Genome-Wide Identification, Characterization, and Expression Analysis of the CYP450 Family Associated with Triterpenoid Saponin in Soapberry (Sapindus mukorossi Gaertn.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Gene Identification and Structural Analysis
2.3. Analyses of Conserved Motifs and Physicochemical Properties
2.4. Analyses of Cis-Acting Elements and Proteins
2.5. Analysis of the Correlations of SmCYP450 Genes with Other Saponin Synthesis Pathway Genes
2.6. Analysis of the Expression Pattern of the SmCYP450 Genes
3. Results
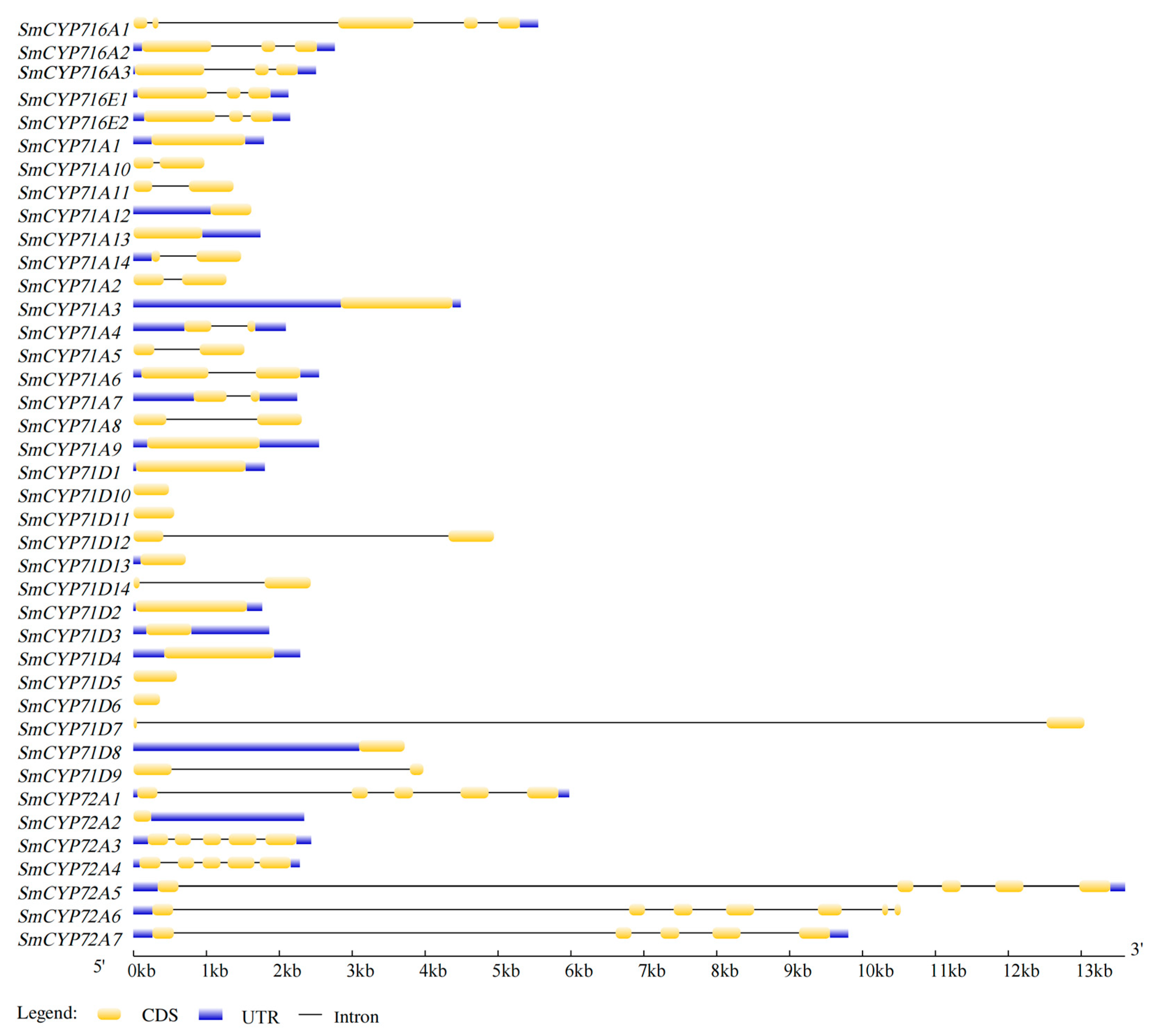
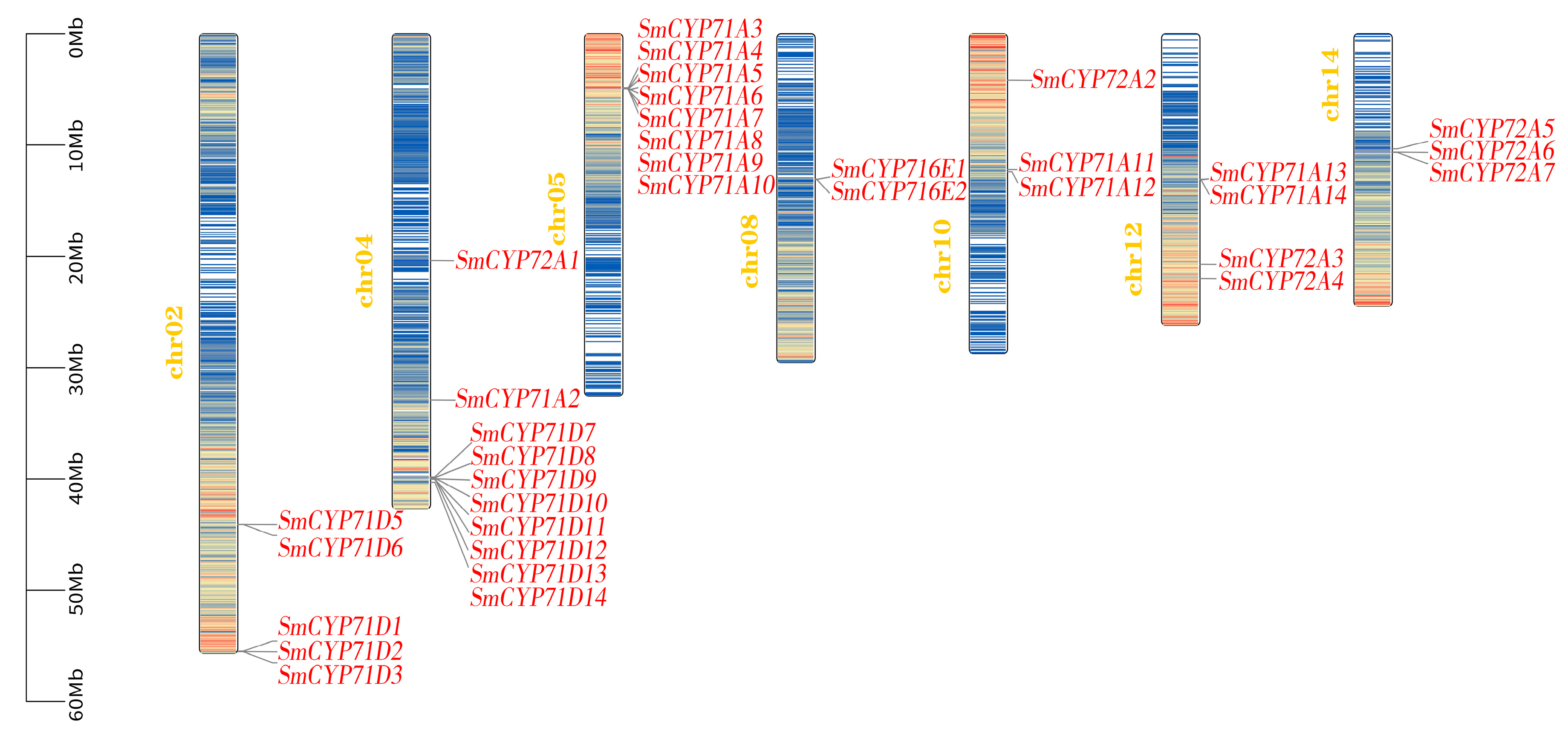
3.1. Identification and Structural Analysis of the SmCYP450 Genes
3.2. Physicochemical Properties of the SmCYP450 Proteins
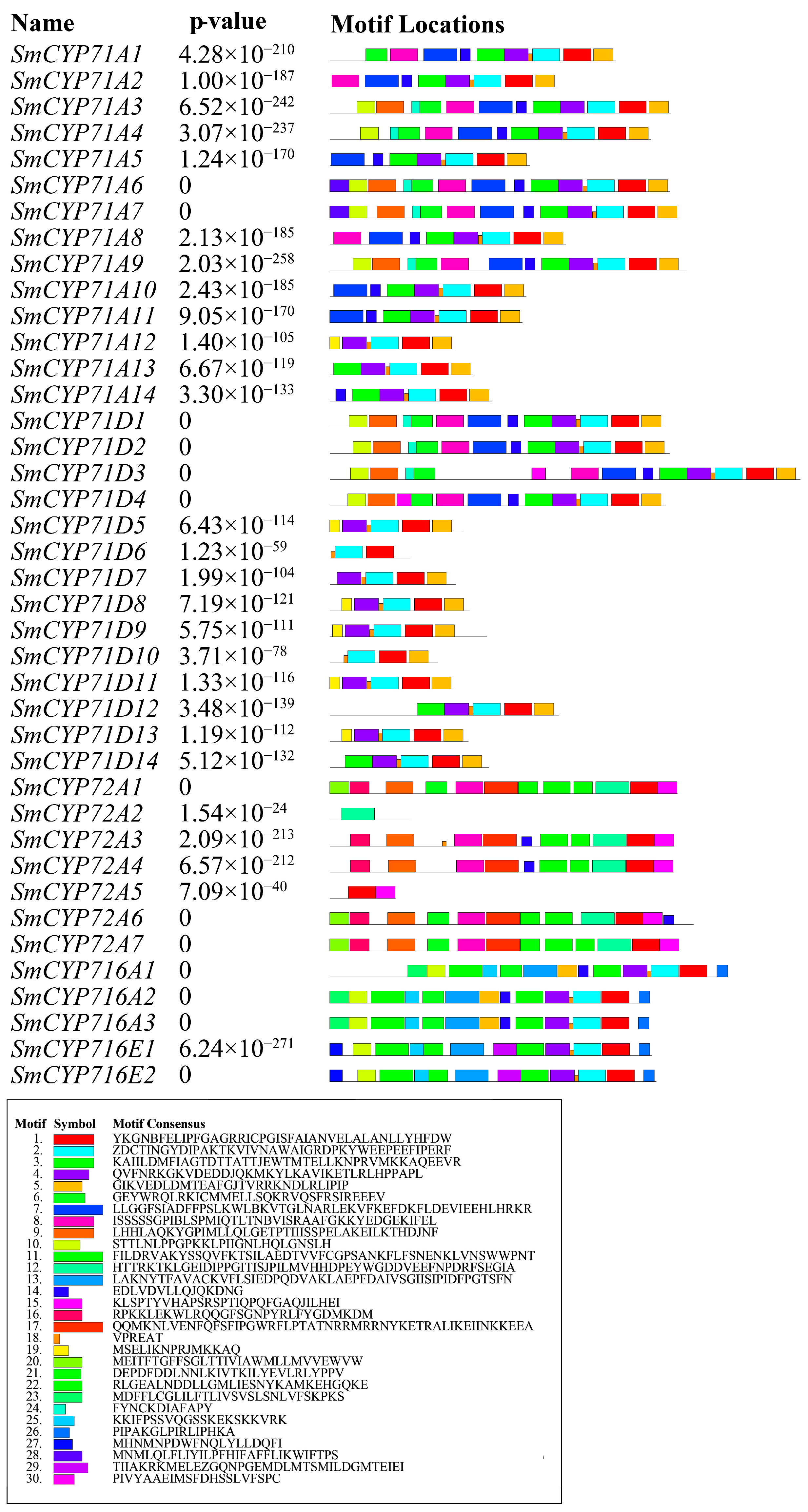
3.3. Conserved Motifs of the SmCYP450 Proteins
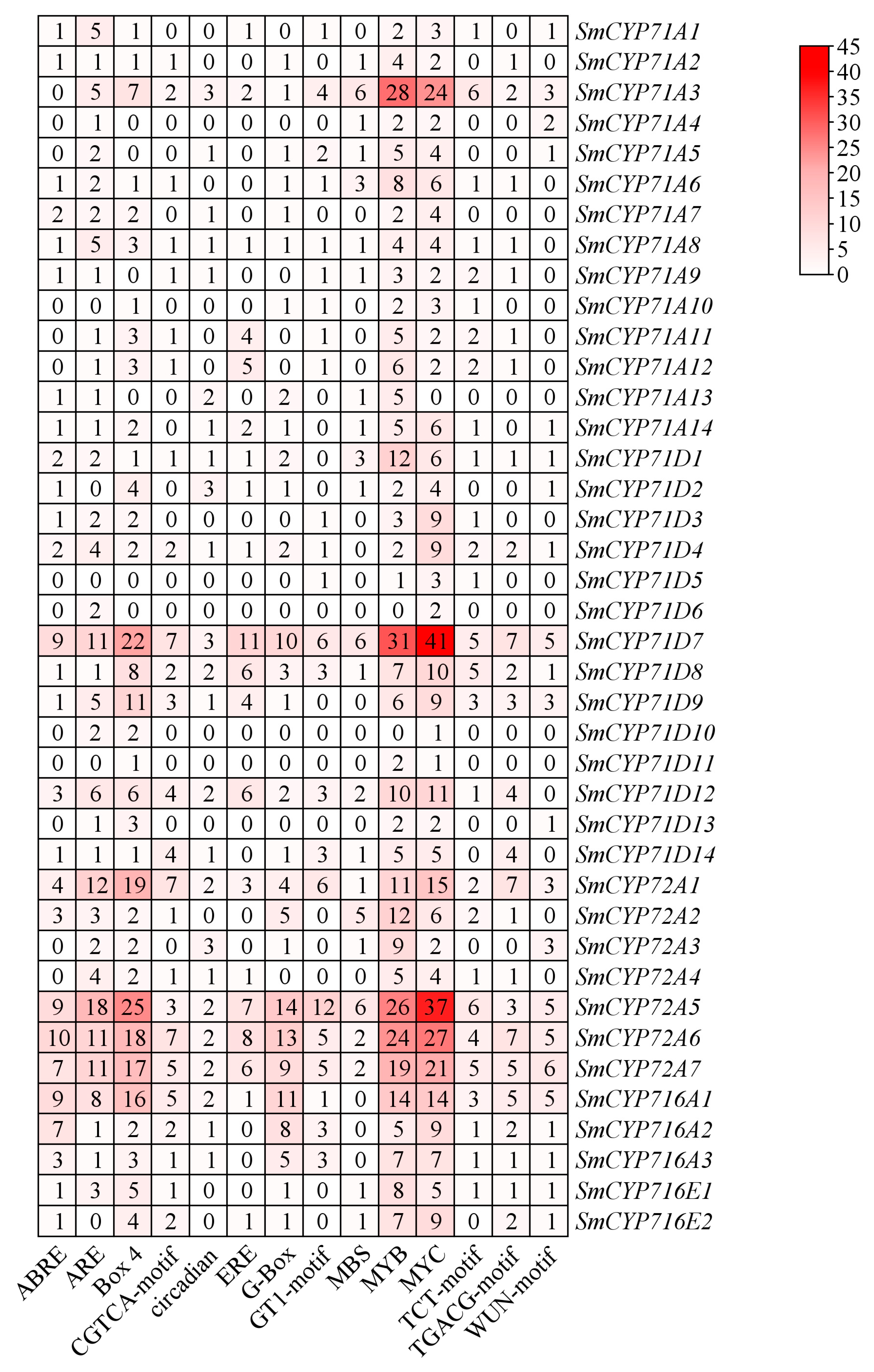
3.4. Analysis of Cis-Acting Elements
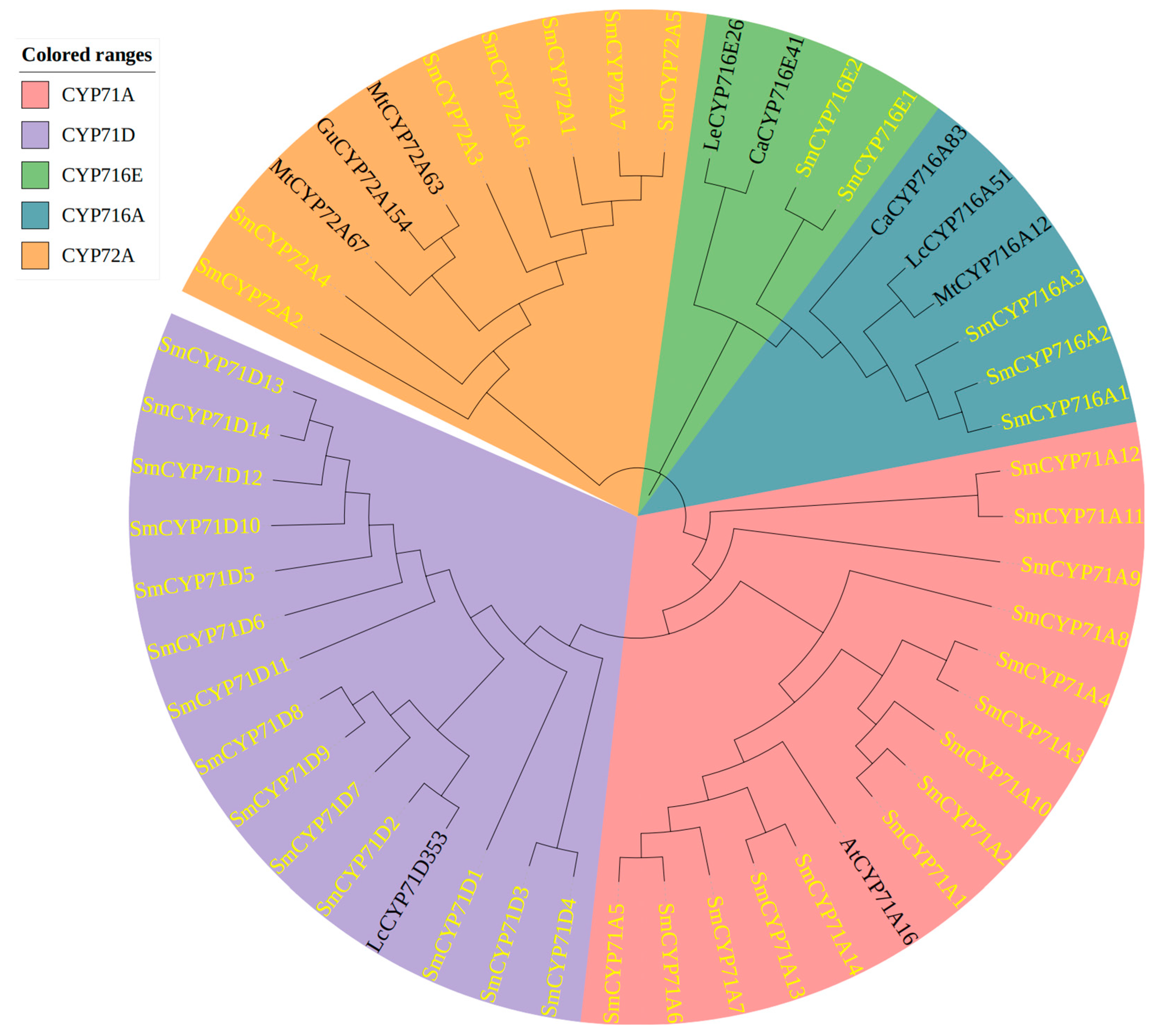
3.5. Phylogenetic Analysis of the SmCYP450 Proteins
3.6. Structural Characterization of SmCYP450 Proteins
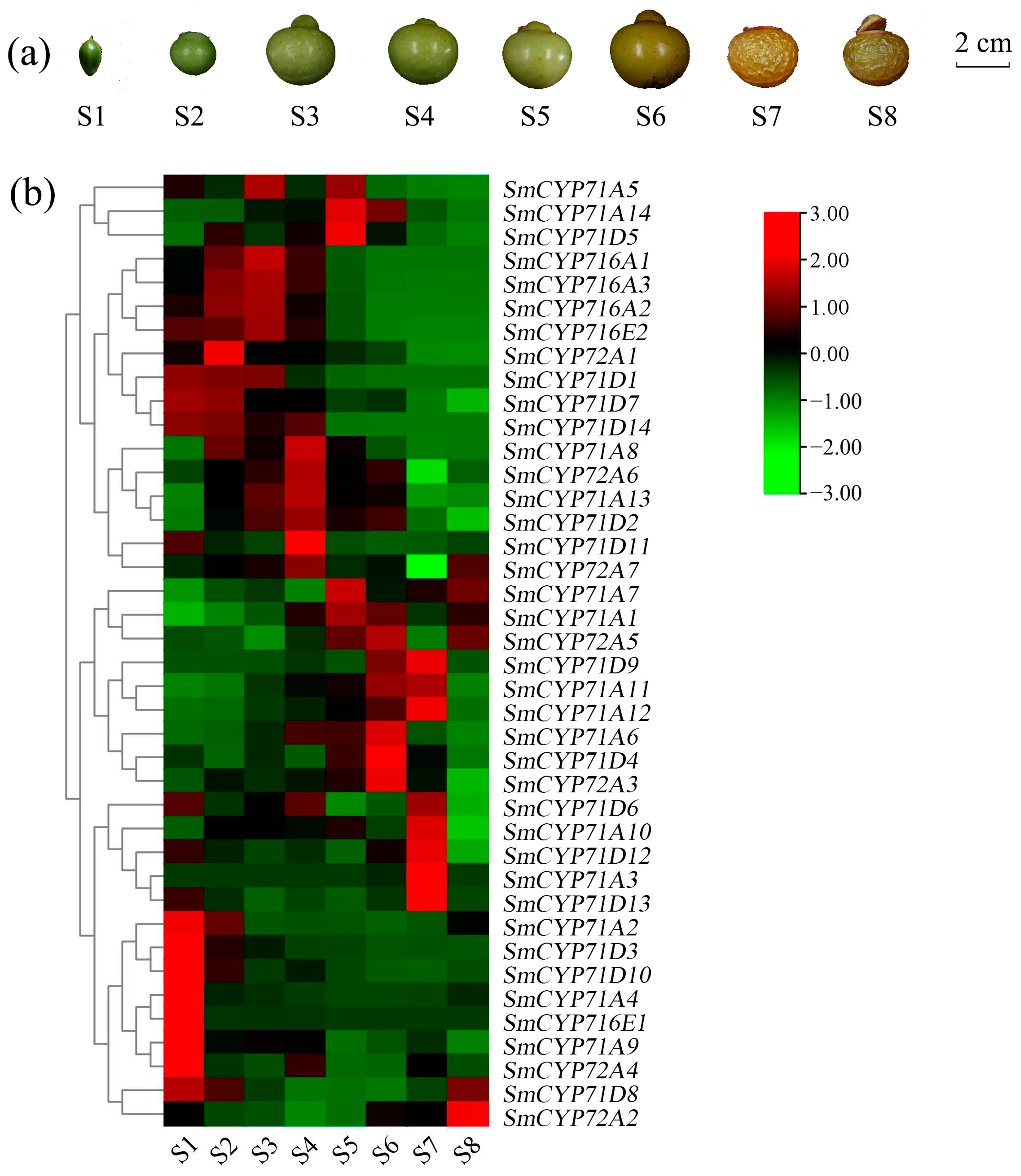
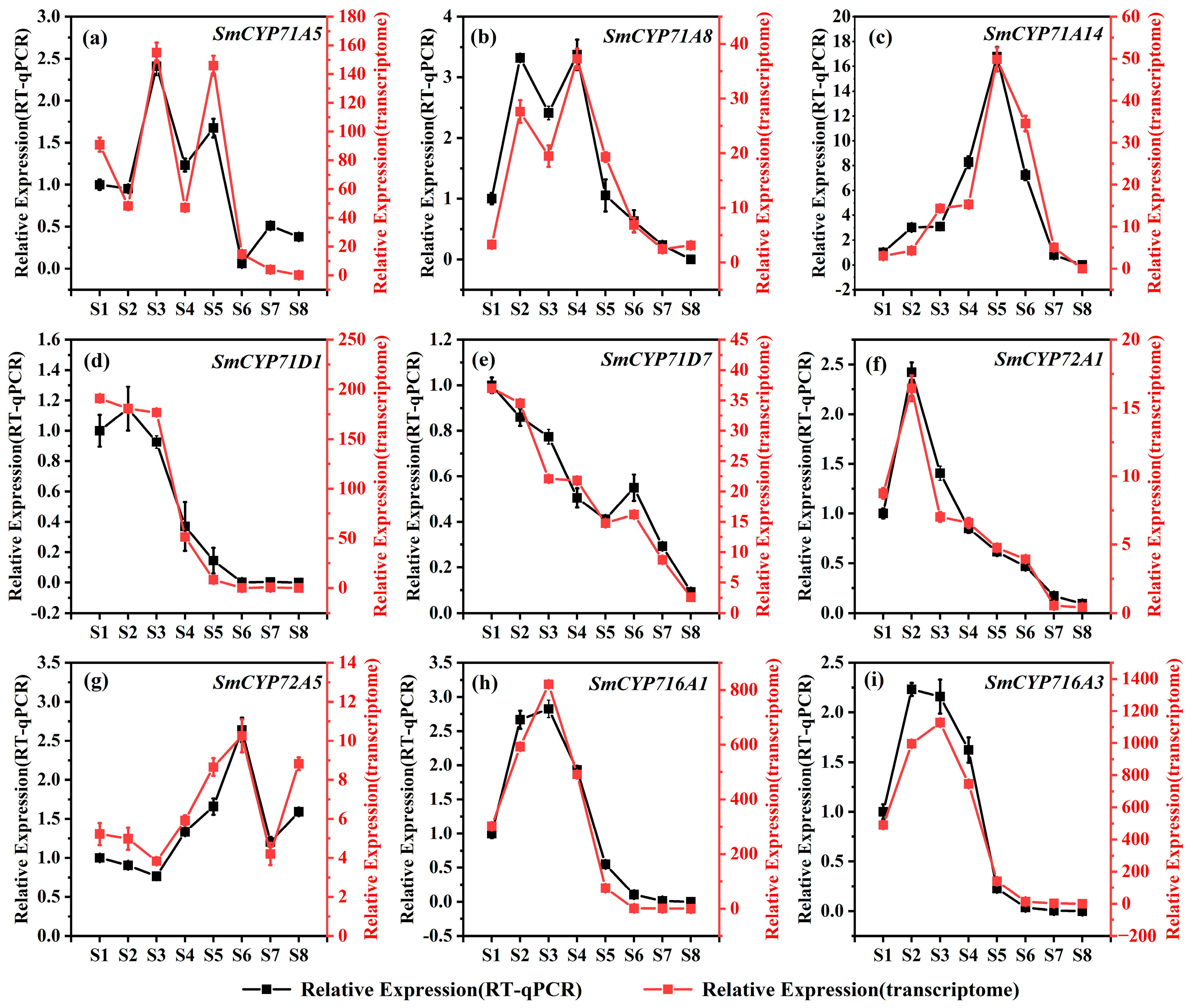
3.7. Expression of SmCYP450 Genes According to Developmental Stages
3.8. Correlations of SmCYP450s with Saponin-Synthesis-Pathway Genes
3.9. Correlation of SmCYP450s Expression with Total and Monomeric Saponin Contents
4. Discussion
4.1. Structures and Conserved Motifs of SmCYP450 Genes
4.2. Phylogenetic Analysis of the SmCYP450 Genes
4.3. Expression Pattern According to Stage of Fruit Development
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, Y.; Jia, L.; Chen, Z.; Gao, Y. Advances on Triterpenoid Saponin of Sapindus mukorossi. Chemistry 2018, 81, 1078–1088. (In Chinese) [Google Scholar] [CrossRef]
