Functional Segregation of Resource Utilization Strategies between Invasive and Native Plants and Invasion Mechanisms in the Water Level Fluctuation Zone: A Case Study of Pengxi River in Three Gorges Reservoir, China
Abstract
1. Introduction
2. Materials and Methods
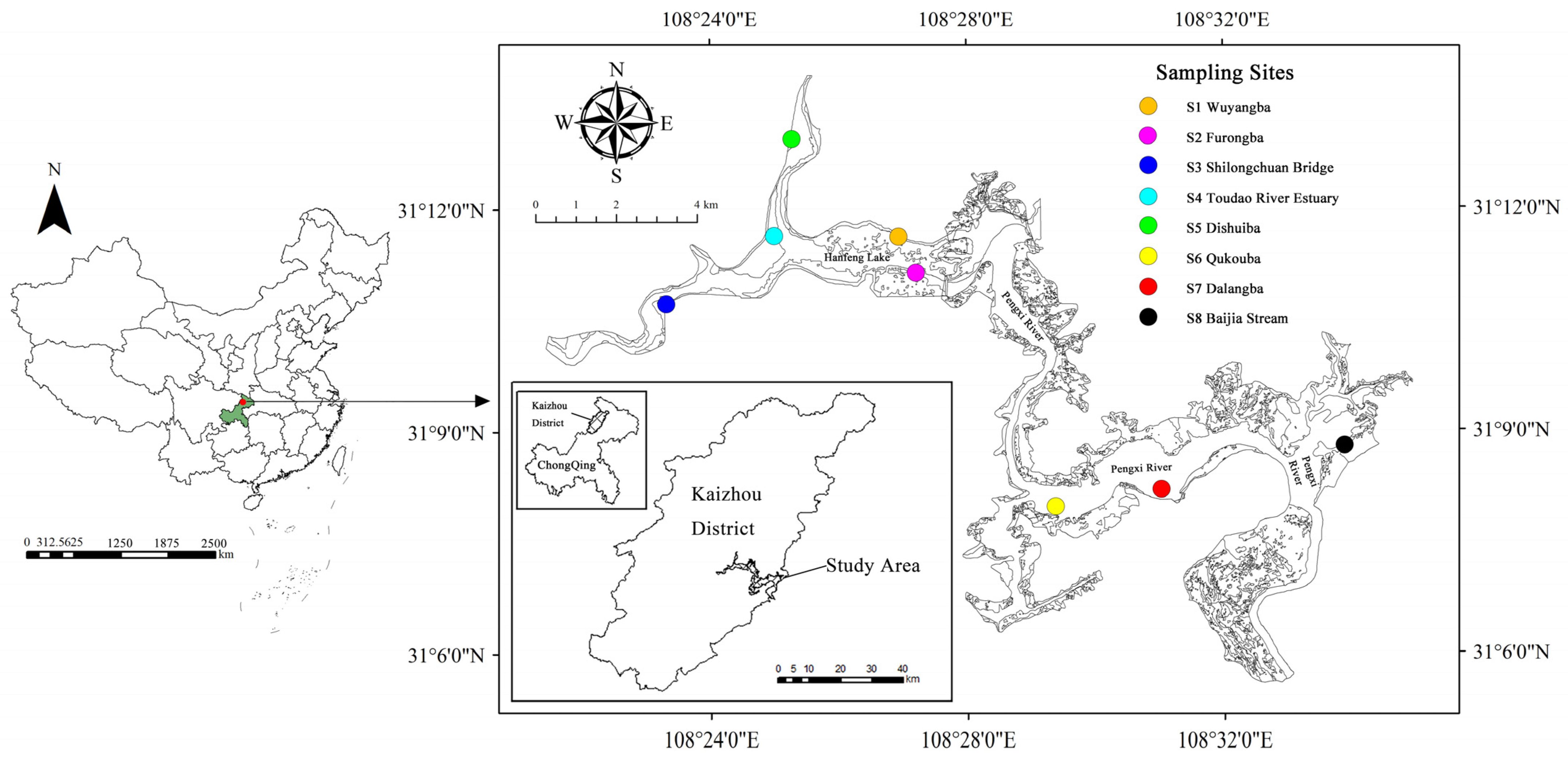
2.1. Study Area
2.2. Community Surveys and Identification of IP
2.3. Sampling and Measurement of Plant Functional Traits
2.4. Statistical Analysis
3. Results
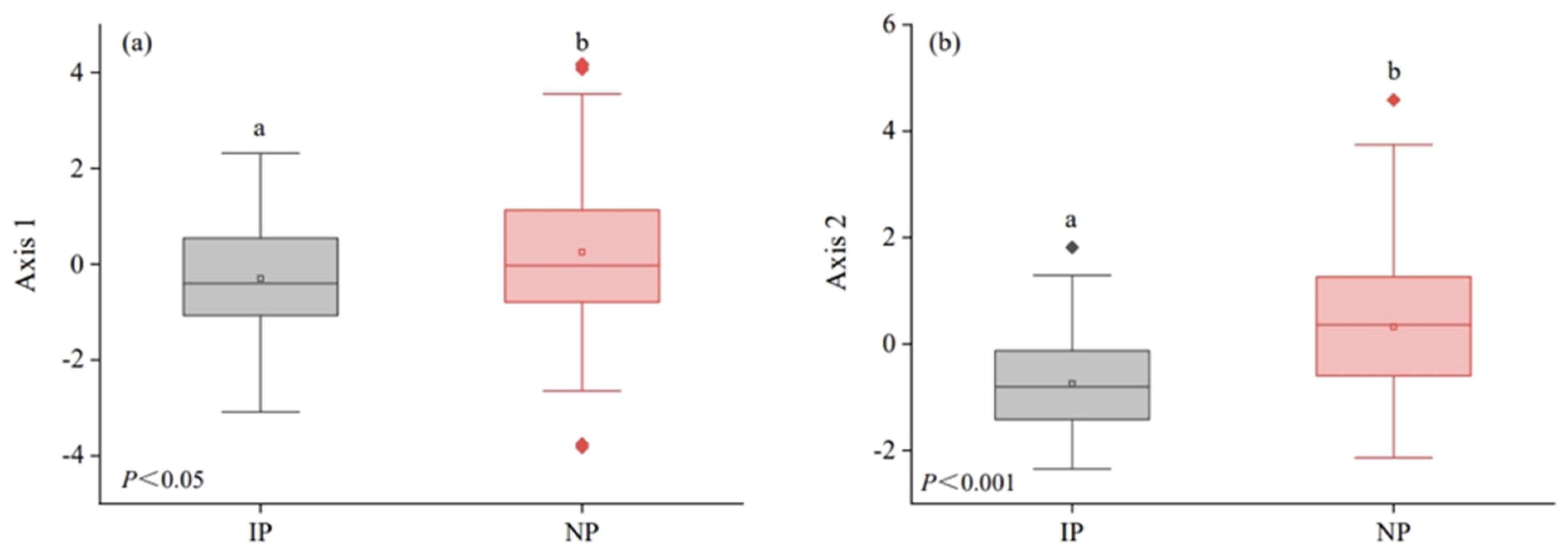
3.1. Differences in Plant Functional Strategies at the Regional Scale
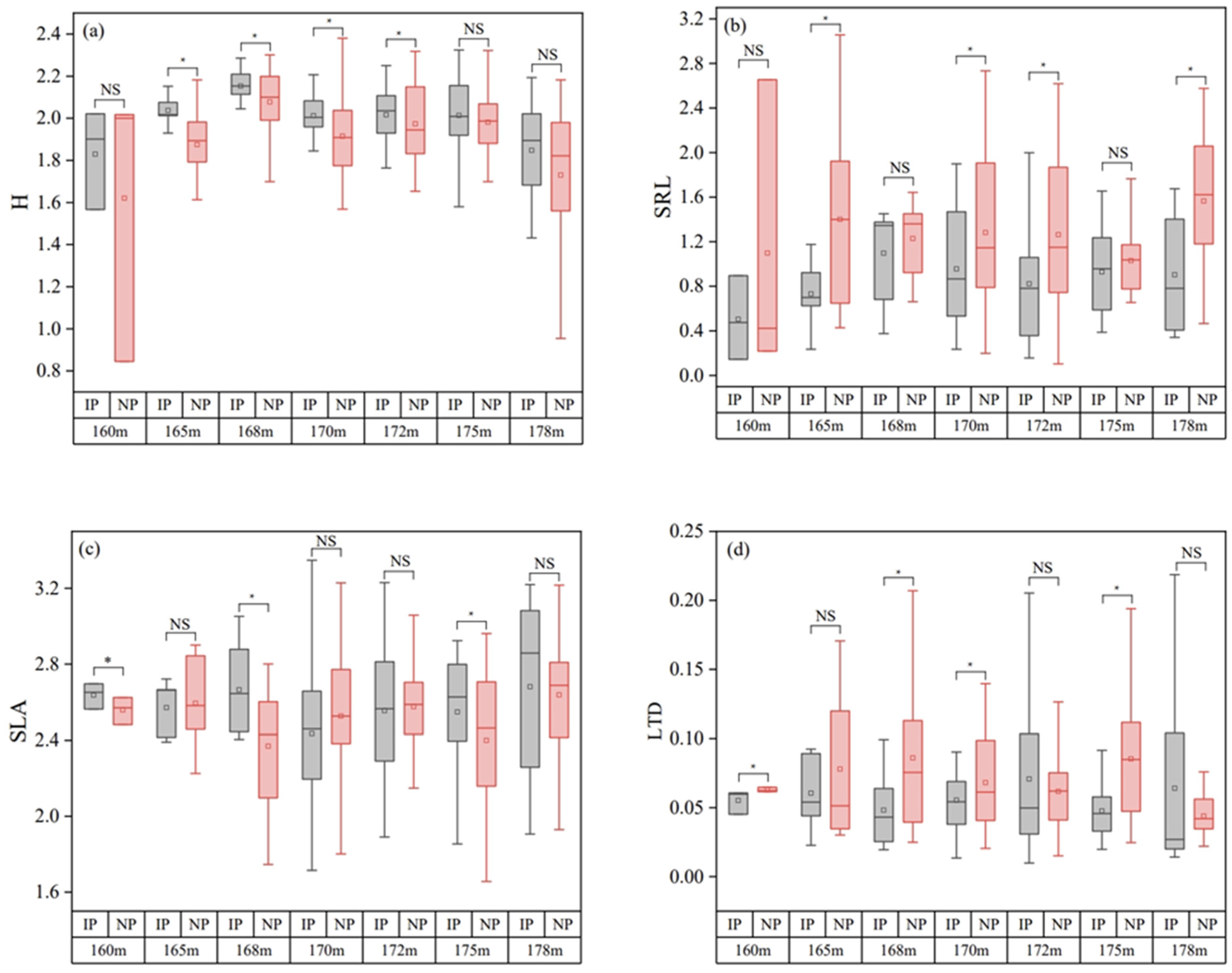
3.2. Differences in Plant Functional Traits at the Regional Scale
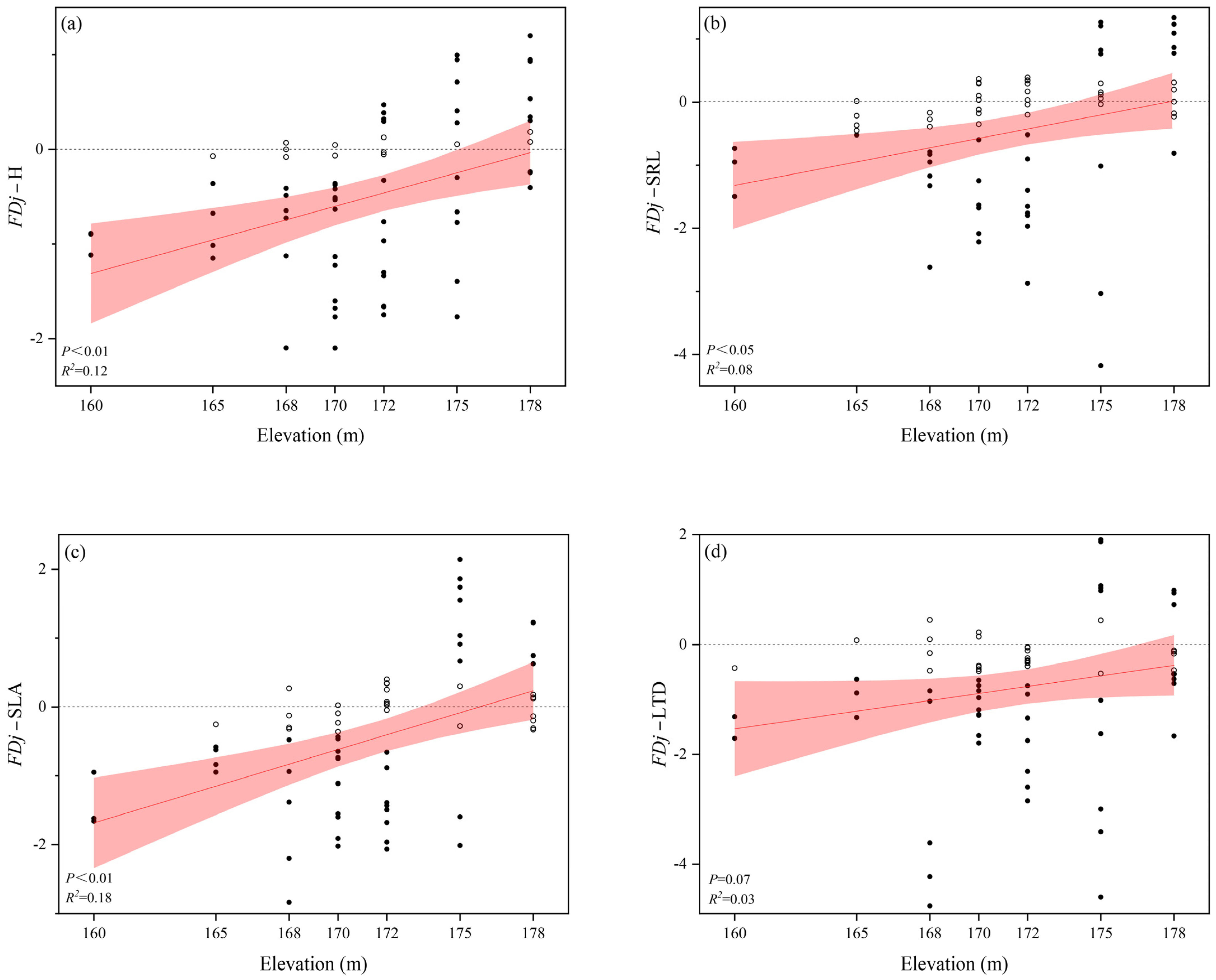
3.3. Response of Functional Traits at the Community Scale to Elevation
3.4. Functional Differences at the Community Scale
4. Discussion
4.1. Spatial Differentiation of IP Species in the WLF Zone
4.2. The Invasion Mechanism of IP in the WLF Zone
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Korpelainen, H.; Pietilainen, M. What Makes a Good Plant Invader? Life 2023, 13, 1596. [Google Scholar] [CrossRef] [PubMed]
- Munro, D.; Steer, J.; Linklater, W. On allegations of invasive species denialism. Conserv. Biol. 2019, 33, 797–802. [Google Scholar] [CrossRef]
- Simberloff, D.; Martin, J.-L.; Genovesi, P.; Maris, V.; Wardle, D.A.; Aronson, J.; Courchamp, F.; Galil, B.; García-Berthou, E.; Pascal, M.; et al. Impacts of biological invasions: What’s what and the way forward. Trends Ecol. Evol. 2013, 28, 58–66. [Google Scholar] [CrossRef]
- Lin, W.; Zhou, G.; Cheng, X.; Xu, R. Fast economic development accelerates biological invasions in China. PLoS ONE 2007, 2, e1208. [Google Scholar] [CrossRef]
- Seebens, H.; Blackburn, T.M.; Dyer, E.E.; Genovesi, P.; Hulme, P.E.; Jeschke, J.M.; Pagad, S.; Pysek, P.; Winter, M.; Arianoutsou, M.; et al. No saturation in the accumulation of alien species worldwide. Nat. Commun. 2017, 8, 14435. [Google Scholar] [CrossRef] [PubMed]
- Violle, C.; Navas, M.-L.; Vile, D.; Kazakou, E.; Fortunel, C.; Hummel, I.; Garnier, E. Let the concept of trait be functional! Oikos 2007, 116, 882–892. [Google Scholar] [CrossRef]
- De Bello, F.; Lavorel, S.; Díaz, S.; Harrington, R.; Cornelissen, J.H.C.; Bardgett, R.D.; Berg, M.P.; Cipriotti, P.; Feld, C.K.; Hering, D.; et al. Towards an assessment of multiple ecosystem processes and services via functional traits. Biodivers. Conserv. 2010, 19, 2873–2893. [Google Scholar] [CrossRef]
- Sharma, P.; Rathee, S.; Ahmad, M.; Siddiqui, M.H.; Alamri, S.; Kaur, S.; Kohli, R.K.; Singh, H.P.; Batish, D.R. Leaf functional traits and resource use strategies facilitate the spread of invasive plant Parthenium hysterophorus across an elevational gradient in western Himalayas. BMC Plant Biol. 2024, 24, 234. [Google Scholar] [CrossRef]
- Hulme, P.E.; Bernard-Verdier, M.; Baltzer, J. Comparing traits of native and alien plants: Can we do better? Funct. Ecol. 2017, 32, 117–125. [Google Scholar] [CrossRef]
