Abstract
Bamboo and wood-mixed forests are management models that remarkably enhance the balance and productivity of bamboo ecosystems. However, the effects of this model on soil nutrients and enzyme activities remain largely unknown. This study compared the soil organic carbon, nitrogen, phosphorus, and enzyme activity, along with the characteristics of fine roots in pure Moso bamboo plantations (CK) and those mixed with Liriodendron chinense (ML), Sassafras tzumu (MS), Cunninghamia lanceolata (MC), and Pseudolarix amabilis (MP). The results showed that mixed forests improve carbon pools in 0–40 cm soil layers, increasing the total organic C(TOC), free particulate organic C (fPOC), occluded particulate organic C (oPOC), hot-water-extractable organic C (DOC), and mineral-associated organic C (MOC). They also increase soil total N, total P, available N, available P, NH4+-N, NO3−-N, inorganic P, organic P, and microbial biomass N. Bacterial and fungal abundances, along with enzyme activities (urease, acid phosphatase, polyphenol oxidase, peroxidase, and β-glucosidase), also improved. MP and MS were the most effective. Moreover, MS and MP supported a higher biomass and length of fine root and increased the nitrogen and phosphorus uptake of Moso bamboo. In conclusion, Sassafras tzumu and Pseudolarix amabilis are optimal for mixed planting, offering substantial benefits to soil nutrient dynamics and preventing soil quality decline in Moso bamboo forests, thereby supporting better nutrient cycling and carbon sequestration. This research offers insights into enhancing soil quality through diversified Moso bamboo forestry.
1. Introduction
Bamboo is a perennial herbaceous plant found primarily in tropical, subtropical, and warm temperate regions [1]. It is known as the “second largest forest in the world”. Moso bamboo, the most prevalent bamboo species in China, occupies approximately 4.43 million ha [2]. It exhibits a unique growth pattern, reaching full height within 2–3 months and expanding by 30–100 cm daily during peak growth periods [3,4]. Moso bamboo offers several benefits, including a short rotation period and high biomass productivity [5], which make it valuable in the food, furniture, and construction industries. It is also recognized as a crucial plant for carbon sequestration [6] and provides substantial social, economic, and ecological benefits [5]. Over recent decades, the Moso bamboo forest area in subtropical China has been increasing at an annual rate of approximately 3%. With the expansion of the area, the problems associated with the unsustainable management of Moso bamboo forests have become increasingly prominent.
Currently, Moso bamboo forests are mainly managed using intensive forest management practices, such as fertilization, deep plowing, and the removal of understory vegetation [7]. While these methods enhance the growth of Moso bamboo and yield remarkable economic benefits, they also negatively affect the soil [8]. The application of phosphorus fertilizer in intensive management reduces the effective inter-root phosphorus content, even though it increases phosphorus levels in the bulk soil [9]. Moreover, intensive management leads to a considerable reduction in soil pH as well as decreases in arbuscular mycorrhizal fungi biomass—measured in neutral lipid fatty acids—and total glomalin-related soil protein content, heightening the risk of soil erosion and organic carbon depletion [8]. Intense harvesting and fertilization practices also diminish carbon stocks in the soil layers [10]. Soil microbial communities are less diverse under intensive management practices compared to conventional methods [11], potentially reducing the abundance of Crearchaeota, which are crucial for NH4+ oxidation and consequently affect nitrification processes and NO2 emissions from the soil [12]. Additionally, prolonged intensive management is associated with reduced rhizome and internode lengths in bamboo seedlings in some species [13]. Given these detrimental effects, restoring soil quality in Moso bamboo forests has become essential.
Bamboo and wood-mixed planting promotes the establishment of multi-storey forests, which helps to enhance the ecological function of single-storey forests [14,15]. Studies have shown that bamboo–tree mixtures can increase the total aboveground biomass, including arborous (tree and Moso bamboo) biomass [16]; enhance litterfall biomass per unit area; improve fungal community structure [17]; and boost the abundance of arbuscular mycorrhizal fungi (AMF) in the soil [18]. The mixing ratio of Moso bamboo to broadleaf trees impacts the physical and chemical properties of the soil, indirectly influencing the soil bacterial community [19]. Current studies show a clear difference between Moso bamboo forest soils and Moso bamboo mixed-forest soils.
C, N, and P are the main soil nutrients and key indicators for evaluating nutrient levels and effectiveness [20]. Soil microorganisms play a functional role in ecosystem nutrient cycling; are highly dynamic; and are associated with soil C, N, and P cycling [21]. The growth environment of soil microorganisms can be affected by changes in root secretions and chemical composition due to changes in vegetation type, which can affect their biomass and soil enzyme activities [22]. The enzymatic activity of the soil is one of the most sensitive indicators of the nutrient content and fertility of the soil [23]. Catalase, β-glucosidase, sucrase, and polyphenol oxidase are associated with the C cycle. They are involved in lignin degradation, humus formation, sucrose hydrolysis, and cellulose catabolism, respectively. Meanwhile, urease and phosphatase are associated with the N cycle and organophosphorus hydrolysis, respectively [9]. Wang et al. [24] reported a remarkable correlation between soil organic carbon (SOC) and total nitrogen (TN) and both soil microbial communities and enzyme activities. Fine roots contribute remarkably to carbon inputs and regulate microbial communities and enzyme activities in the soil [24]. Moreover, fine root morphology is closely related to below-ground resources. Nutrient-poor soils result in long, fine roots and great, fine root biomass [25]. The amount of nutrient uptake by fine roots can also reflect the soil nutrient status. Therefore, understanding the association between soil microorganisms, fine roots, and the soil environment can help select mixed tree species to improve soil quality in Moso bamboo forests. However, the effects of Moso bamboo mixed systems on the storage of soil C, N, and P; soil microorganisms; and enzymatic activities of fine roots have rarely been studied.
This study investigated the soil properties and fine root characteristics of Moso bamboo forests with different mixed species. We hypothesized that: (1) differences in mixed tree species alter soil chemical properties and affect soil microbial and enzymatic activities; and (2) these changes are reflected in the fine roots of Moso bamboo, such as alterations in fine root biomass and nutrient uptake. The objectives of this study were as follows: (1) to study the effects of mixed cultivation of different native tree species with Moso bamboo on soil C, N, and P cycling and to elucidate the mechanisms and drivers of mixing that affect soil microorganisms and enzymes; and (2) to explore the effects of mixing on the root growth and nutrient uptake of Moso bamboo and to analyze the environmental factors that are closely related to root indicators. The findings of this study are expected to enhance our understanding of the effects of mixed cultivation on forest soils and provide a rationale for improving the soil environment of Moso bamboo ecosystems.
2. Materials and Methods
2.1. Site Description
The experimental site was located in Anji County, Huzhou City, Zhejiang Province, China (30°23′–30°53′ N, 119°14′–119°53′ E) at 200–500 m altitude. The region has an average annual temperature of 17.7 °C, an average rainfall of 1300 mm, approximately 1943 h of sunshine per year, a frost-free period of 226–246 d, and a mid-latitude subtropical monsoon climate. Soils in the area are ferric luvisols and come from granitic rock (Figure 1).

Figure 1.
Overview of the study site: pure stands of Moso bamboo (CK), mixed stands of Moso bamboo and Liriodendron chinense (ML), mixed stands of Moso bamboo and Sassafras tzumu (MS), mixed stands of moso bamboo and Cunninghamia lanceolata (MC), and mixed stands of Moso bamboo and Pseudolarix amabilis (MP).
2.2. Experimental Design and Soil and Fine Root Sampling
In July 2018, based on site similarities, we selected five forest stands, i.e., Moso bamboo plantation (CK), Moso bamboo—Liriodendron chinese plantation (ML), Moso bamboo—Sassafras tzumu plantation (MS), Moso bamboo—Cunninghamia lanceolata plantation (MC), and Moso bamboo—Pseudolarix amabilis plantation (MP), with similar elevations, soil types, slopes, and characteristics. Five 20 × 20 m sample plots were established for each stand selection. Five representative Moso bamboo samples were selected from each standard sample plot based on the average plant height and diameter at breast height. Fine roots in 0–20 cm of soil were collected with a root auger in a north–south direction along the lateral roots at a distance of about 0.5 m from the base. They were then transported to the laboratory in sterile plastic bags for freezing and preservation. Soil samples were collected from 25 sample plots at two depths, i.e., 0–20 cm and 20–40 cm, using the five-point sampling method. The samples were mixed to form composite samples from each stratum, sealed in sterile plastic bags, and transferred to the laboratory on ice. The samples were thoroughly mixed and passed through a 2.0 mm sieve. A portion of the sieved sample was stored at −80 °C for DNA extraction.
