Floristic Diversity and Green-Tree Retention in Intensively Managed Temperate Forests: A Case Study in Puebla, Mexico
Abstract
1. Introduction
2. Materials and Methods
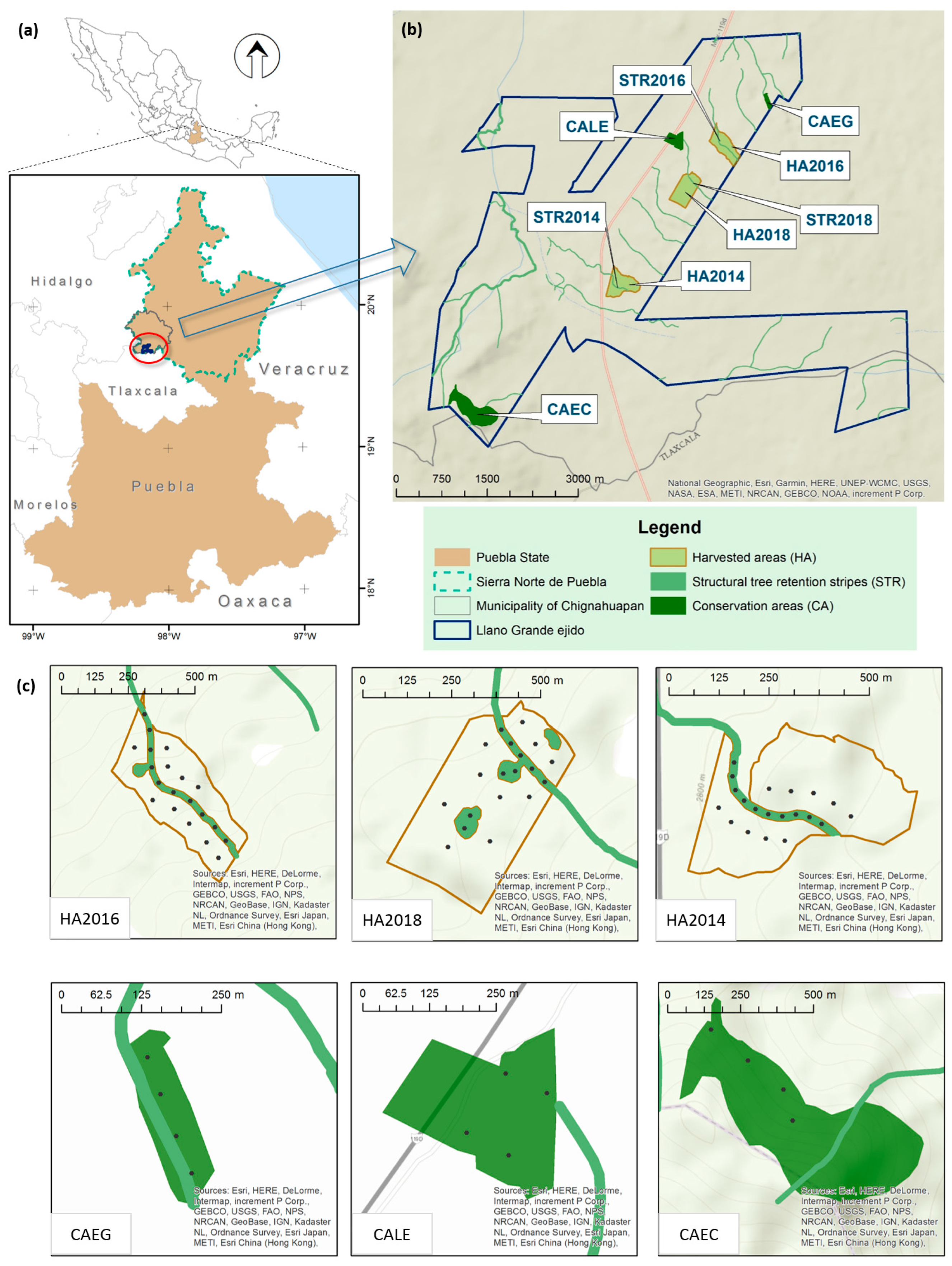
2.1. Study Site
2.2. Floristic Inventory
2.3. Floristic Richness and Diversity
2.4. Floristic Composition
3. Results
3.1. Richness and Diversity Floristics
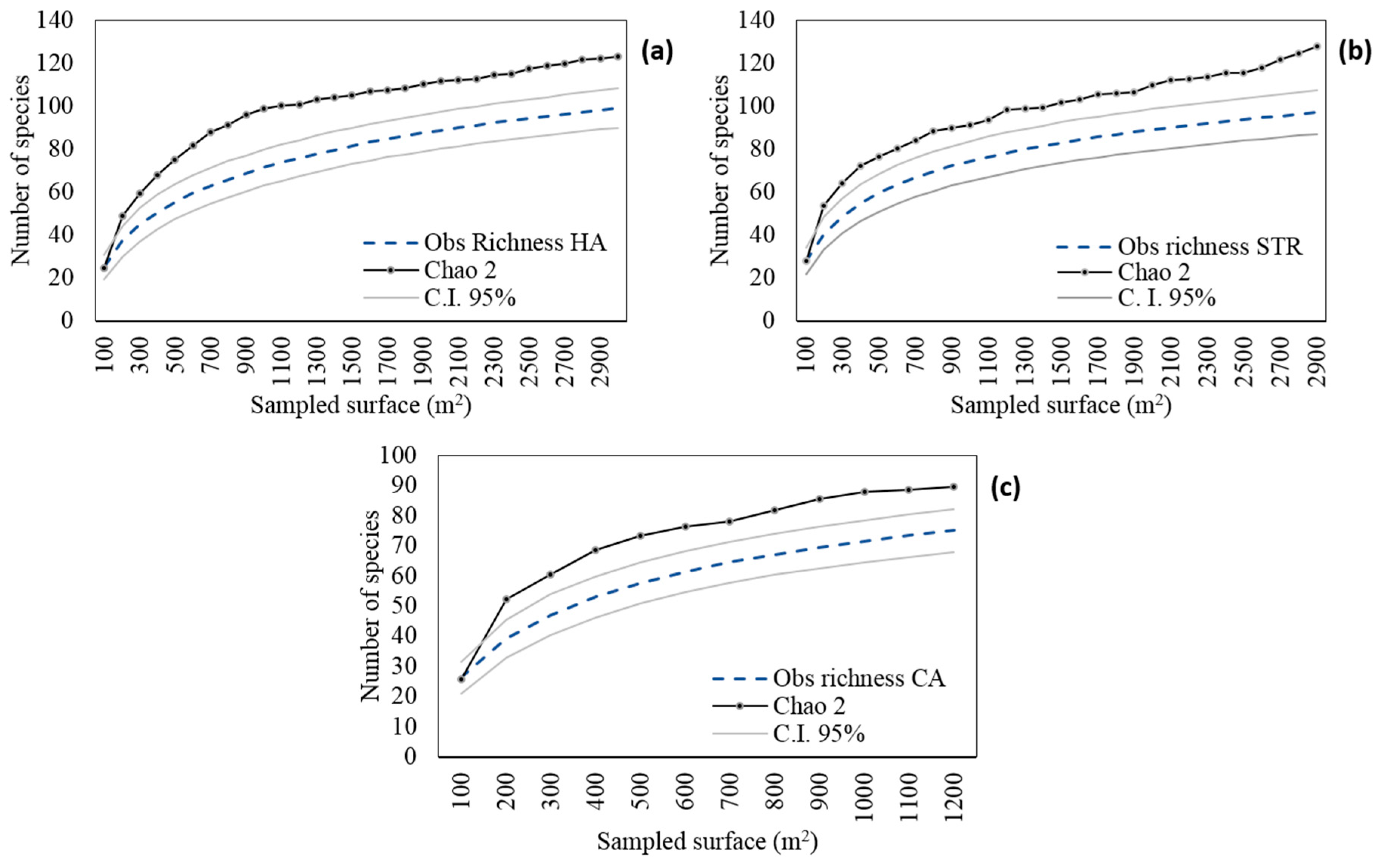
3.1.1. Harvested Areas (HAs)
3.1.2. Structural Tree Retention Stripes (STRs)
3.1.3. Conservation Areas (CAs)
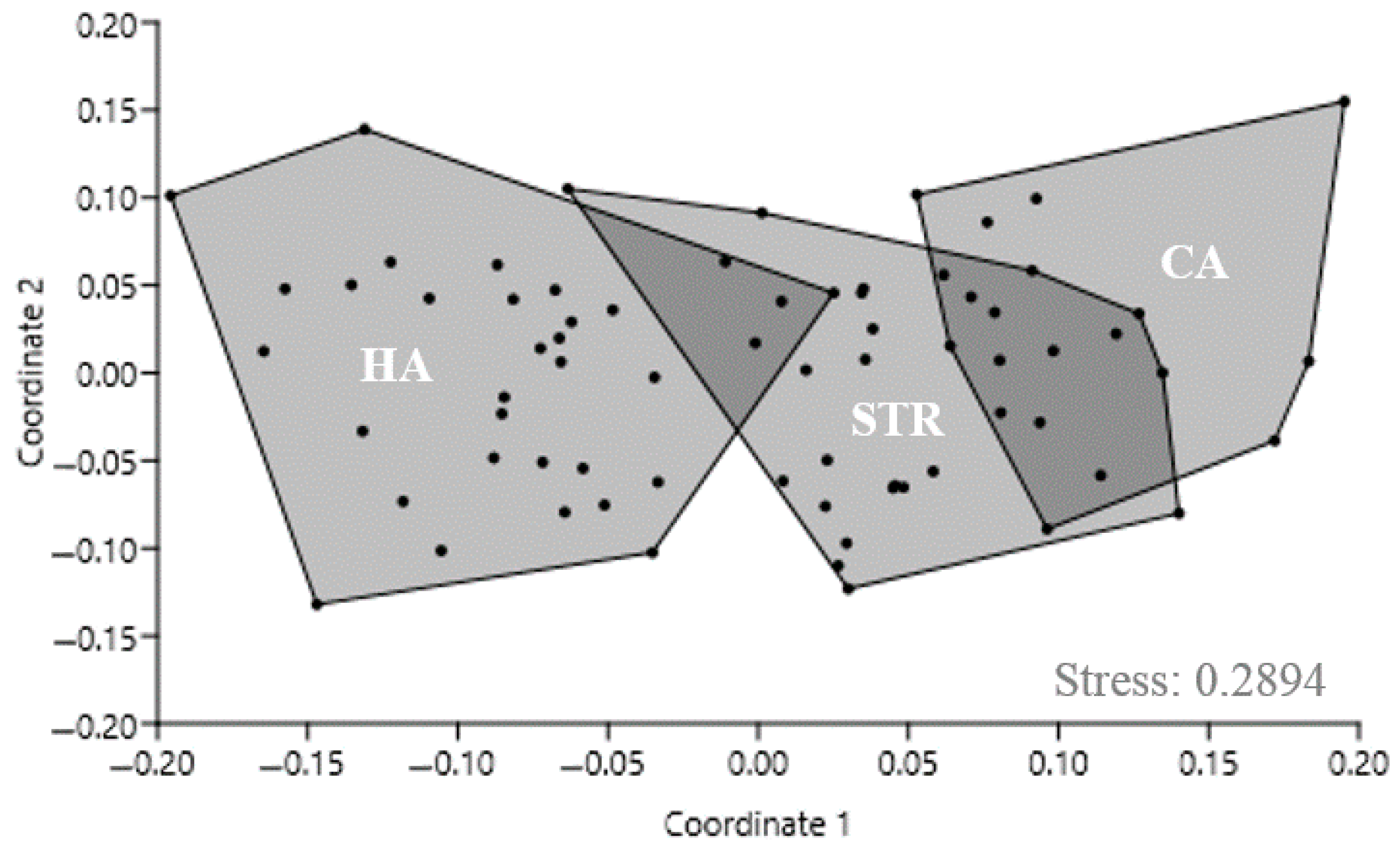
3.2. Floristic Composition
4. Discussion
4.1. Floristic Composition, Richness, and Diversity
4.2. Importance of Forest Retention in Conservation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- CONAFOR (Comisión Nacional Forestal). Sistema Nacional de Monitoreo Forestal. Available online: https://snmf.cnf.gob.mx/principaleindicadoresforestalesciclo-2015-2020/ (accessed on 12 January 2024).
- Villaseñor, J.L.; Ortiz, E. Biodiversidad de las plantas con flores (División Magnoliophyta) en México. Rev. Mex. Biodivers. 2014, 85, S134–S142. [Google Scholar] [CrossRef]
- Villaseñor, J.L. Checklist of the native vascular plants of Mexico. Rev. Mex. Biodivers. 2016, 87, 559–902. [Google Scholar] [CrossRef]