- Mathakutha, R.; Steyn, C.; le Roux, P.C.; Blom, I.J.; Chown, S.L.; Daru, B.H.; Ripley, B.S.; Louw, A.; Greve, M.; Price, J. Invasive species differ in key functional traits from native and non-invasive alien plant species. J. Veg. Sci. 2019, 30, 994–1006. [Google Scholar] [CrossRef]
- Guisan, A.; Petitpierre, B.; Broennimann, O.; Daehler, C.; Kueffer, C. Unifying niche shift studies: Insights from biological invasions. Trends Ecol. Evol. 2014, 29, 260–269. [Google Scholar] [CrossRef]
- Strayer, D.L. Eight questions about invasions and ecosystem functioning. Ecol. Lett. 2012, 15, 1199–1210. [Google Scholar] [CrossRef] [PubMed]
- Bezeng, B.S.; Savolainen, V.; Yessoufou, K.; Papadopulos, A.S.T.; Maurin, O.; Van der Bank, M. A phylogenetic approach towards understanding the drivers of plant invasiveness on Robben Island, South Africa. Bot. J. Linn. Soc. 2013, 172, 142–152. [Google Scholar] [CrossRef]
- Lososová, Z.; de Bello, F.; Chytrý, M.; Kühn, I.; Pyšek, P.; Sádlo, J.; Winter, M.; Zelený, D. Alien plants invade more phylogenetically clustered community types and cause even stronger clustering. Glob. Ecol. Biogeogr. 2015, 24, 786–794. [Google Scholar] [CrossRef]
- Castro, S.A.; Escobedo, V.M.; Aranda, J.; Carvallo, G.O. Evaluating Darwin’s naturalization hypothesis in experimental plant assemblages: Phylogenetic relationships do not determine colonization success. PLoS ONE 2014, 9, e105535. [Google Scholar] [CrossRef]
- Darwin, C. The Origin of Species by Means of Natural Selection, 6th ed.; J.M. Dent & Sons Ltd.: London, UK, 1872. [Google Scholar]
- Strauss, S.Y.; Webb, C.O.; Salamin, N. Exotic taxa less related to native species are more invasive. Proc. Natl. Acad. Sci. USA 2006, 103, 5841–5845. [Google Scholar] [CrossRef] [PubMed]
- Daehler, C.C. Darwin’s naturalization hypothesis revisited. Am. Nat. 2001, 158, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Park, D.S.; Feng, X.; Maitner, B.S.; Ernst, K.C.; Enquist, B.J. Darwin’s naturalization conundrum can be explained by spatial scale. Proc. Natl. Acad. Sci. USA 2020, 117, 10904–10910. [Google Scholar] [CrossRef] [PubMed]
- Thuiller, W.; Gallien, L.; Boulangeat, I.; De Bello, F.; Münkemüller, T.; Roquet, C.; Lavergne, S. Resolving Darwin’s naturalization conundrum: A quest for evidence. Divers. Distrib. 2010, 16, 461–475. [Google Scholar] [CrossRef]
- Carboni, M.; Munkemuller, T.; Gallien, L.; Lavergne, S.; Acosta, A.; Thuiller, W. Darwin’s naturalization hypothesis: Scale matters in coastal plant communities. Ecography 2013, 36, 560–568. [Google Scholar] [CrossRef]
- Leffler, A.J.; James, J.J.; Monaco, T.A.; Sheley, R.L. A new perspective on trait differences between native and invasive exotic plants: Reply. Ecology 2015, 96, 1152–1153. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yu, Y.; Cheng, H.; Du, D. Which factor contributes most to the invasion resistance of native plant communities under the co-invasion of two invasive plant species? Sci. Total Environ. 2022, 813, 152628. [Google Scholar] [CrossRef]