The remainder was divided into two portions. One of these was air-dried and used for the determination of total P (TP), organic P (OP), inorganic P (IP), available P (AP), total organic C (TOC), free particulate organic C (fPOC), occluded particulate organic C(oPOC), mineral-associated organic C (MOC), available N (AN), and total N (TN). Another copy was stored at 4 °C for enzyme activities, microbial biomass C (MBC) microbial biomass N (MBN), hot-water-extractable organic C (DOC), water-soluble organic N (WSON), NH4+-N, and NO3−-N content under field moisture.
2.3. Extraction and Determination of Soil C, N and P
We used the TOC analyzer (MultiN/C3100, Analytik, Jena, Germany) to determine TOC, DOC, fPOC, and WSON. Using the method described by Li et al. [26], 30 mL of 1.8 g·cm3 NaI solution was added to 20.0 g of air-dried soil to determine the fPOC, 30 mL of 0.5% sodium hexametaphosphate was used to determine the MOC, and finally, the oPOC was obtained. The chloroform fumigation extraction method [27] was used to analyze MBC and MBN. An elemental analyzer (Perkin-Elmer 2400, PerkinElmer, Waltham, MA, USA) was used to determine total nitrogen. The soil samples were mixed with 2 mol·L−1 KCL at a ratio of 1:5, shaken for 1 h, and filtered, and the NH4+-N and NO3−-N contents were determined using the indophenol blue method and the phenolate disulphonic acid method [28], respectively. The alkaline hydrolysis diffusion method [29] was used for the determination of soil available nitrogen (AN). The HClO4-H2SO4 digestion method and the aluminum–antimony colorimetric method were used for the determination of TP, while available P was determined by the Bray method by adding 0.5 mol·mol·L−1 NaHCO3 to the soil sample and then determining AP using a spectrophotometer [30]. The soil IP content was measured using the sequential P fractionation method described by [31], and the soil organic P content was the difference between TP and IP.
2.4. Determination of Soil Enzyme Activity and Microbial Abundance Measurements
Polyphenol oxidase activity and peroxidase activity were determined by a microtiter plate enzyme assay [32] using L-DOPA as a calibrated substrate and a hydrolase assay using a multifunctional enzyme marker (Spectra Max M5, Molecular Devices, Sunnyvale, CA, USA). Soil acid phosphatase activity and β-glucosidase activity were determined via the disodium phenylphosphate method and the nitrophenol colourimetric method, respectively. The urease activity was the mass (mg) of soil NH3-N g−1 produced by enzymatic degradation with the addition of urea, then incubated for 24 h at 7 °C.
Soil bacterial and fungal abundance were determined using real-time quantitative fluorescence PCR. The primers used for bacterial gene copy number quantification were 338F (5′-ACTCCTACGGGAGGCAGCAG-3′) and 518R (5′-ATTAC-CGCGGGCTGCTGGG-3′), and the primers used for fungal gene copy number quantification were NS1 (5′-GTAGTCATATGCTTGTCC-3′) and Fung (5′-CATTC- CCCGTTACCCGTTG-3′). qPCR amplification was performed using the instrument CFX 96TM (Bio-Rad, Hercules, CA, USA). The qPCR conditions were as follows: pre-denatured at 94 °C for 2 min; 94 °C for 30 s; 60 °C (bacterial)/55 °C (fungal) for 30 s; and 72 °C for 1 min, with 35 cycles, and the copy number of the target gene was calculated according to the standard curve.
2.5. Determination of Fine Root Characteristics
The roots obtained were placed on a 0.85 mm sieve and rinsed with tap water before being treated in an ultrasonic bath for 1 min. Root volume, mean diameter, and total length were obtained by scanning with a flatbed scanner (Epson Expression) at 400 dpi after the water had been wiped off. To obtain the dry weight of the root system, the analyzed roots were dried at 70 °C to a constant weight. The same methods as for the soil samples were used to determine total nitrogen, phosphorus, and potassium in the root system. The dry weight of the roots was multiplied by the total nitrogen, phosphorus, and potassium content to obtain the root nitrogen, phosphorus, and potassium uptake. Fine root biomass was calculated as the dry weight of fine roots per area using the method described by Ni et al. [9].
2.6. Statistical Analysis
All statistics and analyses were performed using SPSS 26.0 (SPSS Inc., Chicago, IL, USA). Significant differences were calculated for soil properties, soil microbes, soil enzymes, and root-related indices using one-way analysis of variance (ANOVA) with the significance level set at p < 0.05, and multiple comparisons were made using least significant difference (LSD). Correlations between indicators were determined using Pearson correlations, and graphs were generated using Origin Pro (2024).
3. Results
3.1. Soil C Fractions and Content
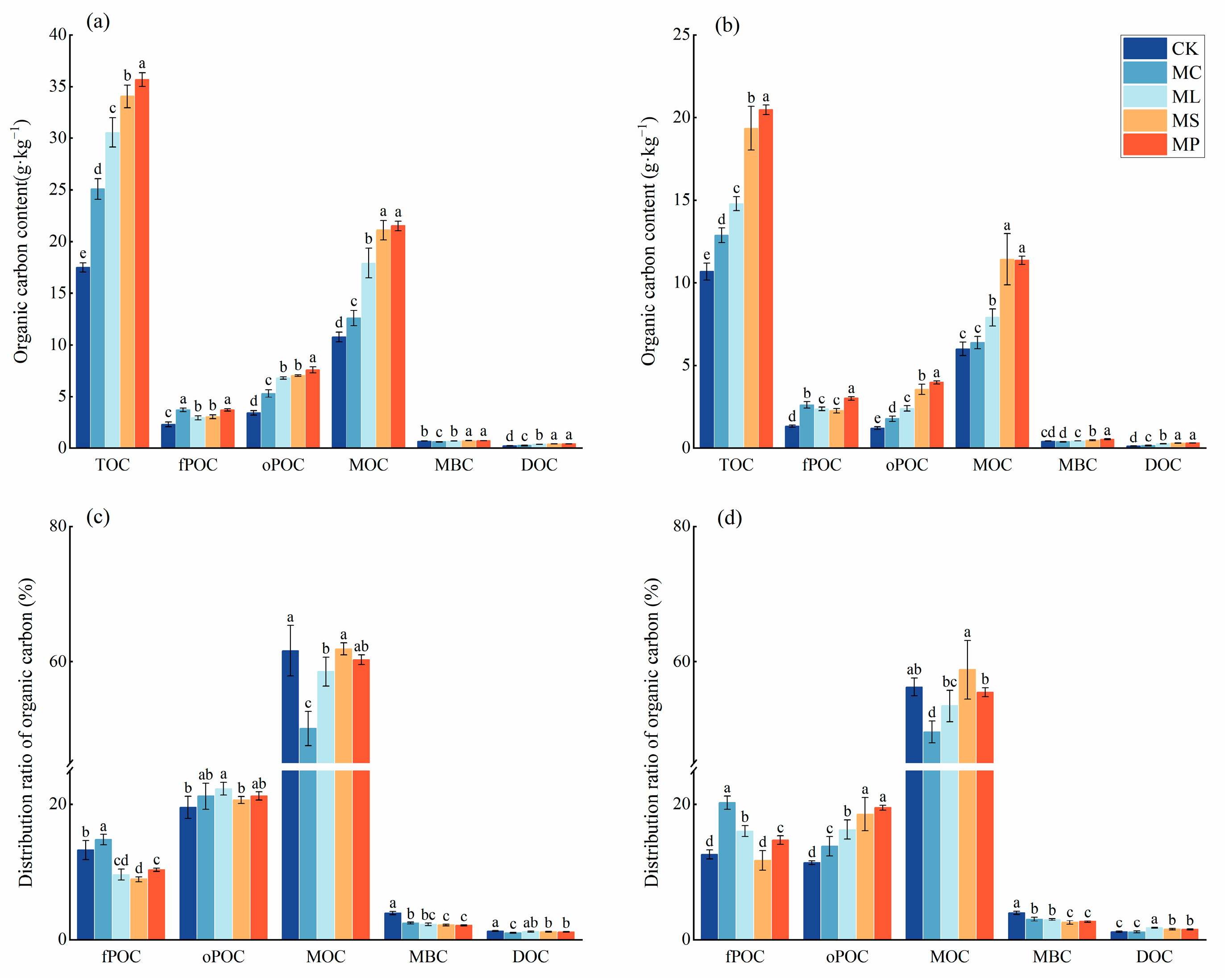
The four treatments significantly (Figure 2, p < 0.05) affected the content of soil carbon fractions compared to CK. All four treatments significantly (p < 0.05) increased the contents of TOC, fPOC, oPOC, and DOC in soil layers from both 0–20 cm and 20–40 cm compared with CK. In the 0–20 cm soil layer, all four treatments significantly (p < 0.05) increased the mineral-associated organic carbon (MOC) content compared to CK. However, in the 20–40 cm soil layer, MC was unable to increase the soil MOC content compared to CK. The remaining three treatments significantly (p < 0.05) increased the soil MOC content. CK, MP, MS, and MC significantly (p < 0.05) increased the MBC content in the 0–20 cm soil layer. Meanwhile, MC had the lowest MBC content in the 20–40 cm soil layer. The MP was optimal for all the carbon fractions in all the soil layers.