- Rzedowski, J. Diversity and origins of the phanerogamic flora of Mexico. In Biological Diversity of Mexico. Origins and Distribution; Ramamoorthy, T.P., Bye, R., Lot, A., Fa, J., Eds.; Oxford University Press: New York, NY, USA, 1993; pp. 129–144. [Google Scholar]
- Valencia-Avalos, S. Diversity of the genus Quercus (Fagaceae) in Mexico. Bol. Soc. Bot. Méx. 2004, 75, 33–53. [Google Scholar] [CrossRef]
- Rzedowski, J. Vegetación de México, 1st ed.; Comisión Nacional para el Conocimiento y Uso de la Biodiversidad (CONABIO): Mexico City, Mexico, 2006; pp. 112–113. [Google Scholar]
- Guerra-De la Cruz, V.; Galicia, L. Tropical and highland temperate forest plantations in Mexico: Pathways for climate change mitigation and ecosystem services delivery. Forests 2017, 8, 489. [Google Scholar] [CrossRef]
- CONAFOR (Comisión Nacional Forestal). Estado que Guarda el Sector Forestal en México 2020, Bosques Para el Bienestar Social y Climático; CONAFOR: Zapopan, Mexico, 2020; 363p. Available online: http://www.conafor.gob.mx:8080/documentos/docs/1/7825El%20Estado%20que%20guarda%20el%20Sector%20Forestal%20en%20M%c3%a9xico%202020.pdf (accessed on 22 September 2023).
- Bray, D.B.; Merino-Pérez, L. The Rise of Community Forestry in Mexico: History, Concepts, and Lessons Learned from Twenty-Five Years of Community Timber Production; The Ford Foundation: Mexico City, Mexico, 2002; Available online: https://ccmss.org.mx/wp-content/uploads/2014/09/the_rise_of_community_forestry_in_mexico.pdf (accessed on 20 September 2023).
