biochemical plant traits. Funct. Ecol. 2023, 37, 1436–1448. [Google Scholar] [CrossRef]
- Zuo, X.; Zhao, S.; Cheng, H.; Hu, Y.; Wang, S.; Yue, P.; Liu, R.; Knapp, A.K.; Smith, M.D.; Yu, Q.; et al. Functional diversity response to geographic and experimental precipitation gradients varies with plant community type. Funct. Ecol. 2021, 35, 2119–2132. [Google Scholar] [CrossRef]
- Des Roches, S.; Post, D.M.; Turley, N.E.; Bailey, J.K.; Hendry, A.P.; Kinnison, M.T.; Schweitzer, J.A.; Palkovacs, E.P. The ecological importance of intraspecific variation. Nat. Ecol. Evol. 2018, 2, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Moran, E.V.; Hartig, F.; Bell, D.M. Intraspecific trait variation across scales: Implications for understanding global change responses. Glob. Chang. Biol. 2016, 22, 137–150. [Google Scholar] [CrossRef] [PubMed]
- Griffin-Nolan, R.J.J.; Sandel, B. Global intraspecific trait-climate relationships for grasses are linked to a species’ typical form and function. Ecography 2023, 2023, e06586. [Google Scholar] [CrossRef]
- Raffard, A.; Cucherousset, J.; Santoul, F.; Di Gesu, L.; Blanchet, S. Climate and intraspecific variation in a consumer species drive ecosystem multifunctionality. Oikos 2023, 2023, e09286. [Google Scholar] [CrossRef]
- Siefert, A.; Violle, C.; Chalmandrier, L.; Albert, C.H.; Taudiere, A.; Fajardo, A.; Aarssen, L.W.; Baraloto, C.; Carlucci, M.B.; Cianciaruso, M.V.; et al. A global meta-analysis of the relative extent of intraspecific trait variation in plant communities. Ecol. Lett. 2015, 18, 1406–1419. [Google Scholar] [CrossRef]
- Gorne, L.D.; Diaz, S.; Minden, V.; Onoda, Y.; Kramer, K.; Muir, C.; Michaletz, S.T.; Lavorel, S.; Sharpe, J.; Jansen, S.; et al. The acquisitive-conservative axis of leaf trait variation emerges even in homogeneous environments. Ann. Bot-Lond. 2022, 129, 709–722. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, W.; Wang, S.; Yang, L.; Gu, J. Intraspecific variations of anatomical, morphological and chemical traits in leaves and absorptive roots along climate and soil gradients: A case study with Ginkgo biloba and Eucommia ulmoides. Plant Soil 2021, 469, 73–88. [Google Scholar] [CrossRef]
- Baez, S.; Homeier, J. Functional traits determine tree growth and ecosystem productivity of a tropical montane forest: Insights from a long-term nutrient manipulation experiment. Glob. Chang. Biol. 2018, 24, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Hurtado, P.; Prieto, M.; Aragon, G.; de Bello, F.; Martinez, I. Intraspecific variability drives functional changes in lichen epiphytic communities across Europe. Ecology 2020, 101, e03017. [Google Scholar] [CrossRef] [PubMed]
- Jung, V.; Albert, C.H.; Violle, C.; Kunstler, G.; Loucougaray, G.; Spiegelberger, T. Intraspecific trait variability mediates the response of subalpine grassland communities to extreme drought events. J. Ecol. 2014, 102, 45–53. [Google Scholar] [CrossRef]
- Benavides, R.; Valladares, F.; Wirth, C.; Mueller, S.; Scherer-Lorenzen, M. Intraspecific trait variability of trees is related to canopy species richness in European forests. Perspect. Plant Ecol. 2019, 36, 24–32. [Google Scholar] [CrossRef]
- Zhang, L.; Cappelli, S.L.; Huang, M.; Liu, X.; Xiao, Y.; Cardou, F.; Rong, Y.; Zhou, S. Intraspecific trait variability mediates the effect of nitrogen addition and warming on aboveground productivity. Oikos 2023, 2023, e09559. [Google Scholar] [CrossRef]
- Fu, H.; Yuan, G.; Jeppesen, E. Trait-based community assembly of submersed macrophytes subjected to nutrient enrichment in freshwater lakes: Do traits at the individual level matter? Ecol. Indic. 2020, 110, 105895. [Google Scholar] [CrossRef]