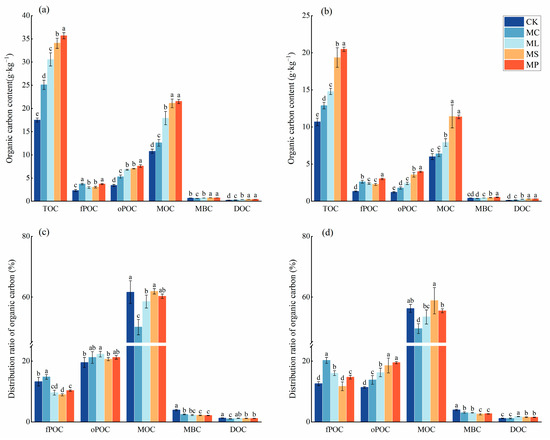
Figure 2.
Contents and proportions of soil organic carbon pools. (a,c) represent 0–20 cm soil; (b,d) represent 20–40 cm soil. Different lowercase letters indicate significant (p < 0.05) differences between treatments. Error bars indicate standard deviations (n = 5).
The ratio of MBC/TOC was the largest, and the ratio of oPOC/TOC was the smallest for CK in both soil layers. The values of DOC/TOC were highest for CK in the upper soils and for ML in the lower soils. The MOC/TOC ratio of MS had the maximum value in both soil layers. fPOC/TOC had the maximum value in the MC and the minimum value in the MS.
3.2. Soil N Fractions and Content
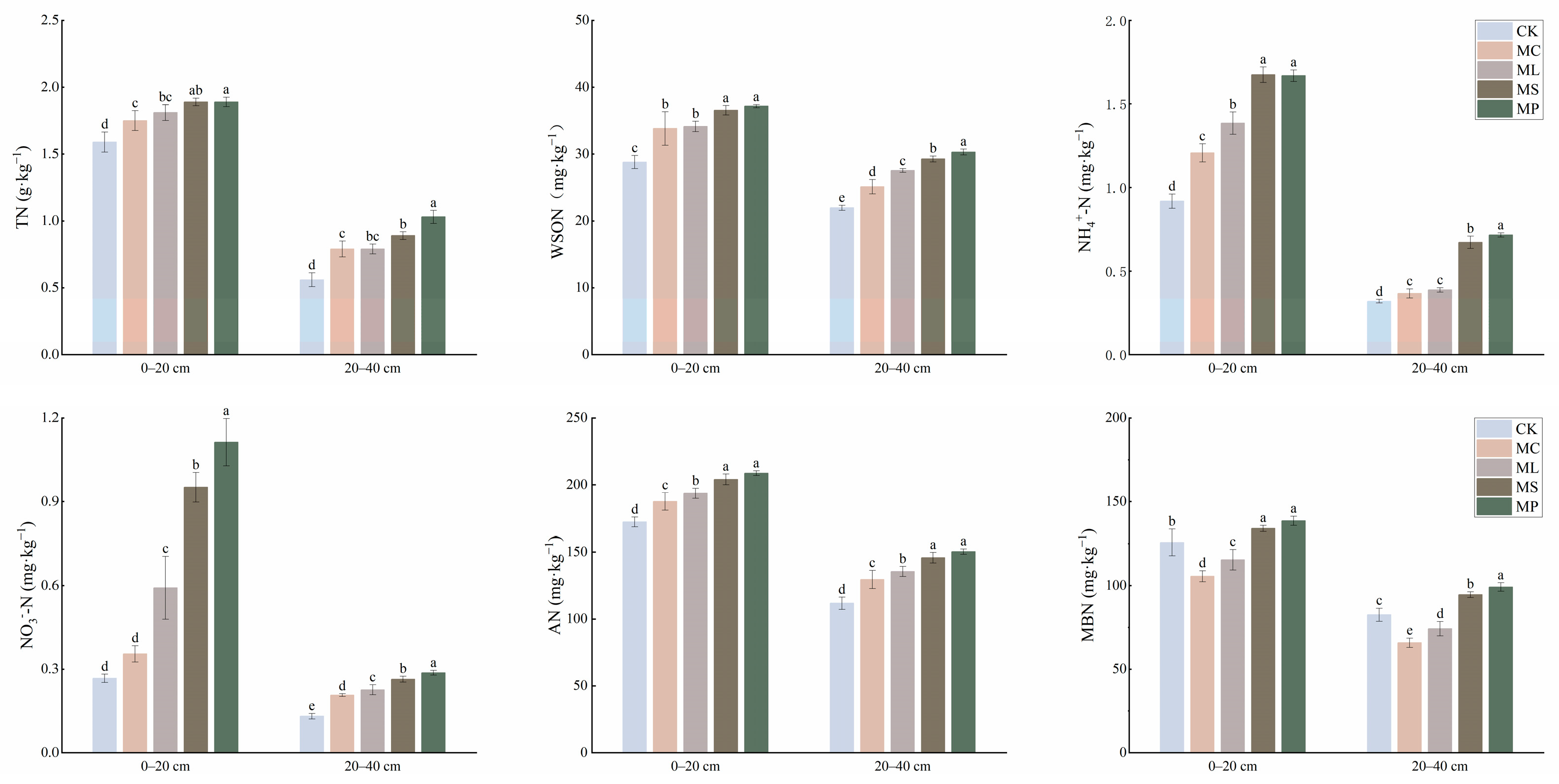
MP, MS, ML, and MC significantly (Figure 3, p < 0.05) increased the contents of total N, WSON, NH4+-N, and alkaline hydrolysis N in both soil horizons compared to CK. All four treatments significantly (p < 0.05) increased the NO3−-N content in the 20–40 cm soil layer. However, only MP, MS, and ML significantly (p < 0.05) increased NO3−-N content in the 0–20 cm soil layer compared with CK. MC and ML significantly (p < 0.05) reduced the MBN content in both the 0–20 cm and 20–40 cm soil layers, and MP and MS significantly (p < 0.05) increased the MBN content.
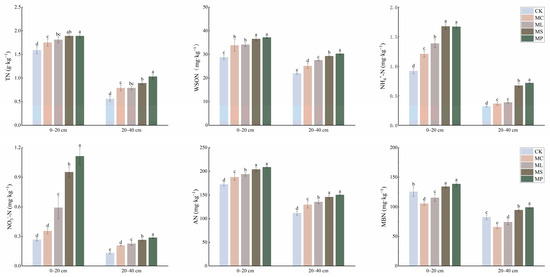
Figure 3.
Effects of different treatments on soil nitrogen pool content in 0–20 cm and 20–40 cm soil. Different lowercase letters indicate significant (p < 0.05) differences between treatments. Error bars indicate standard deviations (n = 5).
In the 0–20 cm soil, MP had the highest WSON/TN, NH4+-N/TN, and NO3−-N/TN; CK had the highest MBN/TN; and AP did not change significantly (Table 1, p < 0.05). WSON/TN and AN/TN were lowest in MP in the 20–40 cm soil. NH4+-N/TN and NO3−-N/TN were lowest and WSON/TN, AN/TN, and MBN/TN were highest in CK in the 20–40 cm soil. In the 0–20 cm soil, NH4+-N/TN and NO3−-N/TN were highest in MS.

Table 1.
Effects of different treatments on the proportion of soil nitrogen pools.