- van Kleunen, M.; Weber, E.; Fischer, M. A meta-analysis of trait differences between invasive and non-invasive plant species. Ecol. Lett. 2010, 13, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Chiu, J.H.; Chong, K.Y.; Lum, S.K.Y.; Wardle, D.A. Trends in the direction of global plant invasion biology research over the past two decades. Ecol. Evol. 2023, 13, e9690. [Google Scholar] [CrossRef]
- Yuan, X.Z.; Zhang, Y.W.; Liu, H.; Xiong, S.; Li, B.; Deng, W. The littoral zone in the Three Gorges Reservoir, China: Challenges and opportunities. Environ. Sci. Pollut. Res. Int. 2013, 20, 7092–7102. [Google Scholar] [CrossRef]
- Sun, R.; Deng, W.q.; Yuan, X.z.; Liu, H.; Zhang, Y.w. Riparian vegetation after dam construction on mountain rivers in China. Ecohydrology 2013, 7, 1187–1195. [Google Scholar] [CrossRef]
- Li, H.; Yu, K.; Ratajczak, Z.; Nippert, J.B.; Tondrob, D.; Xu, D.; Li, W.; Du, G. When variability outperforms the mean: Trait plasticity predicts plant cover and biomass in an alpine wetland. Plant Soil 2016, 407, 401–415. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, L.; Shen, W. Dramatic altitudinal variations in leaf mass per area of two plant growth forms at extreme heights. Ecol. Indic. 2020, 110, 105890. [Google Scholar] [CrossRef]
- Kepa Brian Morgan, T.K.; Sardelic, D.N.; Waretini, A.F. The Three Gorges Project: How sustainable? J. Hydrol. 2012, 460–461, 1–12. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Ma, M.; Ding, Z.; Wu, S.; Jia, W.; Chen, Q.; Yi, X.; Zhang, J.; Li, X.; et al. Dam-induced difference of invasive plant species distribution along the riparian habitats. Sci. Total Environ. 2022, 808, 152103. [Google Scholar] [CrossRef]
- Xiong, W.; Wang, H.; Wang, Q.; Tang, J.F.; Bowler, P.A.; Xie, D.; Pan, L.; Wang, Z.X. Non-native species in the Three Gorges Dam Reservoir: Status and risks. Bioinvasions Rec. 2018, 7, 153–158. [Google Scholar] [CrossRef]
- Wen, Y.; Su, X.; Cai, F.; Qian, R.; Bejarano, M.D.; Wu, S.; Yang, Q.; Liu, X.; Zeng, B. The differences in plant invasion in two types of shorelines under flow regulation of the Three Gorges Reservoir. Sci. Total Environ. 2024, 912, 168892. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Gao, P.; He, X. The water-level fluctuation zone of Three Gorges Reservoir—A unique geomorphological unit. Earth-Sci. Rev. 2015, 150, 14–24. [Google Scholar] [CrossRef]
- Wang, C.; Wei, M.; Wang, S.; Wu, B.; Cheng, H. Erigeron annuus (L.) Pers. and Solidago canadensis L. antagonistically affect community stability and community invasibility under the co-invasion condition. Sci. Total Environ. 2020, 716, 137128. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Chu, P.; Chen, D.; Bai, Y.; Niu, S. Functional correlations between specific leaf area and specific root length along a regional environmental gradient in Inner Mongolia grasslands. Funct. Ecol. 2016, 30, 985–997. [Google Scholar] [CrossRef]
- Mazurczyk, T.; Brooks, R.P. Native biodiversity increases with rising plant invasions in temperate, freshwater wetlands. Wetl. Ecol. Manag. 2021, 30, 139–160. [Google Scholar] [CrossRef]