- Kraft, N.J.B.; Godoy, O.; Levine, J.M. Plant functional traits and the multidimensional nature of species coexistence. Proc. Natl. Acad. Sci. USA 2015, 112, 797–802. [Google Scholar] [CrossRef]
- Violle, C.; Enquist, B.J.; McGill, B.J.; Jiang, L.; Albert, C.H.; Hulshof, C.; Jung, V.; Messier, J. The return of the variance: Intraspecific variability in community ecology. Trends Ecol. Evol. 2012, 27, 244–252. [Google Scholar] [CrossRef]
- Hart, S.P.; Schreiber, S.J.; Levine, J.M. How variation between individuals affects species coexistence. Ecol. Lett. 2016, 19, 825–838. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Chi, Y.; Yang, X.; Li, W.; Lan, Z.; Bai, Y. Direct and indirect effects of nitrogen enrichment and grazing on grassland productivity through intraspecific trait variability. J Appl. Ecol. 2022, 59, 598–610. [Google Scholar] [CrossRef]
- Messier, J.; McGill, B.J.; Enquist, B.J.; Lechowicz, M.J. Trait variation and integration across scales: Is the leaf economic spectrum present at local scales? Ecography 2017, 40, 685–697. [Google Scholar] [CrossRef]
- E-Vojtko, A.; Junker, R.R.; de Bello, F.; Gotzenberger, L. Floral and reproductive traits are an independent dimension within the plant economic spectrum of temperate central Europe. New Phytol. 2022, 236, 1964–1975. [Google Scholar] [CrossRef] [PubMed]
- Lubbe, F.C.; Klimes, A.; Dolezal, J.; Jandova, V.; Mudrak, O.; Janecek, S.; Bartuskova, A.; Klimesova, J. Carbohydrate storage in herbs: The forgotten functional dimension of the plant economic spectrum. Ann. Bot-Lond. 2021, 127, 813–825. [Google Scholar] [CrossRef] [PubMed]
- de Souza, B.C.; Carvalho, E.C.D.; Oliveira, R.S.; de Araujo, F.S.; de Lima, A.L.A.; Rodal, M.J.N. Drought response strategies of deciduous and evergreen woody species in a seasonally dry neotropical forest. Oecologia 2020, 194, 221–236. [Google Scholar] [CrossRef]
- Onoda, Y.; Richards, A.E.; Westoby, M. The relationship between stem biomechanics and wood density is modified by rainfall in 32 Australian woody plant species. New Phytol. 2010, 185, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.B.; Yang, D.; Zhang, K.Y.; Bai, X.L.; Wang, Y.S.D.; Wu, H.D.; Ding, L.Z.; Zhang, Y.J.; Zhang, J.L. Higher water and nutrient use efficiencies in savanna than in rainforest lianas result in no difference in photosynthesis. Tree Physiol. 2022, 42, 145–159. [Google Scholar] [CrossRef] [PubMed]
- Jing, H.; Xiong, X.; Jiang, F.; Pu, X.; Ma, W.; Li, D.; Liu, Z.; Wang, Z. Climate change filtered out resource-acquisitive plants in a temperate grassland in Inner Mongolia, China. Sci. China Life Sci. 2023, 67, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Sun, R.; Li, Y.; Ji, H.; Jia, B.; Xu, Z. Plant economic strategies in two contrasting forests. BMC Plant Biol. 2023, 23, 366. [Google Scholar] [CrossRef]
- Deng, Y.; Deng, X.; Dong, J.; Zhang, W.; Hu, T.; Nakamura, A.; Song, X.; Fu, P.; Cao, M. Detecting growth phase shifts based on leaf trait variation of a canopy dipterocarp tree species (Parashorea chinensis). Forests 2020, 11, 1145. [Google Scholar] [CrossRef]
- Zhao, G.; Chen, F.; Yuan, C.; Yang, J.; Shen, Y.; Zhang, S.; Yang, J.; Ayemele, A.G.; Li, X.; Xu, J. Response strategies of woody seedlings to shading and watering over time after topsoil translocation in dry-hot karst region of China. For. Ecol. Manag. 2022, 519, 120319. [Google Scholar] [CrossRef]
- Xu, H.G.; Liu, S.R. Effects of soil salinization on halophytic vegetation. Inn. M. Pr. 2004, 16, 1–2. [Google Scholar]
- Zhang, C.; Wang, Y.; Jia, X.; Shao, M.a.; An, Z. Impacts of shrub introduction on soil properties and implications for dryland revegetation. Sci. Total Environ. 2020, 742, 140498. [Google Scholar] [CrossRef] [PubMed]