3.3. Soil P Fractions and Content
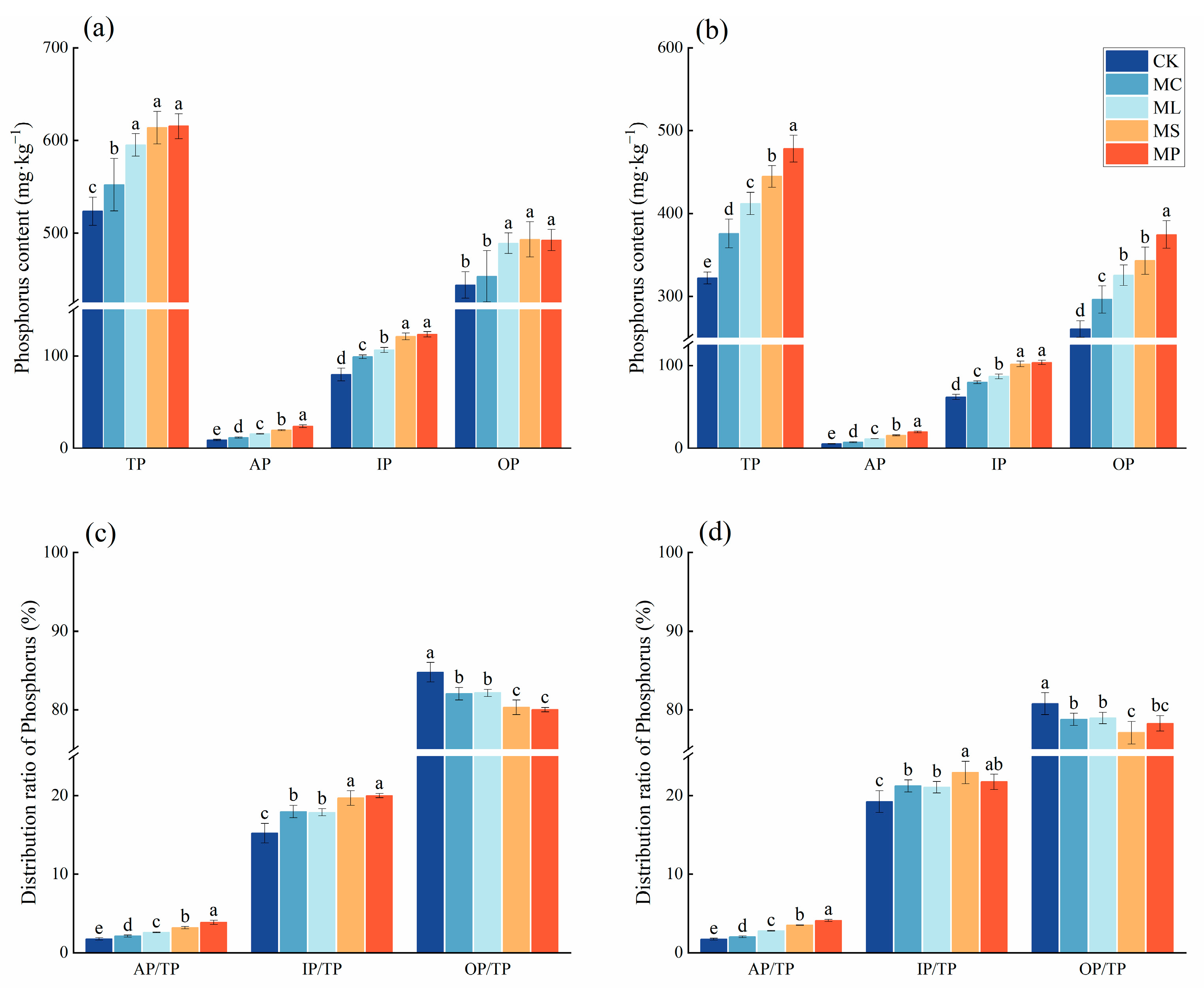
Compared with CK, the four treatments significantly (Figure 4, p < 0.05) increased soil total P, available P, and inorganic P contents in the soil layer of 0–20 cm, while in the soil layer of 20–40 cm, the four treatments not only significantly (p < 0.05) increased soil total P, available P, and inorganic P contents, but also significantly (p < 0.05) increased organic P. MC had no significant effect on increasing the soil organic P content in the 0–20 cm soil layer. The soil total P, available P, inorganic P, and organic P contents were highest in the MP treatment in both soil horizons.
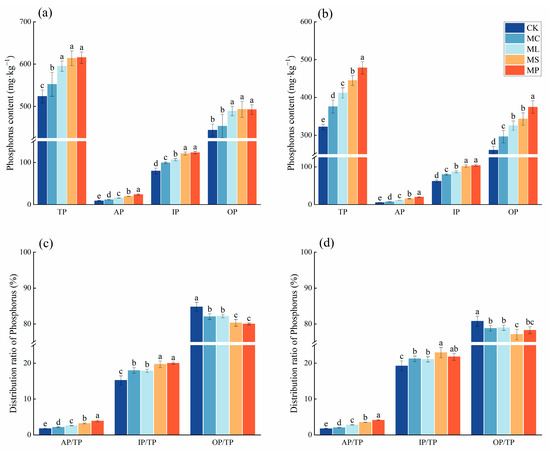
Figure 4.
Contents and proportions of soil phosphorus pools. (a,c) represent 0–20 cm soil; (b,d) represent 20–40 cm soil. Different lowercase letters indicate significant (p < 0.05) differences between treatments. Error bars indicate standard deviations (n = 5).
In both soil layers, all four treatments significantly (p < 0.05) increased the AP/TP and IP/TP ratios and decreased the OP/TP ratio compared to CK. MP had the highest AP/TP and IP/TP ratios and the lowest OP/TP in the 0–20 cm soil layer. In the 20–40 cm soil, MP had the highest AP/TP, and MS had the highest IP/TP and the lowest OP/TP.
3.4. Soil Enzyme Activity and Microbial Abundance
MP, MS, ML, and MC increased the abundance of soil bacteria and fungi, soil urease activity, acid phosphatase activity, and polyphenol oxidase activity compared to CK (Table 2). Soil peroxidase activity and β-glucosidase activity did not change significantly (p < 0.05) under MC treatment compared to CK. This was in contrast with the remaining three treatments, which increased soil peroxidase activity and β-glucosidase activity. Taken together, the MP provided the greatest enhancement of soil bacterial abundance, fungal abundance, and soil enzyme activity.

Table 2.
Effects of different forest stands on soil microbial population and enzyme activities.
3.5. Fine Root Characteristics
Compared with CK, MS and MP significantly (p < 0.05) increased the biomass, nitrogen uptake, and phosphorus uptake of the Moso bamboo roots (Table 3). MC significantly (p < 0.05) decreased the biomass, root length, root volume, phosphorus uptake, and potassium uptake of the Moso bamboo roots. ML decreased the root length. There were no significant changes in the average diameter, root volume, or potassium uptake of the Moso bamboo root system under ML, MS, and MP compared to CK.

Table 3.
Effects of different forest stands on fine root morphology and nutrient uptake.