- Xu, Y.; Chen, Z.; Jia, L.; Weng, X. Advances in Understanding of the Biosynthetic Pathway and Regulatory Mechanism of Triterpenoid Saponins in Plants. Sci. Sin.-Vitae 2021, 51, 525–555. [Google Scholar] [CrossRef]
- Abbas, F.; Ke, Y.; Yu, R.; Yue, Y.; Amanullah, S.; Jahangir, M.M.; Fan, Y. Volatile Terpenoids: Multiple Functions, Biosynthesis, Modulation and Manipulation by Genetic Engineering. Planta 2017, 246, 803–816. [Google Scholar] [CrossRef] [PubMed]
- Laszczyk, M. Pentacyclic Triterpenes of the Lupane, Oleanane and Ursane Group as Tools in Cancer Therapy. Planta Med. 2009, 75, 1549–1560. [Google Scholar] [CrossRef]
- Chen, H.; Wu, B.; Nelson, D.R.; Wu, K.; Liu, C. Computational Identification and Systematic Classification of Novel Cytochrome P450 Genes in Salvia miltiorrhiza. PLoS ONE 2014, 9, e115149. [Google Scholar] [CrossRef]
- Xu, T.-H.; Lv, G.; Xu, Y.-J.; Xie, S.-X.; Zhao, H.-F.; Han, D.; Si, Y.-S.; Li, Y.; Niu, J.-Z.; Xu, D.-M. A Novel Triterpenoid Saponin from Polygala tenuifolia Willd. J. Asian Nat. Prod. Res. 2008, 10, 803–806. [Google Scholar] [CrossRef] [PubMed]
- Thaniarasu, R.; Anitha, A. Phytochemical Screening, Antioxidant Activity and Identification of Active Phytochemical Compounds of Scutellaria wightiana Benth. an Endemic Plant to Peninsular India. Res. J. Biotechnol. 2023, 18, 45–55. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, C. Biosynthesis of Plant Triterpenoid Saponins in Microbial Cell Factories. J. Agric. Food Chem. 2018, 66, 12155–12165. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.-L.; Hu, Z.-F.; Zhang, T.-T.; Gu, A.-D.; Gong, T.; Zhu, P. Progress on the Studies of the Key Enzymes of Ginsenoside Biosynthesis. Molecules 2018, 23, 589. [Google Scholar] [CrossRef]
