Innovative Treatment of Ancient Architectural Wood Using Polyvinyl Alcohol and Methyltrimethoxysilane for Improved Waterproofing, Dimensional Stability, and Self-Cleaning Properties
Abstract
:1. Introduction
2. Experimental Section
2.1. Raw Materials
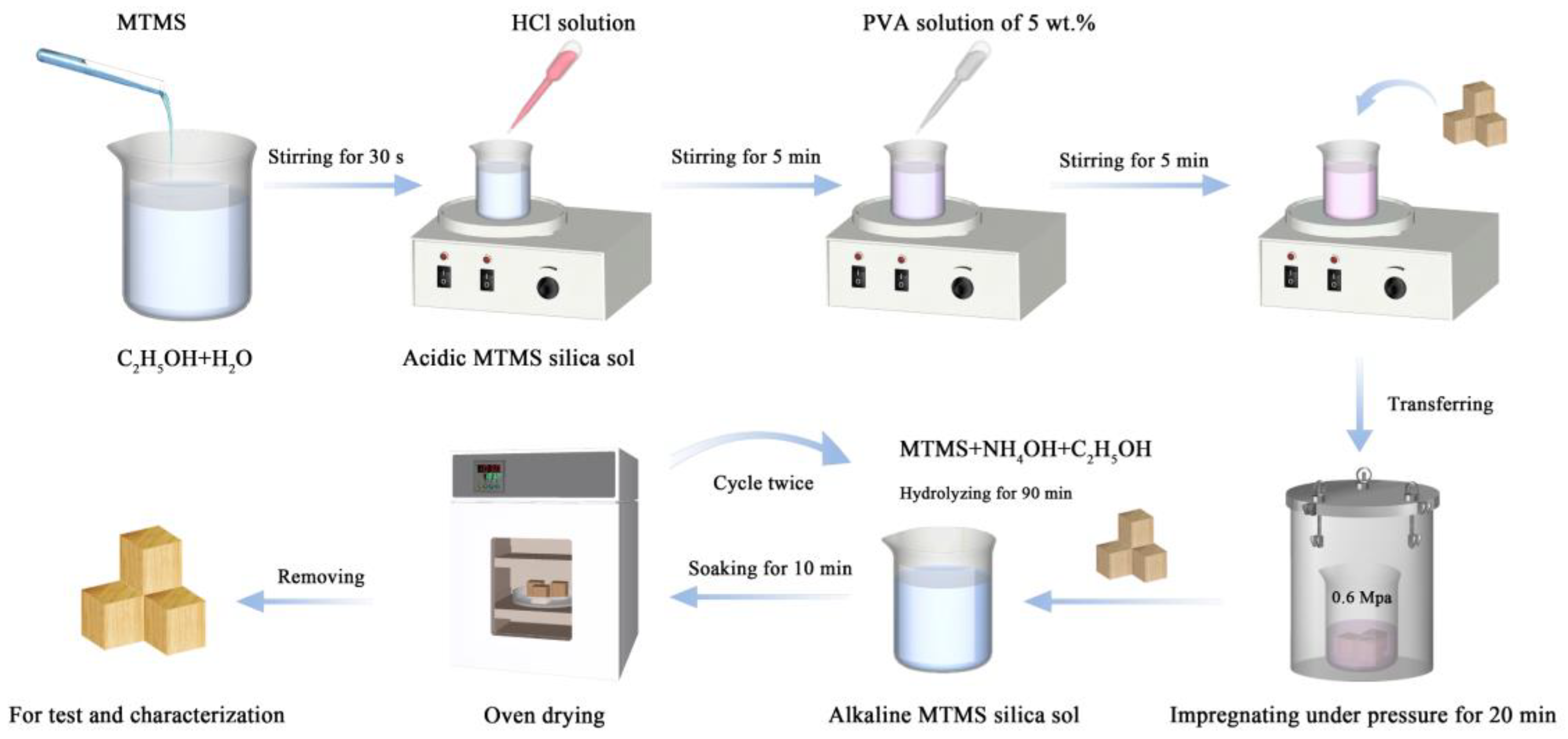
2.2. Preparation of the Samples
2.2.1. Acidic MTMS–Silica Sol/PVA Impregnation
2.2.2. Construction of Superhydrophobic Surface
2.3. Morphological Observation
2.4. FTIR, XRD and XPS Investigation
2.5. Wettability Characterization
2.6. Water Uptake/Moisture Absorption Measurement
2.7. Dimensional Stability Determination
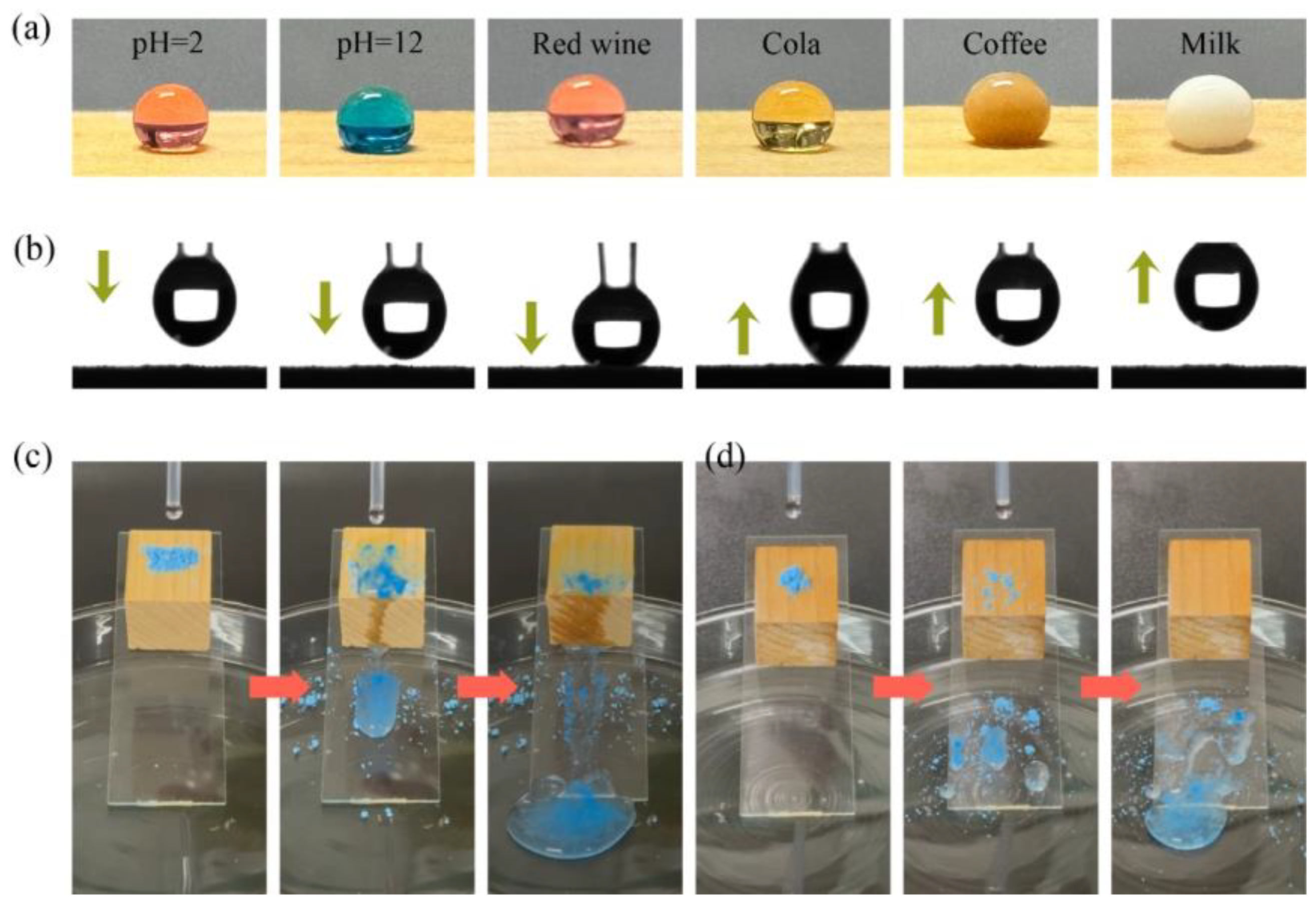
2.8. Anti-Fouling and Self-Cleaning Test
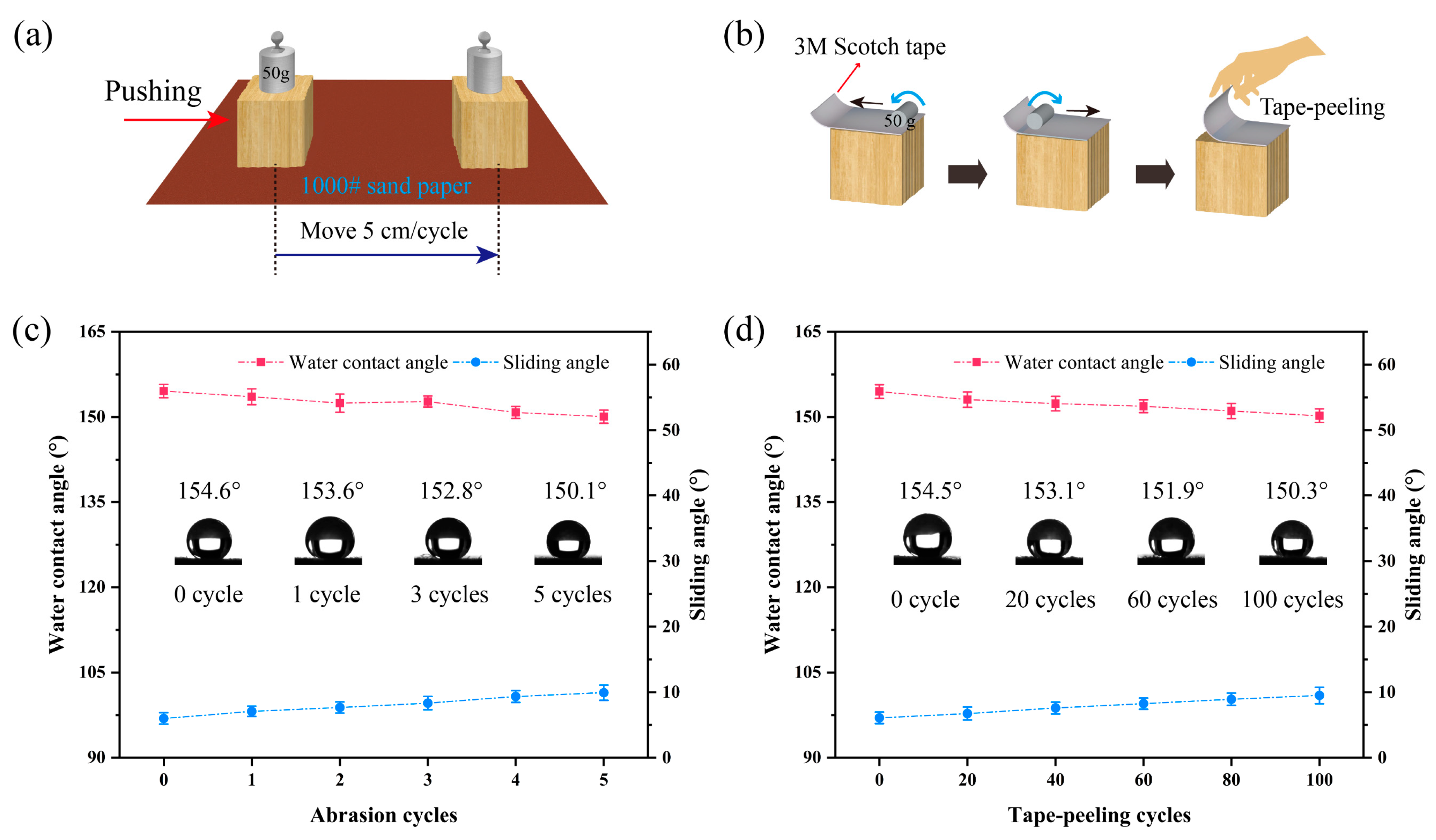
2.9. Superhydrophobic Surface Durability Test
2.10. Thermal Stability Test
2.11. Compressive Strength Test
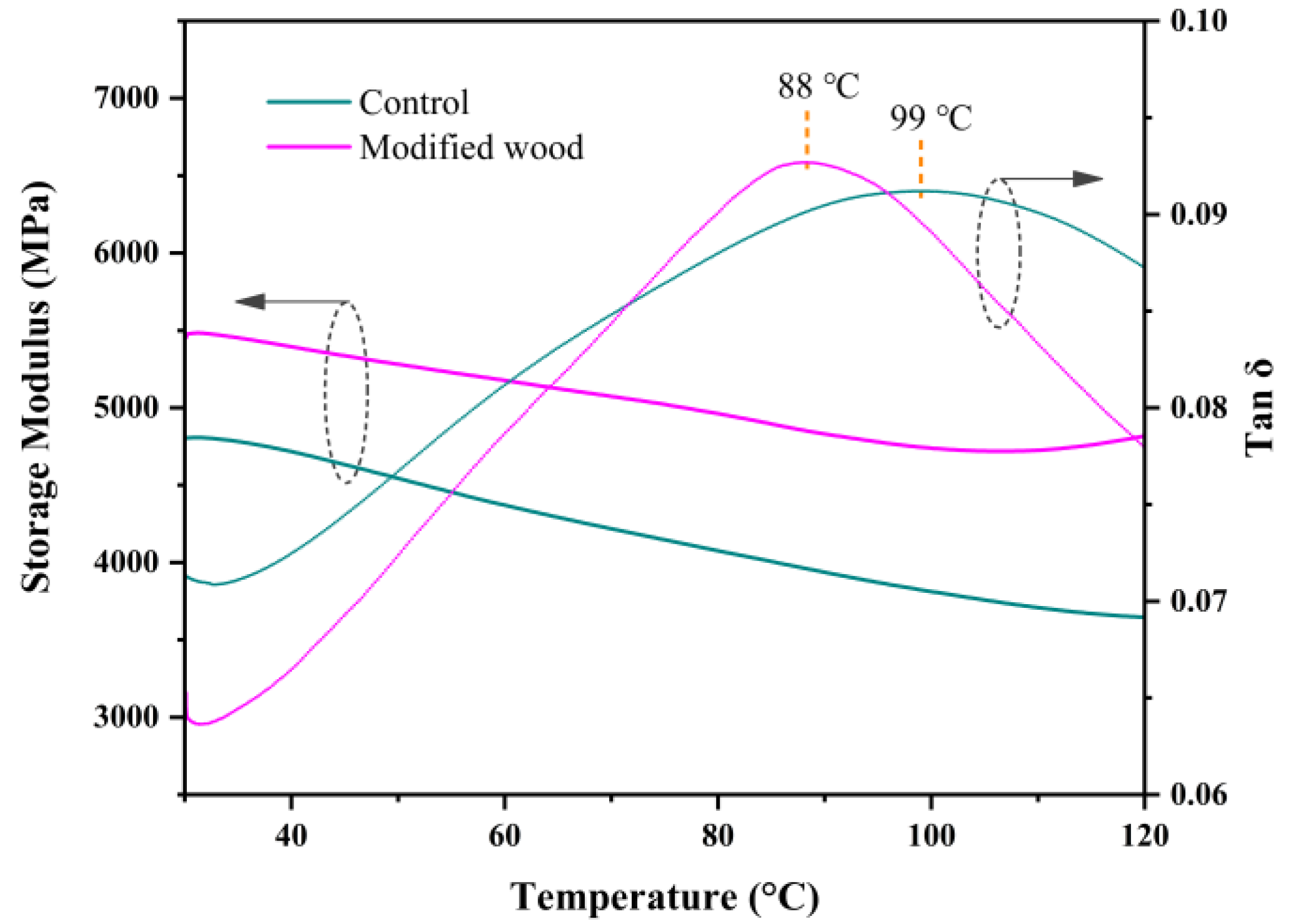
2.12. Dynamic Mechanical Thermal Test
3. Results and Discussion
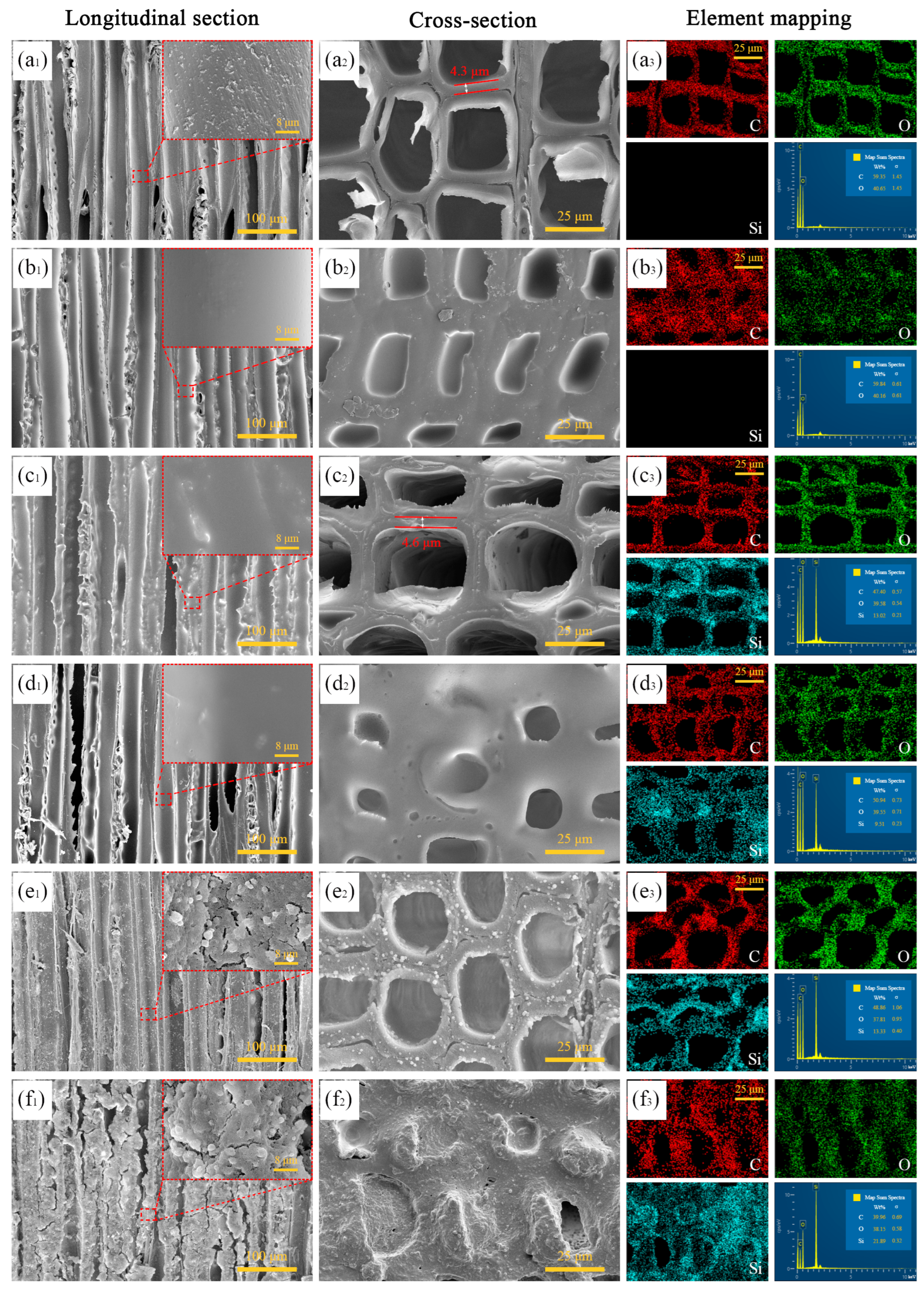
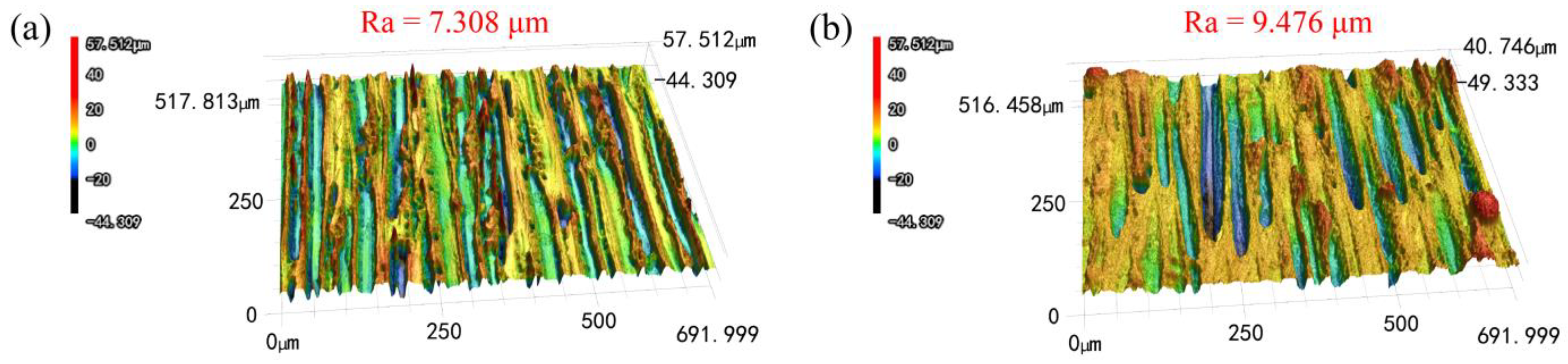
3.1. Morphologies Observation
3.2. FT-IR Analysis
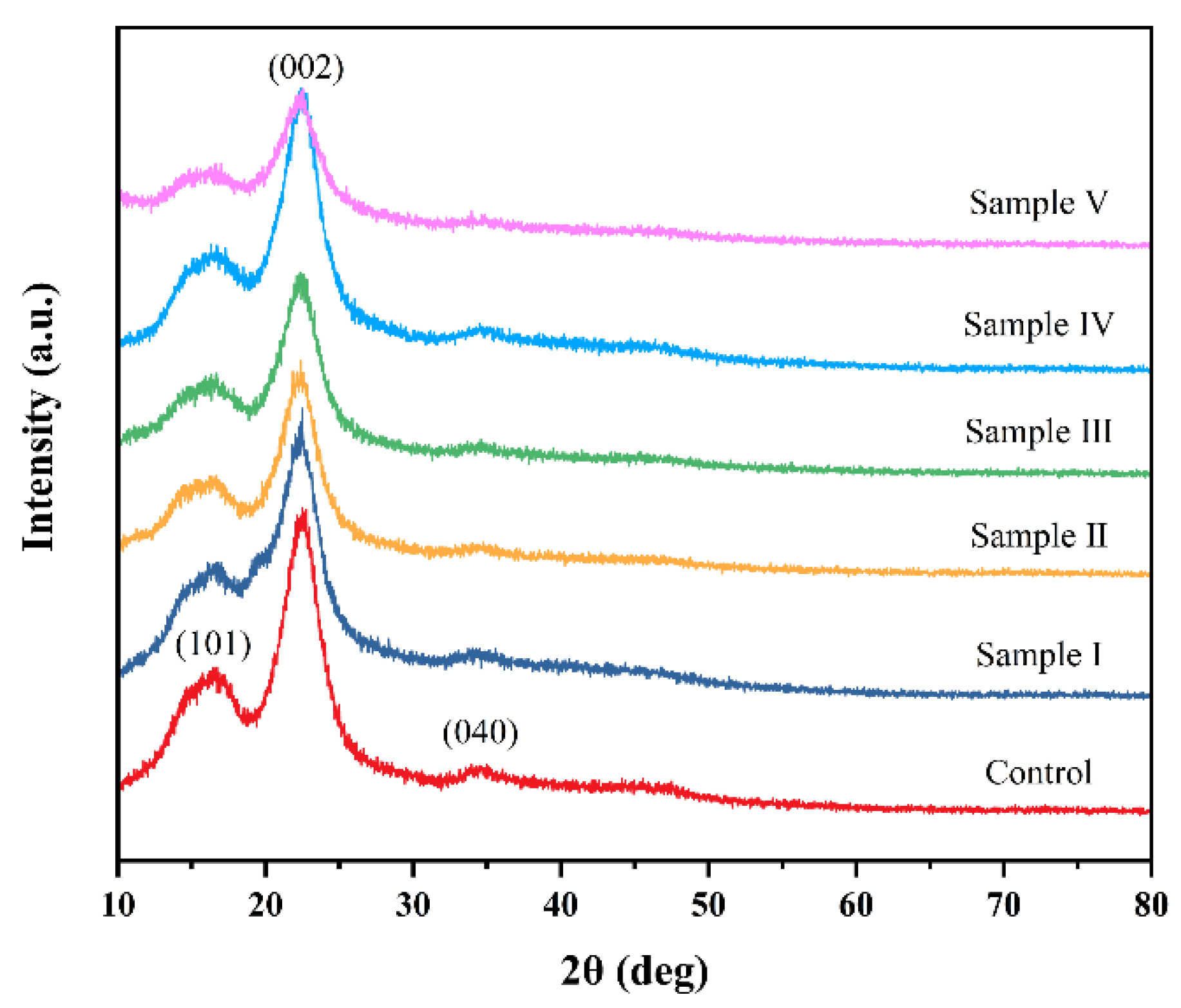
3.3. XRD Analysis
3.4. XPS Analysis
3.5. Modification Mechanism Analysis
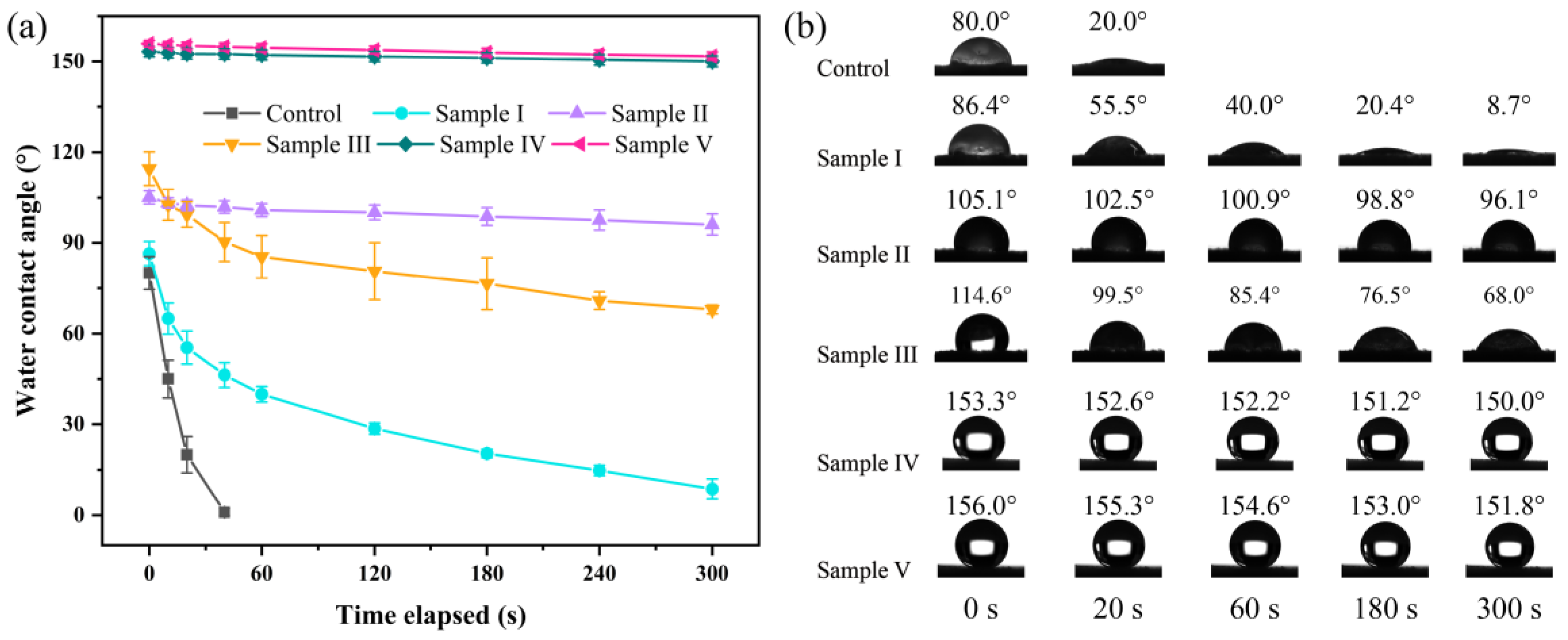
3.6. Dynamic Wettability Analysis
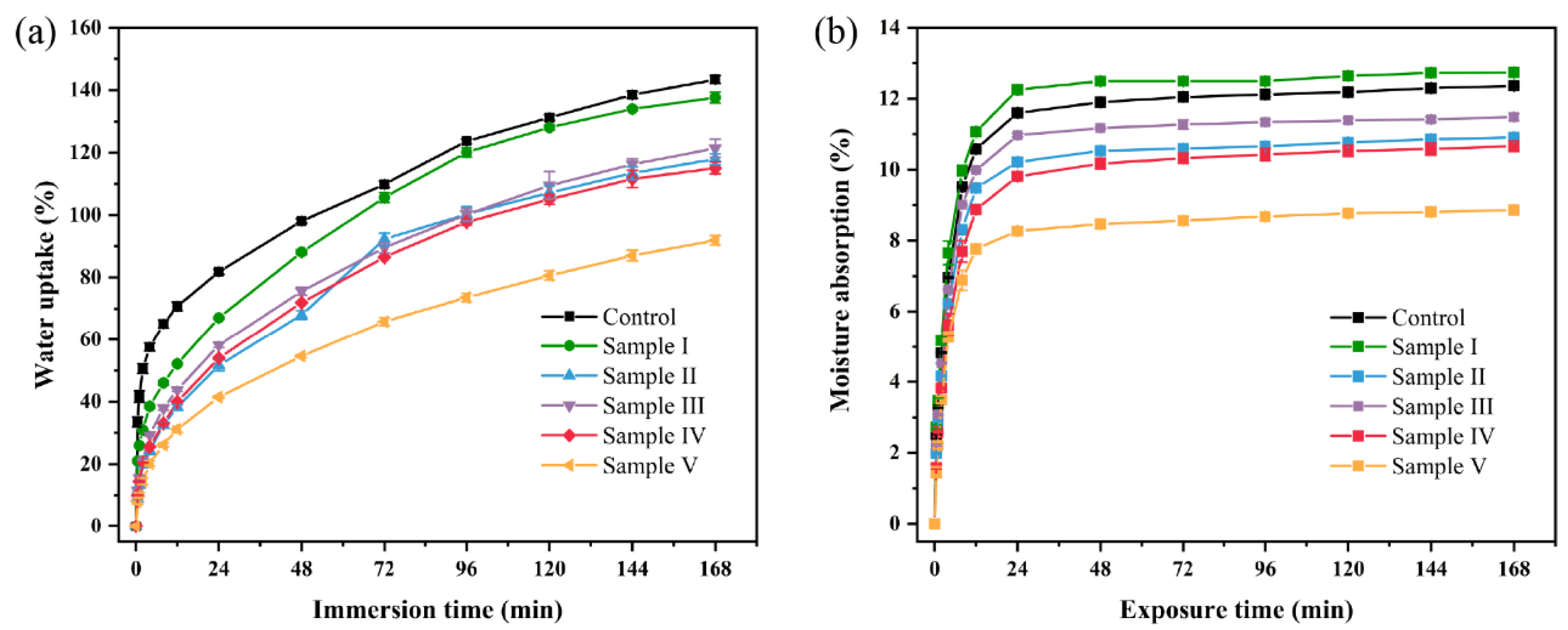
3.7. Water Uptake (WU) and Moisture Absorption (MA) Analysis
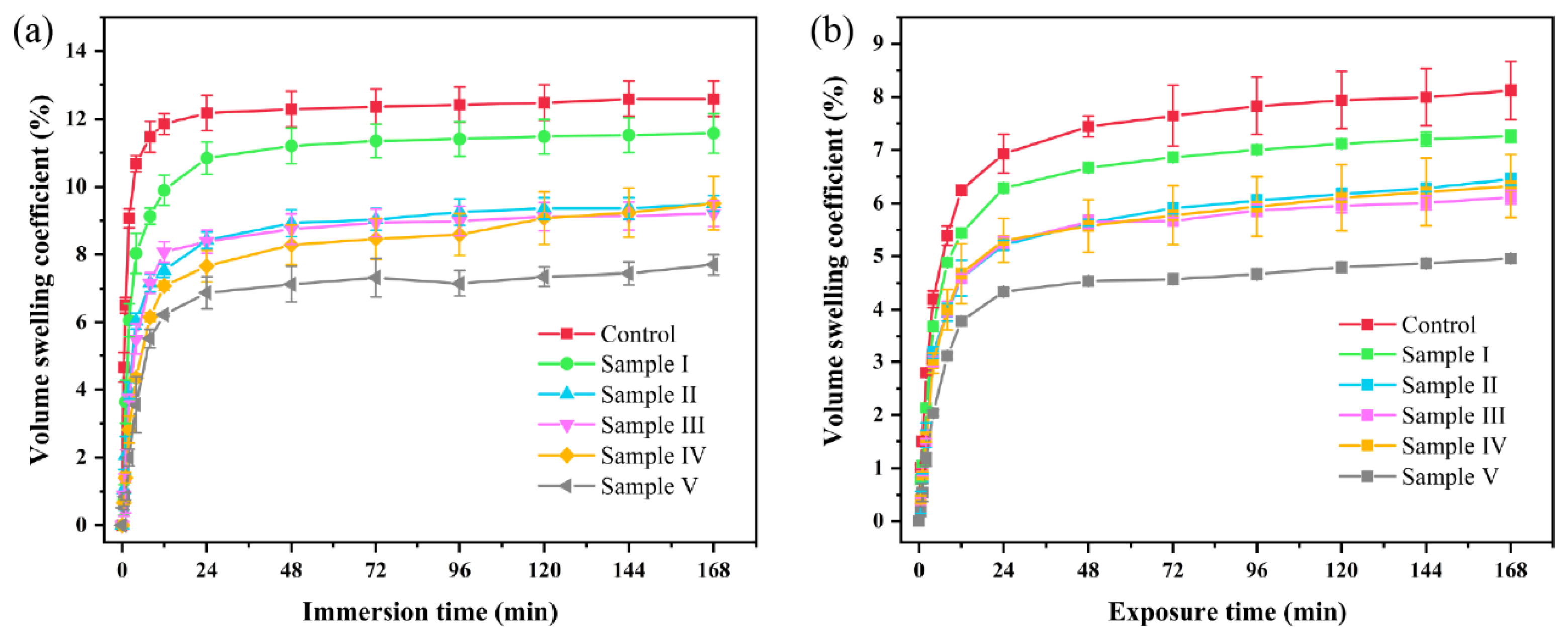
3.8. Dimensional Stability Analysis
3.9. Anti-Fouling and Self-Cleaning Performance Analysis
3.10. Durability Analysis
3.11. Thermostability Analysis
3.12. Compressive Strength Analysis
3.13. Dynamic Mechanical Thermal Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Terlikowski, W. Problems and technical issues in the diagnosis, conservation, and rehabilitation of structures of historical wooden buildings with a focus on wooden historic buildings in Poland. Sustainability 2022, 15, 510. [Google Scholar] [CrossRef]