3.6. Relationship between Soil and Fine Root
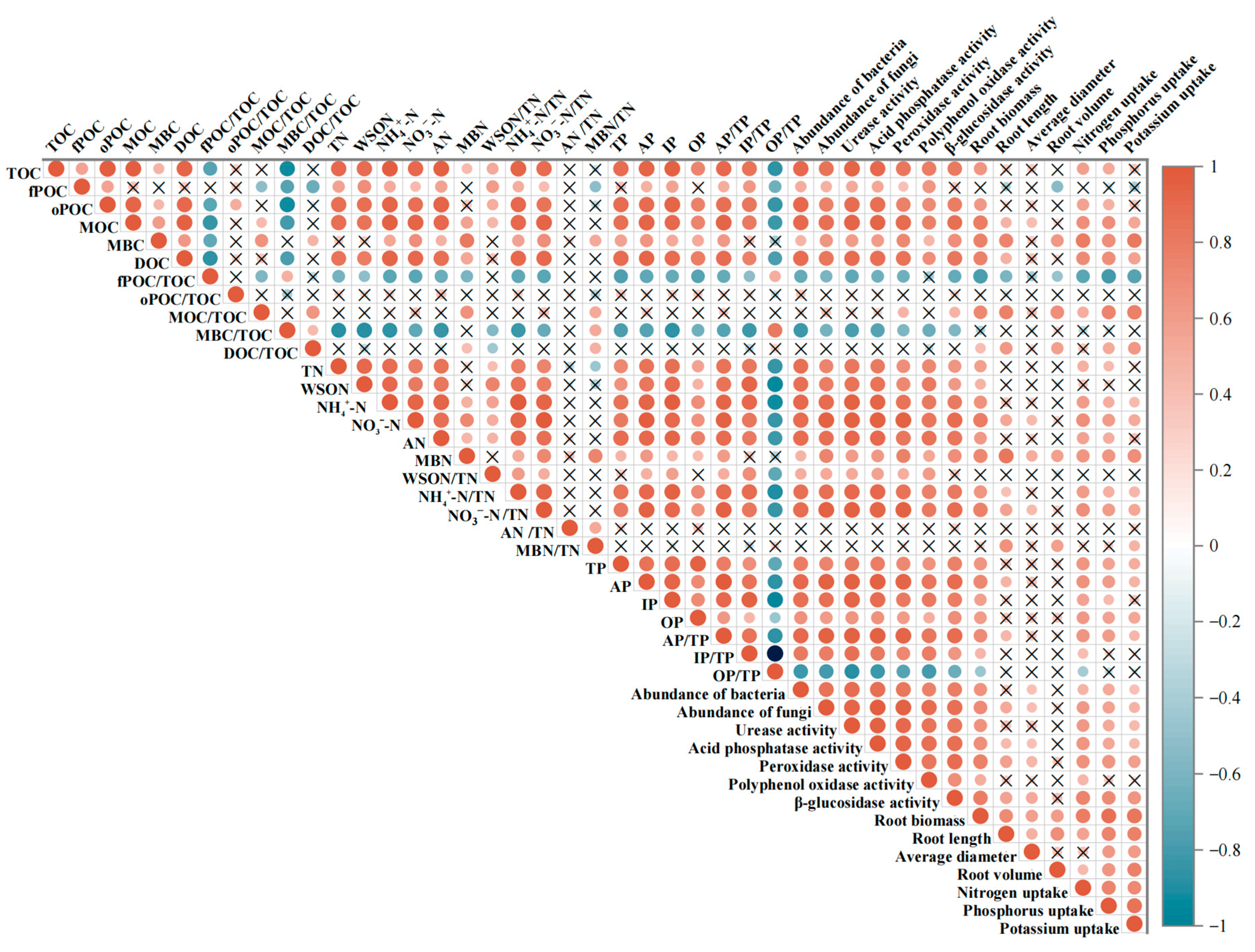
Correlation analysis showed that the Moso bamboo root growth was closely related to changes in soil properties (Figure 5). Root biomass, root nitrogen uptake, and root phosphorus uptake were positively correlated with most soil organic carbon fractions, soil microbial and enzyme indicators, and all soil phosphorus and nitrogen fractions. Root potassium uptake was positively correlated with soil microbial and enzyme activity indicators. Root potassium uptake was more strongly correlated with soil nitrogen- and phosphorus-related indicators in the 0–20 cm soil layer. Root potassium uptake was more closely associated with soil carbon fraction indicators in the 20–40 cm soil layer. Root volume was negatively correlated with fPOC.
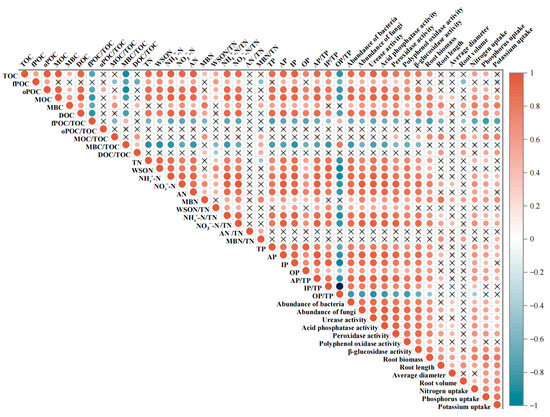
Figure 5.
Relationship between soil and fine root. Orange and blue colors indicate positive and negative correlations, and a cross in the box indicates an insignificant correlation.
4. Discussion
4.1. Effects of the Mixed Cultivation of Moso Bamboo with Other Indigenous Tree Species on Soil C, N, and P
The conversion of Moso bamboo forests to mixed forests significantly (p < 0.05) improved the amounts of stable and unstable carbon in the soil. The increase in tree species led to an increase in aboveground apoptosis and belowground rooting, all of which are favorable behaviors for soil carbon pools [33]. The main source of particulate organic carbon (POC) is plant material [34]. The increase in belowground fine root biomass was also a significant contributor to the increase in POC [35]. This is consistent with the increase in soil fPOC and oPOC content following the introduction of other tree species. The depth of the plant root system had a significant effect on soil fPOC [36]. This may explain the increased contribution of fPOC to TOC in the 0–20 cm soil layer in MC. This was the only shallow-rooted plant among the four mixed species. oPOC can promote long-term carbon sequestration by reducing microbial decomposition through the dense physical structure of micromeres [37]. Mixed stands increased oPOC and oPOC/TOC in the soil. This indicated that mixing is an effective means of increasing the carbon sequestration capacity of Moso bamboo forests. MOC, the carbon fraction with the lowest turnover rate in the soil [38], is an inorganic–organic complex formed from the end products of organic matter decomposition by the adsorption of soil silt and clay fractions through strong ligand exchange, hydrophobicity, and multivalent cation bridges [39]. The MOC content in the soil under all four treatments was higher than that in the pure forest. Among these, MP and MS had the highest MOC content, and there was a greater MOC contribution to TOC. They also showed greater carbon sequestration capacity. DOC and MBC are readily available soil organic carbons, and MBC can be used to characterize SOC turnover and nutrient cycling [40]. This is an important indicator of the role of soil microorganisms that can be directly involved in soil biochemical transformation processes [41]. Although the MBC and DOC contents increased in MP and MS, their MBC/TOC and DOC/TOC ratios were lower. This indicated that the mixing of Moso bamboo with Sassafras tzumu and Pseudolarix amabilis slowed down the conversion of stabilized organic matter to reactive organic carbon fractions and prolonged the retention time of carbon in the soil.
Bamboo forest mixing was favorable for reactive nitrogen pools, including the contents of WSON, NH4+-N, and NO3−-N. These were significantly (p < 0.05) elevated in both soil horizons. Meanwhile, the proportion of reactive nitrogen pools to total soil nitrogen pools increased in the 0–20 cm soil horizon. One potential explanation is that mixing increases the accumulation of plant residues in the soil, thereby enhancing nitrogen mineralization [42]. In the 20–40 cm soil layer, the WSON/TN ratio decreased. Meanwhile, the NH4+-N/TN and NO3−-N/TN ratios increased substantially under the four treatments. This may be related to the enhanced mineralization and leaching of soil organic matter by mixing [43,44]. As an indicator of soil nitrogen supply capacity, alkaline-hydrolyzed nitrogen significantly (p < 0.05) increased under the mixing treatment. Bamboo–wood mixing is an effective means of improving soil nutrients, with phosphorus being the most limiting nutrient in subtropical China [45]. The mixing measure increased soil OP content but decreased the OP/TP ratio. The soil inorganic phosphorus content and the ratio to total phosphorus also increased substantially. This suggests that mixing promotes the mineralization of soil phosphorus-containing organic matter. The study findings showed that there was a significant increase in total and effective soil phosphorus content in both soil horizons under mixing conditions. This has highlighted the positive effect of mixing on phosphorus effectiveness. Bamboo forest mixing has been shown to significantly (p < 0.05) affect soils and can alter soil carbon and nitrogen pools and phosphorus fractions [46,47]. Soil nutrients in mixed Moso bamboo forests were higher than those in pure Moso bamboo forests. This significantly (p < 0.05) increased the effectiveness of C, N, and P in the soil, with considerable potential for carbon sequestration. Moso bamboo mixed with Sassafras tzumu and Pseudolarix amabilis had the most successful results and is an effective means of improving soil degradation in pure Moso bamboo forests.
4.2. Effects of Cultivating Moso Bamboo with Other Indigenous Tree Species on Soil Microbial Biomass, Abundance, and Enzyme Activity
Soil microorganisms play a crucial role in maintaining soil structure and nutrient cycling, and cultivating Moso bamboo with other tree species can alter the soil microbial community. It can also change the microbial composition and functional capacity [18,48]. More stable soils have a greater soil microbial diversity [49]. In our study, mixing significantly (p < 0.05) increased the abundance of fungi and bacteria in the soil and increased the contents of effective elements such as NH4+-N, NO3−-N, AP, and AN. Nutrients have been shown to be one of the main drivers of bacterial and fungal communities [50]. Similar results were obtained in our study, where the effective nutrient content was highly correlated with fungal and bacterial abundance. Pure stands of Moso bamboo often face constraints on effective soil nutrients under intensive management. Mixing can improve the effectiveness of the microbial substrate, thereby reducing the nutrient constraints on the growth of soil microorganisms and improving the stand stability.
