The Relationship between Trait-Based Functional Niche Hypervolume and Community Phylogenetic Structures of Typical Forests across Different Climatic Zones in China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Plots and Species
2.3. Functional Traits
2.4. Phylogenetic Data Generation
2.5. Index
2.6. Data Analysis
3. Results
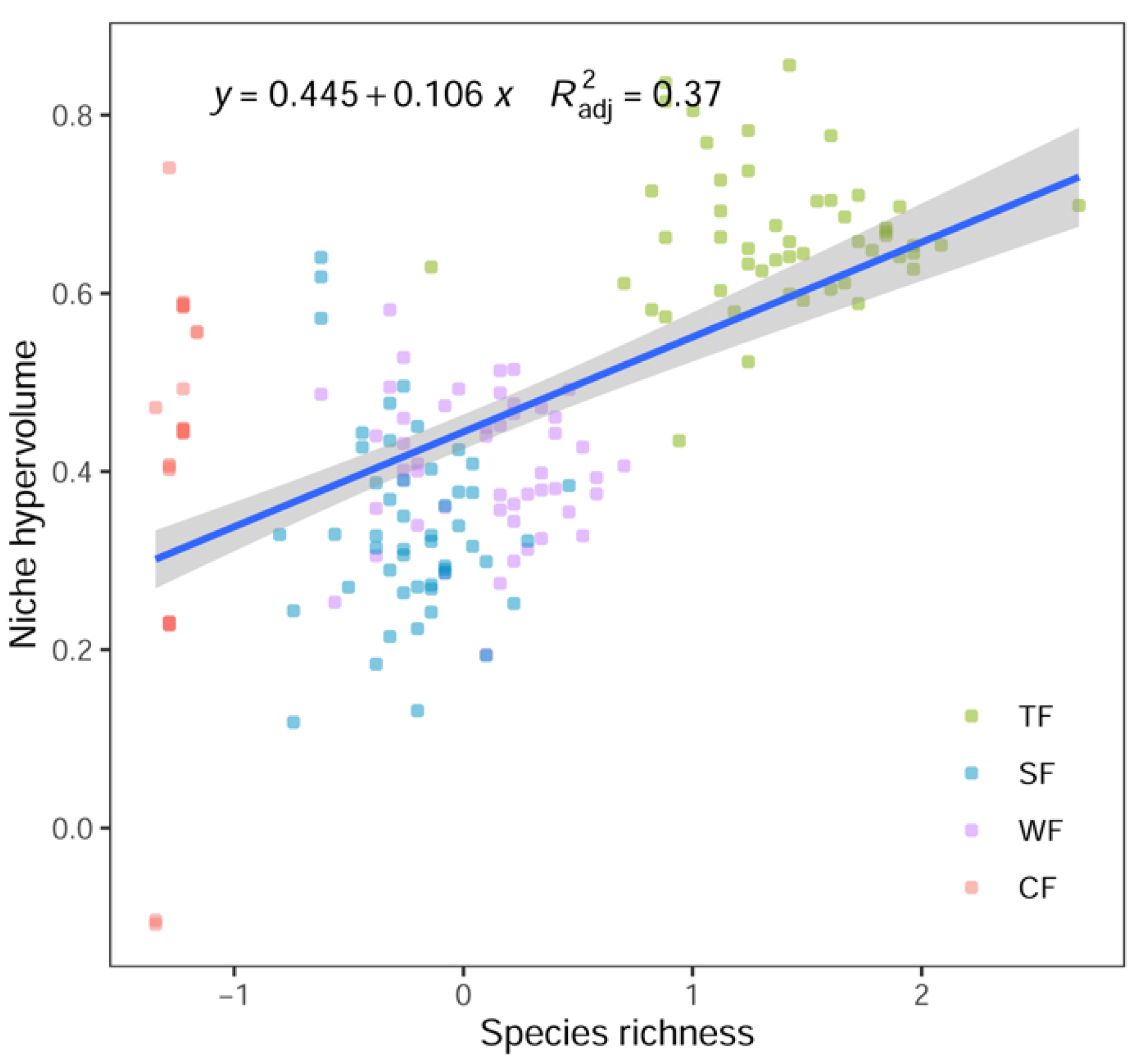
3.1. Relationship between Functional Niche Space and Community Structure Characteristics
3.2. Relationship between FNH of Woody Plants in Different Forest Vegetation Types and Community Phylogenetic Characteristics
4. Discussion
4.1. Community Structure Characteristics and Their Differences in Different Forest Vegetation Types
4.2. Effects of Community Structure Characteristics on the Geographical Differentiation of the Functional Niche Space
4.3. Diversity and Distinction among Different Forest Vegetation Communities
4.4. Effects of Community Phylogenetic Characteristics on the Geographical Differentiation of the Functional Niche of Woody Plants
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Violle, C.; Reich, P.B.; Pacala, S.W.; Enquist, B.J.; Kattge, J. The emergence and promise of functional biogeography. Proc. Natl. Acad. Sci. USA 2014, 111, 13690. [Google Scholar] [CrossRef] [PubMed]
- Petchey, O.L.; Gorman, E.J.O.; Dan, F.B.F. A Functional Guide to Functional Diversity Measures; Oxford University Press: Oxford, UK, 2009. [Google Scholar]
- Webb, C.T.; Hoeting, J.A.; Ames, G.M.; Pyne, M.I.; Leroy Poff, N. A structured and dynamic framework to advance traits-based theory and prediction in ecology. Ecol. Lett. 2010, 13, 267–283. [Google Scholar] [CrossRef]
- Violle, C.; Enquist, B.J.; McGill, B.J.; Jiang, L.; Albert, C.H.; Hulshof, C.; Jung, V.; Messier, J. The return of the variance: Intraspecific variability in community ecology. Trends Ecol. Evol. 2012, 27, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Lamanna, C.; Blonder, B.; Violle, C.; Kraft, N.J.B.; Sandel, B.; Šímová, I.; Donoghue, J.C.; Svenning, J.-C.; McGill, B.J.; Boyle, B.; et al. Functional trait space and the latitudinal diversity gradient. Proc. Natl. Acad. Sci. USA 2014, 111, 13745–13750. [Google Scholar] [CrossRef] [PubMed]
- Mouillot, D.; Graham, N.A.J.; Villéger, S.; Mason, N.W.H.; Bellwood, D.R. A functional approach reveals community responses to disturbances. Trends Ecol. Evol. 2013, 28, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Hallett, L.M.; Stein, C.; Suding, K.N. Functional diversity increases ecological stability in a grazed grassland. Oecologia 2017, 183, 831–840. [Google Scholar] [CrossRef]
- Spasojevic, M.J.; Grace, J.B.; Harrison, S.; Damschen, E.I. Functional diversity supports the physiological tolerance hypothesis for plant species richness along climatic gradients. J. Ecol. 2014, 102, 447–455. [Google Scholar] [CrossRef]
- Gherardi, L.A.; Sala, O.E. Enhanced interannual precipitation variability increases plant functional diversity that in turn ameliorates negative impact on productivity. Ecol. Lett. 2015, 18, 1293–1300. [Google Scholar] [CrossRef] [PubMed]