- Long, K.; Chen, K.; Lin, L.; Fu, F.; Zhong, Y. Deterioration of microstructures and properties in ancient architectural wood from Yingxian Wooden Pagoda (1056 AD) during natural aging. Forests 2023, 14, 393. [Google Scholar] [CrossRef]
- Cogulet, A.; Blanchet, P.; Landry, V. The multifactorial aspect of wood weathering: A review based on a holistic approach of wood degradation protected by clear coating. BioResources 2018, 13, 2116–2138. [Google Scholar] [CrossRef]
- Wang, Q.; Feng, X.; Liu, X. Advances in historical wood consolidation and conservation materials. BioResources 2023, 18, 6680–6723. [Google Scholar] [CrossRef]
- Hua, Y.; Chun, Q.; Mi, Z.; Wu, Y. Experimental research on progressive collapse behavior of Chinese ancient timber buildings with different joint strengthening methods. J. Build. Eng. 2023, 68, 106215. [Google Scholar] [CrossRef]
- Peng, Y.; Wang, C.; Zhang, X.; Zheng, W.; Yu, Y. Inorganic-accelerated aging method: An efficient and simple strategy to obtain antique Chinese fir wood for the restoration of ancient wooden architecture. J. Build. Eng. 2024, 84, 108372. [Google Scholar] [CrossRef]
- Brischke, C.; Bayerbach, R.; Otto Rapp, A. Decay-influencing factors: A basis for service life prediction of wood and wood-based products. Wood Mater. Sci. Eng. 2006, 1, 91–107. [Google Scholar] [CrossRef]
- Guo, J.; Zhou, H.; Stevanic, J.S.; Dong, M.; Yu, M.; Salmén, L.; Yin, Y. Effects of ageing on the cell wall and its hygroscopicity of wood in ancient timber construction. Wood Sci. Technol. 2018, 52, 131–147. [Google Scholar] [CrossRef]
- Zhou, K.; Li, A.; Xie, L.; Wang, P.; Wang, C.-C. The organic–inorganic hybrid sol for the consolidation of decayed wood in architectural heritage. Constr. Build. Mater. 2023, 372, 130847. [Google Scholar] [CrossRef]