- Torres-Rojo, J.M.; Moreno-Sánchez, R.; Mendoza-Briseño, M.A. Sustainable Forest Management in Mexico. Curr. For. Rep. 2016, 2, 93–105. [Google Scholar] [CrossRef]
- López-Hernández, J.A.; Aguirre-Calderón, O.A.; Alanís-Rodríguez, E.; Monárrez-González, J.C.; González-Tagle, M.A.; Jiménez-Pérez, J. Composición y diversidad de especies forestales en bosques templados de Puebla, México. Madera Bosques 2017, 23, 39–51. [Google Scholar] [CrossRef]
- Jardel, P.E. El manejo forestal en México: Estado actual y perspectivas. In Estado de los Bosques de México; Chapela, F., Ed.; Consejo Civil Mexicano para la Silvicultura Sostenible: Mexico City, Mexico, 2012; pp. 69–115. [Google Scholar]
- Soto-Cervantes, J.A.; Padilla-Martínez, J.R.; Domínguez-Calleros, P.A.; Carrillo-Parra, A.; Rodríguez-Laguna, R.; Pompa-García, M.; García-Montiel, E.; Corral-Rivas, J.J. Efecto de cuatro tratamientos silvícolas en la producción maderable en un bosque de Durango. Rev. Mex. Cienc. For. 2021, 12, 56–80. [Google Scholar] [CrossRef]
- Matlack, G.R. Microenvironment variation within and among forest edge sites in the eastern United States. Biol. Conserv. 1993, 66, 185–194. [Google Scholar] [CrossRef]
- Hannerz, M.; Hånell, B. Effects on the flora in Norway spruce forests following clearcutting and shelterwood cutting. For. Ecol. Manag. 1997, 90, 29–49. [Google Scholar] [CrossRef]
- Cabrelli, D.; Rebottaro, S.; Effron, D. Characterization of forest canopy and light microenviroment in stands with management different, using hemispherical photography. Quebracho 2006, 13, 17–25. Available online: http://www.scielo.org.ar/scielo.php?script=sci_arttext&pid=S1851-30262006000100003&lng=es&tlng=en (accessed on 10 May 2024).
- Seliger, A.; Ammer, C.; Kreft, H.; Zerbe, S. Changes of vegetation in coniferous monocultures in the context of conversion to mixed forests in 30 years—Implications for biodiversity restoration. J. Environ. Manag. 2023, 343, 118199. [Google Scholar] [CrossRef] [PubMed]
- Keenan, R.J.; Kimmins, J.P. The ecological effects of clear-cutting. Environ. Rev. 1993, 1, 121–144. [Google Scholar] [CrossRef]
- Brunet, J.; Fritz, Ö.; Richnau, G. Biodiversity in European beech forests—A review with recommendations for sustainable forest management. Ecol. Bull. 2010, 53, 77–94. Available online: http://www.jstor.org/stable/41442021 (accessed on 10 May 2024).
- Lindenmayer, D.B.; Franklin, J.F.; Lõhmus, A.; Baker, S.C.; Bauhus, J.; Beese, W.; Brodie, A.; Kiehl, B.; Kouki, J.; Pastur, G.M.; et al. A major shift to the retention approach for forestry can help resolve some global forest sustainability issues. Conserv. Lett. 2012, 5, 421–431. [Google Scholar] [CrossRef]
- Smith, J.R. Seral Stage, Site Conditions, and the Vulnerability of Understory Plant Communities to Forest Harvesting. Master’s Thesis, Simon Fraser University, Burnaby, BC, Canada, 2005. [Google Scholar]
- Dieler, J.; Uhl, E.; Pimienta, P.; Müller, J.; Rötzer, T.; Pretzsch, H. Effect of forest stand management on species composition, structural diversity, and productivity in the temperate zone of Europe. Eur. J. For. Res. 2017, 136, 739–766. [Google Scholar] [CrossRef]
- Monárrez-González, J.C.; Pérez-Verdín, G.; López-González, C.; Márquez-Linares, M.A.; González-Elizondo, M.D.S. Efecto del manejo forestal sobre algunos servicios ecosistémicos en los bosques templados de México. Madera Bosques 2018, 24, e2421569. [Google Scholar] [CrossRef]
- Pérez-Flores, M.; Martínez-Pastur, G.J.; Cellini, J.M.; Lencinas, M.V. Recovery of understory assemblage along 50 years after shelterwood cut harvesting in Nothofagus pumilio Southern Patagonian forests. For. Ecol. Manag. 2019, 450, 117494. [Google Scholar] [CrossRef]
- Martínez-Pastur, G.J.; Vanha-Majamaa, I.; Franklin, J.F. Ecological perspectives on variable retention forestry. Ecol. Process. 2020, 9, 12. [Google Scholar] [CrossRef]
- Lindenmayer, D.B.; Franklin, J.F. Conserving Forest Biodiversity: A Comprehensive Multiscaled Approach; Island Press: Washington, DC, USA, 2002; 351p. [Google Scholar]
- Franklin, J.F.; Donato, D.C. Variable retention harvesting in the Douglas-fir region. Ecol. Process. 2020, 9, 8. [Google Scholar] [CrossRef]
- Beese, W.J.; Deal, J.; Dunsworth, B.G.; Mitchell, S.J.; Philpott, T.J. Two decades of variable retention in British Columbia: A review of its implementation and effectiveness for biodiversity conservation. Ecol. Process. 2019, 8, 33. [Google Scholar] [CrossRef]
- Scott, R.E.; Neyland, M.G.; McElwee, D.J. Early regeneration results following aggregated retention harvesting of wet eucalypt forests in Tasmania, Australia. For. Ecol. Manag. 2013, 302, 254–263. [Google Scholar] [CrossRef]
- Johnson, S.; Strengbom, J.; Kouki, J. Low levels of tree retention do not mitigate the effects of clearcutting on ground vegetation dynamics. For. Ecol. Manag. 2014, 330, 67–74. [Google Scholar] [CrossRef]
- Franklin, J.F.; Berg, D.R.; Thornburgh, D.A.; Tappeiner, J.C. Alternative silvicultural approaches to timber harvesting: Variable retention harvest systems. In Creating a Forestry for the 21st Century: The Science of Ecosystem Management; Kohn, K.A., Franklin, J.F., Eds.; Island Press: Washington, DC, USA, 1997; Chapter 7; pp. 111–140. [Google Scholar]
- Rosenvald, R.; Lõhmus, A. For what, when, and where is green-tree retention better than clear-cutting? A review of biodiversity aspects. For. Ecol. Manag. 2008, 255, 1–15. [Google Scholar] [CrossRef]
- Gustafsson, L.; Hannerz, M.; Koivula, M.; Shorohova, E.; Vanha-Majamaa, I.; Weslien, J. Research on retention forestry in Northern Europe. Ecol. Process. 2020, 9, 3. [Google Scholar] [CrossRef]
- Gustafsson, L.; Baker, S.C.; Bauhus, J.; Beese, W.J.; Brodie, A.; Kouki, J.; Lindenmayer, D.B.; Lõhmus, A.; Martínez, P.G.; Messier, C.; et al. Retention Forestry to Maintain Multifunctional Forests: A World Perspective. BioScience 2012, 62, 633–645. [Google Scholar] [CrossRef]
- Halpern, C.B.; Halaj, J.; Evans, S.A.; Dovčiak, M. Level and pattern of overstory retention interact to shape long-term responses of understories to timber harvest. Ecol. Appl. 2012, 22, 2049–2064. [Google Scholar] [CrossRef] [PubMed]
- Fedrowitz, K.; Koricheva, J.; Baker, S.C.; Lindenmayer, D.B.; Palik, B.; Rosenvald, R.; Beese, W.; Franklin, J.F.; Kouki, J.; Macdonald, E.; et al. REVIEW: Can retention forestry help conserve biodiversity? A meta-analysis. J. App. Ecol. 2014, 51, 1669–1679. [Google Scholar] [CrossRef] [PubMed]
- Beese, W.J.; Sandford, J.S.; Harrison, M.L.; Filipescu, C.N. Understory vegetation response to alternative silvicultural systems in coastal British Columbia montane forests. For. Ecol. Manag. 2022, 504, 119817. [Google Scholar] [CrossRef]
- Tinya, F.; Kovács, B.; Prättälä, A.; Farkas, P.; Aszalós, R.; Ódor, P. Initial understory response to experimental silvicultural treatments in a temperate oak-dominated forest. Eur. J. For. Res. 2019, 138, 65–77. [Google Scholar] [CrossRef]
- Soler, R.M.; Schindler, S.; Lencinas, M.V.; Peri, P.L.; Martínez-Pastur, G. Why biodiversity increases after variable retention harvesting: A meta-analysis for southern Patagonian forests. For. Ecol. Manag. 2016, 369, 161–169. [Google Scholar] [CrossRef]
- Lencinas, M.V.; Sola, F.J.; Martínez-Pastur, G.J. Variable retention effects on vascular plants and beetles along a regional gradient in Nothofagus pumilio forests. For. Ecol. Manag. 2017, 406, 251–265. [Google Scholar] [CrossRef]
- Morrone, J.J. Regionalización biogeográfica y evolución biótica de México: Encrucijada de la biodiversidad del Nuevo Mundo. Rev. Mex. Biodivers. 2019, 90, e902980. [Google Scholar] [CrossRef]
- Reyes-González, J.A.; Rhodes, A. Conservación de la biodiversidad en el Eje Neovolcánico, Colaboración interinstitucional en un territorio biodiverso y proveedor de servicios ambientales. Territorios 2015, 2, 7–8. Available online: https://www.researchgate.net/publication/309762380_Conservacion_de_la_biodiversidad_en_el_Eje_Neovolcanico_colaboracion_interinstitucional_en_un_territorio_biodiverso_y_proveedor_de_servicios_ambientales (accessed on 4 April 2024).