- Maynard, D.S.; Leonard, K.E.; Drake, J.M.; Hall, D.W.; Crowther, T.W.; Bradford, M.A. Modelling the multidimensional niche by linking functional traits to competitive performance. Proc. R. Soc. Biol. Sci. 2015, 282, 20150516. [Google Scholar] [CrossRef]
- Jiang, F.; Xun, Y.; Cai, H.; Jin, G. Functional traits can improve our understanding of niche- and dispersal-based processes. Oecologia 2018, 186, 783. [Google Scholar] [CrossRef]
- Blonder, B. Hypervolume concepts in niche- and trait-based ecology. Ecography 2018, 41, 1441–1455. [Google Scholar] [CrossRef]
- Li, Y.; Shipley, B.; Price, J.N.; Dantas, V.d.L.; Tamme, R.; Westoby, M.; Siefert, A.; Schamp, B.S.; Spasojevic, M.J.; Jung, V.; et al. Habitat filtering determines the functional niche occupancy of plant communities worldwide. J. Ecol. 2018, 106, 1001–1009. [Google Scholar] [CrossRef]
- Raffard, A.; Santoul, F.; Blanchet, S.; Cucherousset, J. Linking intraspecific variability in trophic and functional niches along an environmental gradient. Freshw. Biol. 2020, 65, 1401–1411. [Google Scholar] [CrossRef]
- Violle, C.; Jiang, L. Towards a trait-based quantification of species niche. J. Plant Ecol. 2009, 2, 87–93. [Google Scholar] [CrossRef]
- Lebrija-Trejos, E.; Pérez-García, E.A.; Meave, J.A.; Bongers, F.; Poorter, L. Functional traits and environmental filtering drive community assembly in a species-rich tropical system. Ecology 2010, 91, 386–398. [Google Scholar] [CrossRef] [PubMed]
- Blonder, B.; Lamanna, C.; Violle, C.; Enquist, B.J. The n-dimensional hypervolume. Glob. Ecol. Biogeogr. 2014, 23, 595–609. [Google Scholar] [CrossRef]
- Diaz, S.; Kattge, J.; Cornelissen, J.H.C.; Wright, I.J.; Lavorel, S.; Dray, S.; Reu, B.; Kleyer, M.; Wirth, C.; Colin Prentice, I.; et al. The global spectrum of plant form and function. Nature 2016, 529, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, X.; Sun, Z.; Bu, W.; Bongers, F.J.; Song, X.; Yang, J.; Sun, Z.; Li, Y.; Li, S.; et al. Functional trait space and redundancy of plant communities decrease toward cold temperature at high altitudes in Southwest China. Sci. China Life Sci. 2023, 66, 1674–7305. [Google Scholar] [CrossRef]
- Lei, L.; Kong, D.; Li, X.; Guo, Z.; Li, G. Plant functional traits, functional diversity, and ecosystem functioning: Current knowledge and perspectives. Biodivers. Sci. 2016, 24, 922–931. [Google Scholar] [CrossRef]
- Diaz, S.; Cabido, M.; Zak, M.; Martínez Carretero, E.; Araníbar, J. Plant functional traits, ecosystem structure and land-use history along a climatic gradient in central-western Argentina. J. Veg. Sci. 1999, 10, 651–660. [Google Scholar] [CrossRef]
- De Bello, F.; Lepš, J.; Sebastià, M.T. Variations in species and functional plant diversity along climatic and grazing gradients. Ecography 2006, 29, 801–810. [Google Scholar] [CrossRef]
