Determining Suitable Sampling Times for Soil CO2 and N2O Emissions Helps to Accurately Evaluate the Ability of Rubber-Based Agroforestry Systems to Cope with Climate Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Experimental Design
2.3. Data Collection
2.3.1. Greenhouse Gas Sampling and Analysis
2.3.2. Environmental Factors Measurement
2.4. Statistical Analysis
3. Results
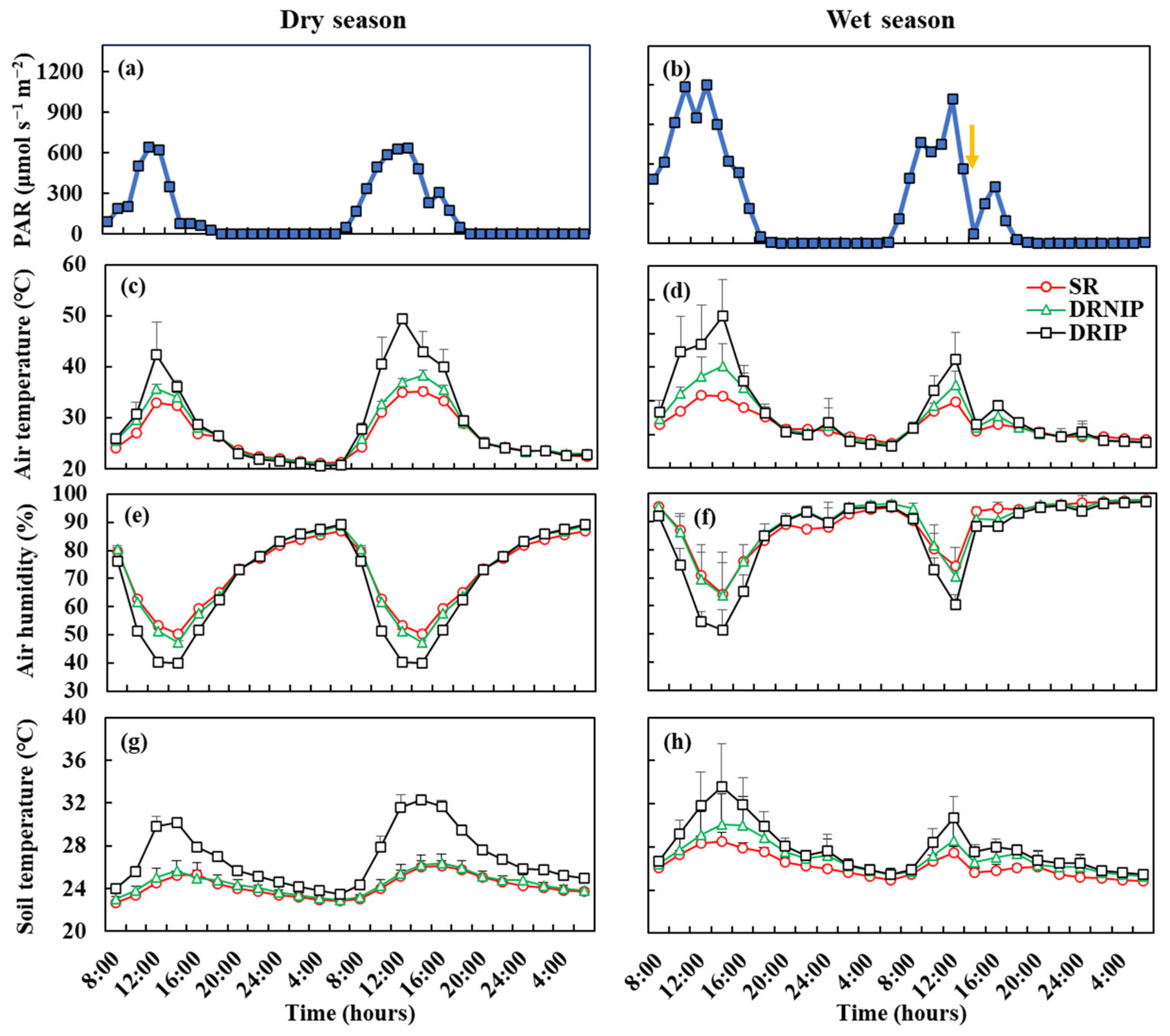
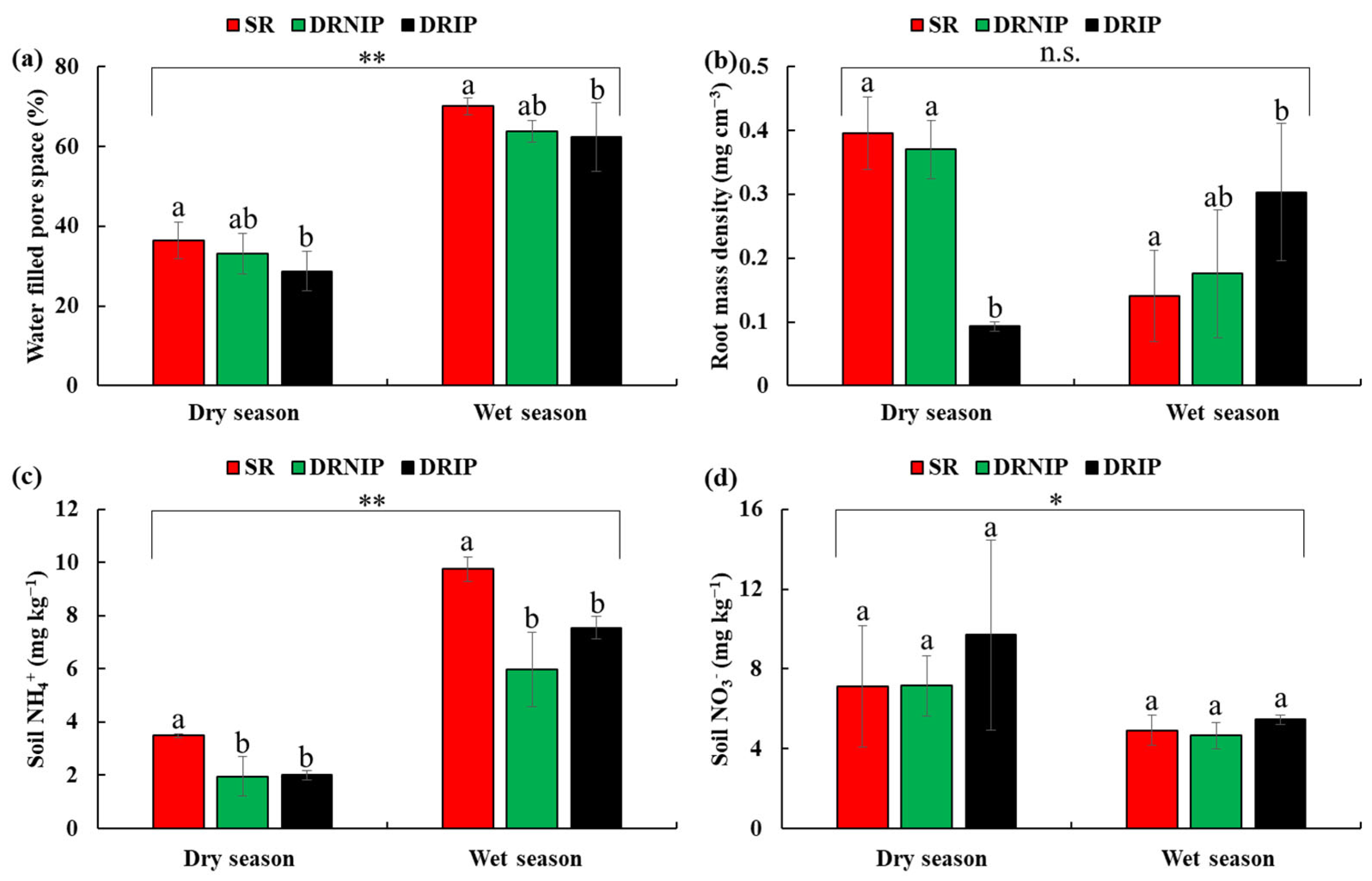