- Haralampidis, K.; Trojanowska, M.; Osbourn, A.E. Biosynthesis of Triterpenoid Saponins in Plants. In History and Trends in Bioprocessing and Biotransformation; Dutta, N.N., Hammar, F., Haralampidis, K., Karanth, N.G., König, A., Krishna, S.H., Kunze, G., Nagy, E., Orlich, B., Osbourn, A.E., et al., Eds.; Advances in Biochemical Engineering/Biotechnology; Springer: Berlin/Heidelberg, Germany, 2002; Volume 75, pp. 31–49. ISBN 978-3-540-42371-3. [Google Scholar]
- Zeng, X.; Luo, T.; Li, J.; Li, G.; Zhou, D.; Liu, T.; Zou, X.; Pandey, A.; Luo, Z. Transcriptomics-Based Identification and Characterization of 11 CYP450 Genes of Panax Ginseng Responsive to MeJA. Acta Biochim. Biophys. Sin. 2018, 50, 1094–1103. [Google Scholar] [CrossRef]
- Osbourn, A.; Goss, R.J.M.; Field, R.A. The Saponins–Polar Isoprenoids with Important and Diverse Biological Activities. Nat. Prod. Rep. 2011, 28, 1261. [Google Scholar] [CrossRef]
- Augustin, J.M.; Kuzina, V.; Andersen, S.B.; Bak, S. Molecular Activities, Biosynthesis and Evolution of Triterpenoid Saponins. Phytochemistry 2011, 72, 435–457. [Google Scholar] [CrossRef]
- Moses, T.; Papadopoulou, K.K.; Osbourn, A. Metabolic and Functional Diversity of Saponins, Biosynthetic Intermediates and Semi-Synthetic Derivatives. Crit. Rev. Biochem. Mol. Biol. 2014, 49, 439–462. [Google Scholar] [CrossRef]
- Ghosh, S. Triterpene Structural Diversification by Plant Cytochrome P450 Enzymes. Front. Plant Sci. 2017, 8, 1886. [Google Scholar] [CrossRef]
- Yao, L.; Lu, J.; Wang, J.; Gao, W.-Y. Advances in Biosynthesis of Triterpenoid Saponins in Medicinal Plants. Chin. J. Nat. Med. 2020, 18, 417–424. [Google Scholar] [CrossRef]