- Wang, F.; Huang, J.; Zhang, N.; Li, Y.; He, S.; Wen, J.; Yin, L.; Liang, Y. Exploring plant characteristics for constructing a pre-border weed risk assessment for China. Biol. Invasions 2024, 26, 909–933. [Google Scholar] [CrossRef]
- Gross, N.; Suding, K.N.; Lavorel, S. Leaf dry matter content and lateral spread predict response to land use change for six subalpine grassland species. J. Veg. Sci. 2007, 18, 289–300. [Google Scholar] [CrossRef]
- Maire, V.; Gross, N.; Borger, L.; Proulx, R.; Wirth, C.; Pontes, L.D.S.; Soussana, J.F.; Louault, F. Habitat filtering and niche differentiation jointly explain species relative abundance within grassland communities along fertility and disturbance gradients. New Phytol. 2012, 196, 497–509. [Google Scholar] [CrossRef]
- Cornelissen, J.H.C.; Lavorel, S.; Garnier, E.; Diaz, S.; Buchmann, N.; Gurvich, D.E.; Reich, P.B.; ter Steege, H.; Morgan, H.D.; van der Heijden, M.G.A.; et al. A handbook of protocols for standardised and easy measurement of plant functional traits worldwide. Aust. J. Bot. 2003, 51, 335–380. [Google Scholar] [CrossRef]
- Gross, N.; Börger, L.; Duncan, R.P.; Hulme, P.E.; Wilson, S. Functional differences between alien and native species: Do biotic interactions determine the functional structure of highly invaded grasslands? Funct. Ecol. 2013, 27, 1262–1272. [Google Scholar] [CrossRef]
- de Oliveira, A.C.P.; Nunes, A.; Rodrigues, R.G.; Branquinho, C. The response of plant functional traits to aridity in a tropical dry forest. Sci. Total Environ. 2020, 747, 141177. [Google Scholar] [CrossRef] [PubMed]
- Daehler, C.C. Performance comparisons of co-occurring native and alien invasive plants: Implications for conservation and restoration. Annu. Rev. Ecol. Evol. Syst. 2003, 34, 183–211. [Google Scholar] [CrossRef]
- Laughlin, D.C.; Leppert, J.J.; Moore, M.M.; Sieg, C.H. A multi-trait test of the leaf-height-seed plant strategy scheme with 133 species from a pine forest flora. Funct. Ecol. 2010, 24, 493–501. [Google Scholar] [CrossRef]
- Diaz, S.; Kattge, J.; Cornelissen, J.H.; Wright, I.J.; Lavorel, S.; Dray, S.; Reu, B.; Kleyer, M.; Wirth, C.; Prentice, I.C.; et al. The global spectrum of plant form and function. Nature 2016, 529, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Lloret, F.; de la Riva, E.G.; Perez-Ramos, I.M.; Maranon, T.; Saura-Mas, S.; Diaz-Delgado, R.; Villar, R. Climatic events inducing die-off in Mediterranean shrublands: Are species’ responses related to their functional traits? Oecologia 2016, 180, 961–973. [Google Scholar] [CrossRef] [PubMed]
- Ordonez, A.; Wright, I.J.; Olff, H. Functional differences between native and alien species: A global-scale comparison. Funct. Ecol. 2010, 24, 1353–1361. [Google Scholar] [CrossRef]
- Tecco, P.A.; DÃ az, S.; Cabido, M.; Urcelay, C. Functional traits of alien plants across contrasting climatic and land-use regimes: Do aliens join the locals or try harder than them? J. Ecol. 2010, 98, 17–27. [Google Scholar] [CrossRef]