3.1. Environmental Factors
3.2. Diurnal Variations in Soil Greenhouse Gases
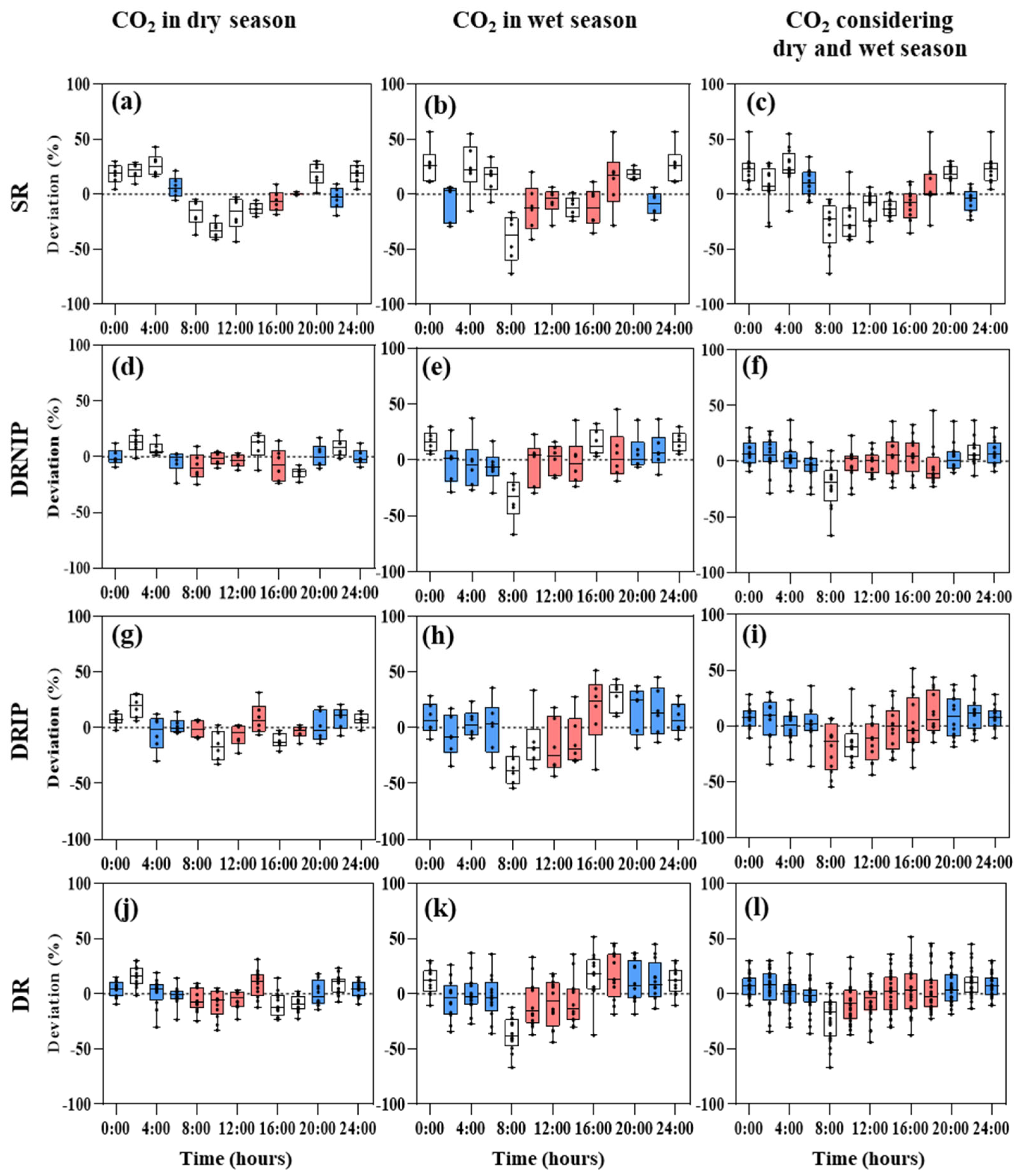
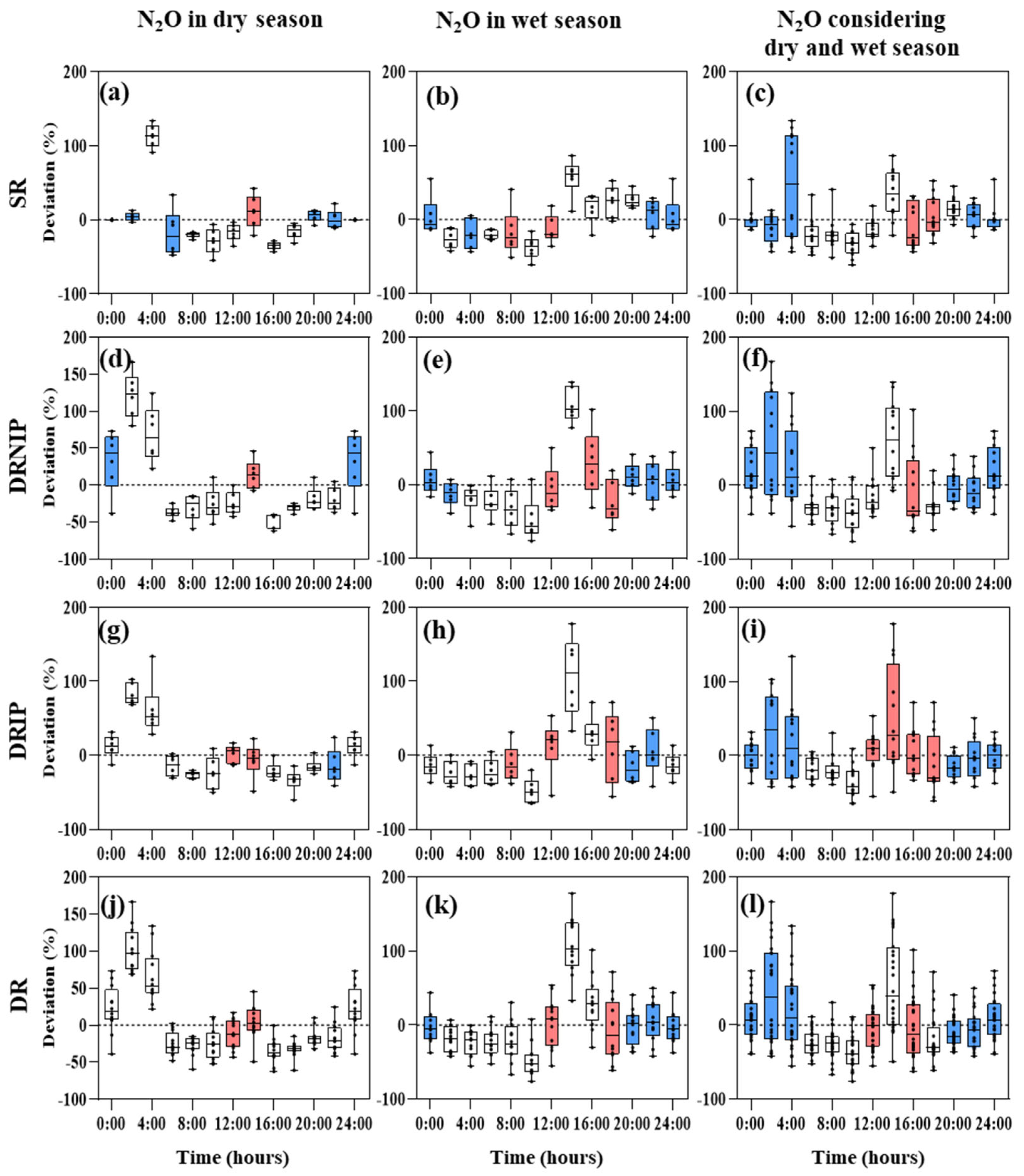
3.3. Suitable Soil Greenhouse Gas Sampling Times