- Shang, Y.; Huang, S. Multi-omics Data-driven Investigations of Metabolic Diversity of Plant Triterpenoids. Plant J. 2019, 97, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Andre, C.M.; Legay, S.; Deleruelle, A.; Nieuwenhuizen, N.; Punter, M.; Brendolise, C.; Cooney, J.M.; Lateur, M.; Hausman, J.; Larondelle, Y.; et al. Multifunctional Oxidosqualene Cyclases and Cytochrome P450 Involved in the Biosynthesis of Apple Fruit Triterpenic Acids. New Phytol. 2016, 211, 1279–1294. [Google Scholar] [CrossRef]
- Babu, P.R.; Rao, K.V.; Reddy, V.D. Structural Organization and Classification of Cytochrome P450 Genes in Flax (Linum usitatissimum L.). Gene 2013, 513, 156–162. [Google Scholar] [CrossRef]
- Liao, W.; Zhao, S.; Zhang, M.; Dong, K.; Chen, Y.; Fu, C.; Yu, L. Transcriptome Assembly and Systematic Identification of Novel Cytochrome P450s in Taxus chinensis. Front. Plant Sci. 2017, 8, 1468. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-M.; Zhang, X.-X.; He, X.; Yang, K.; Huang, X.-R.; Ye, J.-B.; Zhang, W.-W.; Xu, F. Identification and Analysis of CYP450 Family Members in Ginkgo biloba Reveals the Candidate Genes for Terpene Trilactone Biosynthesis. Sci. Hortic. 2022, 301, 111103. [Google Scholar] [CrossRef]
- Cheng, Y.; Liu, H.; Tong, X.; Liu, Z.; Zhang, X.; Li, D.; Jiang, X.; Yu, X. Identification and Analysis of CYP450 and UGT Supergene Family Members from the Transcriptome of Aralia elata (Miq.) Seem Reveal Candidate Genes for Triterpenoid Saponin Biosynthesis. BMC Plant Biol. 2020, 20, 214. [Google Scholar] [CrossRef]
- Xu, Y.; Gao, Y.; Chen, Z.; Zhao, G.; Liu, J.; Wang, X.; Gao, S.; Zhang, D.; Jia, L. Metabolomics Analysis of the Soapberry (Sapindus mukorossi Gaertn.) Pericarp during Fruit Development and Ripening Based on UHPLC-HRMS. Sci. Rep. 2021, 11, 11657. [Google Scholar] [CrossRef]