- FAO (Food and Agriculture Organization of the United States); UNEP (United Nations Environment Programme). The State of the World’s Forests 2020. Forests, Biodiversity and People; FAO and UNEP: Rome, Italy, 2020; 214p. [Google Scholar] [CrossRef]
- Navarro-Martínez, A.; Palmas, S.; Ellis, E.A.; Blanco-Reyes, P.; Vargas-Godínez, C.; Iuit-Jiménez, A.C.; Hernández-Gómez, I.U.; Ellis, P.; Álvarez-Ugalde, A.; Carrera-Quirino, Y.G.; et al. Remnant trees in enrichment planted gaps in Quintana Roo, Mexico: Reasons for retention and effects on seedlings. Forests 2017, 8, 272. [Google Scholar] [CrossRef]
- Merino-Pérez, L. Comunidades forestales en México. Formas de vida, gobernanza y conservación. Rev. Mex. Sociol. 2018, 80, 909–940. Available online: http://www.scielo.org.mx/scielo.php?script=sci_arttext&pid=S0188-25032018000400909&lng=es&tlng=es (accessed on 3 February 2024).
- Chaudhary, A.; Burivalova, Z.; Koh, L.; Hellweg, S. Impact of forest management on species richness: Global meta-analysis and economic trade-offs. Sci. Rep. 2016, 6, 23954. [Google Scholar] [CrossRef] [PubMed]
- Matias, G.; Cagnacci, F.; Rosalino, L.M. FSC forest certification effects on biodiversity: A global review and meta-analysis. Sci. Total Environ. 2024, 908, 168296. [Google Scholar] [CrossRef] [PubMed]
- CONAFOR (Comisión Nacional Forestal); PNUD (Programa de las Naciones Unidas para el Desarrollo). Caso de Éxito 05, Conservación de Biodiversidad en el Ejido Llano Grande; CONAFOR, PNUD: Puebla, Mexico, 2017. Available online: https://www.gob.mx/cms/uploads/attachment/file/159093/05_Llano_Grande__Puebla.pdf (accessed on 24 October 2022).
- INEGI (Instituto Nacional de Estadística y Geografía). Conjunto de Datos Vectoriales Fisiográficos; INEGI: Aguascalientes, Mexico, 2001. [Google Scholar]
- Moctezuma-Peralta, J.V. Estado Actual de los Mamíferos Silvestres de la Sierra Norte de Puebla. Bachelor’s Thesis, Benemérita Universidad Autónoma de Puebla, Puebla, Mexico, 2011. [Google Scholar]
- INEGI (Instituto Nacional de Estadística y Geografía). Compendio de Información Geográfica Municipal, Chignahuapan, Puebla; INEGI: Aguascalientes, Mexico, 2010.
- Barrón-Sevilla, J.A. Biodiversidad y manejo forestal en la Sierra Norte de Puebla. Elementos 2021, 123, 45–49. Available online: https://elementos.buap.mx/directus/storage/uploads/123-A8-p45-Biodiversidad_y_manejo_forestal_en_la_Sierra_Norte_de_Puebla_M.pdf (accessed on 8 April 2022).
- INEGI (Instituto Nacional de Estadística y Geografía). Conjunto de Datos Vectoriales de Uso del Suelo y Vegetación; INEGI: Aguascalientes, Mexico, 2018.
- Salinas-Cruz, E.; González-Guillén, M.d.J.; León-Merino, A.; Rodríguez-Hernández, F.R. La actividad forestal en el desarrollo económico de Chignahuapan, Puebla. Reg. Soc. 2017, 29, 185–218. [Google Scholar] [CrossRef]
- Gámez, V.A. Sistema de Monitoreo de Biodiversidad en Predios Bajo Manejo Forestal en la UMAFOR Chignahuapan—Zacatlán, Puebla. Master’s Thesis, Colegio de Postgraduados, Montecillo, Mexico, 2019. [Google Scholar]
- Mostacedo, B.; Fredericksen, T. Manual de Métodos Básicos de Muestreo y Análisis en Ecología Vegetal; Proyecto de Manejo Forestal Sostenible (BOLFOR): Santa Cruz, Bolivia, 2000; 87p.
- Matteucci, S.D.; Colma, A. Metodología Para el Estudio de la Vegetación; Chesneau, E.V., Ed.; General Secretariat of the Organization of American States: Washington DC, USA, 1982; 159p.
- Hurtado-Reveles, L.; Burgos-Hernández, M.; Vázquez-Sánchez, M.; López-Acosta, J.C. Contribution to the floristic knowledge of the Sierra de los Cardos, Susticacán, Zacatecas, Mexico. Bot. Sci. 2022, 100, 247–262. [Google Scholar] [CrossRef]
- Simpson, M.G. Plant Systematics, 3rd ed.; Academic Press: Burlington, MA, USA, 2019; 774p. [Google Scholar]
- Diéguez-Aranda, U.; Castedo-Dorado, F.; Anta, M.B.; Álvarez-González, J.G.; Rojo-Alboreca, A.; Ruiz-González, A.D. Prácticas de Dasometría; UNICOPIA: Asturias, Spain, 2005; 125p, Available online: https://www.researchgate.net/profile/Alberto-Rojo-Alboreca/publication/305640101_Practicas_de_dasometria/links/5797266408ae33e89faea3f8/Practicas-de-dasometria.pdf (accessed on 10 May 2024).