- Hamed, S.A.A.K.M.; Hassan, M.L. A new mixture of hydroxypropyl cellulose and nanocellulose for wood consolidation. J. Cult. Heritage 2019, 35, 140–144. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, T.; Zhong, H.; Liu, R.; Xu, J. Improved surface properties of a novel self-healing polyurethane-acrylate coating by in situ polymerizations of dihydroxy organo-montmorillonite on ancient wood. Prog. Org. Coat. 2022, 172, 107134. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, R.; Xu, J.; Yuan, X. Protection of wood in ancient timber construction by surface painting of waterborne siloxane-modified polyurethane against water destroy. Wood Mater. Sci. Eng. 2023, 18, 314–321. [Google Scholar] [CrossRef]
- Mok, C.F.; Ching, Y.C.; Muhamad, F.; Abu Osman, N.A.; Hai, N.D.; Hassan, C.R.C. Adsorption of dyes using poly(vinyl alcohol) (PVA) and PVA-based polymer composite adsorbents: A review. J. Polym. Environ. 2020, 28, 775–793. [Google Scholar] [CrossRef]
- Sun, W.; Shen, H.; Cao, J. Modification of wood by glutaraldehyde and poly (vinyl alcohol). Mater. Des. 2016, 96, 392–400. [Google Scholar] [CrossRef]
- Liu, F.; Wang, S.; Zhang, M.; Ma, M.; Wang, C.; Li, J. Improvement of mechanical robustness of the superhydrophobic wood surface by coating PVA/SiO2 composite polymer. Appl. Surf. Sci. 2013, 280, 686–692. [Google Scholar] [CrossRef]
- Van Nguyen, S.; Lee, B.-K. Polyvinyl alcohol/cellulose nanocrystals/alkyl ketene dimer nanocomposite as a novel biodegradable food packing material. Int. J. Biol. Macromol. 2022, 207, 31–39. [Google Scholar] [CrossRef]
- Suleiman, G.S.A.; Zeng, X.; Chakma, R.; Wakai, I.Y.; Feng, Y. Recent advances and challenges in thermal stability of PVA-based film: A review. Polym. Adv. Technol. 2024, 35, e6327. [Google Scholar] [CrossRef]