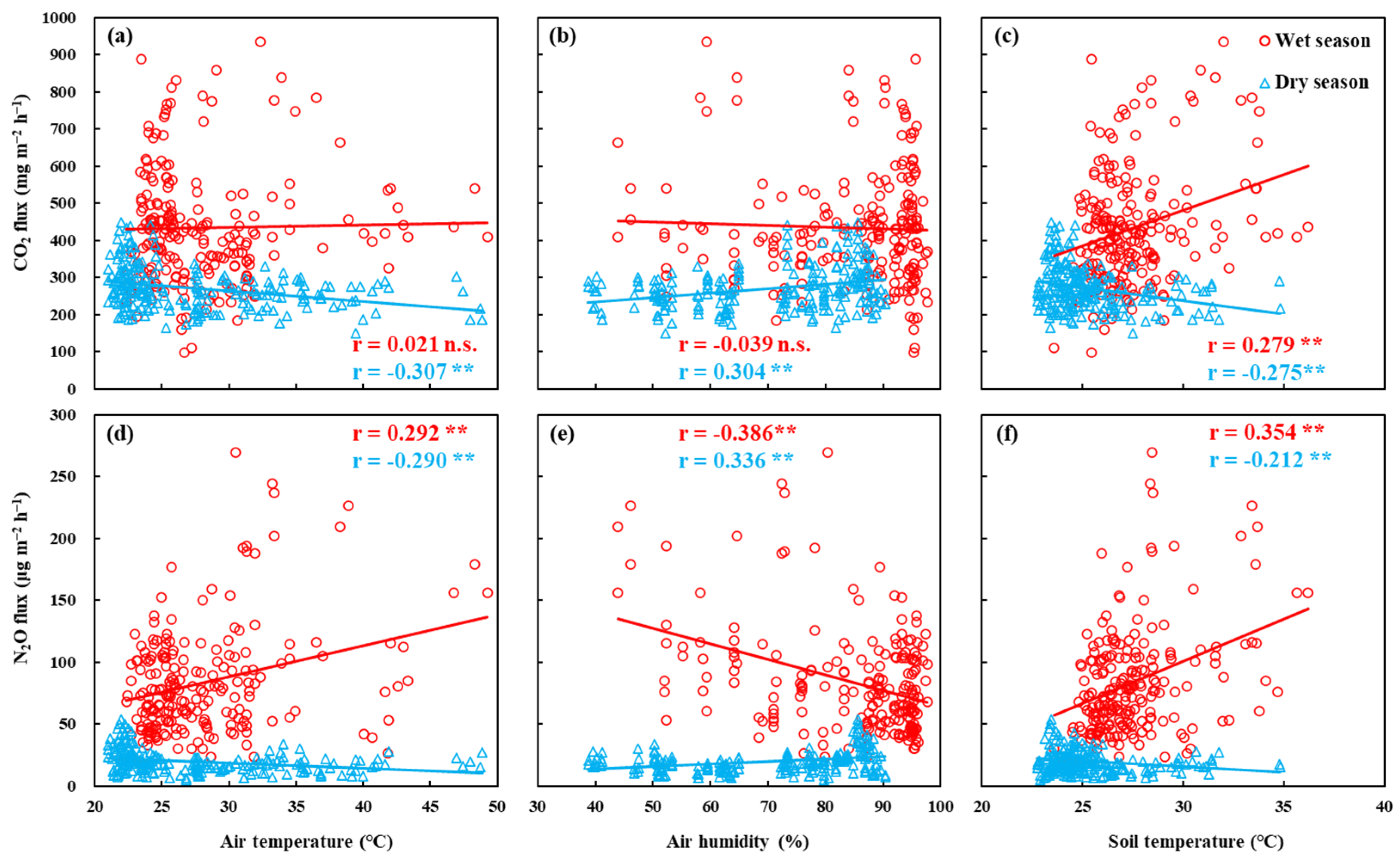
3.4. Correlations between Fluxes and Environmental Factors
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- AR6 Synthesis Report: Climate Change 2023. Available online: https://www.ipcc.ch/report/sixth-assessment-report-cycle/ (accessed on 31 December 2023).
- AR5 Synthesis Report: Climate Change 2014. Available online: https://www.ipcc.ch/report/ar5/syr/ (accessed on 31 December 2023).