- Van der Maarel, E. Transformation of cover-abundance values in phytosociology and its effects on community similarity. Vegetatio 1979, 39, 97–114. [Google Scholar] [CrossRef]
- Pinelo, G. Manual de Inventario Forestal Integrado Para Unidades de Manejo: Reserva de la Biosfera Maya, Petén, Guatemala; WWF/PROARCA: San José, Costa Rica, 2004; 49p. [Google Scholar]
- Lot, A.; Chiang, F. (Eds.) Manual de Herbario. Administración y Manejo de Colecciones, Técnicas de Recolección y Preparación de Ejemplares Botánicos; Consejo Nacional de la Flora de México A.C.: Mexico City, Mexico, 1986; 142p. [Google Scholar]
- Santacruz, G.N.; Espejel, R.A. Los Encinos (Quercus) de Tlaxcala, México; Centro de Investigaciones Interdisciplinarias Sobre el Desarrollo Regional: Tlaxcala, Mexico, 2004; 83p. [Google Scholar]
- Pérez-Bravo, R.; Salazar, G.A.; Mora-Guzmán, E. Orquídeas de Las Lomas-La Manzanilla, Sierra Madre Oriental, Puebla, México. Bol. Soc. Bot. Méx. 2010, 87, 125–129. Available online: https://www.scielo.org.mx/scielo.php?script=sci_arttext&pid=S0366-21282010000200010&lng=es&tlng=es (accessed on 21 August 2023). [CrossRef]
- Martínez, M.; Vargas-Ponce, O.; Rodríguez, A.; Chiang, F.; Ocegueda, S. Solanaceae family in Mexico. Bot. Sci. 2017, 95, 131–145. [Google Scholar] [CrossRef]
- Romero, R.S.; Rojas, Z.E.C.; Rubio, L.L.E. Encinos de México (Quercus, Fagaceae); Facultad de Estudios Superiores “Iztacala”: Mexico City, Mexico, 2017; 298p. [Google Scholar]
- Ramos-Dorantes, D.B.; Villaseñor, J.L.; Ortiz, E.; Gernandt, D.S. Biodiversity, distribution, and conservation status of Pinaceae in Puebla, Mexico. Rev. Mex. Biodiv. 2017, 88, 215–223. [Google Scholar] [CrossRef]
- Miguel-Vázquez, M.I.; Espejo-Serna, M.A.; Ceja-Romero, J.; Cerros-Tlatilpa, R. The angiosperms epiphytes of Puebla, Mexico: Richness and distribution. Bot. Sci. 2020, 98, 585–596. [Google Scholar] [CrossRef]
- Tzompa-Coatl, R.; Cerón-Carpio, A.B.; Mendoza-Ruiz, A.; Ceja-Romero, J. Riqueza específica y distribución de licofitas y helechos por tipos de vegetación, en la Sierra Norte de Puebla, México. Acta Bot. Mex. 2022, 129, e2063. [Google Scholar] [CrossRef]
- Harvey, E.B. Violaceae. In Flora del Bajío y Regiones Adyacentes; Rzedowski, J., de Rzedowski, G.C., Eds.; Instituto de Ecología A.C.: Patzcuaro, Mexico, 1994; Fascicle 31; 44p. [Google Scholar] [CrossRef]
- De Rzedowski, G.C.; Rzedowski, J. Smilacaceae. In Flora del Bajío y Regiones Adyacentes; Rzedowski, J., de Rzedowski, G.C., Eds.; Instituto de Ecología A.C.: Patzcuaro, Mexico, 1994; Fascicle 26; 28p. [Google Scholar] [CrossRef]
- Carranza, E. Salicaceae. In Flora del Bajío y Regiones Adyacentes; Rzedowski, J., de Rzedowski, G.C., Eds.; Instituto de Ecología A.C.: Patzcuaro, Mexico, 1995; Fascicle 37; 26p. [Google Scholar] [CrossRef]
- Carranza, E.; Madrigal, S.X. Betulaceae. In Flora del Bajío y Regiones Adyacentes; Rzedowski, J., de Rzedowski, G.C., Eds.; Instituto de Ecología A.C.: Patzcuaro, Mexico, 1995; Fascicle 39; 28p. [Google Scholar] [CrossRef]
- Rzedowski, J.; de Rzedowski, G.C. (Eds.) Geraniaceae. In Flora del Bajío y Regiones Adyacentes; Instituto de Ecología A.C.: Patzcuaro, Mexico, 1995; Fascicle 40; 42p. [Google Scholar] [CrossRef]
- Carranza, E.G. Garryaceae. In Flora del Bajío y Regiones Adyacentes; Rzedowski, J., de Rzedowski, G.C., Eds.; Instituto de Ecología A.C.: Patzcuaro, Mexico, 1996; Fascicle 49; 22p. [Google Scholar] [CrossRef]
- De Rzedowski, G.C.; Rzedowski, J. Flora Fanerogámica del Valle de México, 2nd ed.; Instituto de Ecología A.C.; Comisión Nacional para el Conocimiento y Uso de la Biodiversidad: Patzcuaro, Mexico, 2005; 1406p. [Google Scholar]
- Pérez-Calix, E. Grossulariaceae. In Flora del Bajío y Regiones Adyacentes; Rzedowski, J., de Rzedowski, G.C., Eds.; Instituto de Ecología A.C.: Patzcuaro, Mexico, 2005; Fascicle 138; 26p. [Google Scholar] [CrossRef]
- Pérez-Calix, E. Oxalidaceae. In Flora del Bajío y Regiones Adyacentes; Rzedowski, J., de Rzedowski, G.C., Eds.; Instituto de Ecología A.C.: Patzcuaro, Mexico, 2009; Fascicle 164; 60p. [Google Scholar] [CrossRef]
- Pérez-Calix, E.; Grajales-Tam, K.M. Caryophyllaceae. In Flora del Bajío y Regiones Adyacentes; Rzedowski, J., de Rzedowski, G.C., Eds.; Instituto de Ecología A.C.: Patzcuaro, Mexico, 2013; Fascicle 180; 125p. [Google Scholar] [CrossRef]
- González, E.M.S.; González, E.M. Ericaceae. In Flora del Bajío y Regiones Adyacentes; Rzedowski, J., de Rzedowski, G.C., Eds.; Instituto de Ecología A.C.: Patzcuaro, Mexico, 2014; Fascicle 183; 128p. [Google Scholar] [CrossRef]
- Martínez, M. Ranunculaceae. In Flora del Bajío y Regiones Adyacentes; Rzedowski, J., de Rzedowski, G.C., Eds.; Instituto de Ecología A.C.: Patzcuaro, Mexico, 2015; Fascicle 190; 76p. [Google Scholar] [CrossRef]
- Pacheco, L.; Sánchez, M.A.; Guzmán, C.L. Ophioglossaceae. In Flora del Bajío y Regiones Adyacentes; Rzedowski, J., de Rzedowski, G.C., Eds.; Instituto de Ecología A.C.: Patzcuaro, Mexico, 2018; Fascicle 208; 26p. [Google Scholar] [CrossRef]
- Velázquez, M.E. Pteridaceae. In Flora del Bajío y Regiones Adyacentes; Rzedowski, J., de Rzedowski, G.C., Eds.; Instituto de Ecología A.C.: Patzcuaro, Mexico, 2019; Fascicle 210; 261p. [Google Scholar] [CrossRef]
- Rzedowski, J. Catálogo preliminar de especies de plantas vasculares de distribución restringida al eje volcánico transversal. In Flora del Bajío y Regiones Adyacentes; Rzedowski, J., Hernández-Ledesma, P., Eds.; Instituto de Ecología A.C.: Patzcuaro, Mexico, 2020; Complementary Fascicle XXXIV; 55p. [Google Scholar] [CrossRef]
- Vigosa-Mercado, J.L.; Ruíz-Sánchez, E. Gramineae, Subfamilia Poöideae. In Flora del Bajío y Regiones Adyacentes; Rzedowski, J., Hernández-Ledesma, P., Eds.; Instituto de Ecología A.C.: Patzcuaro, Mexico, 2020; Fascicle 219; 221p. [Google Scholar] [CrossRef]
- Villers, R.L.; Rojas, G.F.; Tenorio, L.P. Guía Botánica del Parque Nacional Malinche, Tlaxcala-Puebla; Universidad Nacional Autónoma de México: Mexico City, México, 2006; 196p. [Google Scholar]
- Rodríguez-Acosta, M.; Villaseñor, J.L.; Coombes, A.J.; Cerón-Carpio, A.B. Flora del Estado de Puebla, México; Benemérita Universidad Autónoma de Puebla: Puebla, Mexico, 2014; 176p. [Google Scholar]
- CONAFOR (Comisión Nacional Forestal); Turismo de Naturaleza de la Sierra Norte de Puebla A.C.; ASMARF (Asesores en Manejo de Recursos Forestales S.C.). Estudio Florístico de la Cuenca de Abasto de la Región Chignahuapan-Zacatlán, Puebla, México; CONAFOR-Turismo de Naturaleza de la Sierra Norte de Puebla A. C.-ASMARF: Puebla, Mexico, 2016. Available online: http://www.conafor.gob.mx:8080/documentos/docs/22/6249Chignahuapan%20-%20Zacatlan.pdf (accessed on 12 August 2022).