- Schorr, D.; Blanchet, P. Improvement of white spruce wood dimensional stability by organosilanes sol-gel impregnation and heat treatment. Materials 2020, 13, 973. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Xie, L.; Hess, D.W.; Breedveld, V. Fabrication of amphiphobic softwood and hardwood by treatment with non-fluorinated chemicals. Wood Sci. Technol. 2016, 51, 97–113. [Google Scholar] [CrossRef]
- Nenashev, R.N.; Kotova, N.M.; Vishnevskii, A.S.; Vorotilov, K.A. Effect of methyltrimethoxysilane hydrolysis and condensation conditions on the properties of thin polymethylsilsesquioxane films. Inorg. Mater. 2016, 52, 625–629. [Google Scholar] [CrossRef]
- Baatti, A.; Erchiqui, F.; Bébin, P.; Godard, F.; Bussières, D. A two-step Sol-Gel method to synthesize a ladder polymethylsilsesquioxane nanoparticles. Adv. Powder Technol. 2017, 28, 1038–1046. [Google Scholar] [CrossRef]
- Mahadik, D.B.; Jung, H.-N.R.; Han, W.; Cho, H.H.; Park, H.-H. Flexible, elastic, and superhydrophobic silica-polymer composite aerogels by high internal phase emulsion process. Compos. Sci. Technol. 2017, 147, 45–51. [Google Scholar] [CrossRef]
- Wang, X.; Deng, X.; Wu, L.; Deng, Y.; Liu, Q.; Li, M.; Li, Z. Facile preparation of mechanically strong polyvinyl alcohol/MTMS aerogel composites with improved thermal stability. J. Nanoparticle Res. 2021, 23, 261. [Google Scholar] [CrossRef]
- Wang, Q.; Xu, S.; Xing, X.; Wang, N. Progress in fabrication and applications of micro/nanostructured superhydrophobic surfaces. Surf. Innov. 2022, 10, 89–110. [Google Scholar] [CrossRef]
- Lin, H.; Rosu, C.; Jiang, L.; Sundar, V.A.; Breedveld, V.; Hess, D.W. Nonfluorinated superhydrophobic chemical coatings on polyester fabric prepared with kinetically controlled hydrolyzed methyltrimethoxysilane. Ind. Eng. Chem. Res. 2019, 58, 15368–15378. [Google Scholar] [CrossRef]