- Diaz, S.; Cabido, M. Vive la différence: Plant functional diversity matters to ecosystem processes. Trends Ecol. Evol. 2001, 16, 646–655. [Google Scholar] [CrossRef]
- Atkinson, C.L.; Van Ee, B.C.; Pfeiffer, J.M. Evolutionary history drives aspects of stoichiometric niche variation and functional effects within a guild. Ecology 2020, 101, e03100. [Google Scholar] [CrossRef]
- Du, Y.; Mao, L.; Queenborough, S.A.; Freckleton, R.P.; Chen, B.; Ma, K. Phylogenetic constraints and trait correlates of flowering phenology in the angiosperm flora of China. Glob. Ecol. Biogeogr. 2015, 24, 928–938. [Google Scholar] [CrossRef]
- Wills, J.; Herbohn, J.; Wells, J.; Maranguit Moreno, M.O.; Ferraren, A.; Firn, J. Seedling diversity in actively and passively restored tropical forest understories. Ecol. Appl. 2021, 31, e02286. [Google Scholar] [CrossRef] [PubMed]
- Baraloto, C.; Hardy, O.J.; Paine, C.E.T.; Dexter, K.G.; Cruaud, C.; Dunning, L.T.; Gonzalez, M.-A.; Molino, J.-F.; Sabatier, D.; Savolainen, V.; et al. Using functional traits and phylogenetic trees to examine the assembly of tropical tree communities. J. Ecol. 2012, 100, 690–701. [Google Scholar] [CrossRef]
- Donovan, L.A.; Maherali, H.; Caruso, C.M.; Huber, H.; de Kroon, H. The evolution of the worldwide leaf economics spectrum. Trends Ecol. Evol. 2011, 26, 88–95. [Google Scholar] [CrossRef]
- Hu, X.; Chang-Yang, C.H.; Mi, X.; Du, Y.; Chang, Z. Influence of climate, phylogeny, and functional traits on flowering phenology in a subtropical evergreen broad-leaved forest, East China. Biodivers. Sci. 2015, 23, 601–609. [Google Scholar] [CrossRef]
- Wang, H.; Wang, R.; Harrison, S.P.; Prentice, I.C. Leaf morphological traits as adaptations to multiple climate gradients. J. Ecol. 2022, 110, 1344–1355. [Google Scholar] [CrossRef]
- Webb, C.O.; Ackerly, D.D.; McPeek, M.A.; Donoghue, M.J. Phylogenies and community ecology. Annu. Rev. Ecol. Syst. 2002, 33, 475–505. [Google Scholar] [CrossRef]
- Losos, J.B. Phylogenetic niche conservatism, phylogenetic signal and the relationship between phylogenetic relatedness and ecological similarity among species. Ecol. Lett. 2008, 11, 995–1003. [Google Scholar] [CrossRef] [PubMed]
- Winemiller, K.O.; Fitzgerald, D.B.; Bower, L.M.; Pianka, E.R. Functional traits, convergent evolution, and periodic tables of niches. Ecol. Lett. 2015, 18, 737–751. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.; Huang, J.; Xu, Y.; Ding, Y.; Zang, R. Plant functional niches in forests across four climatic zones: Exploring the periodic table of niches based on plant functional traits. Front. Plant Sci. 2020, 11, 00841. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Yu, R.; Zang, R. Differences in functional niche hypervolume among four types of forest vegetation and their environmental determinants across various climatic regions in China. Front. Plant Sci. 2023, 14, 1243209. [Google Scholar] [CrossRef] [PubMed]
- Investigation and Planning Institute of the Ministry of Forestry: Mountain forests in China; China Forestry Publishing House: Beijing, China, 1981.
