Decomposition of Foliar Litter from Dominant Plants of Desert Riparian Forests in Extremely Arid Regions
Abstract
:1. Introduction
2. Materials and Methods
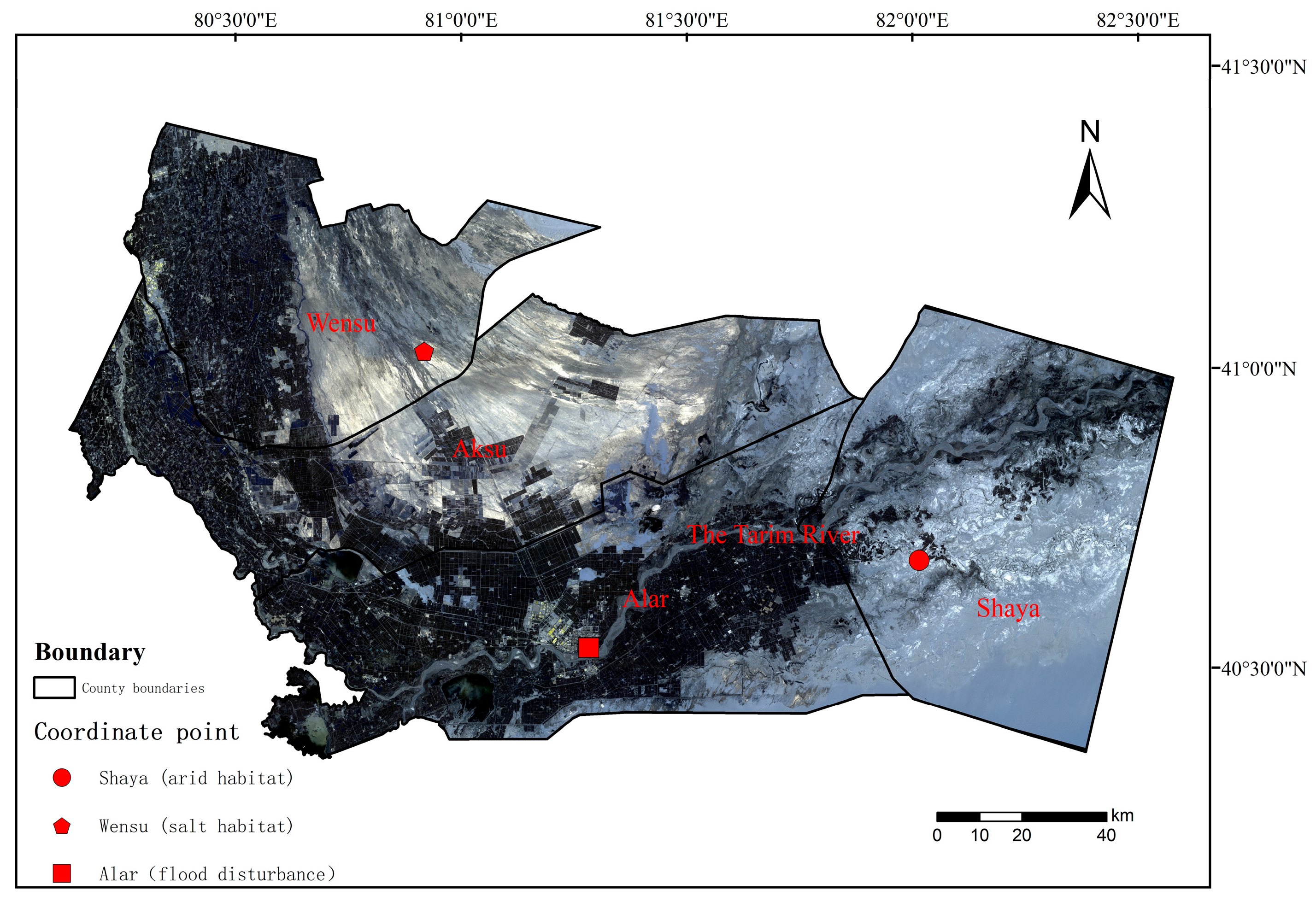
2.1. Experimental Sites and Plant Species
2.2. Experimental Design
2.3. Litter and Soil Properties
2.4. Data Processing and Analyses
3. Results
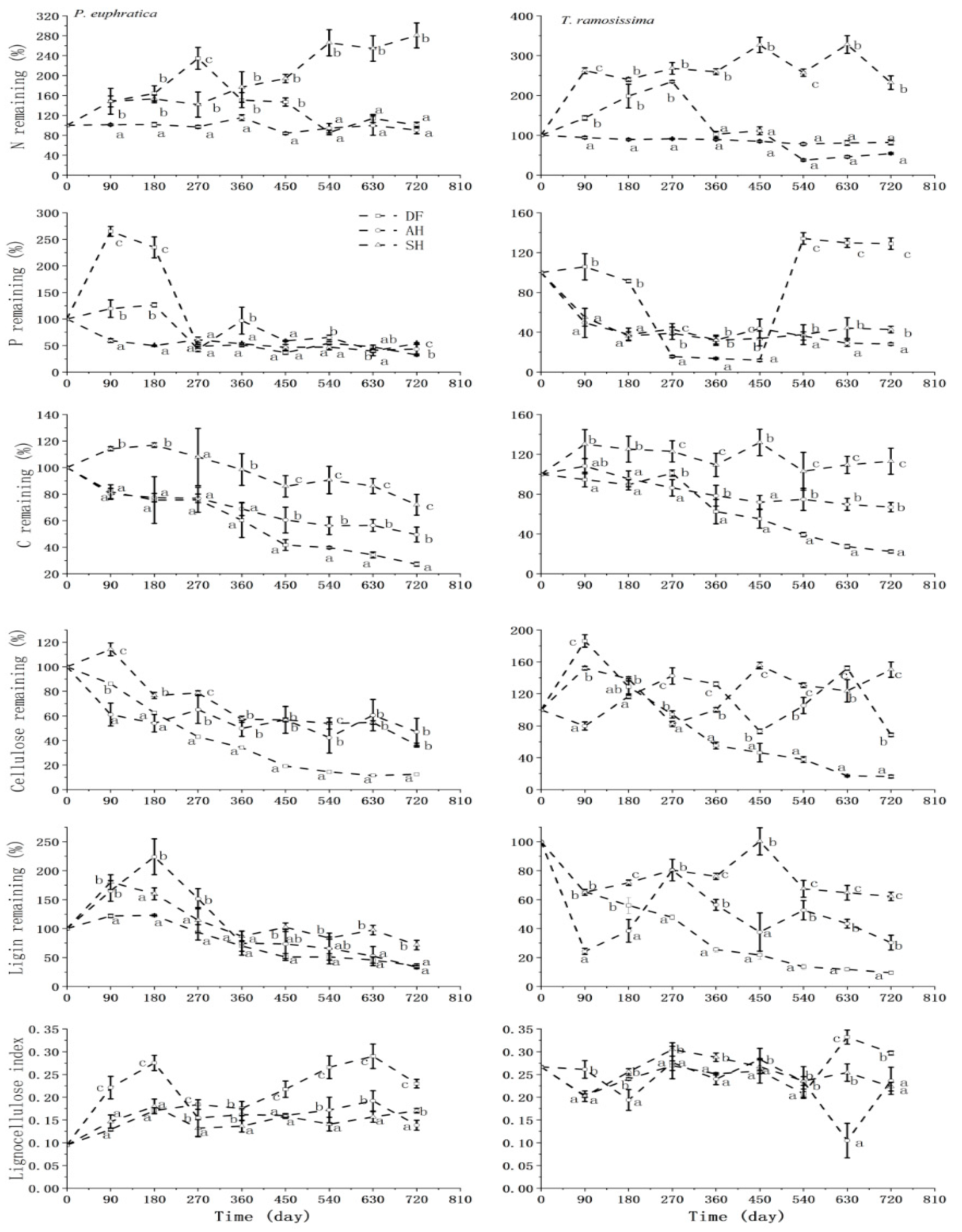
3.1. Nutrient Release Patterns of Litter in Different Habitats
3.2. Dynamic Changes in Total C, Total N, Total P, Cellulose, and Lignin Contents
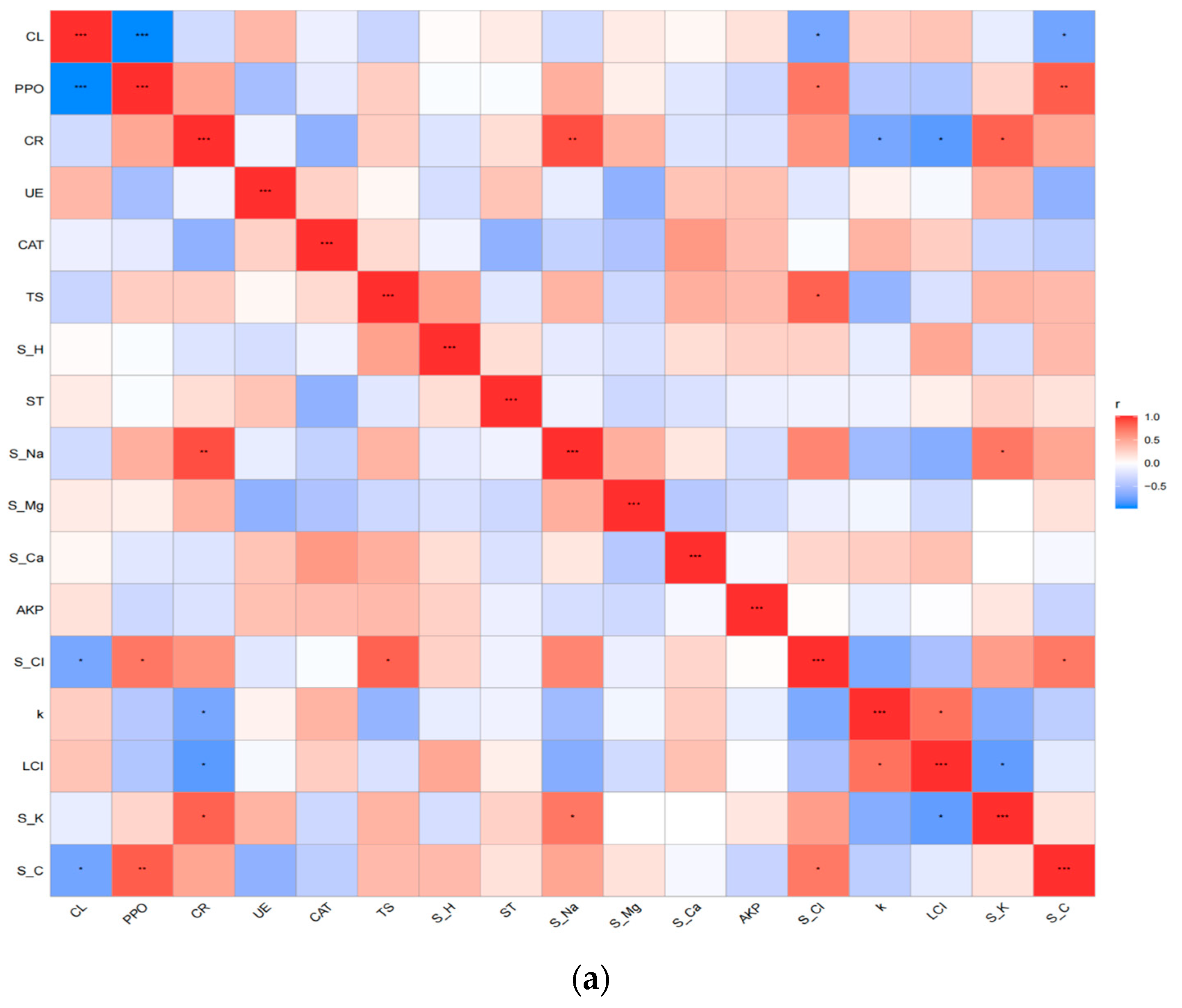
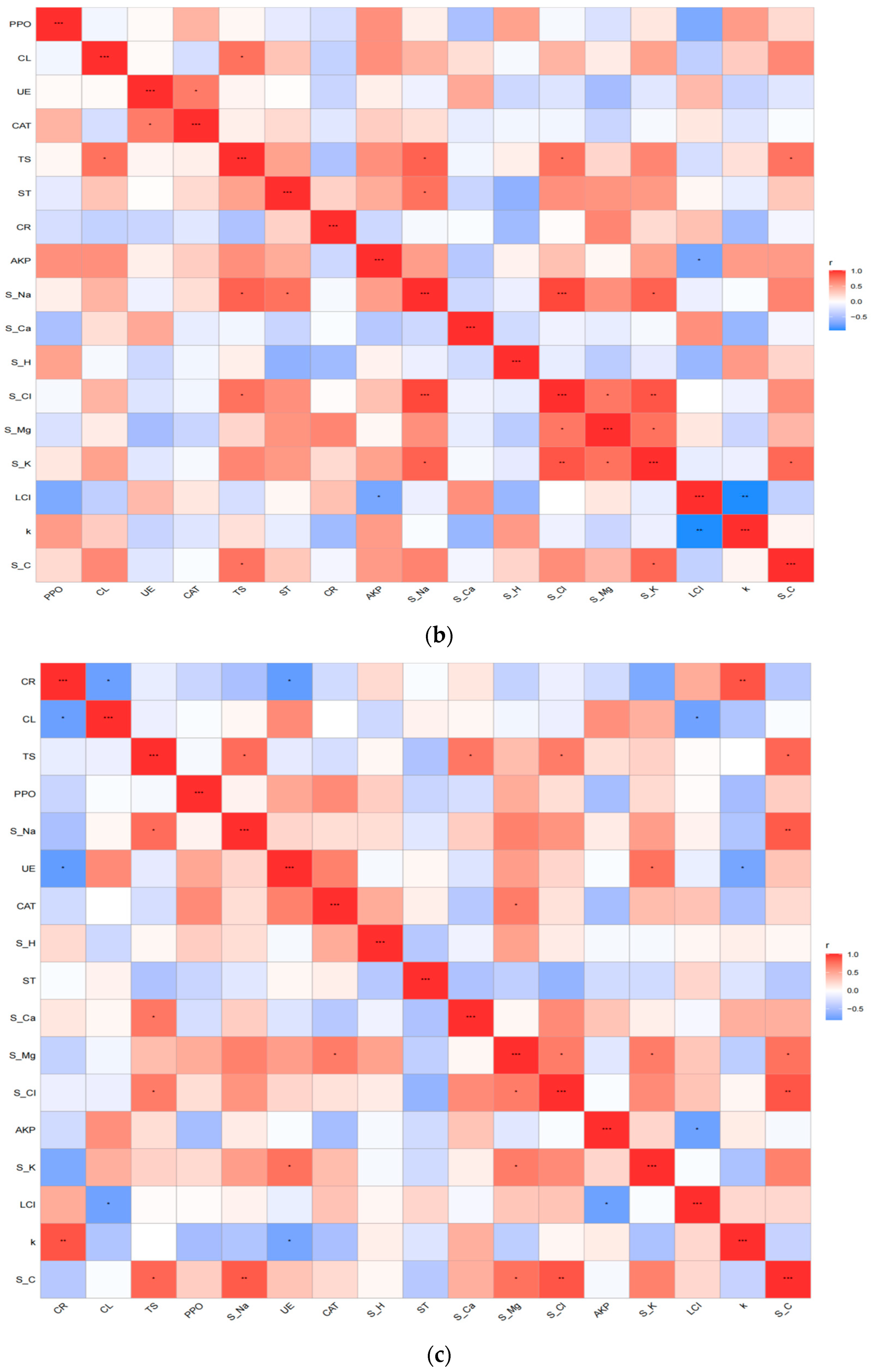
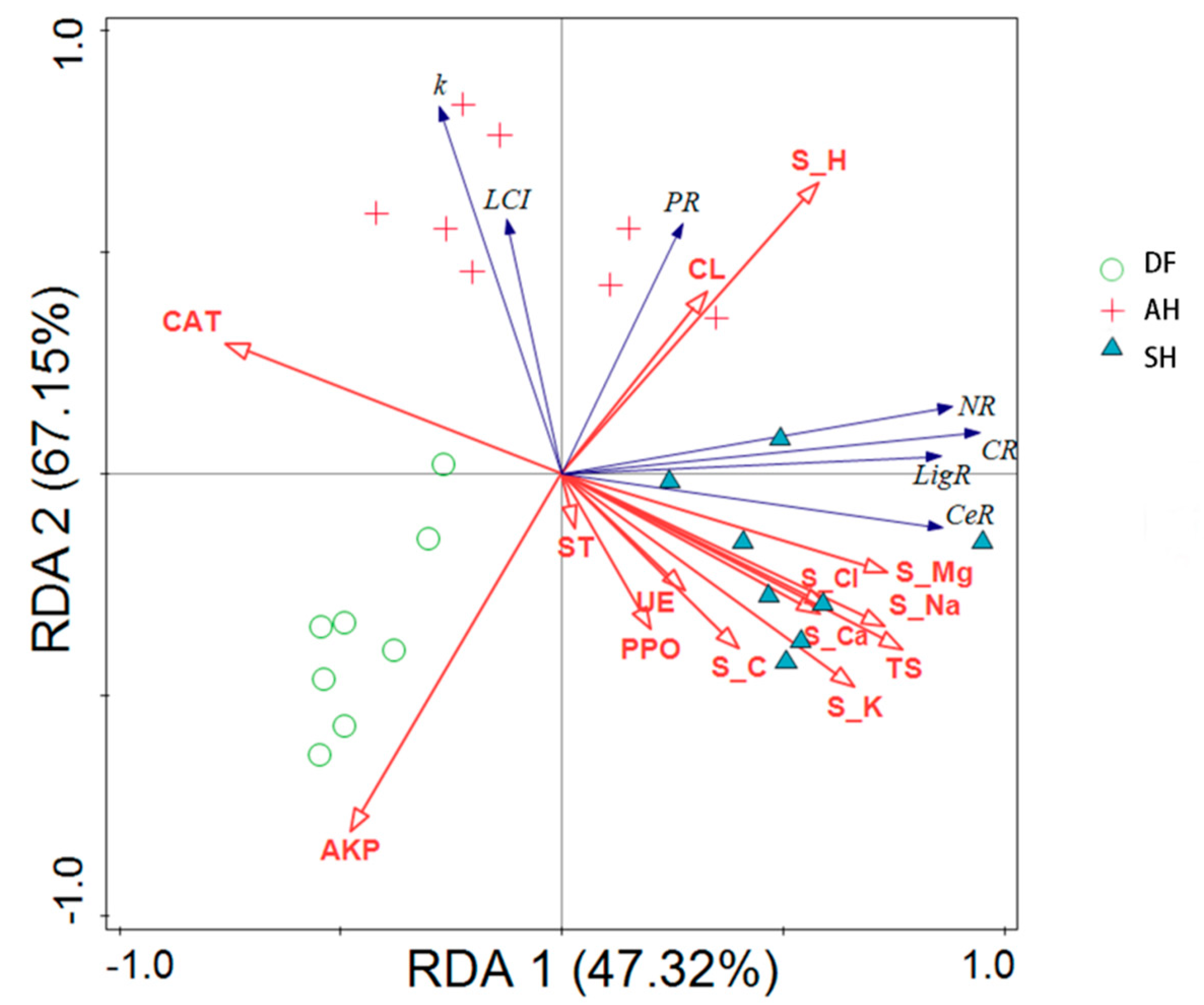
3.3. Comprehensive Analysis of Factors Affecting Litter Decomposition in Different Habitats
4. Discussion
4.1. Effect of Habitat Variability on the Characteristics and Release Processes of Litter Decomposition
4.2. Effect of Habitat Variability on Factors Controlling Litter Decomposition
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bai, X.J.; Zhai, G.Q.; Yan, Z.F.; An, S.S.; Liu, J.Z.; Huo, L.Q.; Dippold, M.A.; Kuzyakov, Y. Effects of microbial groups on soil organic carbon accrual and mineralization during high- and low-quality litter decomposition. Catena 2024, 241, 108051. [Google Scholar] [CrossRef]
- Dong, D.; Badiou, P.; Moore, T.R.; von Sperber, C. Litter decomposition and nutrient dynamics of four macrophytes in intact, restored, and constructed freshwater marshes of Canada. Restor. Ecol. 2024, 32, e14135. [Google Scholar] [CrossRef]