- Funk, J.L.; Standish, R.J.; Stock, W.D.; Valladares, F. Plant functional traits of dominant native and invasive species in mediterranean-climate ecosystems. Ecology 2016, 97, 75–83. [Google Scholar] [CrossRef]
- Helsen, K.; Van Cleemput, E.; Bassi, L.; Graae, B.J.; Somers, B.; Blonder, B.; Honnay, O. Inter- and intraspecific trait variation shape multidimensional trait overlap between two plant invaders and the invaded communities. Oikos 2020, 129, 677–688. [Google Scholar] [CrossRef]
- Montesinos, D. Fast invasives fastly become faster: Invasive plants align largely with the fast side of the plant economics spectrum. J. Ecol. 2021, 110, 1010–1014. [Google Scholar] [CrossRef]
- Tordoni, E.; Petruzzellis, F.; Nardini, A.; Savi, T.; Bacaro, G.; Botta-Dukát, Z. Make it simpler: Alien species decrease functional diversity of coastal plant communities. J. Veg. Sci. 2019, 30, 498–509. [Google Scholar] [CrossRef]
- de la Riva, E.G.; Maranon, T.; Violle, C.; Villar, R.; Perez-Ramos, I.M. Biogeochemical and Ecomorphological Niche Segregation of Mediterranean Woody Species along a Local Gradient. Front. Plant Sci. 2017, 8, 1242. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Harguindeguy, N.; Díaz, S.; Garnier, E.; Lavorel, S.; Poorter, H.; Jaureguiberry, P.; Bret-Harte, M.S.; Cornwell, W.K.; Craine, J.M.; Gurvich, D.E.; et al. Corrigendum to: New handbook for standardised measurement of plant functional traits worldwide. Aust. J. Bot. 2016, 64, 715–716. [Google Scholar] [CrossRef]
- Wahl, S.; Ryser, P. Root tissue structure is linked to ecological strategies of grasses. New Phytol. 2000, 148, 459–471. [Google Scholar] [CrossRef] [PubMed]
- Westoby, M.; Falster, D.S.; Moles, A.T.; Vesk, P.A.; Wright, I.J. Plant ecological strategies: Some leading dimensions of variation between species. Annu. Rev. Ecol. Syst. 2002, 33, 125–159. [Google Scholar] [CrossRef]
- Mao, L.; Chen, S.; Zhang, J.; Zhou, G. Altitudinal patterns of maximum plant height on the Tibetan Plateau. J. Plant Ecol. 2018, 11, 85–91. [Google Scholar] [CrossRef]
- Divisek, J.; Chytry, M.; Beckage, B.; Gotelli, N.J.; Lososova, Z.; Pysek, P.; Richardson, D.M.; Molofsky, J. Similarity of introduced plant species to native ones facilitates naturalization, but differences enhance invasion success. Nat. Commun. 2018, 9, 4631. [Google Scholar] [CrossRef] [PubMed]
- Hejda, M.; Pyšek, P.; Jarošík, V. Impact of invasive plants on the species richness, diversity and composition of invaded communities. J. Ecol. 2009, 97, 393–403. [Google Scholar] [CrossRef]
- Zhang, X.; Oduor, A.M.O.; Liu, Y. Invasive plants have greater growth than co-occurring natives in live soil subjected to a drought-rewetting treatment. Funct. Ecol. 2022, 37, 513–522. [Google Scholar] [CrossRef]
- te Beest, M.; Esler, K.J.; Richardson, D.M. Linking functional traits to impacts of invasive plant species: A case study. Plant Ecol. 2014, 216, 293–305. [Google Scholar] [CrossRef]
