Response of Soil Microbial Community Structure and Diversity to Mixed Proportions and Mixed Tree Species in Bamboo–Broad-Leaved Mixed Forests
Abstract
:1. Introduction
2. Methods
2.1. Site Description
2.2. Filed Sites and Soil Sampling
2.3. Soil Chemical Analysis
2.4. Soil Microbial Community
2.5. Statistical Analyses
3. Results
3.1. Changes in Soil Properties
3.2. Effects of Mixed Tree Species and Mixing Ratios on the Soil Bacterial Community Composition
3.3. Effects of Mixed Tree Species and Mixing Ratio on the Soil Bacterial Diversity and Richness
3.4. Effects of Mixed Tree Species and Mixing Ratio on the Structure of Soil Bacterial Communities
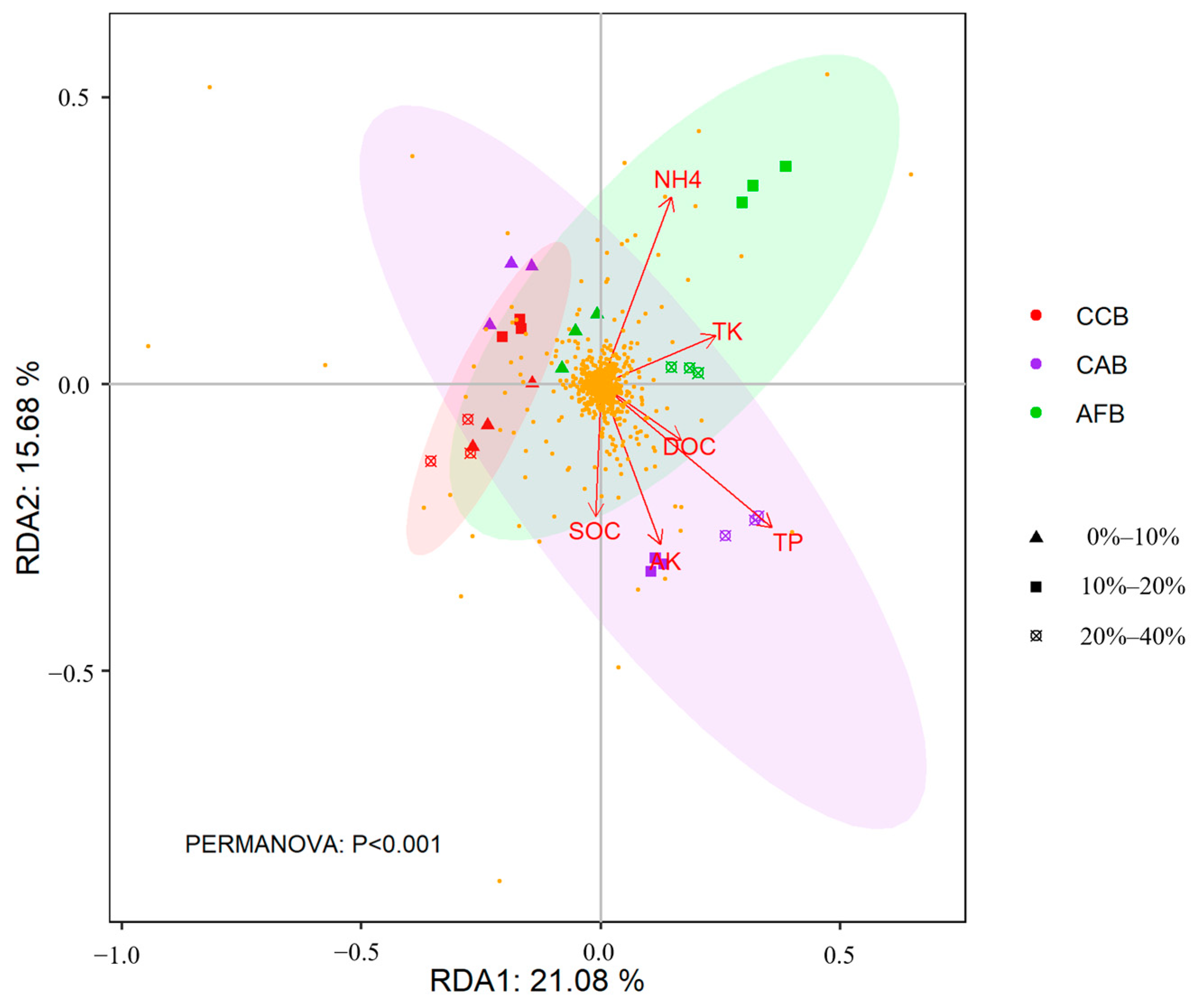
3.5. Effects of Soil Properties on the Composition of Soil Bacterial Communities
4. Discussion
4.1. Effects of Mixed Tree Species and Mixing Ratios on Soil Properties
4.2. Effects of Mixed Tree Species and Mixing Ratio on the Soil Bacterial Community
4.3. Effects of Soil Properties on the Soil Bacterial Community
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Prescott, C.E.; Grayston, S.J. Tree species influence on microbial communities in litter and soil: Current knowledge and research needs. For. Ecol. Manag. 2013, 309, 19–27. [Google Scholar] [CrossRef]
- Gillespie, L.M.; Hättenschwiler, S.; Milcu, A.; Wambsganss, J.; Shihan, A.; Fromin, N. Tree species mixing affects soil microbial functioning indirectly via root and litter traits and soil parameters in European forests. Funct. Ecol. 2021, 35, 2190–2204. [Google Scholar] [CrossRef]
- Richards, A.E.; Forrester, D.I.; Bauhus, J.; Scherer-Lorenzen, M. The influence of mixed tree plantations on the nutrition of individual species: A review. Tree Physiol. 2010, 30, 1192–1208. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.K.; Bardgett, R.D.; Smith, P.; Reay, D. Microorganisms and climate change: Terrestrial feedbacks and mitigation options. Nat. Rev. Microbiol. 2010, 8, 779–790. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, C.; Yang, J.; Liao, J.; Chen HY, H.; Ruan, H. Elevated CO2 shifts soil microbial communities from K- to r-strategists. Glob. Ecol. Biogeogr. 2021, 30, 961–972. [Google Scholar] [CrossRef]
- Fanin, N.; Kardol, P.; Farrell, M.; Nilsson, M.C.; Gundale, M.J.; Wardle, D.A. The ratio of Gram-positive to Gram-negative bacterial PLFA markers as an indicator of carbon availability in organic soils. Soil Biol. Biochem. 2019, 128, 111–114. [Google Scholar] [CrossRef]
- Yang, D.; Hu, L.; Song, X.; Wang, C. Effects of changing precipitation on litter quality and decomposition of different plant functional groups in an alpine meadow. Chin. J. Plant Ecol. 2021, 45, 1314–1328. (In Chinese) [Google Scholar] [CrossRef]