- PPG I (Pteridophyte Phylogeny Group I). A community-derived classification for extant lycophytes and ferns. J. Syst. Evol. 2016, 54, 563–603. [Google Scholar] [CrossRef]
- Christenhusz, M.J.M.; Reveal, J.L.; Farjon, A.; Gardner, M.F.; Mill, R.R.; Chase, M.W. A new classification and linear sequence of extant gymnosperms. Phytotaxa 2011, 19, 55–70. [Google Scholar] [CrossRef]
- The Angiosperm Phylogeny Group; Chase, M.W.; Christenhusz, M.J.; Fay, M.F.; Byng, J.W.; Judd, W.S.; Soltis, D.E.; Mabberley, D.J.; Sennikov, A.N.; Soltis, P.S.; et al. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG IV. Bot. J. Linn. Soc. 2016, 181, 1–20. [Google Scholar] [CrossRef]
- DOF (Diario Oficial de la Federación). Norma Oficial Mexicana NOM-059-SEMARNAT-2010, Protección Ambiental-Especies Nativas de México de Flora y Fauna Silvestres-Categorías de Riesgo y Especificaciones para su Inclusión, Exclusión o Cambio-Lista de Especies en Riesgo; Secretaría de Medio Ambiente y Recursos Naturales: Mexico City, Mexico, 2010.
- International Union for Conservation of Nature (IUCN). The IUCN Red List of Threatened Species. Available online: https://www.iucnredlist.org (accessed on 8 December 2023).
- Villaseñor, J.L.; Espinosa-Garcia, F.J. The alien flowering plants of Mexico. Divers. Distrib. 2004, 10, 113–123. [Google Scholar] [CrossRef]
- CONABIO (Comisión Nacional para el Conocimiento y Uso de la Biodiversidad). Malezas de México. Available online: http://www.conabio.gob.mx/malezasdemexico/2inicio/paginas/lista-plantas-abr2006.htm (accessed on 8 April 2024).
- Colwell, R.K.; Coddington, J.A. Estimating terrestrial biodiversity through extrapolation. Phil. Trans. R. Soc. Lond. B 1994, 345, 101–118. [Google Scholar] [CrossRef] [PubMed]
- López-Gómez, A.M.; Williams-Linera, G. Evaluación de métodos no paramétricos para la estimación de riqueza de especies de plantas leñosas en cafetales. Bot. Sci. 2006, 78, 7–15. [Google Scholar] [CrossRef]
- Colwell, R. Statistical Estimation of Species Richness and Shared Species from Samples. Version 9. 2013. User’s Guide and Application. Available online: http://purl.oclc.org/estimates (accessed on 14 March 2023).
- Chao, A.; Jost, L. Coverage-based rarefaction and extrapolation: Standardizing samples by completeness rather than size. Ecology 2012, 93, 2533–2547. [Google Scholar] [CrossRef] [PubMed]
- Del Río, M.; Montes, F.; Cañellas, I.; Montero, G. Revisión: Índices de diversidad estructural en masas forestales. Investig. Agrar. Sist. Recur. For. 2003, 12, 159–176. Available online: https://www.researchgate.net/publication/28061992_Indices_de_diversidad_estructural_en_masas_forestales (accessed on 10 February 2024).
- Moreno, C.E. Métodos para Medir la Biodiversidad; M&T-Manuales y Tesis SEA: Zaragoza, Spain, 2001; Volume 1, 84p. [Google Scholar]
- Bravo-Nuñez, E. Sobre la cuantificación de la diversidad ecológica. Hidrobiológica 1991, 1, 87–93. Available online: https://hidrobiologica.izt.uam.mx/hidrobiologica/index.php/revHidro/article/view/523 (accessed on 5 June 2023).
- Jost, L.; González-Oreja, J. Midiendo la diversidad biológica: Más allá del índice de Shannon. Acta Zool. Lilloana 2012, 56, 3–14. Available online: https://lillo.org.ar/revis/zoo/2012/v56n1_2/v56n1_2a01.pdf (accessed on 14 March 2023).
- Burgos-Hernández, M.; Castillo-Campos, G.; Tenorio, M.D.C.V. Flora potencialmente útil de la selva tropical en la parte central de Veracruz, México: Consideraciones para su conservación. Acta Bot. Mex. 2014, 109, 55–77. [Google Scholar] [CrossRef]
- Clarke, K.R. Non-parametric multivariate analyses of changes in community structure. Aust. J. Ecol. 1993, 18, 117–143. [Google Scholar] [CrossRef]
- López-González, E.; Hidalgo, S.R. Escalamiento Multidimensional No Métrico. Un ejemplo con R empleando el algoritmo SMACOF. Estud. Sobre Educ. 2016, 18, 9–35. [Google Scholar] [CrossRef]
- Linares, G. Escalamiento multidimensional: Conceptos y enfoques. Investig. Oper. 2023, 22, 173–183. Available online: https://revistas.uh.cu/invoperacional/article/view/7038/6007 (accessed on 5 March 2023).
- Somerfield, P.J.; Clarke, K.R.; Gorley, R.N. A generalized analysis of similarities (ANOSIM) statistic for designs with ordered factors. Aust. Ecol. 2021, 46, 901–910. [Google Scholar] [CrossRef]
- Calderón-Patrón, J.M.; Moreno, C.E. Diversidad beta como disimilitud: Su partición en componentes de recambio y diferencias en riqueza. In La Biodiversidad en un Mundo Cambiante: Fundamentos Teóricos y Metodológicos para su Estudio; Moreno, C.E., Ed.; Universidad Autónoma del Estado de Hidalgo: Mexico City, Mexico, 2019; Chapter 9; pp. 203–222. [Google Scholar]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- Villaseñor, J.L.; Meave, J.A. Floristics in Mexico today: Insights into a better understanding of biodiversity in a megadiverse country. Bot. Sci. 2022, 100, S14–S33. [Google Scholar] [CrossRef]
- Handal-Silva, A.; Cantú-Montemayor, B.; Villarreal, O.A.; López, P.A.; López-Reyes, L.; Cruz-Angón, A.; Camacho-Rico, F. La Biodiversidad en Puebla: Estudio de Estado; Comisión Nacional para el Conocimiento y Uso de la Biodiversidad-Gobierno del Estado de Puebla-Benemérita Universidad Autónoma de Puebla: Puebla, Mexico, 2011; 440p.