- Eissenstat, D.M.; Wells, C.E.; Yanai, R.D.; Whitbeck, J.L. Building roots in a changing environment: Implications for root longevity. New Phytol. 2000, 147, 33–42. [Google Scholar] [CrossRef]
- Grotkopp, E.; Rejmanek, M. High seedling relative growth rate and specific leaf area are traits of invasive species: Phylogenetically independent contrasts of woody angiosperms. Am. J. Bot. 2007, 94, 526–532. [Google Scholar] [CrossRef] [PubMed]
- Quan, G.M.; Mao, D.J.; Zhang, J.E.; Xie, J.F.; Xu, H.Q.; An, M. Response of invasive Chromolaena odorata and two coexisting weeds to contrasting irradiance and nitrogen. Photosynthetica 2015, 53, 419–429. [Google Scholar] [CrossRef]
- Gioria, M.; Osborne, B.A. Resource competition in plant invasions: Emerging patterns and research needs. Front. Plant Sci. 2014, 5, 501. [Google Scholar] [CrossRef]
- Herms, D.A.; Mattson, W.J. The Dilemma of Plants—To Grow or Defend. Q. Rev. Biol. 1992, 67, 478. [Google Scholar] [CrossRef]
- Henn, J.J.; Yelenik, S.; Damschen, E.I. Environmental gradients influence differences in leaf functional traits between native and non-native plants. Oecologia 2019, 191, 397–409. [Google Scholar] [CrossRef]
- Geng, Y.; Wang, L.; Jin, D.; Liu, H.; He, J.S. Alpine climate alters the relationships between leaf and root morphological traits but not chemical traits. Oecologia 2014, 175, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Xia, M.; Wei, X.; Chang, W.; Liu, Y.; Wang, Z. Anatomical traits associated with absorption and mycorrhizal colonization are linked to root branch order in twenty-three Chinese temperate tree species. New Phytol. 2008, 180, 673–683. [Google Scholar] [CrossRef]
- Li, W.; Wang, L.; Qian, S.; He, M.; Cai, X.; Ding, J. Root characteristics explain greater water use efficiency and drought tolerance in invasive Compositae plants. Plant Soil 2022, 483, 209–223. [Google Scholar] [CrossRef]
- Simberloff, D.; Vitule, J.R.S. A call for an end to calls for the end of invasion biology. Oikos 2014, 123, 408–413. [Google Scholar] [CrossRef]
- Cavender-Bares, J.; Keen, A.; Miles, B. Phylogenetic Structure of Floridian Plant Communities Depends on Taxonomic and Spatial Scale. Ecology 2006, 87, S109–S122. [Google Scholar] [CrossRef] [PubMed]
- Loiola, P.P.; de Bello, F.; Chytrý, M.; Götzenberger, L.; Carmona, C.P.; Pyšek, P.; Lososová, Z.; Swenson, N. Invaders among locals: Alien species decrease phylogenetic and functional diversity while increasing dissimilarity among native community members. J. Ecol. 2018, 106, 2230–2241. [Google Scholar] [CrossRef]
- Zhang, B.; Hastings, A.; Grosholz, E.D.; Zhai, L. The comparison of dispersal rate between invasive and native species varied by plant life form and functional traits. Mov. Ecol. 2023, 11, 73. [Google Scholar] [CrossRef] [PubMed]
- Kovács-Hostyánszki, A.; Szigeti, V.; Miholcsa, Z.; Sándor, D.; Soltész, Z.; Török, E.; Fenesi, A. Threats and benefits of invasive alien plant species on pollinators. Basic Appl. Ecol. 2022, 64, 89–102. [Google Scholar] [CrossRef]