- Wang, Y.G.; Zhu, H.; Li, Y. Spatial heterogeneity of soil moisture, microbial biomass carbon and soil respiration at stand scale of an arid scrubland. Environ. Earth Sci. 2013, 70, 3217–3224. [Google Scholar] [CrossRef]
- White, D.S.; Howes, B.L. Nitrogen incorporation into decomposing litter of Spartina alterniflora. Limnol. Oceanogr. 1994, 39, 133–140. [Google Scholar] [CrossRef]
- Kobayashi, T.; Oguro, M.; Taki, H.; Kurokawa, H. Decomposability of leaf and wood litter are not correlated across species: Effects of litter traits on decomposition in field and laboratory conditions. Oikos 2024, 2024, e10045. [Google Scholar] [CrossRef]
- Vanderbilt, K.L.; White, C.S.; Hopkins, O.; Craig, J.A. Aboveground decomposition in arid environments: Results of a long-term study in central New Mexico. J. Arid. Environ. 2008, 72, 696–709. [Google Scholar] [CrossRef]
- Elumeeva, T.G.; Makarov, M.I.; Kadulin, M.S.; Zamaletdinova, K.N.; Malysheva, T.I.; Gulov, D.M.; Akhmetzhanova, A.A.; Chepurnova, M.A.; Onipchenko, V.G. Organic Matter Content and Standard Material Decomposition Rate in Soils of High-Mountain Plant Communities of the Teberda National Park. Eurasian Soil Sci. 2023, 56, 1940–1954. [Google Scholar] [CrossRef]
- Austin, A.T. Has water limited our imagination for aridland biogeochemistry? Trends Ecol. Evol. 2011, 26, 229–235. [Google Scholar] [CrossRef]