- Xinjiang Forest Editorial Committee: Xinjiang Forest Xinjiang People’s Publishing House; China Forestry Publishing House: Urumqi/Beijing, China, 1989.
- Condit, R. Research in Large, Long-Term Tropical Forest Plots. Trends Ecol. Evol. 1995, 10, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Harguindeguy, N.; Diaz, S.; Garnier, E.; Lavorel, S.; Poorter, H.; Jaureguiberry, P.; Bret-Harte, M.S.; Cornwell, W.K.; Craine, J.M.; Gurvich, D.E.; et al. New handbook for standardised measurement of plant functional traits worldwide. Aust. J. Bot. 2013, 61, 167–234. [Google Scholar] [CrossRef]
- Laughlin, D.C.; Leppert, J.J.; Moore, M.M.; Sieg, C.H. A multi-trait test of the leaf-height-seed plant strategy scheme with 133 species from a pine forest flora. Funct. Ecol. 2010, 24, 493–501. [Google Scholar] [CrossRef]
- Mo, F.; Zhang, J.; Wang, J.; Cheng, Z.-G.; Sun, G.-J.; Ren, H.-X.; Zhao, X.-Z.; Cheruiyot, W.K.; Kavagi, L.; Wang, J.-Y.; et al. Phenological evidence from China to address rapid shifts in global flowering times with recent climate change. Agric. For. Meteorol. 2017, 246, 22–30. [Google Scholar] [CrossRef]
- Rafferty, N.E.; Nabity, P.D. A global test for phylogenetic signal in shifts in flowering time under climate change. J. Ecol. 2017, 105, 627–633. [Google Scholar] [CrossRef]
- R Core Team, T.R. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Garnier, E.; Navas, M.L.; Grigulis, K. Plant Functional Diversity: Organism Traits, Community Structure, and Ecosystem Properties; Oxford University Press: Oxford, UK, 2016. [Google Scholar]
- Brown, J.H. Why are there so many species in the tropics? J. Biogeogr. 2014, 41, 8–22. [Google Scholar] [CrossRef]
- Zang, R.; Yang, Y.; Jiang, Y. Community structure and tree species diversity characteristics in a tropical montane rain forest in Bawangling Nature Reserve, Hainan Island. Acta Phytoecol. Sin. 2001, 25, 270–275. [Google Scholar]
- Aros-Mualin, D.; Noben, S.; Karger, D.N.; Carvajal-Hernández, C.I.; Kessler, M. Functional diversity in Ferns is driven by species richness rather than by environmental constraints. Front. Plant Sci. 2021, 11, 615723. [Google Scholar] [CrossRef] [PubMed]
- Kraft, N.J.B.; Godoy; Levine, J.M. Plant functional traits and the multidimensional nature of species coexistence. Proc. Natl. Acad. Sci. USA 2015, 112, 797–802. [Google Scholar] [CrossRef]
- Gauzere, P.; Morin, X.; Violle, C.; Caspeta, I.; Ray, C.; Blonder, B. Vacant yet invasible niches in forest community assembly. Funct. Ecol. 2020, 34, 1945–1955. [Google Scholar] [CrossRef]
- Mayfield, M.M.; Boni, M.F.; Daily, G.C.; Ackerly, D. Species and functional diversity of native and human-dominated plant communities. Ecology 2005, 86, 2365–2372. [Google Scholar] [CrossRef]
- Biswas, S.R.; Mallik, A.U. Disturbance effects on species diversity and functional diversity in riparian and upland plant communities. Ecology 2010, 91, 28–35. [Google Scholar] [CrossRef]
- Huang, L.; Xue, W.; Herben, T. Temporal niche differentiation among species changes with habitat productivity and light conditions. J. Veg. Sci. 2019, 30, 438–447. [Google Scholar] [CrossRef]
- Sapijanskas, J.; Paquette, A.; Potvin, C.; Kunert, N.; Loreau, M. Tropical tree diversity enhances light capture through crown plasticity and spatial and temporal niche differences. Ecology 2014, 95, 2479–2492. [Google Scholar] [CrossRef]
- Silvertown, J. Plant coexistence and the niche. Trends Ecol. Evol. 2004, 19, 605–611. [Google Scholar] [CrossRef]