- Wang, X.-P.; Fu, X.; Shi, M.-M.; Zhao, Z.-T.; Li, S.-J.; Tu, T.-Y. Invasive Asteraceae plants can enhance community stability by changing pollination network structure, yet cause intense pollen disturbance to native plants in an oceanic island community. Biol. Invasions 2023, 25, 3603–3618. [Google Scholar] [CrossRef]
- Sun, J.; Yuan, X.; Liu, H.; Liu, G. Emergy and eco-exergy evaluation of wetland reconstruction based on ecological engineering approaches in the three Gorges Reservoir, China. Ecol. Indic. 2020, 122, 107278. [Google Scholar] [CrossRef]
- Huang, Y.; Ge, Y.; Wang, Q.; Zhou, H.; Liu, W.; Christie, P. Allelopathic Effects of Aqueous Extracts of Alternanthera philoxeroides on the Growth of Zoysia matrella. Pol. J. Environ. Stud. 2017, 26, 97–105. [Google Scholar] [CrossRef]
- Brock, M.T.; Weinig, C.; Galen, C. A comparison of phenotypic plasticity in the native dandelion Taraxacum ceratophorum and its invasive congener T. officinale. New Phytol. 2005, 166, 173–183. [Google Scholar] [CrossRef]
- Richards, C.L.; Pennings, S.C.; Donovan, L.A. Habitat range and phenotypic variation in salt marsh plants. Plant Ecol. 2005, 176, 263–273. [Google Scholar] [CrossRef]
- Masoodi, A.; Khan, F. Invasion of alligator weed (Alternanthera philoxeroides) in Wular Lake, Kashmir, India. Aquat. Invasions 2012, 7, 143–146. [Google Scholar] [CrossRef]
- Keddy, P.A. Assembly and response rules: Two goals for predictive community ecology. J. Veg. Sci. 1992, 3, 157–164. [Google Scholar] [CrossRef]
- Kraft, N.J.B.; Adler, P.B.; Godoy, O.; James, E.C.; Fuller, S.; Levine, J.M.; Fox, J. Community assembly, coexistence and the environmental filtering metaphor. Funct. Ecol. 2014, 29, 592–599. [Google Scholar] [CrossRef]
- Callaway, R.M.; Brooker, R.W.; Choler, P.; Kikvidze, Z.; Lortie, C.J.; Michalet, R.; Paolini, L.; Pugnaire, F.I.; Newingham, B.; Aschehoug, E.T.; et al. Positive interactions among alpine plants increase with stress. Nature 2002, 417, 844–848. [Google Scholar] [CrossRef] [PubMed]
- Li, S.P.; Cadotte, M.W.; Meiners, S.J.; Hua, Z.S.; Shu, H.Y.; Li, J.T.; Shu, W.S. The effects of phylogenetic relatedness on invasion success and impact: Deconstructing Darwin’s naturalisation conundrum. Ecol. Lett. 2015, 18, 1285–1292. [Google Scholar] [CrossRef]
- de Bello, F.; Price, J.N.; Munkemuller, T.; Liira, J.; Zobel, M.; Thuiller, W.; Gerhold, P.; Gotzenberger, L.; Lavergne, S.; Leps, J.; et al. Functional species pool framework to test for biotic effects on community assembly. Ecology 2012, 93, 2263–2273. [Google Scholar] [CrossRef]
- Mayfield, M.M.; Levine, J.M. Opposing effects of competitive exclusion on the phylogenetic structure of communities. Ecol. Lett. 2010, 13, 1085–1093. [Google Scholar] [CrossRef] [PubMed]
- Leishman, M.R.; Thomson, V.P.; Cooke, J. Native and exotic invasive plants have fundamentally similar carbon capture strategies. J. Ecol. 2009, 98, 28–42. [Google Scholar] [CrossRef]
- Park, D.S.; Potter, D. A test of Darwin’s naturalization hypothesis in the thistle tribe shows that close relatives make bad neighbors. Proc. Natl. Acad. Sci. USA 2013, 110, 17915–17920. [Google Scholar] [CrossRef]