- Luna-Bautista, L.; Hernández-de la Rosa, P.; Velázquez-Martínez, A.; Gómez-Guerrero, A.; Acosta-Mireles, M. Understory in the composition and diversity of managed forest areas in Santa Catarina Ixtepeji, Oaxaca. Rev. Chapingo Ser. Cienc. For. Ambiente 2015, 21, 109–121. [Google Scholar] [CrossRef]
- Rendón-Pérez, M.A.; Hernández-de la Rosa, P.; Velázquez-Martínez, A.; Alcántara-Carbajal, J.L.; Reyes-Hernández, V.J. Composición, diversidad y estructura de un bosque manejado del centro de México. Madera Bosques 2021, 27, e2712127. [Google Scholar] [CrossRef]
- Leyva-López, J.C.; Velázquez-Martínez, A.; Ángeles-Pérez, G. Patrones de diversidad de la regeneración natural en rodales mezclados de pinos. Rev. Chapingo Ser. Cienc. For. Ambiente 2010, 16, 227–239. [Google Scholar] [CrossRef]
- Hernández-Salas, J.; Aguirre-Calderón, O.A.; Alanís-Rodríguez, E.; Jiménez-Pérez, J.; Treviño-Garza, E.J.; González-Tagle, M.A.; Luján-Álvarez, C.; Olivas-García, J.M.; Domínguez-Pereda, A. Efecto del manejo forestal en la diversidad y composición arbórea de un bosque templado del noroeste de México. Rev. Chapingo Ser. Cienc. For. Ambiente 2013, 19, 189–199. [Google Scholar] [CrossRef]
- Dávila-Lara, M.A.; Aguirre-Calderón, O.A.; Jurado-Ybarra, E.; Treviño-Garza, E.; González-Tagle, M.A.; Trincado, G. Estructura y diversidad de especies arbóreas en bosques templados de San Luis Potosí, México. Ecosist. Recur. Agropec. 2019, 6, 399–409. [Google Scholar] [CrossRef]
- Silva-García, J.E.; Aguirre-Calderón, O.A.; Alanís-Rodríguez, E.; Jurado-Ybarra, E.; Jiménez-Pérez, J.; Vargas-Larreta, B. Estructura y diversidad de especies arbóreas en un Bosque templado del Noroeste de México. Polibotánica 2021, 52, 89–102. [Google Scholar] [CrossRef]
- Martínez-Calderón, V.M.; Sosa-Ramírez, J.; Siqueiros-Delgado, M.E.; Díaz-Núñez, V. Composición, diversidad y estructura de especies leñosas en los bosques templados de Monte Grande, Sierra Fría, Aguascalientes, México. Acta Bot. Mex. 2021, 128, e1829:1–e1829:20. [Google Scholar] [CrossRef]
- Velasco-Luis, M.U.; Velázquez-Martínez, A.; Hernández-de-la-Rosa, P.; Fierros-González, A.M.; Vera-Castillo, J.A.G. Caracterización de un bosque templado en un gradiente altitudinal en Oaxaca, México. Madera Bosques 2023, 29, e2912465. [Google Scholar] [CrossRef]
- Roberts, D.L.; Dixon, K.W. Orchids. Curr. Biol. 2008, 18, 325–329. [Google Scholar] [CrossRef]
- Flores-Armillas, V.H.; Botello, F.; Sánchez-Cordero, V.; García-Barrios, R.; Jaramillo, F.; Gallina-Tessaro, S. Caracterización del hábitat del venado cola blanca (Odocoileus virginianus mexicanus) en los bosques templados del Corredor Biológico Chichinautzin y modelación de su hábitat potencial en Eje Transvolcánico Mexicano. Therya 2013, 4, 377–393. [Google Scholar] [CrossRef]
- Santibañez-Andrade, G.; Castillo-Argüero, S.; Martínez-Orea, Y. Evaluación del estado de conservación de la vegetación de los bosques de una cuenca heterogénea del Valle de México. Bosque 2015, 36, 299–313. [Google Scholar] [CrossRef]
- Hartshorn, G.S. Biogeografía de los bosques neotropicales. In Ecología y Conservación de Bosques Neotropicales; Guariguata, M.R., Kattan, G.H., Eds.; Libro Universitario Regional: Cartago, Costa Rica, 2002; pp. 59–81. [Google Scholar]
- Gámez, N.; Escalante, T.; Rodríguez, G.; Linaje, M.; Morrone, J.J. Caracterización biogeográfica de la Faja Volcánica Transmexicana y análisis de los patrones de distribución de su mastofauna. Rev. Mex. Biodivers. 2012, 83, 258–272. Available online: http://www.scielo.org.mx/scielo.php?script=sci_arttext&pid=S1870-34532012000100028&lng=es&tlng=es (accessed on 10 May 2024). [CrossRef]
- Suárez-Mota, M.E.; Téllez-Valdés, O. Red de áreas prioritarias para la conservación de la biodiversidad del Eje Volcánico Transmexicano analizando su riqueza florística y variabilidad climática. Polibotánica 2014, 38, 67–93. Available online: http://www.scielo.org.mx/scielo.php?script=sci_arttext&pid=S1405-27682014000200004&lng=es&tlng=es (accessed on 10 May 2024).