- Cao, S.K.; Feng, Q.; Su, Y.H.; Chang, Z.Q.; Xi, H.Y. Research on the water use efficiency and foliar nutrient status of Populus euphratica and Tamarix ramosissima in the extreme arid region of China (EI). Environ. Earth Sci. 2011, 62, 1597–1607. Available online: http://ir.casnw.net/handle/362004/22117 (accessed on 15 June 2023). [CrossRef]
- Chen, Y.; Li, W.; Xu, C.; Ye, Z.; Chen, Y. Desert riparian vegetation and groundwater in the lower reaches of the Tarim River basin. Environ. Earth Sci. 2015, 73, 547–558. [Google Scholar] [CrossRef]
- De Graaff, M.A.; Throop, H.L.; Verburg, P.S.J.; Arnone, J.A.; Campos, X. A Synthesis of Climate and Vegetation Cover Effects on Biogeochemical Cycling in Shrub-Dominated Drylands. Ecosystems 2014, 17, 931–945. [Google Scholar] [CrossRef]
- Liu, S.; Hu, R.; Cai, G.; Lin, S.; Zhao, J.; Li, Y. The role of UV-B radiation and precipitation on straw decomposition and topsoil C turnover. Soil Biol. Biochem. 2014, 77, 197–202. [Google Scholar] [CrossRef]
- Martínez-Yrízar, A.; Núez, S.; Búrquez, A. Leaf litter decomposition in a southern Sonoran Desert ecosystem, northwestern Mexico: Effects of habitat and litter quality. Acta Oecologica 2007, 32, 291–300. [Google Scholar] [CrossRef]
- Lin, Y.; King, J.Y. Effects of UV Exposure and Litter Position on Decomposition in a California Grassland. Ecosystems 2014, 17, 158–168. [Google Scholar] [CrossRef]
- Lin, Z.; Chen, L.; Dai, Y.; Chen, S.; Li, C.; Yuan, T.; Ma, X.; Liu, A. Effects of Enhanced UV-B Radiation on Decomposition and Nutrient Release Rates of Litter from Cunninghamia lanceolata (Lamb.) Hook. Forests 2024, 15, 686. [Google Scholar] [CrossRef]