- Tang, W.; Cheng, Y.; Jian, Y.; Sun, Y.; Xiao, J.; Yi, L.; Zhang, H.; Xu, T.; Zhang, Y.; Liu, J.; et al. Synergetic strategy to fabricate superhydrophobic wood by MTMS for improving dimensional stability, durability and self-cleaning ability. Mater. Lett. 2023, 343, 134348. [Google Scholar] [CrossRef]
- Yang, T.; Zhong, H.; Xu, C.; Luo, D.; Mei, C. Fabrication and mechanism analysis of wood polymer composites with improved hydrophobicity, dimensional stability and mechanical strength. Cellulose 2023, 30, 3099–3112. [Google Scholar] [CrossRef]
- Long, M.; Yi, L.; Yan, L.; Xu, Z.; Zheng, J. Comparative analysis of microstructure, combustion characteristics and mechanical properties of Pinus massoniana lamb. under natural aging conditions. Therm. Sci. Eng. Prog. 2024, 48, 102425. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, D.; Sun, Z.; Song, J.; Deng, X. Robust superhydrophobicity: Mechanisms and strategies. Chem. Soc. Rev. 2021, 50, 4031–4061. [Google Scholar] [CrossRef]
- Köhler, R.; Sauerbier, P.; Weber, M.; Wander, R.-C.; Wieneke, S.; Viöl, W. Water-repellent characteristics of beech wood coated with parylene-N. Polymers 2021, 13, 2076. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, K.; Luo, F.; Lu, M.; Xiao, F.; Du, X.; Zhang, S.; Liang, L.; Lu, M. Significantly enhanced thermal conductivity in polyvinyl alcohol composites enabled by dopamine modified graphene nanoplatelets. Compos. Part A Appl. Sci. Manuf. 2019, 117, 134–143. [Google Scholar] [CrossRef]
- Chandrakala, H.N.; Ramaraj, B.; Shivakumaraiah; Madhu, G.M.; Siddaramaiah. The influence of zinc oxide–cerium oxide nanoparticles on the structural characteristics and electrical properties of polyvinyl alcohol films. J. Mater. Sci. 2012, 47, 8076–8084. [Google Scholar] [CrossRef]