- Lal, R. Farming systems to return land for nature: It’s all about soil health and re-carbonization of the terrestrial biosphere. Farm. Syst. 2023, 1, 100002. [Google Scholar] [CrossRef]
- Paustian, K.; Lehmann, J.; Ogle, S.; Reay, D.; Robertson, G.P.; Smith, P. Climate-smart soils. Nature 2016, 532, 49–57. [Google Scholar] [CrossRef]
- Tian, H.; Lu, C.; Ciais, P.; Michalak, A.M.; Canadell, J.G.; Saikawa, E.; Huntzinger, D.N.; Gurney, K.R.; Sitch, S.; Zhang, B.; et al. The terrestrial biosphere as a net source of greenhouse gases to the atmosphere. Nature 2016, 531, 225–228. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Kumar, A.; Kumar, M.; Bhat, J.A. Editorial: Greenhouse gas emissions and terrestrial ecosystems. Front. Environ. Sci. 2022, 10, 834444. [Google Scholar] [CrossRef]
- Singh, A.K.; Liu, W.; Zakari, S.; Wu, J.; Yang, B.; Jiang, X.J.; Zhu, X.; Zou, X.; Zhang, W.; Chen, C.; et al. A global review of rubber plantations: Impacts on ecosystem functions, mitigations, future directions, and policies for sustainable cultivation. Sci. Total Environ. 2021, 796, 148948. [Google Scholar] [CrossRef]
- Qi, D.; Wu, Z.; Yang, C.; Xie, G.; Li, Z.; Yang, X.; Li, D. Can intercropping with native trees enhance structural stability in young rubber (Hevea brasiliensis) agroforestry system? Eur. J. Agron. 2021, 130, 126353. [Google Scholar] [CrossRef]
- Chen, C.; Liu, W.; Wu, J.; Jiang, X.; Zhu, X. Can intercropping with the cash crop help improve the soil physico-chemical properties of rubber plantations? Geoderma 2019, 335, 149–160. [Google Scholar] [CrossRef]
- Rodrigo, V.H.L.; Silva, T.U.K.; Munasinghe, E.S. Improving the spatial arrangement of planting rubber (Hevea brasiliensis Muell. Arg.) For long-term intercropping. Field Crops Res. 2004, 89, 327–335. [Google Scholar] [CrossRef]
- Jessy, M.D.; Shankar, M.; Nair, N.U. A cropping system for reduction of gestation period and enhanced yield of rubber trees (Hevea brasiliensis). Rubber Sci. 2013, 26, 210–216. [Google Scholar]
- Oliosi, G.; Oliveira, M.G.; Partelli, F.L. Microclimate and development of black pepper intercropped with rubber tree. Agrofor. Syst. 2021, 95, 1635–1645. [Google Scholar] [CrossRef]
- FAOATAT. Available online: https://www.fao.org/faostat/en/#data (accessed on 31 December 2022).
- Zeng, X.; Cai, M.; Lin, W. Improving planting pattern for intercropping in the whole production span of rubber tree. Afr. J. Biotechnol. 2012, 11, 8484–8490. [Google Scholar] [CrossRef]
- Zhou, L.; Lin, W.; Zeng, X.; Cai, M.; Chen, J.; Huang, J.; Pan, J.; Zheng, D.; Zhou, J. Investigation on a new rubber based farming system in China. In Proceedings of the CRRI and IRRDB International Rubber Conference, Siem Reap, Cambodia, 24–25 November 2016. [Google Scholar]
- Huang, J.; Pan, J.; Zhou, L.; Zheng, D.; Yuan, S.; Chen, J.; Li, J.; Gui, Q.; Lin, W. An improved double-row rubber (Hevea brasiliensis) plantation system increases land use efficiency by allowing intercropping with yam bean, common bean, soybean, peanut, and coffee: A 17-year case study on Hainan island, China. J. Clean. Prod. 2020, 263, 121493. [Google Scholar] [CrossRef]
- Dong, T. Response of Intercropped Ficus Hirta Vahl Growth and Yield to Nitrogen Fertilization in Different Rubber Plantations; China Agricultural University: Beijing, China, 2023. (In Chinese) [Google Scholar]
- Rao, X.; Liu, C.; Tang, J.; Nie, Y.; Liang, M.; Shen, W.; Siddique, K.H.M. Rubber-leguminous shrub systems stimulate soil N2O but reduce CO2 and CH4 emissions. For. Ecol. Manag. 2021, 480, 118665. [Google Scholar] [CrossRef]