- Litalien, A.; Zeeb, B. Curing the earth: A review of anthropogenic soil salinization and plant-based strategies for sustainable mitigation. Sci. Total Environ. 2020, 698, 134235. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Xu, C.; Allen, C.D.; Hartmann, H.; Wei, X.; Yakir, D.; Wu, X.; Yu, P. Nature-based framework for sustainable afforestation in global drylands under changing climate. Glob. Chang. Biol. 2022, 28, 2202–2220. [Google Scholar] [CrossRef] [PubMed]
- Dhehibi, B.; Fouzai, A.; Frija, A.; Adhim, M.A.; M’Hamed, H.C.; Ouerghemmi, H.; Rekik, M. Assessing complementary synergies for integrated crop-livestock systems under conservation agriculture in Tunisian dryland farming systems. Front. Sustain. Food Syst. 2023, 6, 1022213. [Google Scholar] [CrossRef]
- Su, N.; Zhao, Y.; Ding, G.; Duan, W. Relationships between key dryland ecosystem services: A case study in Ordos, China. Front. Earth Sci. 2022, 10, 937491. [Google Scholar] [CrossRef]
- Camara, A.S. Do woody plants create ‘fertile islands’ in dryland New Zealand? N. Z. J. Ecol. 2021, 45, 1–7. [Google Scholar] [CrossRef]
- Bao, S.D. Soil Agrochemical Analysis, 3rd ed.; Agriculture Press: Beijing, China, 2000. [Google Scholar]
- Carmona, C.P.; de Bello, F.; Mason, N.W.H.; Leps, J. Traits without borders: Integrating functional diversity across scales. Trends Ecol. Evol. 2016, 31, 382–394. [Google Scholar] [CrossRef]
- Carmona, C.P.; de Bello, F.; Mason, N.W.H.; Leps, J. Trait probability density (TPD): Measuring functional diversity across scales based on TPD with R. Ecology 2019, 100, e02876. [Google Scholar] [CrossRef] [PubMed]
- Duong, T. ks: Kernel density estimation and kernel discriminant analysis for multivariate data in R. J. Stat. Softw. 2007, 21, 1–16. [Google Scholar] [CrossRef]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Du, L.; Tian, S.C.; Zhao, N.; Zhang, B.; Mu, X.H.; Tang, L.S.; Zheng, X.J.; Li, Y. Evaluating soil acidification risk and its effects on biodiversity-ecosystem multifunctionality relationships in the drylands of China. For. Ecosyst. 2024, 11, 100178. [Google Scholar] [CrossRef]
- Batjes. World Soil Property Estimates for Broad-Scale Modelling (WISE30sec). (ISRIC Report; No. 2015/01). ISRIC—World Soil Information. 2015. Available online: https://edepot.wur.nl/400244 (accessed on 1 July 2015).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 1 July 2015).
- Dray, S.; Dufour, A.-B. The ade4 package: Implementing the duality diagram for ecologists. J. Stat. Softw. 2007, 22, 1–20. [Google Scholar] [CrossRef]
- Lefcheck, J.S. PIECEWISESEM: Piecewise structural equation modelling in R for ecology, evolution, and systematics. Methods Ecol. Evol. 2016, 7, 573–579. [Google Scholar] [CrossRef]
- Shipley, B. Confirmatory path analysis in a generalized multilevel context. Ecology 2009, 90, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Xie, T.; Yang, Y.; Chen, S.; Chen, F.; Huang, W.; Chen, J. Discussion of the “warming and wetting” trend and its future variation in the drylands of Northwest China under global warming. Sci. China Earth Sci. 2023, 66, 1241–1257. [Google Scholar] [CrossRef]
- Mason, N.W.H.; Mouillot, D.; Lee, W.G.; Wilson, J.B. Functional richness, functional evenness and functional divergence: The primary components of functional diversity. Oikos 2005, 111, 112–118. [Google Scholar] [CrossRef]
- Reich, P.B. The world-wide ‘fast-slow’ plant economics spectrum: A traits manifesto. J. Ecol. 2014, 102, 275–301. [Google Scholar] [CrossRef]
- Wright, I.J.; Reich, P.B.; Westoby, M. Strategy shifts in leaf physiology, structure and nutrient content between species of high- and low-rainfall and high- and low-nutrient habitats. Funct. Ecol. 2001, 15, 423–434. [Google Scholar] [CrossRef]
- LaForgia, M.L.; Harrison, S.P.; Latimer, A.M. Invasive species interact with climatic variability to reduce success of natives. Ecology 2020, 101, e03022. [Google Scholar] [CrossRef] [PubMed]