- Cai, Z.; Lin, J.; Hong, X. Transparent superhydrophobic hollow films (TSHFs) with superior thermal stability and moisture resistance. RSC Adv. 2018, 8, 491–498. [Google Scholar] [CrossRef]
- Jitianu, A.; Doyle, J.; Amatucci, G.; Klein, L.C. Methyl modified siloxane melting gels for hydrophobic films. J. Sol-Gel Sci. Technol. 2009, 53, 272–279. [Google Scholar] [CrossRef]
- Zanini, M.; Lavoratti, A.; Lazzari, L.K.; Galiotto, D.; Baldasso, C.; Zattera, A.J. Obtaining hydrophobic aerogels of unbleached cellulose nanofibers of the species Eucalyptus sp. and Pinus elliottii. J. Nanomater. 2018, 2018, 4646197. [Google Scholar] [CrossRef]
- Xing, Y.; Xue, Y.; Song, J.; Sun, Y.; Huang, L.; Liu, X.; Sun, J. Superhydrophobic coatings on wood substrate for self-cleaning and EMI shielding. Appl. Surf. Sci. 2018, 436, 865–872. [Google Scholar] [CrossRef]
- Yang, H.; Wang, D.; Han, Y.; Tian, P.; Gao, C.; Yang, X.; Mu, H.; Zhang, M. Preparation and properties of modified poplar impregnated with PVA-nano silica sol composite dispersion system. J. Wood Chem. Technol. 2022, 42, 211–221. [Google Scholar] [CrossRef]
- Čabalová, I.; Výbohová, E.; Igaz, R.; Kristak, L.; Kačík, F.; Antov, P.; Papadopoulos, A.N. Effect of oxidizing thermal modification on the chemical properties and thermal conductivity of Norway spruce (Picea abies L.) wood. Wood Mater. Sci. Eng. 2021, 17, 366–375. [Google Scholar] [CrossRef]
- Nzokou, P.; Kamdem, D.P. X-ray photoelectron spectroscopy study of red oak- (Quercus rubra), black cherry- (Prunus serotina) and red pine- (Pinus resinosa) extracted wood surfaces. Surf. Interface Anal. 2005, 37, 689–694. [Google Scholar] [CrossRef]