- Yue, K.; Yang, W.; Peng, Y.; Huang, C.; Wu, F. Effects of streams on lignin degradation during foliar litter decomposition in an alpine forest. Chin. J. Plant Ecol. 2016, 40, 893–901. [Google Scholar] [CrossRef]
- Bao, S.D. Soil Agrochemical Analysis, 3rd ed.; China Agriculture Press: Beijing, China, 2000. [Google Scholar]
- Sun, Z.; Mou, X.; Liu, J.S. Effects of Flooding Regimes on the Decomposition and Nutrient Dynamics of Calamagrostis angustifolia Litter in the Sanjiang Plain of China. Environ. Earth Sci. 2011, 66, 2235–2246. [Google Scholar] [CrossRef]
- Tong, Z.; Li, H.; Zhang, R.; Ma, L.; Dong, J.; Wang, T. Co-downregulation of the hydroxycinnamoyl-CoA:shikimate hydroxycinnamoyl transferase and coumarate 3-hydroxylase significantly increases cellulose content in transgenic alfalfa (Medicago sativa L.). Plant Sci. 2015, 239, 230–237. [Google Scholar] [CrossRef]
- Anderson, J.T.; Smith, L.M. The effect of flooding regimes on decomposition of Polygonum pensylvanicum in playa wetlands (Southern Great Plains, USA). Aquat. Bot. 2002, 74, 97–108. [Google Scholar] [CrossRef]
- Smolander, K.A. Polyphenol oxidase, tannase and proteolytic activity in relation to tannin concentration in the soil organic horizon under silver birch and Norway spruce. Soil Biol. Biochem. 2009, 41, 2085–2093. [Google Scholar] [CrossRef]
- Özçelik, R.; Altinkaya, H. Comparison of tree volume equations for brutian pine stands in Eğirdir district. Turk. J. For. 2019, 20, 149–156. [Google Scholar] [CrossRef]