- Kramer-Walter, K.R.; Laughlin, D.C. Root nutrient concentration and biomass allocation are more plastic than morphological traits in response to nutrient limitation. Plant Soil 2017, 416, 539–550. [Google Scholar] [CrossRef]
- Li, W.-Q.; Huang, Y.; Chen, F.-S.; Liu, Y.-Q.; Lin, X.-F.; Zong, Y.; Wu, G.-Y.; Yu, Z.; Fang, X.-M. Mixing with broad-leaved trees shapes the rhizosphere soil fungal communities of coniferous tree species in subtropical forests. For. Ecol. Manag. 2021, 480, 118664. [Google Scholar] [CrossRef]
- Xu, Q.; Liang, C.; Chen, J.; Li, Y.; Qin, H.; Fuhrmann, J.J. Rapid bamboo invasion (expansion) and its effects on biodiversity and soil processes +. Glob. Ecol. Conserv. 2021, 21, e00787. [Google Scholar] [CrossRef]
- Ni, H.; Su, W. Spatial distribution of fine root traits in relation to soil properties and aggregate stability of intensively managed Moso bamboo (Phyllostachys edulis) plantations in subtropical China. Plant Soil 2024, 498, 487–503. [Google Scholar] [CrossRef]
- Ni, H.; Su, W.; Fan, S.; Haoyu, C. Effects of intensive management practices on rhizosphere soil properties, root growth, and nutrient uptake in Moso bamboo plantations in subtropical China. For. Ecol. Manag. 2021, 493, 119083. [Google Scholar] [CrossRef]
- Yuen, J.Q.; Fung, T.; Ziegler, A.D. Carbon stocks in bamboo ecosystems worldwide: Estimates and uncertainties. For. Ecol. Manag. 2017, 393, 113–138. [Google Scholar] [CrossRef]
- Shen, Q.L. Characteristics and Evolution of Ammonia-Oxidizing and Nitrogen-Fixing Bacteria in Moso Bamboo (Phyllostachys Pubescens) Forest Soils. Master’s Dissertation, Zhejiang A&F University, Hangzhou, China, 2015. [Google Scholar]
- Guo, X.; Chen, H.Y.; Meng, M.; Biswas, S.R.; Ye, L.; Zhang, J. Effects of land use change on the composition of soil microbial communities in a managed subtropical forest. J. Trop. Ecol. 2016, 373, 93–99. [Google Scholar] [CrossRef]
- Liu, C.; Zheng, C.; Wang, L.; Zhang, J.; Wang, Q.; Shao, S.; Qin, H.; Xu, Q.; Liang, C.; Chen, J. Moso bamboo invasion changes the assembly process and interactive relationship of soil microbial communities in a subtropical broadleaf forest. For. Ecol. Manag. 2023, 536, 120901. [Google Scholar] [CrossRef]
- Schulp, C.J.E.; Naburus, G.J.; Verburg, P.H.; Waal, R.W. Effect of tree species on carbon stock in forest floor and mineral soil and implication for soil carbon inventories. For. Ecol. Manag. 2008, 256, 482–490. [Google Scholar] [CrossRef]