Soil enzymes play an important role in the maintenance of soil health and nutrient cycling [23]. They are important indicators of biochemical processes and microbial activity. Decreases in soil enzyme activities are common during intensive management of Moso bamboo [9]. The present study showed that C cycle enzyme activities, that is, peroxidase, polyphenol oxidase, and β-glucosidase, significantly (p < 0.05) increased in the soil after mixing. A positive correlation between C cycle enzymes and nitrogen content was previously demonstrated [51], and the same results were obtained in this study. Phosphorus limitation due to nitrogen loading affects the soil carbon cycle [52]. Soil microorganisms can reduce phosphorus limitation by allocating excess nitrogen for the production of phosphatases [53]. In our study, changes in acid phosphatase activity were highly consistent with changes in NH4+-N, NO3−-N, and WSON content. They were also significantly (p < 0.05) and positively correlated with the phosphorus fraction content. Increasing urease activity can promote soil organic nitrogen decomposition [54]. The NH4+-N and NO3−-N contents increased with increasing urease activity. Mixing breaks the soil nitrogen limitation to which pure Moso bamboo forests are susceptible, increasing carbon acquisition. This is conducive to improving the soil carbon sequestration capacity. The increase in acid phosphatase and urease activities implied that nitrogen and phosphorus cycling in the soil was improved by mixing.
Mixing Moso bamboo with other tree species is an effective means of improving soil microorganisms in Moso bamboo forests. This can increase the soil microbial abundance, the C-cycling and N-cycling enzyme activities, and the soil carbon sequestration potential. Understanding the relationship between enzyme activities and C, N, and P fractions can help to determine the effects of mixing on soil microorganisms.
4.3. Root Growth and Nutrient Uptake
Soil resource gradients, stand age, and plant species are important factors that influence root traits [55,56]. Peng, C [57] found that the root biomass and length density of Moso bamboo mixed with Cunninghamia lanceolata increased as the proportion of Moso bamboo increased. This is consistent with the decrease in root biomass, root length, and root volume of Moso bamboo after mixing with Cunninghamia lanceolata. The adaptive response of the root system to soil nutrient availability is related to the trade-offs allocated to the root structure for resource uptake [58]. In our study, root growth and nutrient uptake were closely related to soil nutrients. MOC, MBC, and DOC were soil carbon fractions significantly (p < 0.05) and positively correlated with root biomass. Organic carbon stocks and mechanisms of chemical stabilization and physical protection in Moso bamboo forests are primarily driven by root traits [42]. Root-sourced DOC can enter the MOC pool via a rapid and efficient microbial pathway [59]. Organic matter exudation from dead root formation can bind particles such as microagglomerates into macroscopic agglomerates, increasing soil erosion resistance. Therefore, a higher root biomass represents a higher soil binding capacity. In this study, MP and MS were the most accurate indicators of root biomass and carbon fraction.
Changes in soil NO3−-N and available P are important mechanisms for changes in root morphology [60]. In this study, there was a positive correlation between nitrogen and phosphorus uptake by roots and soil nitrogen and phosphorus fractions. Nitrogen and phosphorus uptake by hairy vetch roots significantly (p < 0.05) increased in MS and MP, but decreased in MC. Cunninghamia lanceolata, the only shallow-rooted plant in the mixed forest, may have relatively intense nutrient competition with the Moso bamboo root system, resulting in reduced nutrient uptake. Sassafras tzumu and Pseudolarix amabilis are deep-rooted plants that have spatial complementarity with Moso bamboo in the underground root system. This avoids the occurrence of direct competition for soil nutrients. Greater root biomass and nutrient uptake were also observed in Moso bamboo when it was mixed with the deep-rooted plant Choerospondias axillaris. Strong negative correlations of root biomass and morphology with soil nitrogen fractions and soil enzymes and positive correlations of root growth parameters with acid phosphatase have been reported for Moso bamboo [9]. In our study, root growth parameters, especially nitrogen uptake and phosphorus uptake, showed positive correlations with most of the environmental factors. This may be because mixing enhances soil nutrients and provides a positive ecosystem-wide impact. Examining the relationship between environmental factors and root growth is conducive to further understanding the mechanisms of plant–environment interactions and helps us to better understand changes in plant growth status and the environment.
5. Conclusions
Different stand structures significantly influence soil carbon (C), nitrogen (N), and phosphorus (P) contents, enhancing nutrient effectiveness. This in turn boosts soil microbial abundance and enzyme activity, which affects root growth and nutrient uptake. Soil microbial abundance is strongly correlated with enzyme activities and environmental factors, and root growth interacts with these factors in the two soil layers at depths of 0–20 cm and 20–40 cm. Among the four mixed species, Moso bamboo combined with Sassafras tzumu and Pseudolarix amabilis showed improved soil C, N, and P pools, along with enhanced microbial abundance and enzyme activities. Mixing also positively impacts the fine roots of Moso bamboo. Consequently, we recommend Sassafras tzumu and Pseudolarix amabilis as the preferred species for mixing with Moso bamboo. Further research should investigate the effects of changed litterfall and root secretions on soil due to mixed forests.
Author Contributions
Conceptualization, X.D. and C.Y.; methodology, Y.N.; software, Y.N.; investigation, X.Z., Z.W., Z.C., H.G., Y.B. and A.W.; resources, X.D.; data curation, Y.N. and C.Y.; writing—original draft preparation, Y.N.; writing—review and editing, Y.B. and L.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by (1) the Key Research and Development Program of Zhejiang Province (2020C02008) (2) Fundamental Research Funds for the Central Non-profit Research Institution of Chinese Academy of Forestry (Grant No. CAFYBB2023XB002) (3) the National Natural Science Foundation of China (Grant No. 31600448).
Data Availability Statement
The datasets are available from the corresponding author upon reasonable request.
Conflicts of Interest
Author Chuanbao Yang was employed by the company Suzhou Baoyu Agricultural Technology Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
References
- Song, X.; Zhou, G.; Jiang, H.; Yu, S.; Fu, J.; Li, W.; Wang, W.; Ma, Z.; Peng, C. Carbon Sequestration by Chinese Bamboo Forests and Their Ecological Benefits: Assessment of Potential, Problems, and Future Challenges. Environ. Rev. 2011, 19, 418–428. [Google Scholar] [CrossRef]