- Jane, J.; Veronica, A.M.; Cynthia, C.; Nancy, B. Crop and Soil Responses to Using Corn Stover as a Bioenergy Feedstock: Observations from the Northern US Corn Belt. Agriculture 2013, 3, 72–89. [Google Scholar] [CrossRef]
- Aerts, R. Nitrogen Partitioning between Resorption and Decomposition Pathways: A Trade-Off between Nitrogen Use Efficiency and Litter Decomposibility? Oikos 1997, 80, 603–606. [Google Scholar] [CrossRef]
- Wang, D.J.; Yuan, F.; Xie, W.; Zuo, J.; Zhou, H. Effects of Leaf Size and Defensive Traits on the Contribution of Soil Fauna to Litter Decomposition. Forests 2024, 15, 481. [Google Scholar] [CrossRef]
- Rani, I.U.; Padmaja, G.; Rao, P.C. Integrated effect of organic manures and inorganic fertilizers on soil phosphatase activity and yield of maize-spinach cropping system. Agric. Sci. Dig.—A Res. J. 2014, 34, 31–35. [Google Scholar] [CrossRef]
- Zhou, J.; Miao, Y.; Guo, L.; Zhang, T.; Nie, Z.; Luo, X.; Yang, F.; Wang, Z. Evaluating the Enzyme Activities and Soil Physicochemical Properties of Four Typical Halophytic Communities in Saline-Sodic Soil. Agronomy 2024, 14, 141. [Google Scholar] [CrossRef]
- Khamzina, A.; Lamers, J.P.A.; Martius, C. Above- and belowground litter stocks and decay at a multi-species afforestation site on arid, saline soil. Nutr. Cycl. Agroecosystems 2016, 104, 187–199. [Google Scholar] [CrossRef]
- Zheng, Y.; Hu, Z.; Pan, X.; Chen, X.; Httenschwiler, S. Carbon and nitrogen transfer from litter to soil is higher in slow than rapid decomposing plant litter: A synthesis of stable isotope studies. Soil Biol. Biochem. 2021, 156, 108196. [Google Scholar] [CrossRef]