- Mendez-Toribio, M.; Ibarra-Manriquez, G.; Paz, H.; Lebrija-Trejos, E. Atmospheric and soil drought risks combined shape community assembly of trees in a tropical dry forest. J. Ecol. 2020, 108, 1347–1357. [Google Scholar] [CrossRef]
- Wang, X.G.; Lu, X.T.; Zhang, H.Y.; Dijkstra, F.A.; Jiang, Y.G.; Wang, X.B.; Lu, J.Y.; Wuyunna; Wang, Z.W.; Han, X.G. Changes in soil C:N:P stoichiometry along an aridity gradient in drylands of northern China. Geoderma 2020, 361, 114087. [Google Scholar] [CrossRef]
- Wertin, T.M.; Reed, S.C.; Belnap, J. C3 and C4 plant responses to increased temperatures and altered monsoonal precipitation in a cool desert on the Colorado Plateau, USA. Oecologia 2015, 177, 997–1013. [Google Scholar] [CrossRef] [PubMed]
- Andrews, H.M.; Krichels, A.H.; Homyak, P.M.; Piper, S.; Aronson, E.L.; Botthoff, J.; Greene, A.C.; Jenerette, G.D. Wetting-induced soil CO2 emission pulses are driven by interactions among soil temperature, carbon, and nitrogen limitation in the Colorado Desert. Glob. Chang. Biol. 2023, 29, 3205–3220. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wen, X. Divergent responses of nitrogen availability to aridity in drylands. Plant Soil 2023, 482, 111–125. [Google Scholar] [CrossRef]
- Zhang, X.; Hasi, M.; Li, A.; Tan, Y.; Daryanto, S.; Wang, L.; Zhang, X.; Chen, S.; Huang, J. Nitrogen addition amplified water effects on species composition shift and productivity increase. J. Plant Ecol. 2021, 14, 816–828. [Google Scholar] [CrossRef]
- Zhou, X.B.; Zhang, Y.M. Review on the ecological effects of N deposition in arid and semi-arid areas. Acta Ecol. Sin. 2009, 29, 3835–3845. [Google Scholar]
- Zhang, Q.; Wang, Q.; Zhu, J.; Xu, L.; Li, M.; Rengel, Z.; Xiao, J.; Hobbie, E.A.; Piao, S.; Luo, W.; et al. Higher soil acidification risk in southeastern Tibetan Plateau. Sci. Total Environ. 2021, 755, 143372. [Google Scholar] [CrossRef]
- Lohbeck, M.; Poorter, L.; Lebrija-Trejos, E.; Martinez-Ramos, M.; Meave, J.A.; Paz, H.; Perez-Garcia, E.A.; Eunice Romero-Perez, I.; Tauro, A.; Bongers, F. Successional changes in functional composition contrast for dry and wet tropical forest. Ecology 2013, 94, 1211–1216. [Google Scholar] [CrossRef] [PubMed]
- Neyret, M.; Bentley, L.P.; Oliveras, I.; Marimon, B.S.; Marimon-Junior, B.H.; de Oliveira, E.A.; Passos, F.B.; Castro Ccoscco, R.; dos Santos, J.; Reis, S.M.; et al. Examining variation in the leaf mass per area of dominant species across two contrasting tropical gradients in light of community assembly. Ecol. Evol. 2016, 6, 5674–5689. [Google Scholar] [CrossRef] [PubMed]
- Wood, C.M.; McKinney, S.T.; Loftin, C.S. Intraspecific functional diversity of common species enhances community stability. Ecol. Evol. 2017, 7, 1553–1560. [Google Scholar] [CrossRef]
- Mayfield, M.M.; Levine, J.M. Opposing effects of competitive exclusion on the phylogenetic structure of communities. Ecol. Lett. 2010, 13, 1085–1093. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, L.; Tian, S.; Zhao, N.; Zhang, B.; Mu, X.; Tang, L.; Zheng, X.; Li, Y. Intraspecific Trait Variation Regulates Biodiversity and Community Productivity of Shrublands in Drylands. Forests 2024, 15, 911. https://doi.org/10.3390/f15060911
Du L, Tian S, Zhao N, Zhang B, Mu X, Tang L, Zheng X, Li Y. Intraspecific Trait Variation Regulates Biodiversity and Community Productivity of Shrublands in Drylands. Forests. 2024; 15(6):911. https://doi.org/10.3390/f15060911
Chicago/Turabian StyleDu, Lan, Shengchuan Tian, Nan Zhao, Bin Zhang, Xiaohan Mu, Lisong Tang, Xinjun Zheng, and Yan Li. 2024. "Intraspecific Trait Variation Regulates Biodiversity and Community Productivity of Shrublands in Drylands" Forests 15, no. 6: 911. https://doi.org/10.3390/f15060911