- Tao, S.L.; Guo, Q.H.; Li, C.; Wang, Z.H.; Fang, J.Y. Global patterns and determinants of forest canopy height. Ecology 2016, 97, 3265. [Google Scholar] [CrossRef]
- Cadotte, M.W.; Dinnage, R.; Tilman, D. Phylogenetic diversity promotes ecosystem stability. Ecology 2012, 93, S223–S233. [Google Scholar] [CrossRef]
- Flynn, D.F.B.; Mirotchnick, N.; Jain, M.; Palmer, M.I.; Naeem, S. Functional and phylogenetic diversity as predictors of biodiversity-ecosystem-function relationships. Ecology 2011, 92, 1573–1581. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Liu, X.; Cadotte, M.W.; Zhou, S. Functional and phylogenetic diversity explain different components of diversity effects on biomass production. Oikos 2020, 129, 1185–1195. [Google Scholar] [CrossRef]
- Nagalingum, N.S.; Knerr, N.; Laffan, S.W.; González-Orozco, C.E.; Thornhill, A.H.; Miller, J.T.; Mishler, B.D. Continental scale patterns and predictors of fern richness and phylogenetic diversity. Front. Genet. 2015, 6, 132. [Google Scholar] [CrossRef]
- Qian, H.; Jin, Y.; Ricklefs, R.E. Phylogenetic diversity anomaly in angiosperms between eastern Asia and eastern North America. Proc. Natl. Acad. Sci. USA 2017, 114, 11452–11457. [Google Scholar] [CrossRef] [PubMed]
- Weigelt, P.; Daniel Kissling, W.; Kisel, Y.; Fritz, S.A.; Karger, D.N.; Kessler, M.; Lehtonen, S.; Svenning, J.-C.; Kreft, H. Global patterns and drivers of phylogenetic structure in island floras. Sci. Rep. 2015, 5, 12213. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zang, R. Tropical forests are vulnerable in terms of functional redundancy. Biol. Conserv. 2021, 262, 109326. [Google Scholar] [CrossRef]
- Myers, J.A.; Chase, J.M.; Jiménez, I.; Jørgensen, P.M.; Araujo-Murakami, A.; Paniagua-Zambrana, N.; Seidel, R. Beta-diversity in temperate and tropical forests reflects dissimilar mechanisms of community assembly. Ecol. Lett. 2013, 16, 151–157. [Google Scholar] [CrossRef]
- Yao, L.; Ding, Y.; Xu, H.; Deng, F.; Yao, L.; Ai, X.; Zang, R. Patterns of diversity change for forest vegetation across different climatic regions—A compound habitat gradient analysis approach. Glob. Ecol. Conserv. 2020, 23, e01106. [Google Scholar] [CrossRef]


| Niche Hypervolume | Abundance | Richness | MPH | Area_DBH | |
|---|---|---|---|---|---|
| Abundance | 0.053 | 1 | |||
| Richness | 0.610 *** | 0.581 *** | 1 | ||
| MPH | −0.118 | −0.800 *** | −0.578 *** | 1 | |
| Area_DBH | 0.029 | −0.766 *** | −0.410 *** | 0.971 *** | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, J.; Yu, R.; Ding, Y.; Xu, Y.; Yao, J.; Zang, R. The Relationship between Trait-Based Functional Niche Hypervolume and Community Phylogenetic Structures of Typical Forests across Different Climatic Zones in China. Forests 2024, 15, 954. https://doi.org/10.3390/f15060954
Huang J, Yu R, Ding Y, Xu Y, Yao J, Zang R. The Relationship between Trait-Based Functional Niche Hypervolume and Community Phylogenetic Structures of Typical Forests across Different Climatic Zones in China. Forests. 2024; 15(6):954. https://doi.org/10.3390/f15060954
Chicago/Turabian StyleHuang, Jihong, Ruoyun Yu, Yi Ding, Yue Xu, Jie Yao, and Runguo Zang. 2024. "The Relationship between Trait-Based Functional Niche Hypervolume and Community Phylogenetic Structures of Typical Forests across Different Climatic Zones in China" Forests 15, no. 6: 954. https://doi.org/10.3390/f15060954