- Querejeta, J.I.; Schlaeppi, K.; Lopéz-García, L.; Ondoo, S.; Alguacil, M.D.M. Lower relative abundance of ectomycorrhizal fungi under a warmer and drier climate is linked to enhanced soil organic matter decomposition. New Phytol. 2021, 232, 1399–1413. [Google Scholar] [CrossRef]
- Glenn, E.P.; Morino, K.; Nagler, P.L.; Murray, R.S.; Pearlstein, S.; Hultine, K.R. Roles of saltcedar (Tamarix spp.) and capillary rise in salinizing a non-flooding terrace on a flow-regulated desert river. J. Arid. Environ. 2012, 79, 56–65. [Google Scholar] [CrossRef]
- Glenn, E.P.; Nagler, P.L.; Morino, K.; Hultine, K.R. Phreatophytes under stress: Transpiration and stomatal conductance of saltcedar (Tamarix spp.) in a high-salinity environment. Plant Soil 2013, 371, 655–672. [Google Scholar] [CrossRef]
- Sun, Q.; Jia, R.; Qin, J.; Wang, Y.; Lu, X.; Yang, P.; Bai, Y. Grassland management regimes regulate soil phosphorus fractions and conversion between phosphorus pools in semiarid steppe ecosystems. Biogeochemistry 2023, 163, 33–48. [Google Scholar] [CrossRef]
- Crews, T.E.; Kitayama, K.; Fownes, J.H.; Riley, R.H.; Herbert, D.A.; Mueller-Dombois, D.; Vitousek, P.M. Changes in Soil Phosphorus Fractions and Ecosystem Dynamics across a Long Chronosequence in Hawaii. Ecology 1995, 76, 1407–1424. [Google Scholar] [CrossRef]
- Qin, Z.-X.; Zhang, Y.-T.; Zhou, Z.-F.; Shi, X.-J.; Guo, T. Characteristics of Mineralization and Nitrification in Neutral Purple Paddy Soil from a Long-Term Fertilization Experiment. Entia Agric. Sin. 2013, 46, 3392–3400. [Google Scholar]
