Tree Rings Elucidate Differential Drought Responses in Stands of Three Mexican Pines
Abstract
1. Introduction
2. Materials and Methods
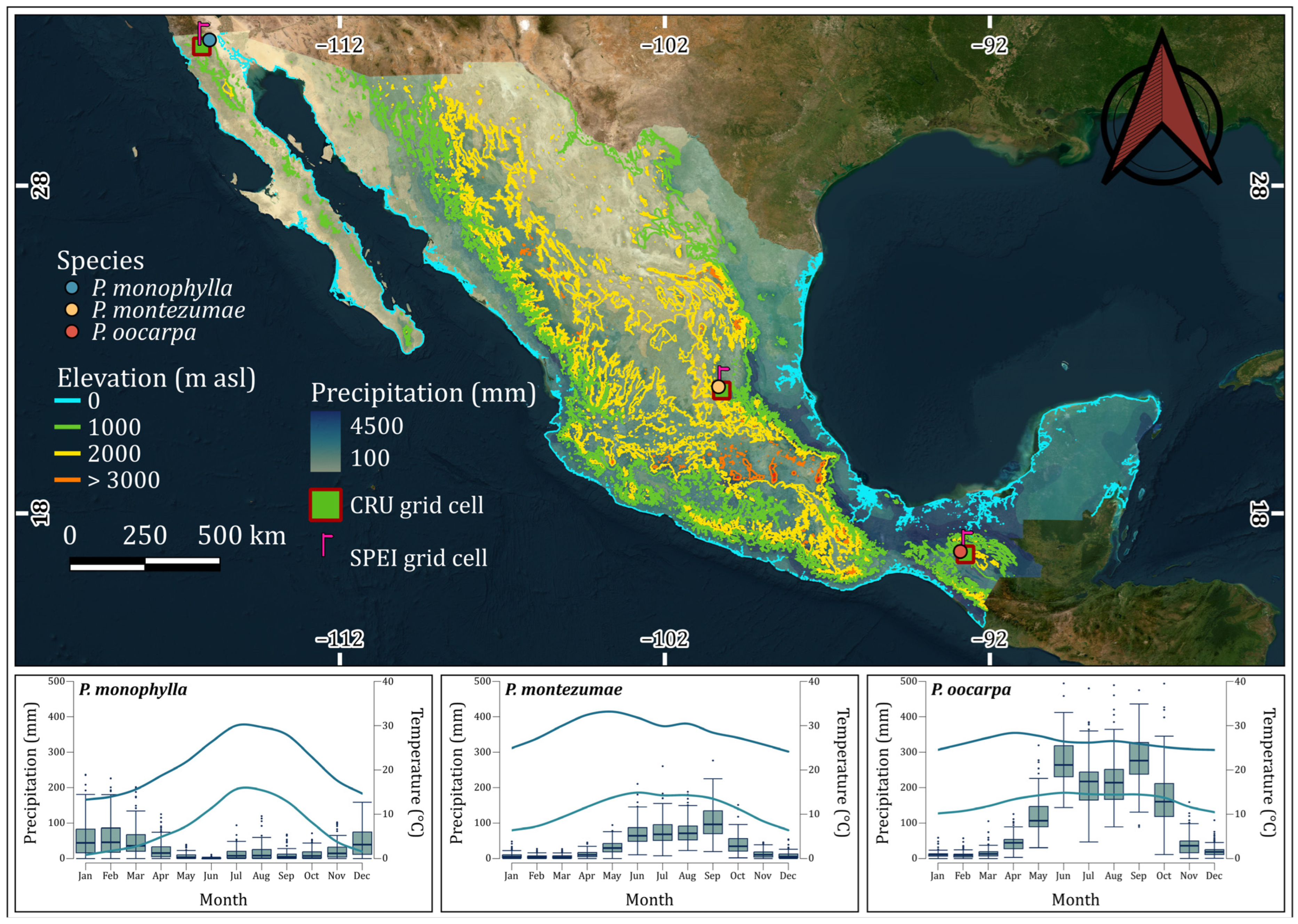