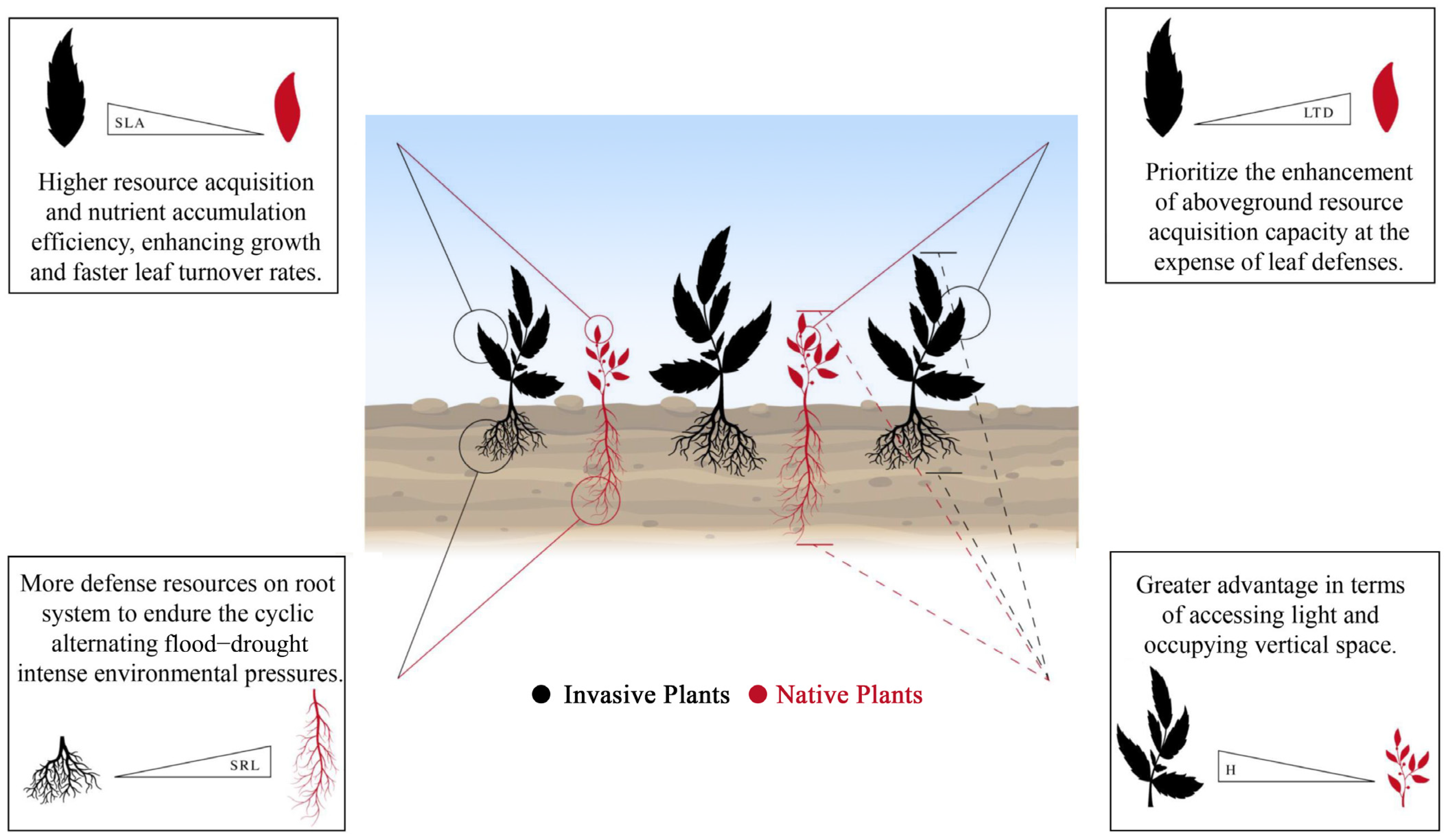
| Functional Traits | Abbreviation | Unit | Plant Characteristics Represented | Definitions and Calculation Methods |
|---|---|---|---|---|
| Height | H | cm | Ability to acquire sunlight resources | Vertical height of the highest point of the plant from the ground in its natural state |
| Specific leaf area | SLA | cm2·g−1 | Ability of plants to absorb and store nutrients | Leaf area/leaf dry mass |
| Leaf dry matter content | LDMC | g·g−1 | The extent to which dry matter synthesized by plants is put into leaf construction | Leaf dry mass/saturated fresh mass |
| Stem dry matter content | SDMC | g·g−1 | The extent to which dry matter synthesized by plants is put into stem construction | Stem dry mass/saturated fresh mass |
| Specific root length | SRL | cm·g−1 | The potential absorption rate of water and nutrients by plant root represents morphological indicators of underground competitiveness. | Root length/root dry mass |
| Leaf tissue density | LTD | g·cm−3 | The accumulation status of leaf biomass, related to the stretching and defense forces of tissues. | Leaf dry mass/(leaf area × leaf thickness) |
| Functional Traits (at the Community Scale) | Community Type | Slope | R2 | p |
|---|---|---|---|---|
| CW−H | IP | 0.034 | 0.066 | <0.001 *** |
| NP | 0.019 | 0.048 | <0.001 *** | |
| CW−SRL | IP | <0.001 | 0.004 | 0.901 |
| NP | 0.014 | 0.003 | 0.190 | |
| CW−SLA | IP | 0.070 | 0.170 | <0.001 *** |
| NP | 0.026 | 0.039 | <0.01 ** | |
| CW−LTD | IP | −0.009 | 0.048 | <0.05 * |
| NP | −0.006 | 0.008 | 0.093 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, L.; Yuan, X.; Sun, K.; Li, P. Functional Segregation of Resource Utilization Strategies between Invasive and Native Plants and Invasion Mechanisms in the Water Level Fluctuation Zone: A Case Study of Pengxi River in Three Gorges Reservoir, China. Forests 2024, 15, 959. https://doi.org/10.3390/f15060959
Cheng L, Yuan X, Sun K, Li P. Functional Segregation of Resource Utilization Strategies between Invasive and Native Plants and Invasion Mechanisms in the Water Level Fluctuation Zone: A Case Study of Pengxi River in Three Gorges Reservoir, China. Forests. 2024; 15(6):959. https://doi.org/10.3390/f15060959
Chicago/Turabian StyleCheng, Lideng, Xingzhong Yuan, Kuo Sun, and Peiwu Li. 2024. "Functional Segregation of Resource Utilization Strategies between Invasive and Native Plants and Invasion Mechanisms in the Water Level Fluctuation Zone: A Case Study of Pengxi River in Three Gorges Reservoir, China" Forests 15, no. 6: 959. https://doi.org/10.3390/f15060959
APA StyleCheng, L., Yuan, X., Sun, K., & Li, P. (2024). Functional Segregation of Resource Utilization Strategies between Invasive and Native Plants and Invasion Mechanisms in the Water Level Fluctuation Zone: A Case Study of Pengxi River in Three Gorges Reservoir, China. Forests, 15(6), 959. https://doi.org/10.3390/f15060959