- Zhang, K.; Shi, Y.; Cui, X.; Yue, P.; Li, K.; Liu, X.; Tripathi, B.M.; Chu, H.; Lozupone, C. Salinity Is a Key Determinant for Soil Microbial Communities in a Desert Ecosystem. Msystems 2019, 4, 10–1128. [Google Scholar] [CrossRef]
- Ahsanul, M.I.; Masum, M.B.; Hanafi, M.I.; Hussin, W.M.R.W.; Bhuiyan, M.K.A.; Sukeri, M.S.B.M.; Kamal, A.H.M. Microbiota and soil fauna mediate litter decomposition and associated carbon and nitrogen dynamics in mangrove blue carbon ecosystems: Insights from a coastal lagoon in Malaysia. Hydrobiologia 2024, 851, 2469–2486. [Google Scholar]

| Habitats | Location | Dominant Species | |
|---|---|---|---|
| Longitude | Latitude | ||
| DF | 81°19′13″ | 36°56′84″ | P. euphratica + T. ramosissima + Glycyrrhiza inflata |
| SH | 80°57′4″ | 41°1′17″ | P. euphratica + T. ramosissima + Halostachys caspica |
| AH | 82°0′18″ | 40°41′6″ | P. euphratica + T. ramosissima + Alhagi sparsifolia |
| Indexes | DBH (cm) | Height of Populus euphratica (cm) | Crown Width (m) | Canopy | Ground Water (m) | pH | SOC (g/kg) | Total N (g/kg) | Total P (g/kg) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Habitats | E-W | N-S | |||||||||
| DF | 15.26 | 8.23 | 3.15 | 3.13 | 0.76 | --- | 8.39 ± 0.23 | 19.59 ± 7.00 | 1.19 ± 0.64 | 0.21 ± 0.05 | |
| SH | 42.99 | 10.56 | 8.02 | 7.62 | 0.53 | 2.23 | 8.66 ± 0.20 | 5.06 ± 1.67 | 1.39 ± 0.20 | 0.41 ± 0.02 | |
| AH | 45.02 | 11.43 | 10.84 | 7.31 | 0.46 | 5.78 | 7.88 ± 0.23 | 7.35 ± 0.38 | 1.22 ± 0.56 | 0.14 ± 0.08 | |