- Sariyildiz, T. Effects of tree species and topography on fine and small root decom-position rates of three common tree species (Alnus glutinosa, Picea orientalis and Pinus sylvestris) in Turkey. For. Ecol. Manag. 2015, 335, 71–86. [Google Scholar] [CrossRef]
- Liu, J.; Dang, P.; Gao, Y.; Zhu, H.; Zhu, H.; Zhao, F.; Zhao, Z. Effects of tree species and soil properties on the composition and diversity of the soil bacterial community following afforestation. For. Ecol. Manag. 2018, 427, 342–349. [Google Scholar] [CrossRef]
- Ding, X.; Liu, G.; Fu, S.; Chen, H.Y. Tree species composition and nutrient availability affect soil microbial diversity and composition across forest types in subtropical China. Catena 2021, 201, 105224. [Google Scholar] [CrossRef]
- Wang, H.; Liu, S.; Wang, J.; Shi, Z.; Lu, L.; Zeng, J.; Ming, A.; Tang, J.; Yu, H. Effects of tree species mixture on soil organic carbon stocks and greenhouse gas fluxes in subtropical plantations in China. For. Ecol. Manag. 2013, 300, 4–13. [Google Scholar] [CrossRef]
- Zhang, W.P.; Fornara, D.; Yang, H.; Yu, R.P.; Callaway, R.M.; Li, L. Plant litter strengthens positive biodiversity-ecosystem functioning relationships over time. Trends Ecol. Evol. 2023, 38, 473–484. [Google Scholar] [CrossRef] [PubMed]
- Jing, X.; Classen, A.; Li, D.; Lin, L.; Lu, M.; Sanders, N.J.; Wang, Y.; Feng, W. Unraveling microbial community structure–function relationships in the horizontal and vertical spatial dimensions in extreme environments. Ecography 2024, e07118. [Google Scholar] [CrossRef]
- Yang, J.; Ding, D.; Zhang, X.; Gu, H. A comparative analysis of soil physicochemical properties and microbial community structure among four shelterbelt species in the northeast China plain. Microbiol. Spectr. 2024, 12, e0368323. [Google Scholar] [CrossRef]
- Huang, C.; Liao, Y.; Ding, Q. Two sample pooling strategies revealed different root-associated fungal diversity of Rhododendron species. Acta Microbiol. Sin. 2017, 57, 571–581. (In Chinese) [Google Scholar]
- Li, Z.C.; Fu, M.Y.; Xie, J.Z.; Li, R.G.; Xiao, J.H. Study on the productivity maintenance of bamboo and broad-leave tree mixed stands. J. Bamboo Res. 2003, 22, 32–37. (In Chinese) [Google Scholar]
- Zhang, M.; Fan, S.; Guan, F.; Yan, X.; Yin, Z. Soil bacterial community structure of mixed bamboo and broad-leaved forest based on tree crown width ratio. Sci. Rep. 2020, 10, 6522. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wu, Z.; Zong, Y.; Li, W.; Chen, F.; Wang, G.G.; Li, J.; Fang, X. Mixing with coniferous tree species alleviates rhizosphere soil phosphorus limitation of broad-leaved trees in subtropical plantations. Soil Biol. Biochem. 2022, 175, 108853. [Google Scholar] [CrossRef]