- Tang, X.; Liu, S.; Zhou, G.; Zhang, D.; Zhou, C. Soil-atmospheric exchange of CO2, CH4, and N2O in three subtropical forest ecosystems in southern China. Glob. Chang. Biol. 2006, 12, 546–560. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y. Quick measurement of CH4, CO2 and N2O emissions from a short-plant ecosystem. Adv. Atmos. Sci. 2003, 20, 842–844. [Google Scholar]
- Reeves, S.; Wang, W. Optimum sampling time and frequency for measuring N2O emissions from a rain-fed cereal cropping system. Sci. Total Environ. 2015, 530–531, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Alves, B.J.R.; Smith, K.A.; Flores, R.A.; Cardoso, A.S.; Oliveira, W.R.D.; Jantalia, C.P.; Urquiaga, S.; Boddey, R.M. Selection of the most suitable sampling time for static chambers for the estimation of daily mean N2O flux from soils. Soil. Biol. Biochem. 2012, 46, 129–135. [Google Scholar] [CrossRef]
- Reeves, S.; Wang, W.; Salter, B.; Halpin, N. Quantifying nitrous oxide emissions from sugarcane cropping systems: Optimum sampling time and frequency. Atmos. Environ. 2016, 136, 123–133. [Google Scholar] [CrossRef]
- Cueva, A.; Bullock, S.H.; López-Reyes, E.; Vargas, R. Potential bias of daily soil CO2 efflux estimates due to sampling time. Sci. Rep. 2017, 7, 11925. [Google Scholar] [CrossRef]
- Yang, H.; Liu, S.; Li, Y.; Xu, H. Diurnal variations and gap effects of soil CO2, N2O and CH4 fluxes in a typical tropical montane rainforest in Hainan island, China. Ecol. Res. 2018, 33, 379–392. [Google Scholar] [CrossRef]
- Wu, Y.; Whitaker, J.; Toet, S.; Bradley, A.; Davies, C.A.; Mcnamara, N.P. Diurnal variability in soil nitrous oxide emissions is a widespread phenomenon. Glob. Chang. Biol. 2021, 27, 4950–4966. [Google Scholar] [CrossRef] [PubMed]
- Lammirato, C.; Wallman, M.; Weslien, P.; Klemedtsson, L.; Rütting, T. Measuring frequency and accuracy of annual nitrous oxide emission estimates. Agric. For. Meteorol. 2021, 310, 108624. [Google Scholar] [CrossRef]
- Tian, S.; Ning, T.; Chi, S.; Wang, Y.; Wang, B.; Han, H.; Li, C.; Li, Z. Diurnal variations of the greenhouse gases emission and their optimal observation duration under different tillage systems. Acta Ecol. Sin. 2012, 32, 879–888. [Google Scholar] [CrossRef]
- Wang, X.; Feng, Y.; Yu, L.; Shu, Y.; Tan, F.; Gou, Y.; Luo, S.; Yang, W.; Li, Z.; Wang, J. Sugarcane/soybean intercropping with reduced nitrogen input improves crop productivity and reduces carbon footprint in China. Sci. Total Environ. 2020, 719, 137517. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Li, X.; Xu, L.; Wang, M.; Zhang, J.; Jiang, M.; Wang, G. Diurnal variation of soil CO2 efflux and its optimal measuring time-window of temperate meadow steppes in western Songnen plain, China. Chin. Geogr. Sci. 2020, 26, 518–526. [Google Scholar] [CrossRef]
- Liu, X.J.; Mosier, A.R.; Halvorson, A.D.; Reule, C.A.; Zhang, F.S. Dinitrogen and N2O emissions in arable soils: Effect of tillage, n source and soil moisture. Soil Biol. Biochem. 2007, 39, 2362–2370. [Google Scholar] [CrossRef]
- Savage, K.; Phillips, R.; Davidson, E. High temporal frequency measurements of greenhouse gas emissions from soils. Biogeosciences 2014, 11, 2709–2720. [Google Scholar] [CrossRef]
- Duan, B.; Cai, T.; Man, X.; Xiao, R.; Gao, M.; Ge, Z.; Mencuccini, M. Different variations in soil CO2, CH4, and N2O fluxes and their responses to edaphic factors along a boreal secondary forest successional trajectory. Sci. Total Environ. 2022, 838, 155983. [Google Scholar] [CrossRef]