- Xu, Y.; Zhao, G.; Ji, X.; Liu, J.; Zhao, T.; Gao, Y.; Gao, S.; Hao, Y.; Gao, Y.; Wang, L.; et al. Metabolome and Transcriptome Analysis Reveals the Transcriptional Regulatory Mechanism of Triterpenoid Saponin Biosynthesis in Soapberry (Sapindus mukorossi Gaertn.). J. Agric. Food Chem. 2022, 70, 7095–7109. [Google Scholar] [CrossRef]
- Rezaei, A.R. Studies On Cytochrome C Oxidase Subunit II Gene in Salmo trutta Caspius. Banat. J. Biotechnol. 2015, 6, 5–12. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Stecher, G.; Tamura, K.; Kumar, S. Molecular Evolutionary Genetics Analysis (MEGA) for macOS. Mol. Biol. Evol. 2020, 37, 1237–1239. [Google Scholar] [CrossRef]
- Lescot, M. PlantCARE, a Database of Plant Cis-Acting Regulatory Elements and a Portal to Tools for in Silico Analysis of Promoter Sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef]
- Guex, N.; Peitsch, M.C. SWISS-MODEL and the Swiss-Pdb Viewer: An Environment for Comparative Protein Modeling. Electrophoresis 1997, 18, 2714–2723. [Google Scholar] [CrossRef]
- Lopes, C.T.; Franz, M.; Kazi, F.; Donaldson, S.L.; Morris, Q.; Bader, G.D. Cytoscape Web: An Interactive Web-Based Network Browser. Bioinformatics 2010, 26, 2347–2348. [Google Scholar] [CrossRef]
- Xu, Y.; Jia, L.; Chen, Z.; Zhao, G.; Hao, Y.; Weng, X.; Chen, Z.; Jia, L. Reference Genes Selection and Validation for RT-qPCR in Sapindus mukorossi. Biotechnol. Bull. 2022, 38, 80–89. (In Chinese) [Google Scholar] [CrossRef]
- Kou, X.; Zhang, L.; Yang, S.; Li, G.; Ye, J. Selection and Validation of Reference Genes for Quantitative RT-PCR Analysis in Peach Fruit under Different Experimental Conditions. Sci. Hortic. 2017, 225, 195–203. [Google Scholar] [CrossRef]
- Paquette, S.M.; Jensen, K.; Bak, S. A Web-Based Resource for the Arabidopsis P450, Cytochromes B5, NADPH-Cytochrome P450 Reductases, and Family 1 Glycosyltransferases (http://www.P450.Kvl.Dk). Phytochemistry 2009, 70, 1940–1947. [Google Scholar] [CrossRef]