- Allen, S.E. Chemical Analysis of Ecological Materials; Blackwell Scientific Publications: Oxford, UK, 1989. [Google Scholar]
- Zhang, M.Y.; Zhang, W.Y.; Bai, S.H.; Niu, Y.; Hu, D.N.; Ji, H.R.; Xu, Z.H. Minor increases in Phyllostachys edulis (moso bamboo) biomass despite evident alterations of soil bacterial community structure after phosphorus fertilization alone: Based on field studies at different altitudes. For. Ecol. Manag. 2019, 451, 117561. [Google Scholar] [CrossRef]
- Lin, Y.T.; Jangid, K.; Whitman, W.B.; Coleman, D.C.; Chiu, C.Y. Change in bacterial community structure in response to disturbance of natural hardwood and secondary coniferous forest soils in central taiwan. Microb. Ecol. 2011, 61, 429–437. [Google Scholar] [CrossRef]
- Xi, Y.; Yan, J.; Li, M.; Ying, S.; Shi, Z. Gut microbiota dysbiosis increases the risk of visceral gout in goslings through translocation of gut-derived lipopolysaccharide. Poult. Sci. 2019, 98, 5361–5373. [Google Scholar] [CrossRef]
- Wen, Z.L.; Yang, M.K.; Du, M.H.; Zhong, Z.Z.; Lu, Y.T.; Wang, G.H.; Hua, X.M.; Fazal, A.; Mu, C.H.; Yan, S.F.; et al. Enrichments/Derichments of Root-Associated Bacteria Related to Plant Growth and Nutrition Caused by the Growth of an EPSPS-Transgenic Maize Line in the Field. Front. Microbiol. 2019, 10, 1335. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed]
- Blaško, R.; Forsmark, B.; Gundale, M.J.; Lundmark, T.; Nordin, A. Impacts of tree species identity and species mixing on ecosystem carbon and nitrogen stocks in a boreal forest. For. Ecol. Manag. 2020, 458, 117783. [Google Scholar] [CrossRef]
- Chen, X.; Chen HY, H.; Chang, S.X. Meta-analysis shows that plant mixtures increase soil phosphorus availability and plant productivity in diverse ecosystems. Nat. Ecol. Evol. 2022, 6, 1112–1121. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.; Zhang, S.; Lu, Y.; Froese, R.E.; Xu, X.; Zeng, J.; Ming, A.; Xianzhao, L.; Xie, Y.; Li, Q. Thinning effects on forest evolution in Masson pine (Pinus massoniana Lamb.) conversion from pure plantations into mixed forests. For. Ecol. Manag. 2020, 477, 118503. [Google Scholar] [CrossRef]
- Song, Q.N.; Ouyang, M.; Yang, Q.P.; Lu, H.; Yang, G.Y.; Chen, F.S.; Shi, J.M. Degradation of litter quality and decline of soil nitrogen mineralization after moso bamboo (Phyllostachys pubscens) expansion to neighboring broadleaved forest in subtropical China. Plant Soil 2016, 404, 113–124. [Google Scholar] [CrossRef]
- Lu, J.; Scheu, S. Response of soil microbial communities to mixed beech-conifer forests varies with site conditions. Soil Biol. Biochem. 2021, 155, 108155. [Google Scholar] [CrossRef]