| Habitats | CO32− g/kg | Cl− g/kg | SO42− g/kg | Na+ g/kg | TS g/kg | ST °C | S-H % |
|---|---|---|---|---|---|---|---|
| AH | 0.01 ± 0.00 | 1.12 ± 0.65 | 23.59 ± 2.45 | 3.69 ± 2.00 | 24.34 ± 9.46 | 13.92 ± 13.83 | 1.48 ± 1.01 |
| (28.18%) a | (58.19%) a | (10.45%) | (54.23%) a | (38.85%) a | (99.32%) a | (67.91%) a | |
| SH | 0.02 ± 0.02 | 8.44 ± 9.19 | 17.82 ± 9.09 | 43.18 ± 24.18 | 72.91 ± 20.75 | 13.76 ± 9.80 | 14.01 ± 5.06 |
| (87.26%) b | (108.85%) b | (50.99%) | (55.99%) b | (28.46%) b | (71.24%) a | (36.15%) b | |
| DF | 0.01 ± 0.00 | 0.70 ± 0.24 | ---- | 2.48 ± 0.82 | 20.06 ± 7.82 | 11.56 ± 9.02 | 15.98 ± 4.81 |
| (10.12%) a | (34.78%) a | ---- | (33.03%) a | (38.97%) a | (78.05%) a | (30.10%) b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, X.; Chen, F.; Yang, J.; Zhou, Z.; Han, L.; Lyu, R. Decomposition of Foliar Litter from Dominant Plants of Desert Riparian Forests in Extremely Arid Regions. Forests 2024, 15, 949. https://doi.org/10.3390/f15060949
Jiang X, Chen F, Yang J, Zhou Z, Han L, Lyu R. Decomposition of Foliar Litter from Dominant Plants of Desert Riparian Forests in Extremely Arid Regions. Forests. 2024; 15(6):949. https://doi.org/10.3390/f15060949
Chicago/Turabian StyleJiang, Xuewei, Fei Chen, Jingjing Yang, Zhengli Zhou, Lu Han, and Ruiheng Lyu. 2024. "Decomposition of Foliar Litter from Dominant Plants of Desert Riparian Forests in Extremely Arid Regions" Forests 15, no. 6: 949. https://doi.org/10.3390/f15060949