- Song, X.; Peng, C.; Ciais, P.; Li, Q.; Xiang, W.; Xiao, W.; Zhou, G.; Deng, L. Nitrogen Addition Increased CO2 Uptake More than Non-CO 2 Greenhouse Gases Emissions in a Moso Bamboo Forest. Sci. Adv. 2020, 6, eaaw5790. [Google Scholar] [CrossRef]
- Li, P.; Zhou, G.; Du, H.; Lu, D.; Mo, L.; Xu, X.; Shi, Y.; Zhou, Y. Current and Potential Carbon Stocks in Moso Bamboo Forests in China. J. Environ. Manag. 2015, 156, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Guo, L.; Ramakrishnan, M.; Fei, Z.; Vinod, K.K.; Ding, Y.; Jiao, C.; Gao, Z.; Zha, R.; Wang, C.; et al. Rapid Growth of Moso Bamboo (Phyllostachys edulis): Cellular Roadmaps, Transcriptome Dynamics, and Environmental Factors. Plant Cell 2022, 34, 3577–3610. [Google Scholar] [CrossRef] [PubMed]
- Buckingham, K.; Jepson, P.; Wu, L.; Ramanuja Rao, I.V.; Jiang, S.; Liese, W.; Lou, Y.; Fu, M. The Potential of Bamboo Is Constrained by Outmoded Policy Frames. Ambio 2011, 40, 544–548. [Google Scholar] [CrossRef] [PubMed]
- Yen, T.-M.; Lee, J.-S. Comparing Aboveground Carbon Sequestration between Moso Bamboo (Phyllostachys heterocycla) and China Fir (Cunninghamia lanceolata) Forests Based on the Allometric Model. For. Ecol. Manag. 2011, 261, 995–1002. [Google Scholar] [CrossRef]
- Liu, J.; Jiang, P.; Wang, H.; Zhou, G.; Wu, J.; Yang, F.; Qian, X. Seasonal Soil CO2 Efflux Dynamics after Land Use Change from a Natural Forest to Moso Bamboo Plantations in Subtropical China. For. Ecol. Manag. 2011, 262, 1131–1137. [Google Scholar] [CrossRef]
- Qin, H.; Chen, J.; Wu, Q.; Niu, L.; Li, Y.; Liang, C.; Shen, Y.; Xu, Q. Intensive Management Decreases Soil Aggregation and Changes the Abundance and Community Compositions of Arbuscular Mycorrhizal Fungi in Moso Bamboo (Phyllostachys pubescens) Forests. For. Ecol. Manag. 2017, 400, 246–255. [Google Scholar] [CrossRef]
- Ni, H.; Su, W.; Fan, S.; Chu, H. Effects of Intensive Management Practices on Rhizosphere Soil Properties, Root Growth, and Nutrient Uptake in Moso Bamboo Plantations in Subtropical China. For. Ecol. Manag. 2021, 493, 119083. [Google Scholar] [CrossRef]
- Li, C.; Shi, Y.; Zhou, G.; Zhou, Y.; Xu, L.; Tong, L.; Liu, X. Effects of Different Management Approaches on Soil Carbon Dynamics in Moso Bamboo Forest Ecosystems. Catena 2018, 169, 59–68. [Google Scholar] [CrossRef]
- Xu, Q.; Jiang, P.; Xu, Z. Soil Microbial Functional Diversity under Intensively Managed Bamboo Plantations in Southern China. J. Soils Sediments 2008, 8, 177–183. [Google Scholar] [CrossRef]
- Li, Q.; Song, X.; Gu, H.; Gao, F. Nitrogen Deposition and Management Practices Increase Soil Microbial Biomass Carbon but Decrease Diversity in Moso Bamboo Plantations. Sci. Rep. 2016, 6, 28235. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Wen, X.; Wu, Z.; Zhong, H.; Zhang, X. Deciphering the Ramet System of a Bamboo Plant in Response to Intensive Management. Forests 2022, 13, 1968. [Google Scholar] [CrossRef]
- Gärtner, S.; Reif, A. The Impact of Forest Transformation on Stand Structure and Ground Vegetation in the Southern Black Forest, Germany. Plant Soil 2004, 264, 35–51. [Google Scholar] [CrossRef]
- Kaitaniemi, P.; Lintunen, A. Neighbor Identity and Competition Influence Tree Growth in Scots Pine, Siberian Larch, and Silver Birch. Ann. For. Sci. 2010, 67, 604. [Google Scholar] [CrossRef]
- Chen, X.; Chen, X.; Huang, S.; Fang, D. Impacts of Moso Bamboo (Phyllostachys pubescens) Invasion on Species Diversity and Aboveground Biomass of Secondary Coniferous and Broad-Leaved Mixed Forest. Front. Plant Sci. 2022, 13, 1001785. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Xu, X.; Wu, Z.; Huang, Z.; Gao, G.; Zhang, J.; Zhang, X. Native Bamboo (Indosasa shibataeoides McClure) Invasion of Broadleaved Forests Promotes Soil Organic Carbon Sequestration in South China Karst. Forests 2023, 14, 2135. [Google Scholar] [CrossRef]
- Qin, H.; Niu, L.; Wu, Q.; Chen, J.; Li, Y.; Liang, C.; Xu, Q.; Fuhrmann, J.J.; Shen, Y. Bamboo Forest Expansion Increases Soil Organic Carbon through Its Effect on Soil Arbuscular Mycorrhizal Fungal Community and Abundance. Plant Soil 2017, 420, 407–421. [Google Scholar] [CrossRef]
- Zhang, M.-M.; Fan, S.-H.; Guan, F.-Y.; Yan, X.-R.; Yin, Z.-X. Soil Bacterial Community Structure of Mixed Bamboo and Broad-Leaved Forest Based on Tree Crown Width Ratio. Sci. Rep. 2020, 10, 6522. [Google Scholar] [CrossRef]
- Bai, Y.; Chen, S.; Shi, S.; Qi, M.; Liu, X.; Wang, H.; Wang, Y.; Jiang, C. Effects of Different Management Approaches on the Stoichiometric Characteristics of Soil C, N, and P in a Mature Chinese Fir Plantation. Sci. Total Environ. 2020, 723, 137868. [Google Scholar] [CrossRef]
- Xu, H.; Qu, Q.; Chen, Y.; Wang, M.; Liu, G.; Xue, S.; Yang, X. Disentangling the Direct and Indirect Effects of Cropland Abandonment on Soil Microbial Activity in Grassland Soil at Different Depths. Catena 2020, 194, 104774. [Google Scholar] [CrossRef]
- Cai, X.; Lin, Z.; Penttinen, P.; Li, Y.; Li, Y.; Luo, Y.; Yue, T.; Jiang, P.; Fu, W. Effects of Conversion from a Natural Evergreen Broadleaf Forest to a Moso Bamboo Plantation on the Soil Nutrient Pools, Microbial Biomass and Enzyme Activities in a Subtropical Area. For. Ecol. Manag. 2018, 422, 161–171. [Google Scholar] [CrossRef]
- Moghimian, N.; Hosseini, S.M.; Kooch, Y.; Darki, B.Z. Impacts of Changes in Land Use/Cover on Soil Microbial and Enzyme Activities. Catena 2017, 157, 407–414. [Google Scholar] [CrossRef]
- Wang, L.; Pang, X.; Li, N.; Qi, K.; Huang, J.; Yin, C. Effects of Vegetation Type, Fine and Coarse Roots on Soil Microbial Communities and Enzyme Activities in Eastern Tibetan Plateau. Catena 2020, 194, 104694. [Google Scholar] [CrossRef]
- Tripathi, S.K.; Singh, K.P.; Singh, P.K. Temporal Changes in Spatial Pattern of Fine-root Mass and Nutrient Concentrations in Indian Bamboo Savanna. Appl. Veg. Sci. 1999, 2, 229–238. [Google Scholar] [CrossRef]
- Li, J.; Shangguan, Z.; Deng, L. Free Particulate Organic Carbon Plays Critical Roles in Carbon Accumulations during Grassland Succession since Grazing Exclusion. Soil Tillage Res. 2022, 220, 105380. [Google Scholar] [CrossRef]
- Tietz, A.; Kirschner, A.; Langergraber, G.; Sleytr, K.; Haberl, R. Characterisation of Microbial Biocoenosis in Vertical Subsurface Flow Constructed Wetlands. Sci. Total Environ. 2007, 380, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, J.; Chang, S.X.; Jiang, P.; Zhou, G.; Shen, Z.; Wu, J.; Lin, L.; Wang, Z.; Shen, M. Converting Native Shrub Forests to Chinese Chestnut Plantations and Subsequent Intensive Management Affected Soil C and N Pools. For. Ecol. Manag. 2014, 312, 161–169. [Google Scholar] [CrossRef]
- Bremner, J.B.; Smith, R.J.; Tarrant, G.J. A Meisenheimer Rearrangement Approach to Bridgehead Hydroxylated Tropane Alkaloid Derivatives. Tetrahedron Lett. 1996, 37, 97–100. [Google Scholar] [CrossRef]
- Li, F.-M.; Song, Q.-H.; Jjemba, P.K.; Shi, Y.-C. Dynamics of Soil Microbial Biomass C and Soil Fertility in Cropland Mulched with Plastic Film in a Semiarid Agro-Ecosystem. Soil Biol. Biochem. 2004, 36, 1893–1902. [Google Scholar] [CrossRef]
- Malik, M.A.; Marschner, P.; Khan, K.S. Addition of Organic and Inorganic P Sources to Soil—Effects on P Pools and Microorganisms. Soil Biol. Biochem. 2012, 49, 106–113. [Google Scholar] [CrossRef]