- Miller, H.G. Dynamics of nutrient cycling in plantation ecosystems. In Nutrition of Plantation Forests; Bowen, G.D., Nambiar, E.K.S., Eds.; Academic Press: London, UK, 1984; pp. 53–78. [Google Scholar]
- Clark, P.W.; D’Amato, A.W. Long-term development of transition hardwood and Pinus strobus—Quercus mixedwood forests with implications for future adaptation and mitigation potential. For. Ecol. Manag. 2021, 501, 119654. [Google Scholar] [CrossRef]
- Kooch, Y.; Tarighat, F.S.; Hosseini, S.M. Tree species effects on soil chemical, biochemical and biological features in mixed Caspian lowland forests. Trees 2016, 31, 863–872. [Google Scholar] [CrossRef]
- Lu, X.; Xiang, W.H.; Ren, H.; Peng, C.H. Litter biomass and its carbon and nitrogen storage in four subtropical forests in central Southern China. Chin. J. Ecol. 2012, 31, 2234–2240. [Google Scholar]
- Xu, Y.M.; Gong, Y.J.; Xi, D.; Jiong, L.; Kuang, Y.W.; Wang, F.G. Chemical Element Contents in Leaves of 16 Dominant Species from Evergreen Broad-leaved Forest at Nanling Nature Reserve. For. Res. 2013, 26, 759–765. [Google Scholar]
- Zhang, Y.; Li, Y.; Wang, L.; Tang, Y.; Chen, J.; Hu, Y.; Fu, X.; Le, Y. Soil microbiological variability under different successional stages of the Chongming Dongtan wetland and its effect on soil organic carbon storage. Ecol. Eng. 2013, 52, 308–315. [Google Scholar] [CrossRef]
- Cheng, J.; Jing, G.; Wei, L.; Jing, Z. Long-term grazing exclusion effects on vegetation characteristics, soil properties and bacterial communities in the semi-arid grasslands of China. Ecol. Eng. 2016, 97, 170–178. [Google Scholar] [CrossRef]
- Rivest, M.; Whalen, J.K.; Rivest, D. Tree diversity is not always a strong driver of soil microbial diversity: A 7-yr-old diversity experiment with trees. Ecosphere 2019, 10, e02685. [Google Scholar] [CrossRef]
- Haghverdi, K.; Kooch, Y. Effects of diversity of tree species on nutrient cycling and soil-related processes. Catena 2019, 178, 335–344. [Google Scholar] [CrossRef]
- Luo, W.; Zhang, Q.; Wang, P.; Luo, J.; She, C.; Guo, X.; Yuan, J.; Sun, Y.; Guo, R.; Li, Z.; et al. Unveiling the impacts moso bamboo invasion on litter and soil properties: A meta-analysis. Sci. Total Environ. 2024, 909, 168532. [Google Scholar] [CrossRef] [PubMed]
- Jangid, K.; Williams, M.; Franzluebbers, A.J.; Sanderlin, J.S.; Reeves, J.; Jenkins, M.; Endale, D.M.; Coleman, D.C.; Whitman, W.B. Relative impacts of land-use, management intensity and fertilization upon soil microbial community structure in agricultural systems. Soil Biol. Biochem. 2008, 40, 2843–2853. [Google Scholar] [CrossRef]