- Nelson, D.R.; Kamataki, T.; Waxman, D.J.; Guengerich, F.P.; Estabrook, R.W.; Feyereisen, R.; Gonzalez, F.J.; Coon, M.J.; Gunsalus, I.C.; Gotoh, O.; et al. The P450 Superfamily: Update on New Sequences, Gene Mapping, Accession Numbers, Early Trivial Names of Enzymes, and Nomenclature. DNA Cell Biol. 1993, 12, 1–51. [Google Scholar] [CrossRef] [PubMed]
- Kunii, M.; Kitahama, Y.; Fukushima, E.O.; Seki, H.; Muranaka, T.; Yoshida, Y.; Aoyama, Y. β-Amyrin Oxidation by Oat CYP51H10 Expressed Heterologously in Yeast Cells: The First Example of CYP51-Dependent Metabolism Other than the 14-Demethylation of Sterol Precursors. Biol. Pharm. Bull. 2012, 35, 801–804. [Google Scholar] [CrossRef]
- Nelson, D.; Werck-Reichhart, D. A P450-centric View of Plant Evolution. Plant J. 2011, 66, 194–211. [Google Scholar] [CrossRef]
- Moses, T.; Thevelein, J.M.; Goossens, A.; Pollier, J. Comparative Analysis of CYP93E Proteins for Improved Microbial Synthesis of Plant Triterpenoids. Phytochemistry 2014, 108, 47–56. [Google Scholar] [CrossRef]
- Fukushima, E.O.; Seki, H.; Sawai, S.; Suzuki, M.; Ohyama, K.; Saito, K.; Muranaka, T. Combinatorial Biosynthesis of Legume Natural and Rare Triterpenoids in Engineered Yeast. Plant Cell Physiol. 2013, 54, 740–749. [Google Scholar] [CrossRef]
- Ma, Y.; Cui, G.; Chen, T.; Ma, X.; Wang, R.; Jin, B.; Yang, J.; Kang, L.; Tang, J.; Lai, C.; et al. Expansion within the CYP71D Subfamily Drives the Heterocyclization of Tanshinones Synthesis in Salvia miltiorrhiza. Nat. Commun. 2021, 12, 685. [Google Scholar] [CrossRef]