- Ouyang, L.; Huang, Y.; Cao, J. Hygroscopicity and characterization of wood fibers modified by alkoxysilanes with different chain lengths. BioResources 2014, 9, 7222–7233. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, L.; Shen, Y.; Wang, L.; Ding, Y. One-step fabrication of self-healing and durable superhydrophobic cotton fabrics based on silica aerogel. J. Nanosci. Nanotechnol. 2018, 18, 7721–7731. [Google Scholar] [CrossRef]
- Sun, T.; Zhang, W.; Liu, Y.; Xu, W.; Sun, J.; Wang, J.; Qin, C.; Dai, L. Preparation of high-hydrophobic polyvinyl alcohol-based composite aerogel fibers containing organosilicon for effective thermal insulation. Polym. Adv. Technol. 2024, 35, e6198. [Google Scholar] [CrossRef]
- Luo, X.; Akram, M.Y.; Yuan, Y.; Nie, J.; Zhu, X. Silicon dioxide/poly(vinyl alcohol) composite hydrogels with high mechanical properties and low swellability. J. Appl. Polym. Sci. 2018, 136, 46895. [Google Scholar] [CrossRef]
- Guo, B.; Liu, Y.; Zhang, Q.; Wang, F.; Wang, Q.; Liu, Y.; Li, J.; Yu, H. Efficient flame-retardant and smoke-suppression properties of Mg–Al-layered double-hydroxide nanostructures on wood substrate. ACS Appl. Mater. Interfaces 2017, 9, 23039–23047. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Huang, S.; Zhang, Y.; Shi, L.; Luo, Y.; Deng, X.; Liu, Q.; Li, Z. Flame retardant polyurethane sponge/MTMS aerogel composites with improved mechanical properties under ambient pressure drying. J. Nanoparticle Res. 2020, 22, 221. [Google Scholar] [CrossRef]
- Islam, M.S.; Hamdan, S.; Talib, Z.A.; Ahmed, A.S.; Rahman, M.R. Tropical wood polymer nanocomposite (WPNC): The impact of nanoclay on dynamic mechanical thermal properties. Compos. Sci. Technol. 2012, 72, 1995–2001. [Google Scholar] [CrossRef]
- Panigrahi, R.; Chakraborty, S.; Ye, J.; Lim, G.S.; Lim, F.C.H.; Yam, J.K.H.; Wu, L.Y.; Chng, S.; Prawirasatya, M.; van Herk, A.M.; et al. Elucidating the role of interfacial hydrogen bonds on glass transition temperature change in a poly(vinyl alcohol)/SiO2 polymer-nanocomposite by noncovalent interaction characterization and atomistic molecular dynamics simulations. Macromol. Rapid Commun. 2020, 41, 2000240. [Google Scholar] [CrossRef]