- Araújo, J.F.; de Castro, A.P.; Costa, M.M.; Togawa, R.C.; Júnior, G.J.; Quirino, B.F.; Bustamante, M.M.; Williamson, L.L.; Handelsman, J.; Krüger, R.H. Characterization of Soil Bacterial Assemblies in Brazilian Savanna-Like Vegetation Reveals Acidobacteria Dominance. Microb. Ecol. 2012, 64, 760–770. [Google Scholar] [CrossRef]
- Meng, H.; Li, K.; Nie, M.; Wan, J.R.; Quan, Z.X.; Fang, C.M.; Chen, J.K.; Gu, J.D.; Li, B. Responses of bacterial and fungal communities to an elevation gradient in a subtropical montane forest of China. Appl. Microbiol. Biotechnol. 2013, 97, 2219–2230. [Google Scholar] [CrossRef]
- Cox, F.; Barsoum, N.; Lilleskov, E.A.; Bidartondo, M.I. Nitrogen availability is a primary determinant of conifer mycorrhizas across complex environmental gradients. Ecol. Lett. 2010, 13, 1103–1113. [Google Scholar] [CrossRef]
- Li, W.; Sheng, H.; Liu, Y.; Chen, W. Responses of soil bacterial compositions to concentrations of nitrogen forms in the process of Moso bamboo invasion. Ecol. Res. 2019, 34, 743–752. [Google Scholar] [CrossRef]
- Kim, H.S.; Lee, S.H.; Jo, H.Y.; Finneran, K.T.; Kwon, M.J. Diversity and composition of soil Acidobacteria and Proteobacteria communities as a bacterial indicator of past land-use change from forest to farmland. Sci. Total Environ. 2021, 797, 148944. [Google Scholar] [CrossRef] [PubMed]
- Kalam, S.; Basu, A.; Ahmad, I.; Sayyed, R.Z.; El-Enshasy, H.A.; Dailin, D.J.; Suriani, N.L. Recent Understanding of Soil Acidobacteria and Their Ecological Significance: A Critical Review. Front. Microbiol. 2020, 11, 580024. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q. Effects of Tree Species Diversity on Soil Enzyme Acticity and Microorganism Diversity. Master’s Dissertation, Central South University of Forestry & Technology, Changsha, China, 2019. [Google Scholar]
- Dai, Z.M.; Su, W.Q.; Chen, H.H.; Barberan, A.; Zhao, H.C.; Yu, M.J.; Yu, L.; Brookes, P.C.; Schadt, C.W.; Chang, S.X.; et al. Long-term nitrogen fertilization decreases bacterial diversity and favors the growth of Actinobacteria and Proteobacteria in agro-ecosystems across the globe. Glob. Change Biol. 2018, 24, 3452–3461. [Google Scholar] [CrossRef] [PubMed]
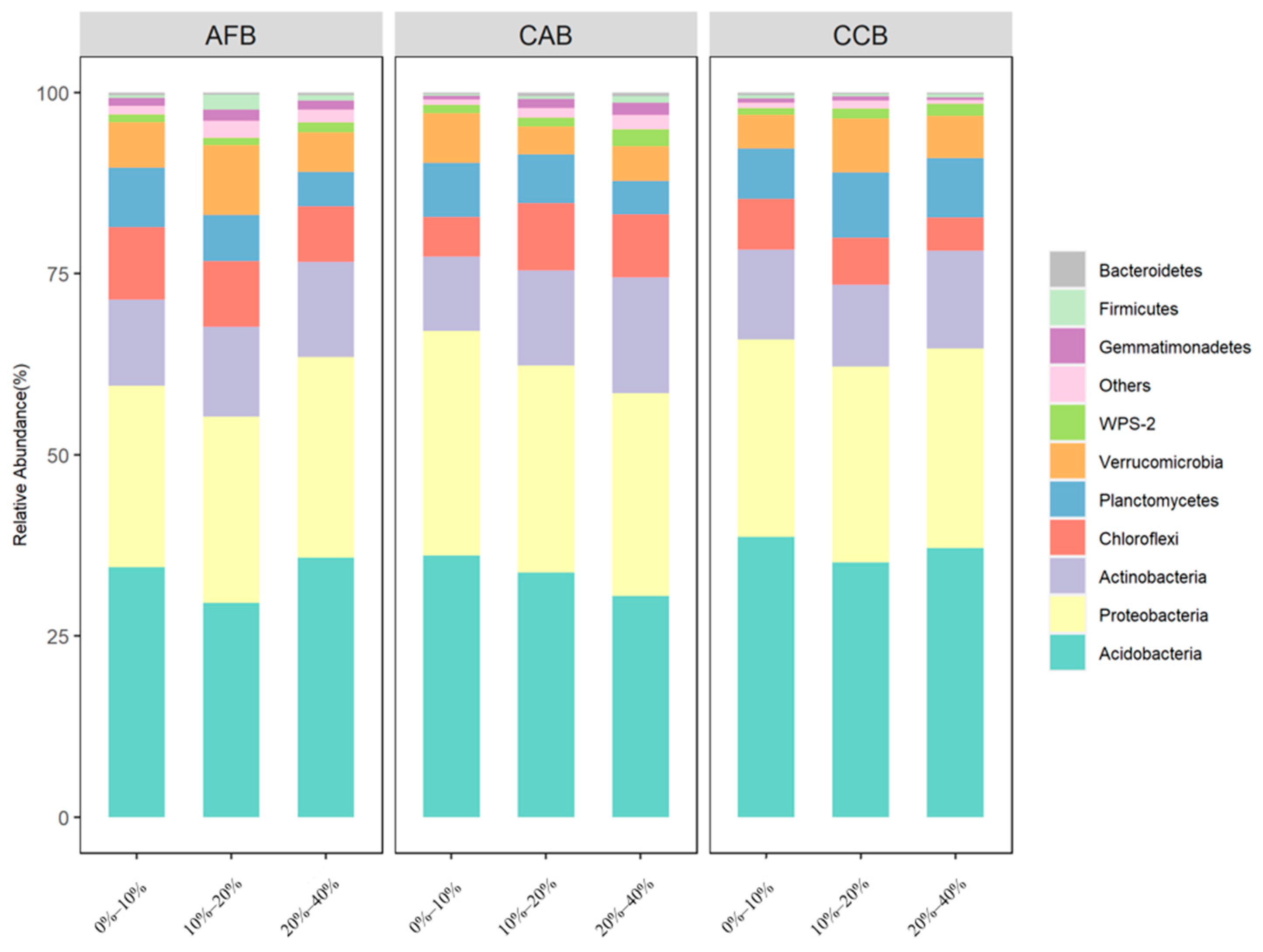
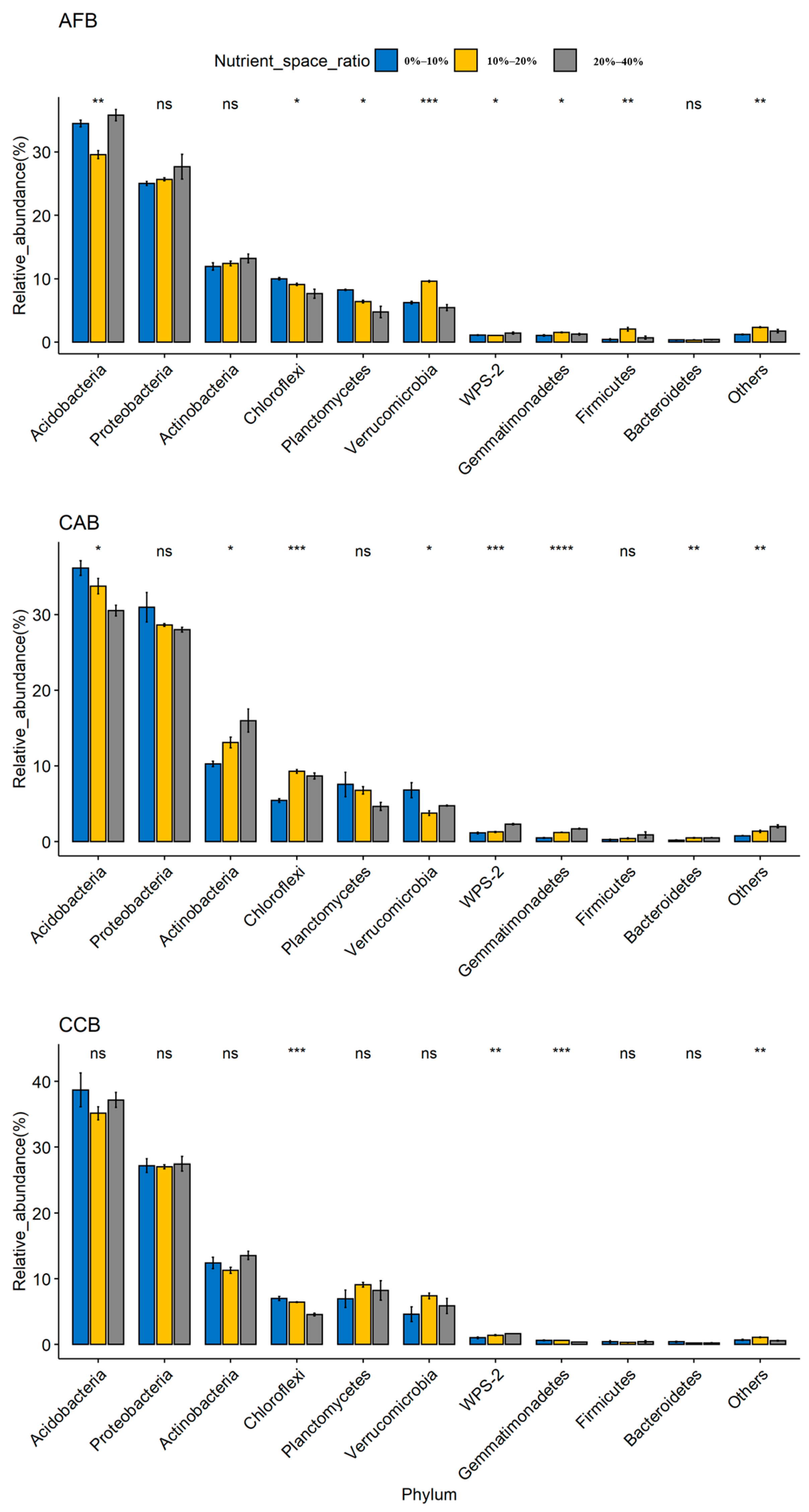

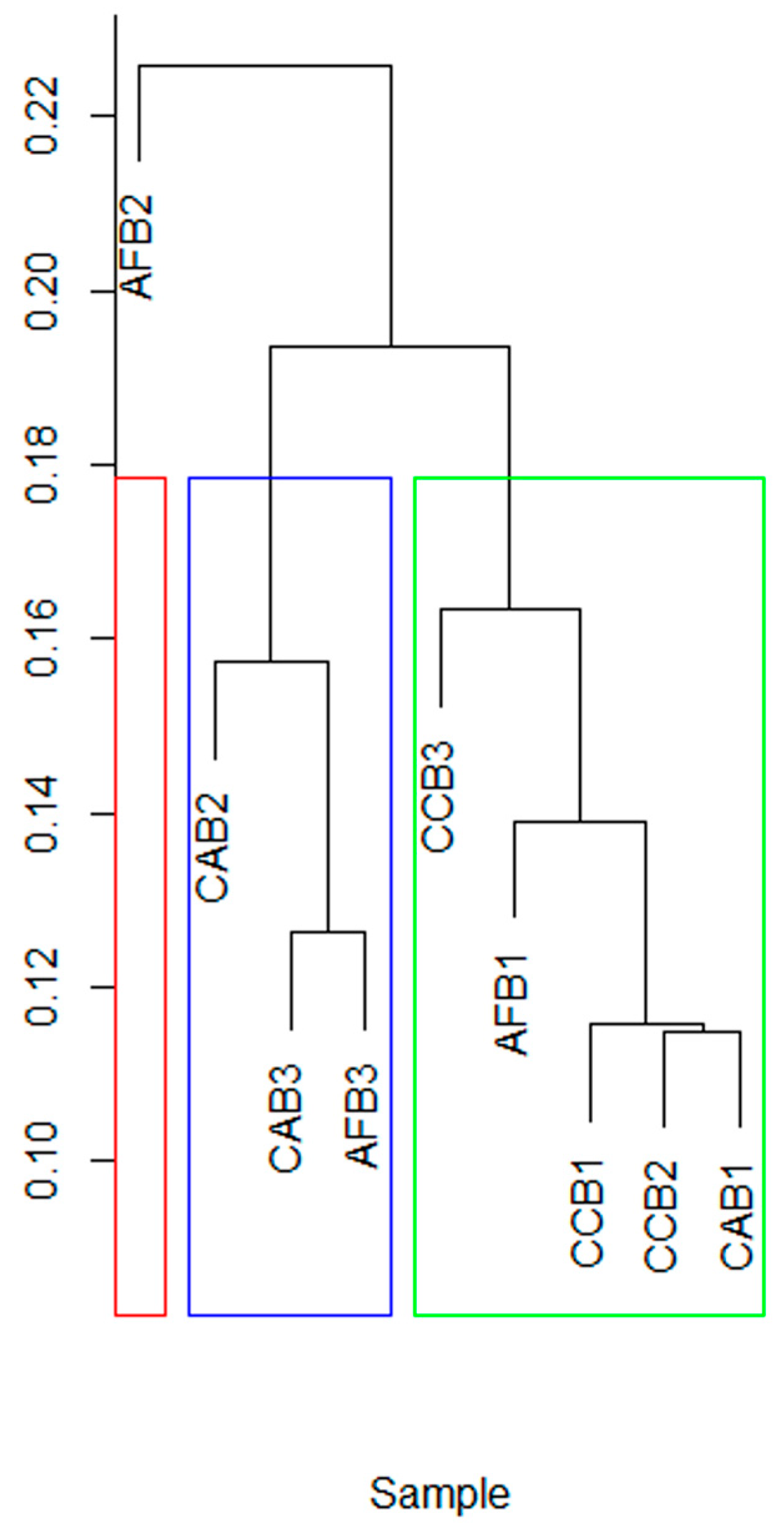
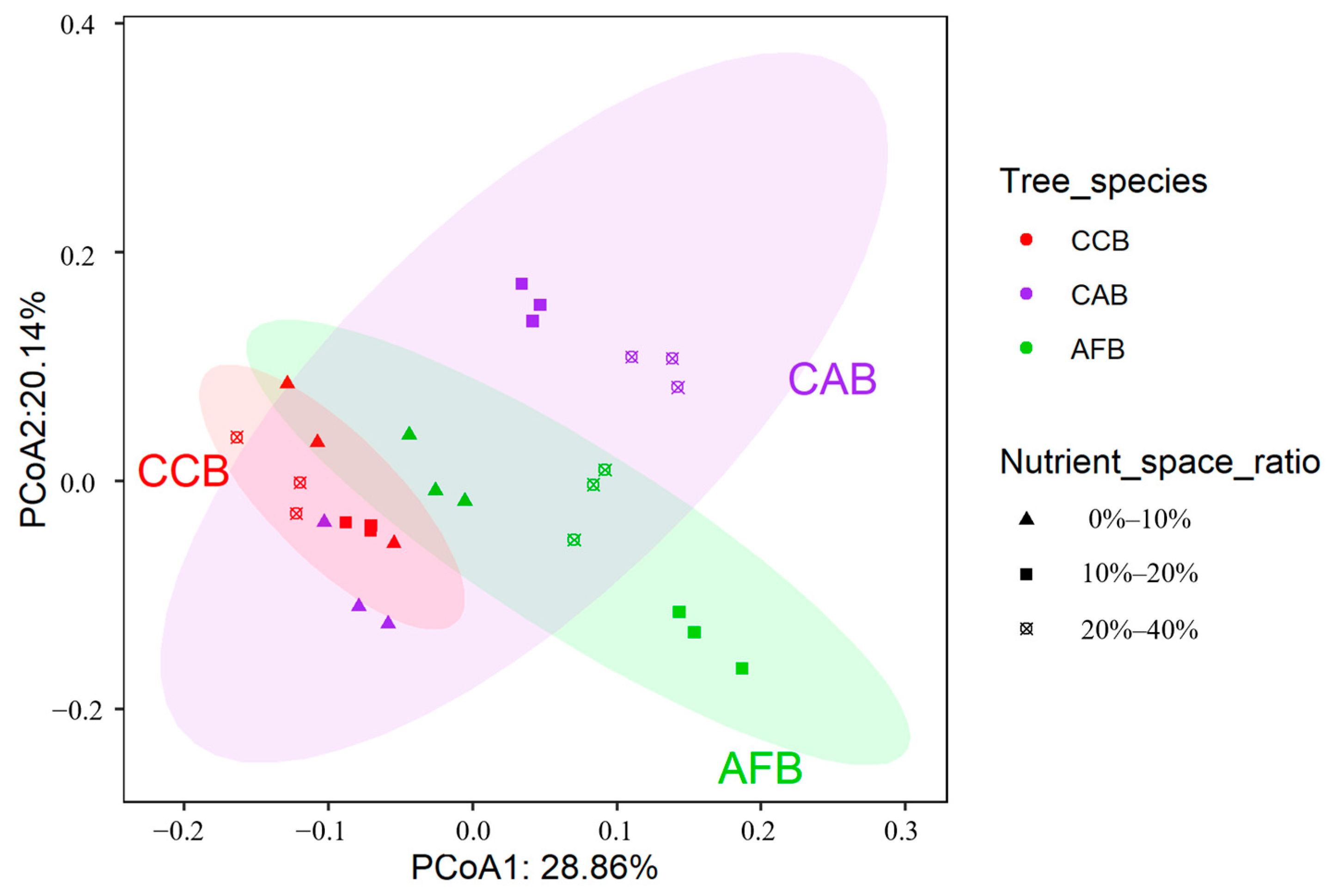
| Nutrient Index | AFB | CAB | CCB | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 0%–10% | 10%–20% | 20%–40% | 0%–10% | 10%–20% | 20%–40% | 0%–10% | 10%–20% | 20%–40% | |