- Muñoz, A.; Alfaro, A.M.; Gutiérrez, R.E.; Morales, M.S. Especies exóticas invasoras: Impactos sobre las poblaciones de flora y fauna, los procesos ecológicos y la economía. In Capital Natural de México, Vol. II: Estado de Conservación y Tendencias de Cambio; Dirzo, R., González, R., March, I.J., Eds.; CONABIO: Mexico City, Mexico, 2009; pp. 277–318. [Google Scholar]
- Mormul, R.P.; Vieira, D.S.; Bailly, D.; Fidanza, K.; Batista da Silva, V.F.; Júnio da Graça, W.; Pontara, V.; Bueno, M.L.; Thomaz, S.M.; Mendes, R.S. Invasive alien species records are exponentially rising across the Earth. Biol. Invasions 2022, 24, 3249–3261. [Google Scholar] [CrossRef]
- Rzedowski, J.; Huerta, L. Vegetación de México; Limusa: Mexico City, Mexico, 1978; 504p. [Google Scholar]
- Holmes, M.A. Mycoheterotrophic plants as indicators of post-agricultural forest regeneration: Abundance of Hypopitys monotropa and Monotropa uniflora in post-agricultural forests changes through time. Botany 2023, 102, 160–167. [Google Scholar] [CrossRef]
- Koob, J. Case Studies in Mycoheterotrophy: Physiological Plasticity and Mycorrhizal Associates of the Mixotrophic Orchid Epipactis helleborine (L.) Crantz, and Mycorrhizal Specificity in Two Color Forms of Hypopitys monotropa Crantz. Ph.D. Thesis, College of Environmental Science and Forestry, Siracusa, Italy, 2021. [Google Scholar]
- Martínez-Pastur, G.; Peri, P.L.; Fernández, M.C.; Staffieri, G.; Lencinas, M.V. Changes in understory species diversity during the Nothofagus pumilio forest management cycle. J. For. Res. 2002, 7, 165–174. [Google Scholar] [CrossRef]
- Mori, A.S.; Kitagawa, R. Retention forestry as a major paradigm for safeguarding forest biodiversity in productive landscapes: A global meta-analysis. Biol. Conserv. 2014, 175, 65–73. [Google Scholar] [CrossRef]
- Battles, J.J.; Shlisky, A.J.; Barrett, R.H.; Heald, R.C.; Allen-Diaz, B.H. The effects of forest management on plant species diversity in a Sierran conifer forest. For. Ecol. Manag. 2001, 146, 211–222. [Google Scholar] [CrossRef]
- Gustienė, D.; Varnagirytė-Kabašinskienė, I.; Stakėnas, V. Ground vegetation in Pinus sylvestris forests at different successional stages following clear cuttings: A case study. Plants 2022, 11, 2651. [Google Scholar] [CrossRef] [PubMed]
- Bartels, S.F.; Macdonald, S.E. Dynamics and recovery of forest understory biodiversity over 17 years following varying levels of retention harvesting. J. Appl. Ecol. 2023, 60, 725–736. [Google Scholar] [CrossRef]
- Krömer, T.; García-Franco, J.G.; Toledo-Aceves, T. Epífitas vasculares como bioindicadores de la calidad forestal: Impacto antrópico sobre su diversidad y composición. In Bioindicadores: Guardianes de Nuestro Futuro Ambiental; González-Zuarth, C.A., Vallarino, A., Pérez-Jiménez, J.C., Low-Pfeng, A.M., Eds.; Instituto Nacional de Ecología y Cambio Climático (INECC); El Colegio de la Frontera Sur (ECOSUR): San Cristobal de las Casas, Mexico, 2014; Chapter 29; pp. 605–623. [Google Scholar]
- Susan-Tepetlan, T.M.; Velázquez-Rosas, N.; Krömer, T. Cambios en las características funcionales de epífitas vasculares de bosque mesófilo de montaña y vegetación secundaria en la región central de Veracruz, México. Bot. Sci. 2015, 93, 153–163. [Google Scholar] [CrossRef]
- Jiménez-Bautista, L. Impacto del Aprovechamiento Forestal Sobre las Epífitas en un Bosque de Pino-Encino en la Sierra Norte de Oaxaca, México. Master’s Thesis, El Colegio de la Frontera Sur (ECOSUR), Lerma, Mexico, 2014. [Google Scholar]
- Redding, T.E.; Hope, G.D.; Fortin, M.-J.; Schmidt, M.G.; Bailey, W.G. Spatial patterns of soil temperature and moisture across subalpine forest-clearcut edges in the southern interior of British Columbia. Can. J. Soil Sci. 2003, 83, 121–130. [Google Scholar] [CrossRef]
- Castañeda Díaz, S. Sucesión Ecológica en Fragmentos Forestales con Vegetación Secundaria en Españita, Tlaxcala. Ph.D. Thesis, Colegio de Postgraduados, Texcoco, Mexico, 2015. [Google Scholar]



| Family | No. Genera/No. Species | Genus | No. Species |
|---|---|---|---|
| Asteraceae | 15/36 | Ageratina | 6 |
| Poaceae | 9/10 | Quercus | 6 |
| Orchidaceae | 7/7 | Pseudognaphalium | 4 |
| Rosaceae | 6/7 | Roldana | 4 |
| Ericaceae | 6/7 | Salvia | 4 |
| Others | 52/71 | Others | 114 |
| Harvested areas (HAs) | Family | No. Species | Genus | No. Species |
| Asteraceae | 32 | Quercus | 6 | |
| Poaceae | 7 | Ageratina | 4 | |
| Fagaceae | 6 | Roldana | 4 | |
| Rosaceae | 5 | Pseudognaphalium | 4 | |
| Others | 53 | Others | 84 | |
| Tree structural retention (STRs) | Asteraceae | 21 | Quercus | 5 |
| Poaceae | 7 | Roldana | 4 | |
| Ericaceae | 7 | Ageratina | 4 | |
| Rosaceae | 6 | - | ||
| Lamiaceae | 6 | - | ||
| Others | 50 | Others | 84 | |
| Conservation areas (CAs) | Asteraceae | 13 | Ageratina | 4 |
| Rosaceae | 6 | Roldana | 3 | |
| Ericaceae | 5 | Quercus | 3 | |
| Polypodiaceae | 4 | Salvia | 3 | |
| Others | 47 | Others | 62 |
| HAs | STRs | CAs | |
|---|---|---|---|
| HAs | 99 | 0.0003 | 0.0003 |
| STRs | 0.528 | 97 | 0.0015 |
| CAs | 0.719 | 0.256 | 75 |
| Species | Contribution (%) | Accumulated (%) | Mean HAs | Mean STEs | Mean CAs |
|---|---|---|---|---|---|
| Abies religiosa | 2.37 | 2.37 | 0.03 | 0.86 | 0.75 |
| Chimaphila umbellata | 2.30 | 4.68 | 0.07 | 0.86 | 0.67 |
| Geranium seemannii | 2.14 | 6.81 | 0.83 | 0.14 | 0.25 |
| Monnina ciliolata | 1.91 | 8.72 | 0.20 | 0.69 | 0.50 |
| Baccharis salicifolia | 1.85 | 10.57 | 0.70 | 0.03 | 0.00 |
| Roldana angulifolia | 1.84 | 12.41 | 0.30 | 0.62 | 0.92 |
| Baccharis conferta | 1.81 | 14.22 | 0.67 | 0.38 | 0.00 |
| Vaccinium leucanthum | 1.76 | 15.98 | 0.37 | 0.93 | 0.83 |
| Roldana barba-johannis | 1.75 | 17.73 | 0.47 | 0.72 | 0.42 |
| Bromus carinatus | 1.72 | 19.45 | 0.43 | 0.59 | 0.33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Pardo, B.E.; Velázquez-Martínez, A.; Burgos-Hernández, M.; Reyes-Hernández, V.J. Floristic Diversity and Green-Tree Retention in Intensively Managed Temperate Forests: A Case Study in Puebla, Mexico. Forests 2024, 15, 920. https://doi.org/10.3390/f15060920
Pérez-Pardo BE, Velázquez-Martínez A, Burgos-Hernández M, Reyes-Hernández VJ. Floristic Diversity and Green-Tree Retention in Intensively Managed Temperate Forests: A Case Study in Puebla, Mexico. Forests. 2024; 15(6):920. https://doi.org/10.3390/f15060920
Chicago/Turabian StylePérez-Pardo, Brenda E., Alejandro Velázquez-Martínez, Mireya Burgos-Hernández, and Valentín J. Reyes-Hernández. 2024. "Floristic Diversity and Green-Tree Retention in Intensively Managed Temperate Forests: A Case Study in Puebla, Mexico" Forests 15, no. 6: 920. https://doi.org/10.3390/f15060920
APA StylePérez-Pardo, B. E., Velázquez-Martínez, A., Burgos-Hernández, M., & Reyes-Hernández, V. J. (2024). Floristic Diversity and Green-Tree Retention in Intensively Managed Temperate Forests: A Case Study in Puebla, Mexico. Forests, 15(6), 920. https://doi.org/10.3390/f15060920