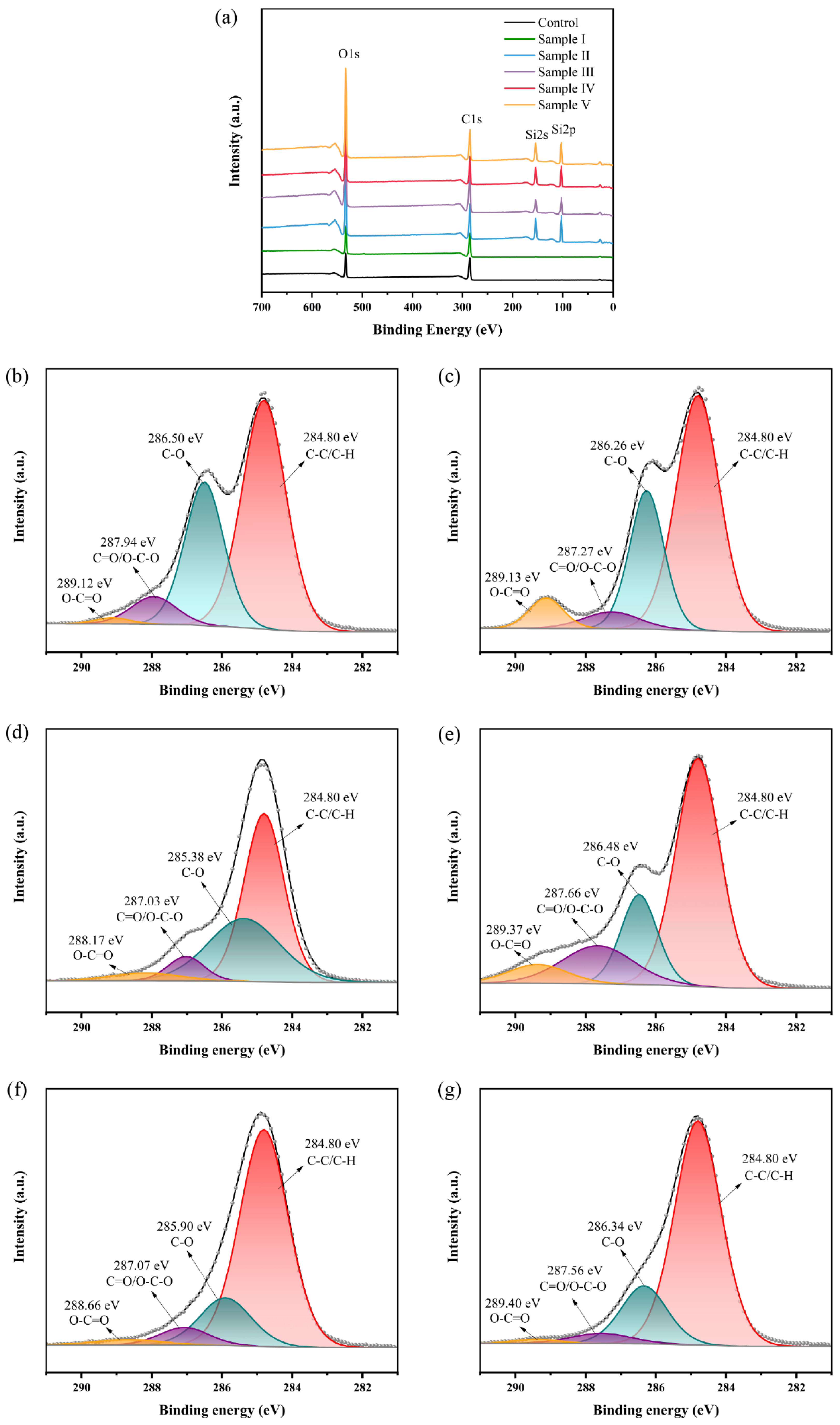



| Sample Label | Elements (at.%) | Ratio | Carbon Components (at.%) | |||||
|---|---|---|---|---|---|---|---|---|
| C | O | Si | C/Si | C1 | C2 | C3 | C4 | |
| Control | 74.47 | 25.53 | - | - | 58.53 | 32.98 | 7.24 | 1.25 |
| Sample I | 69.41 | 30.59 | - | - | 60.24 | 28.11 | 5.51 | 6.14 |
| Sample II | 29.41 | 32.58 | 38.01 | 0.77 | 52.98 | 35.48 | 7.05 | 4.49 |
| Sample III | 39.92 | 34.00 | 26.08 | 1.53 | 58.65 | 19.11 | 15.75 | 6.49 |
| Sample IV | 27.28 | 33.00 | 39.72 | 0.69 | 74.21 | 17.19 | 5.99 | 2.61 |
| Sample V | 29.03 | 32.34 | 38.63 | 0.75 | 75.15 | 19.19 | 4.26 | 1.40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, S.; Tang, W.; Tong, J.; Cao, K.; Yu, H.; Xie, L. Innovative Treatment of Ancient Architectural Wood Using Polyvinyl Alcohol and Methyltrimethoxysilane for Improved Waterproofing, Dimensional Stability, and Self-Cleaning Properties. Forests 2024, 15, 978. https://doi.org/10.3390/f15060978
Zheng S, Tang W, Tong J, Cao K, Yu H, Xie L. Innovative Treatment of Ancient Architectural Wood Using Polyvinyl Alcohol and Methyltrimethoxysilane for Improved Waterproofing, Dimensional Stability, and Self-Cleaning Properties. Forests. 2024; 15(6):978. https://doi.org/10.3390/f15060978
Chicago/Turabian StyleZheng, Shaojiang, Wei Tang, Jihui Tong, Kehao Cao, Houjie Yu, and Linkun Xie. 2024. "Innovative Treatment of Ancient Architectural Wood Using Polyvinyl Alcohol and Methyltrimethoxysilane for Improved Waterproofing, Dimensional Stability, and Self-Cleaning Properties" Forests 15, no. 6: 978. https://doi.org/10.3390/f15060978