| pH | 4.63 ± 0.12 Ab | 4.93 ± 0.06 Aa | 4.71 ± 0.03 Aab | 4.80 ± 0.04 Aa | 4.51 ± 0.05 Cb | 4.41 ± 0.02 Cb | 4.64 ± 0.02 Aa | 4.74 ± 0.05 Ba | 4.53 ± 0.02 Bb |
| SOC (g/kg) | 23.87 ± 0.22 Ca | 25.36 ± 0.19 Ca | 22.08 ± 0.08 Cc | 35.61 ± 0.65 Ab | 46.50 ± 0.28 Aa | 31.32 ± 0.08 Ac | 27.73 ± 0.19 Bb | 30.83 ± 0.16 Ba | 27.43 ± 0.15 Bb |
| DOC (mg/kg) | 134.67 ± 5.51 Ba | 128.33 ± 6.66 Aab | 119.33 ± 4.16 Bc | 168.33 ± 7.23 Ab | 126.33 ± 4.93 Bc | 201.67 ± 9.61 Aa | 134.33 ± 6.03 Ba | 111.33 ± 4.62 Bb | 116.67 ± 4.93 Bb |
| TN (g/kg) | 1.82 ± 0.07 Ca | 1.89 ± 0.004 Ca | 1.79 ± 0.09 Ba | 2.44 ± 0.05 Ab | 2.69 ± 0.05 Aa | 2.31 ± 0.08 Ab | 2.08 ± 0.03 Bab | 2.189 ± 0.08 Ba | 1.99 ± 0.02 Bb |
| NO3− (mg/kg) | 84.53 ± 6.19 Aa | 13.37 ± 1.30 Bc | 56.30 ± 1.51 Ab | 6.75 ± 0.58 Bb | 15.13 ± 1.15 Ba | 16.93 ± 1.53 Ca | 12.50 ± 1.15 Bc | 28.77 ± 2.50 Ab | 39.70 ± 11.97 Ba |
| NH4+ (mg/kg) | 6.78 ± 0.62 Aab | 11.97 ± 0.97 Aa | 6.55 ± 0.49 Bc | 5.56 ± 0.26 Ba | 4.39 ± 0.37 Ba | 3.84 ± 1.46 Aa | 5.30 ± 0.49 Ba | 5.20 ± 0.16 Bb | 6.98 ± 0.33 Aa |
| TP (g/kg) | 0.300 ± 0.008 Ab | 0.332 ± 0.002 Ba | 0.337 ± 0.004 Ba | 0.253 ± 0.001 Bc | 0.497 ± 0.003 Aa | 0.397 ± 0.006 Ab | 0.253 ± 0.002 Bb | 0.224 ± 0.002 Cb | 0.254 ± 0.002 Ca |
| AP (mg/kg) | 8.91 ± 0.36 Aa | 2.43 ± 0.09 Bc | 4.61 ± 0.09 Ab | 1.80 ± 0.05 Cc | 5.22 ± 0.16 Aa | 2.44 ± 0.09 Bb | 2.37 ± 0.15 Ba | 1.77 ± 0.10 Cb | 1.93 ± 0.20 Cb |
| TK (g/kg) | 35.91 ± 0.42 Aa | 30.07 ± 0.10 Ab | 35.60 ± 0.94 Aa | 24.96 ± 0.63 Cb | 30.43 ± 0.96 Aa | 22.87 ± 0.90 Bc | 28.67 ± 0.44 Ba | 20.67 ± 0.76 Bb | 13.64 ± 0.27 Cc |
| AK (mg/kg) | 46.05 ± 1.51 Aa | 46.90 ± 0.91 Ba | 41.19 ± 2.30 Bb | 40.89 ± 6.18 Ac | 59.64 ± 0.26 Aa | 51.01 ± 1.66 Ab | 48.10 ± 1.35 Aa | 32.12 ± 0.83 Cb | 51.29 ± 5.89 Aa |
| C:N | 13.14 ± 0.41 Ba | 13.43 ± 0.13 Ba | 12.39 ± 0.66 Aa | 14.59 ± 0.11 Ab | 17.29 ± 0.36 Aa | 13.57 ± 0.49 Ab | 13.32 ± 0.12 Aa | 14.14 ± 0.57 Ba | 13.82 ± 0.17 Aa |
| C:P | 79.67 ± 2.62 Ca | 76.43 ± 1.09 Ba | 65.59 ± 0.65 Cb | 140.66 ± 2.39 Ba | 93.48 ± 0.27 Cb | 79.01 ± 1.29 Bc | 109.46 ± 0.13 Ab | 137.62 ± 1.97 Aa | 107.96 ± 0.55 Ab |
| Nutrient Space Ratio | Tree Species | Soil Bacterial Richness | Soil Bacterial Diversity | ||
|---|---|---|---|---|---|
| ACE | Chao1 | Shannon_Index | Simpson_Index | ||
| 0%–10% | AFB | 1092 ± 3 Aa | 1096 ± 4 Aa | 5.83 ± 0.011 Aa | 0.0078 ± 0.0002 Aa |
| CAB | 1060 ± 11 Aa | 1067 ±17 Aa | 5.69 ± 0.005 Ba | 0.0084 ± 0.0002 Ba | |
| CCB | 1063 ± 5 Aa | 1070 ± 11 Aa | 5.65 ± 0.057 Ba | 0.0099 ± 0.0007 Ba | |
| 10%–20% | AFB | 1067 ± 10 Aa | 1075 ± 11 Aa | 5.834 ± 0.020 Aa | 0.0071 ± 0.0001 Aa |