- Jovani-Sancho, A.J.; O’Reilly, P.; Anshari, G.; Chong, X.Y.; Crout, N.; Evans, C.D.; Evers, S.; Gan, J.Y.; Gibbins, C.N.; Gusmayanti, E.; et al. CH4 and N2O emissions from smallholder agricultural systems on tropical peatlands in southeast Asia. Glob. Chang. Biol. 2023, 29, 4279–4297. [Google Scholar] [CrossRef]
- Ravi, S.; D’Odorico, P.; Over, T.M.; Zobeck, T.M. On the effect of air humidity on soil susceptibility to wind erosion: The case of air-dry soils. Geophys. Res. Lett. 2004, 31, L09501. [Google Scholar] [CrossRef]
- Lakhal, E.; Ayyoub, A. Effect of air relative humidity harvest on soil moisture content under moroccan climatic conditions. J. Eng. Res. Appl. 2015, 4, 74–82. [Google Scholar] [CrossRef]
- Metya, A.; Datye, A.; Chakraborty, S.; Tiwari, Y.K.; Sarma, D.; Bora, A.; Gogoi, N. Diurnal and seasonal variability of CO2 and CH4 concentration in a semi-urban environment of western India. Sci. Rep. 2021, 11, 2931. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Guan, L.; Chen, B.; Yang, C.; Lan, G.; Xie, G.; Zhou, Z. Components of soil respiration and its monthly dynamics in rubber plantation ecosystems. In Proceedings of the 2013 Fourth International Conference on Digital Manufacturing & Automation, Qindao, China, 29–30 June 2013; pp. 361–369. [Google Scholar] [CrossRef]
- Li, L.; Hao, Y.; Zheng, Z.; Wang, W.; Biederman, J.A.; Wang, Y.; Wen, F.; Qian, R.; Xu, C.; Zhang, B.; et al. Heavy rainfall in peak growing season had larger effects on soil nitrogen flux and pool than in the late season in a semiarid grassland. Agric. Ecosyst. Environ. 2022, 326, 107785. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, H.; Wang, Z.; Zhang, W.; Guo, C.; Wen, X.; Liu, Y. Optimizing manual sampling schedule for estimating annual soil CO2 efflux in a young exotic pine plantation in subtropical China. Eur. J. Soil. Biol. 2012, 52, 41–47. [Google Scholar] [CrossRef]
- Scheer, C.; Rowlings, D.W.; Firrel, M.; Deuter, P.; Morris, S.; Grace, P.R. Impact of nitrification inhibitor (DMPP) on soil nitrous oxide emissions from an intensive broccoli production system in sub-tropical Australia. Soil Biol. Biochem. 2014, 77, 243–251. [Google Scholar] [CrossRef]
- Savage, K.E.; Davidson, E.A. A comparison of manual and automated systems for soil CO2 flux measurements: Trade-offs between spatial and temporal resolution. J. Exp. Bot. 2003, 54, 891–899. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xian, Y.; Li, J.; Zhang, Y.; Shen, Y.; Wang, X.; Huang, J.; Sui, P. Determining Suitable Sampling Times for Soil CO2 and N2O Emissions Helps to Accurately Evaluate the Ability of Rubber-Based Agroforestry Systems to Cope with Climate Stress. Forests 2024, 15, 950. https://doi.org/10.3390/f15060950
Xian Y, Li J, Zhang Y, Shen Y, Wang X, Huang J, Sui P. Determining Suitable Sampling Times for Soil CO2 and N2O Emissions Helps to Accurately Evaluate the Ability of Rubber-Based Agroforestry Systems to Cope with Climate Stress. Forests. 2024; 15(6):950. https://doi.org/10.3390/f15060950
Chicago/Turabian StyleXian, Yuanran, Junlin Li, Yan Zhang, Yanyan Shen, Xiuquan Wang, Jianxiong Huang, and Peng Sui. 2024. "Determining Suitable Sampling Times for Soil CO2 and N2O Emissions Helps to Accurately Evaluate the Ability of Rubber-Based Agroforestry Systems to Cope with Climate Stress" Forests 15, no. 6: 950. https://doi.org/10.3390/f15060950