- Saiya-Cork, K.R.; Sinsabaugh, R.L.; Zak, D.R. The Effects of Long Term Nitrogen Deposition on Extracellular Enzyme Activity in an Acer Saccharum Forest Soil. Soil Biol. Biochem. 2002, 34, 1309–1315. [Google Scholar] [CrossRef]
- Guo, L.B.; Halliday, M.J.; Siakimotu, S.J.M.; Gifford, R.M. Fine Root Production and Litter Input: Its Effects on Soil Carbon. Plant Soil 2005, 272, 1–10. [Google Scholar] [CrossRef]
- Yang, C.; Ni, H.; Zhong, Z.; Zhang, X.; Bian, F. Changes in Soil Carbon Pools and Components Induced by Replacing Secondary Evergreen Broadleaf Forest with Moso Bamboo Plantations in Subtropical China. Catena 2019, 180, 309–319. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiao, L.; Guan, D.; Chen, Y.; Motelica-Heino, M.; Peng, Y.; Lee, S.Y. The Role of Mangrove Fine Root Production and Decomposition on Soil Organic Carbon Component Ratios. Ecol. Indic. 2021, 125, 107525. [Google Scholar] [CrossRef]
- Sheng, H.; Zhou, P.; Zhang, Y.; Kuzyakov, Y.; Zhou, Q.; Ge, T.; Wang, C. Loss of Labile Organic Carbon from Subsoil Due to Land-Use Changes in Subtropical China. Soil Biol. Biochem. 2015, 88, 148–157. [Google Scholar] [CrossRef]
- Han, X.; Zhao, F.; Tong, X.; Deng, J.; Yang, G.; Chen, L.; Kang, D. Understanding Soil Carbon Sequestration Following the Afforestation of Former Arable Land by Physical Fractionation. Catena 2017, 150, 317–327. [Google Scholar] [CrossRef]
- Budge, K.; Leifeld, J.; Hiltbrunner, E.; Fuhrer, J. Litter Quality and PH Are Strong Drivers of Carbon Turnover and Distribu-Tion in Alpine Grassland Soils. Biogeosciences 2010, 7. [Google Scholar] [CrossRef]
- Tong, X.; Xu, M.; Wang, X.; Bhattacharyya, R.; Zhang, W.; Cong, R. Long-Term Fertilization Effects on Organic Carbon Fractions in a Red Soil of China. Catena 2014, 113, 251–259. [Google Scholar] [CrossRef]
- McGILL, W.B.; Cannon, K.R.; Robertson, J.A.; Cook, F.D. Dynamics of Soil Microbial Biomass and Water-Soluble Organic c in Breton l after 50 Years of Cropping to Two Rotations. Can. J. Soil Sci. 1986, 66, 1–19. [Google Scholar] [CrossRef]
- Naorem, A.; Maverick, J.; Singh, P.; Udayana, S.K. Microbial Community Structure in Organic Farming and Their Management. In Advances in Organic Farming; Elsevier: Amsterdam, The Netherlands, 2021; pp. 47–58. ISBN 9780128223581. [Google Scholar]
- Yang, C.; Wang, A.; Zhu, Z.; Lin, S.; Bi, Y.; Du, X. Impact of Extensive Management System on Soil Properties and Carbon Sequestration under an Age Chronosequence of Moso Bamboo Plantations in Subtropical China. For. Ecol. Manag. 2021, 497, 119535. [Google Scholar] [CrossRef]
- Hansen, E.M.; Munkholm, L.J.; Melander, B.; Olesen, J.E. Can Non-Inversion Tillage and Straw Retainment Reduce N Leaching in Cereal-Based Crop Rotations? Soil Tillage Res. 2010, 109, 1–8. [Google Scholar] [CrossRef]
- Mancinelli, R.; Campiglia, E.; Di Tizio, A.; Marinari, S. Soil Carbon Dioxide Emission and Carbon Content as Affected by Conventional and Organic Cropping Systems in Mediterranean Environment. Appl. Soil Ecol. 2010, 46, 64–72. [Google Scholar] [CrossRef]
- Elser, J.J.; Bracken, M.E.S.; Cleland, E.E.; Gruner, D.S.; Harpole, W.S.; Hillebrand, H.; Ngai, J.T.; Seabloom, E.W.; Shurin, J.B.; Smith, J.E. Global Analysis of Nitrogen and Phosphorus Limitation of Primary Producers in Freshwater, Marine and Terrestrial Ecosystems. Ecol. Lett. 2007, 10, 1135–1142. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; Conant, R.T.; Zhou, G.; Wang, Y.; Wang, N.; Li, Y.; Zhang, K. Effects of Moso Bamboo Encroachment into Native, Broad-Leaved Forests on Soil Carbon and Nitrogen Pools. Sci. Rep. 2016, 6, 31480. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Mo, Q.; Wang, H.; Zhang, Z.; Huang, G.; Ye, Q.; Zou, Q.; Kong, F.; Liu, Y.; Geoff Wang, G. Moso Bamboo (Phyllostachys edulis (Carriere) J. Houzeau) Invasion Affects Soil Phosphorus Dynamics in Adjacent Coniferous Forests in Subtropical China. Ann. For. Sci. 2018, 75, 24. [Google Scholar] [CrossRef]
- Chen, Z.; Li, Y.; Chang, S.X.; Xu, Q.; Li, Y.; Ma, Z.; Qin, H.; Cai, Y. Linking Enhanced Soil Nitrogen Mineralization to Increased Fungal Decomposition Capacity with Moso Bamboo Invasion of Broadleaf Forests. Sci. Total Environ. 2021, 771, 144779. [Google Scholar] [CrossRef] [PubMed]
- Chaer, G.; Fernandes, M.; Myrold, D.; Bottomley, P. Comparative Resistance and Resilience of Soil Microbial Communities and Enzyme Activities in Adjacent Native Forest and Agricultural Soils. Microb. Ecol. 2009, 58, 414–424. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Li, Q.; Gai, X.; Zhang, X.; Zhong, Z.; Bian, F.; Yang, C. Effects of On- and off-Year Management Practices on the Soil Organic C Fractions and Microbial Community in a Moso Bamboo (Phyllostachys edulis) Forest in Subtropical China. Front. Plant Sci. 2022, 13, 1020344. [Google Scholar] [CrossRef]
- Bowles, T.M.; Acosta-Martínez, V.; Calderón, F.; Jackson, L.E. Soil Enzyme Activities, Microbial Communities, and Carbon and Nitrogen Availability in Organic Agroecosystems across an Intensively-Managed Agricultural Landscape. Soil Biol. Biochem. 2014, 68, 252–262. [Google Scholar] [CrossRef]
- Luo, M.; Moorhead, D.L.; Ochoa-Hueso, R.; Mueller, C.W.; Ying, S.C.; Chen, J. Nitrogen Loading Enhances Phosphorus Limitation in Terrestrial Ecosystems with Implications for Soil Carbon Cycling. Funct. Ecol. 2022, 36, 2845–2858. [Google Scholar] [CrossRef]
- Allison, S.D.; Vitousek, P.M. Responses of Extracellular Enzymes to Simple and Complex Nutrient Inputs. Soil Biol. Biochem. 2005, 37, 937–944. [Google Scholar] [CrossRef]
- Xing, S.; Chen, C.; Zhou, B.; Zhang, H.; Nang, Z.; Xu, Z. Soil Soluble Organic Nitrogen and Active Microbial Characteristics under Adjacent Coniferous and Broadleaf Plantation Forests. J. Soils Sediments 2010, 10, 748–757. [Google Scholar] [CrossRef]
- Roumet, C.; Birouste, M.; Picon-Cochard, C.; Ghestem, M.; Osman, N.; Vrignon-Brenas, S.; Cao, K.-F.; Stokes, A. Root Structure–Function Relationships in 74 Species: Evidence of a Root Economics Spectrum Related to Carbon Economy. New Phytol. 2016, 210, 815–826. [Google Scholar] [CrossRef] [PubMed]
- Ostonen, I.; Helmisaari, H.-S.; Borken, W.; Tedersoo, L.; Kukumägi, M.; Bahram, M.; Lindroos, A.-J.; Nöjd, P.; Uri, V.; Merilä, P.; et al. Fine Root Foraging Strategies in Norway Spruce Forests across a European Climate Gradient. Glob. Change Biol. 2011, 17, 3620–3632. [Google Scholar] [CrossRef]
- Peng, C.; Tu, J.; Yang, M.; Meng, Y.; Li, M.; Ai, W. Root Stoichiometric Dynamics and Homeostasis of Invasive Species Phyllostachys Edulis and Native Species Cunninghamia Lanceolata in a Subtropical Forest in China. J. For. Res. 2021, 32, 2001–2010. [Google Scholar] [CrossRef]
- Bloom, A.J.; Chapin, F.S., III; Mooney, H.A. Resource Limitation in Plants-an Economic Analogy. Annu. Rev. Ecol. Syst. 1985, 16, 363–392. [Google Scholar] [CrossRef]
- Sokol, N.W.; Bradford, M.A. Microbial Formation of Stable Soil Carbon Is More Efficient from Belowground than Aboveground Input. Nat. Geosci. 2019, 12, 46–53. [Google Scholar] [CrossRef]
- Chen, G.-T.; Tu, L.-H.; Peng, Y.; Hu, H.-L.; Hu, T.-X.; Xu, Z.-F.; Liu, L.; Tang, Y. Effect of Nitrogen Additions on Root Morphology and Chemistry in a Subtropical Bamboo Forest. Plant Soil 2017, 412, 441–451. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).