| CAB | 1079 ± 3 Aa | 1084 ± 4 Aa | 5.825 ± 0.016 Ab | 0.0081 ± 0.0003 Ba | |
| CCB | 1072 ± 3 Aa | 1076 ± 4 Aa | 5.65 ± 0.058 Ba | 0.0074 ± 0.0001 ABb | |
| 20%–40% | AFB | 1177 ± 9 Aa | 1082 ± 10 Aa | 5.822 ± 0.0786 Aa | 0.0078 ± 0.0011 ABa |
| CAB | 1089 ± 3 Aa | 1095 ± 4 Aa | 5.950 ± 0.037 Ac | 0.0058 ± 0.0005 Bb | |
| CCB | 990 ± 5 Bb | 998 ± 7 Bb | 5.65 ± 0.059 Ba | 0.0099 ± 0.0003 Aa | |
| Df | Sums of Squares | Mean Squares | F.Model | Variation (R2) | Pr (>F) | ||
|---|---|---|---|---|---|---|---|
| Bacteria | Tree species | 2 | 0.2767 | 0.1383 | 4.2594 | 0.2620 | 0.001 |
| Residuals | 24 | 0.7794 | 0.0325 | 0.7380 | |||
| Total | 26 | 1.0561 | 1 | ||||
| Bacteria | Mixing ratio | 2 | 0.1979 | 0.0990 | 2.7680 | 0.1874 | 0.003 |
| Residuals | 24 | 0.8581 | 0.0358 | 0.8126 | |||
| Total | 26 | 1.0561 | 1 | ||||
| Bacteria | Tree species × Mixing ratio | 8 | 0.7929 | 0.0991 | 6.7800 | 0.7508 | 0.001 |
| Residuals | 18 | 0.2631 | 0.0146 | 0.2492 | |||
| Total | 26 | 1.0561 | 1 |
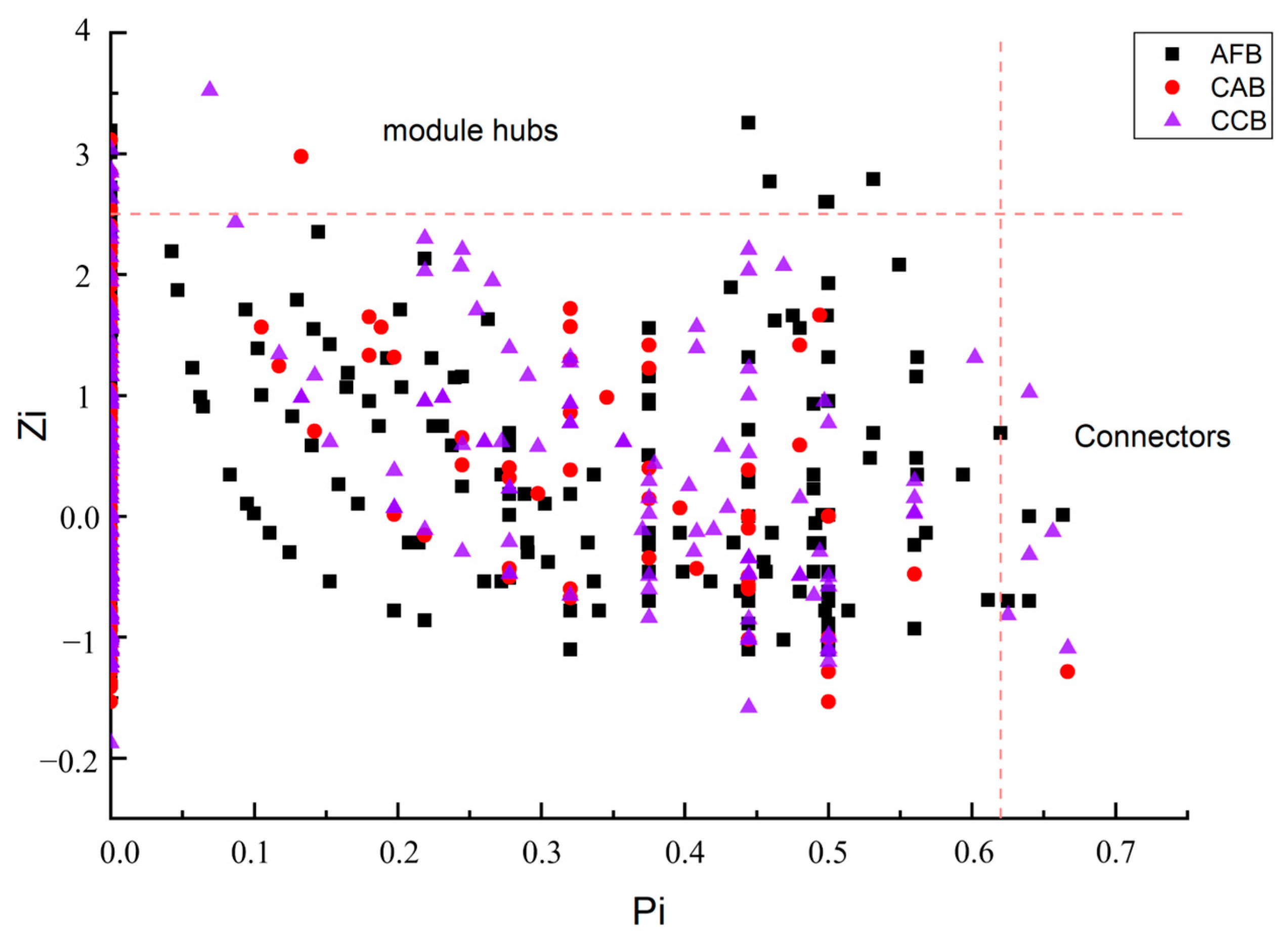
| Network Structure Attributes | AFB | CAB | CCB | |
|---|---|---|---|---|
| Empirical networks | Similarity threshold | 0.940 | 0.960 | 0.950 |
| Total nodes | 628 | 479 | 508 | |
| Total links | 2374 | 1038 | 1008 | |
| R2 of power law | 0.909 | 0.903 | 0.912 | |
| Average path distance (GD) | 8.297 | 8.400 | 6.545 | |
| Average clustering coefficient (avgCC) | 0.301 | 0.273 | 0.245 | |
| Modularity and the number of modules | 0.590 (55) | 0.785 (56) | 0.741 (68) | |
| Module hubs | 13 | 5 | 7 | |
| Connectors | 4 | 1 | 5 | |
| Random networks | Average path distance (GD) | 3.352 +/− 0.026 | 0.046 +/− 0.000 | 4.142 +/− 0.052 |
| Average clustering coefficient (avgCC) | 0.050 +/− 0.005 | 0.019 +/− 0.003 | 0.017 +/− 0.003 | |
| Modularity (M) | 0.305 +/− 0.004 | 0.470 +/− 0.005 | 0.502 +/− 0.005 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Guan, F.; Fan, S.; Zhang, X. Response of Soil Microbial Community Structure and Diversity to Mixed Proportions and Mixed Tree Species in Bamboo–Broad-Leaved Mixed Forests. Forests 2024, 15, 921. https://doi.org/10.3390/f15060921
Zhang M, Guan F, Fan S, Zhang X. Response of Soil Microbial Community Structure and Diversity to Mixed Proportions and Mixed Tree Species in Bamboo–Broad-Leaved Mixed Forests. Forests. 2024; 15(6):921. https://doi.org/10.3390/f15060921
Chicago/Turabian StyleZhang, Meiman, Fengying Guan, Shaohui Fan, and Xuan Zhang. 2024. "Response of Soil Microbial Community Structure and Diversity to Mixed Proportions and Mixed Tree Species in Bamboo–Broad-Leaved Mixed Forests" Forests 15, no. 6: 921. https://doi.org/10.3390/f15060921