- Yano, R.; Takagi, K.; Takada, Y.; Mukaiyama, K.; Tsukamoto, C.; Sayama, T.; Kaga, A.; Anai, T.; Sawai, S.; Ohyama, K.; et al. Metabolic Switching of Astringent and Beneficial Triterpenoid Saponins in Soybean Is Achieved by a Loss-of-function Mutation in Cytochrome P450 72A69. Plant J. 2017, 89, 527–539. [Google Scholar] [CrossRef]
- Liu, Q.; Khakimov, B.; Cárdenas, P.D.; Cozzi, F.; Olsen, C.E.; Jensen, K.R.; Hauser, T.P.; Bak, S. The Cytochrome P450 CYP72A552 Is Key to Production of Hederagenin-based Saponins That Mediate Plant Defense against Herbivores. New Phytol. 2019, 222, 1599–1609. [Google Scholar] [CrossRef]
- Biazzi, E.; Carelli, M.; Tava, A.; Abbruscato, P.; Losini, I.; Avato, P.; Scotti, C.; Calderini, O. CYP72A67 Catalyzes a Key Oxidative Step in Medicago truncatula Hemolytic Saponin Biosynthesis. Mol. Plant 2015, 8, 1493–1506. [Google Scholar] [CrossRef]
- Miettinen, K.; Pollier, J.; Buyst, D.; Arendt, P.; Csuk, R.; Sommerwerk, S.; Moses, T.; Mertens, J.; Sonawane, P.D.; Pauwels, L.; et al. The Ancient CYP716 Family Is a Major Contributor to the Diversification of Eudicot Triterpenoid Biosynthesis. Nat. Commun. 2017, 8, 14153. [Google Scholar] [CrossRef]
- Tamura, K.; Seki, H.; Suzuki, H.; Kojoma, M.; Saito, K.; Muranaka, T. CYP716A179 Functions as a Triterpene C-28 Oxidase in Tissue-cultured Stolons of Glycyrrhiza uralensis. Plant Cell Rep. 2017, 36, 437–445. [Google Scholar] [CrossRef]
- Suzuki, H.; Fukushima, E.O.; Umemoto, N.; Ohyama, K.; Seki, H.; Muranaka, T. Comparative Analysis of CYP716A Subfamily Enzymes for the Heterologous Production of C-28 Oxidized Triterpenoids in Transgenic Yeast. Plant Biotechnol. 2018, 35, 131–139. [Google Scholar] [CrossRef] [PubMed]
- D’Adamo, S.; Schiano Di Visconte, G.; Lowe, G.; Szaub-Newton, J.; Beacham, T.; Landels, A.; Allen, M.J.; Spicer, A.; Matthijs, M. Engineering the Unicellular Alga Phaeodactylum tricornutum for High-value Plant Triterpenoid Production. Plant Biotechnol. J. 2019, 17, 75–87. [Google Scholar] [CrossRef]
- Zhao, M.; Lin, Y.; Wang, Y.; Li, X.; Han, Y.; Wang, K.; Sun, C.; Wang, Y.; Zhang, M. Transcriptome Analysis Identifies Strong Candidate Genes for Ginsenoside Biosynthesis and Reveals Its Underlying Molecular Mechanism in Panax ginseng C.A. Meyer. Sci. Rep. 2019, 9, 615. [Google Scholar] [CrossRef]
- Seki, H.; Sawai, S.; Ohyama, K.; Mizutani, M.; Ohnishi, T.; Sudo, H.; Fukushima, E.O.; Akashi, T.; Aoki, T.; Saito, K.; et al. Triterpene Functional Genomics in Licorice for Identification of CYP72A154 Involved in the Biosynthesis of Glycyrrhizin. Plant Cell 2011, 23, 4112–4123. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, C.; Zhou, M.; Fan, J.; Gao, Y.; Xu, Y.; Jia, L.; An, X.; Chen, Z.; Wang, L. Genome-Wide Identification, Characterization, and Expression Analysis of the CYP450 Family Associated with Triterpenoid Saponin in Soapberry (Sapindus mukorossi Gaertn.). Forests 2024, 15, 926. https://doi.org/10.3390/f15060926
Zheng C, Zhou M, Fan J, Gao Y, Xu Y, Jia L, An X, Chen Z, Wang L. Genome-Wide Identification, Characterization, and Expression Analysis of the CYP450 Family Associated with Triterpenoid Saponin in Soapberry (Sapindus mukorossi Gaertn.). Forests. 2024; 15(6):926. https://doi.org/10.3390/f15060926
Chicago/Turabian StyleZheng, Chunyuan, Mingzhu Zhou, Jialin Fan, Yuhan Gao, Yuanyuan Xu, Liming Jia, Xinmin An, Zhong Chen, and Lianchun Wang. 2024. "Genome-Wide Identification, Characterization, and Expression Analysis of the CYP450 Family Associated with Triterpenoid Saponin in Soapberry (Sapindus mukorossi Gaertn.)" Forests 15, no. 6: 926. https://doi.org/10.3390/f15060926