2.1. Study Area and Species Analyzed
2.2. Field Sampling and Dendrochronological Methods
2.3. Climate Growth Relationships
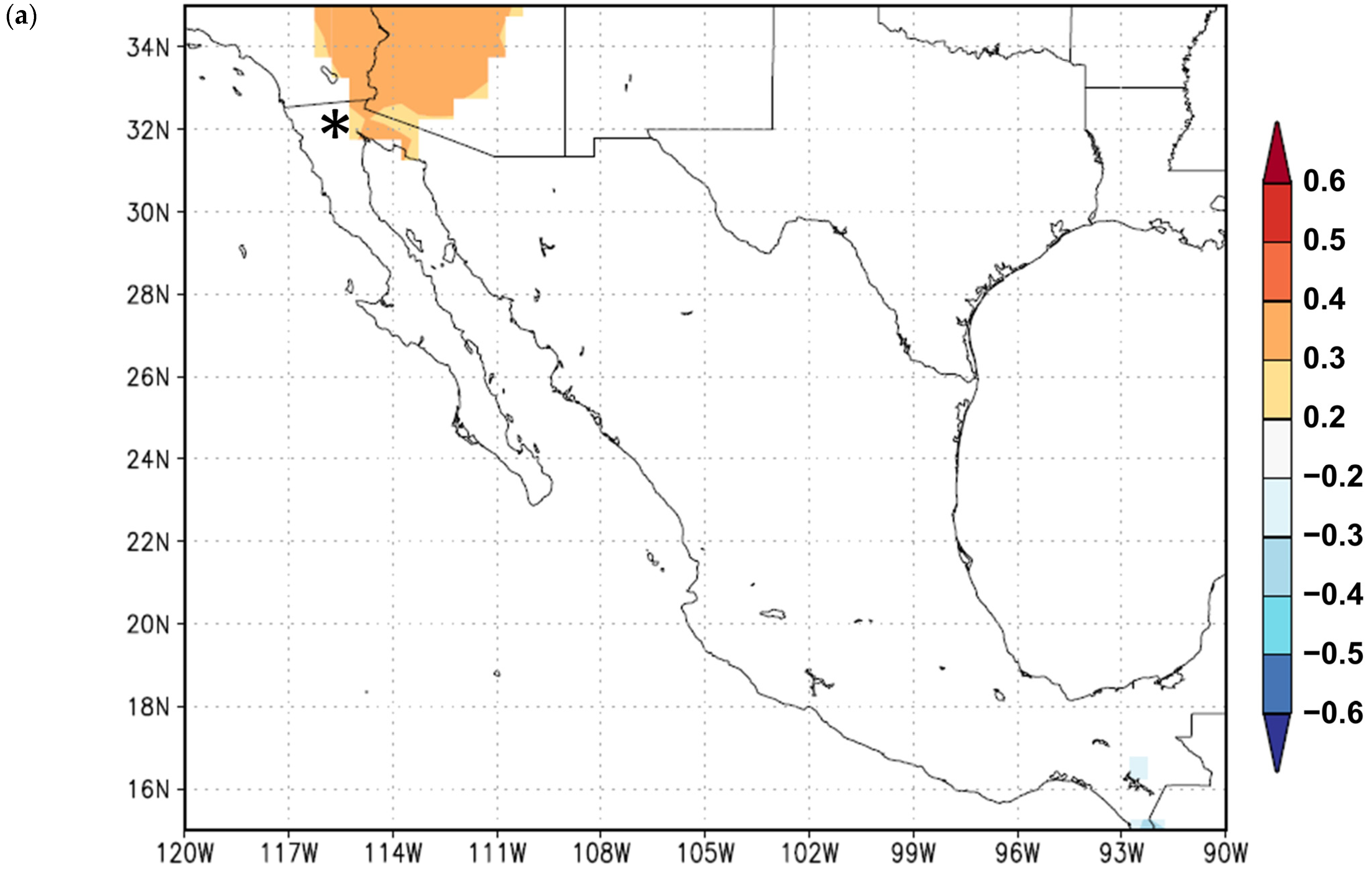
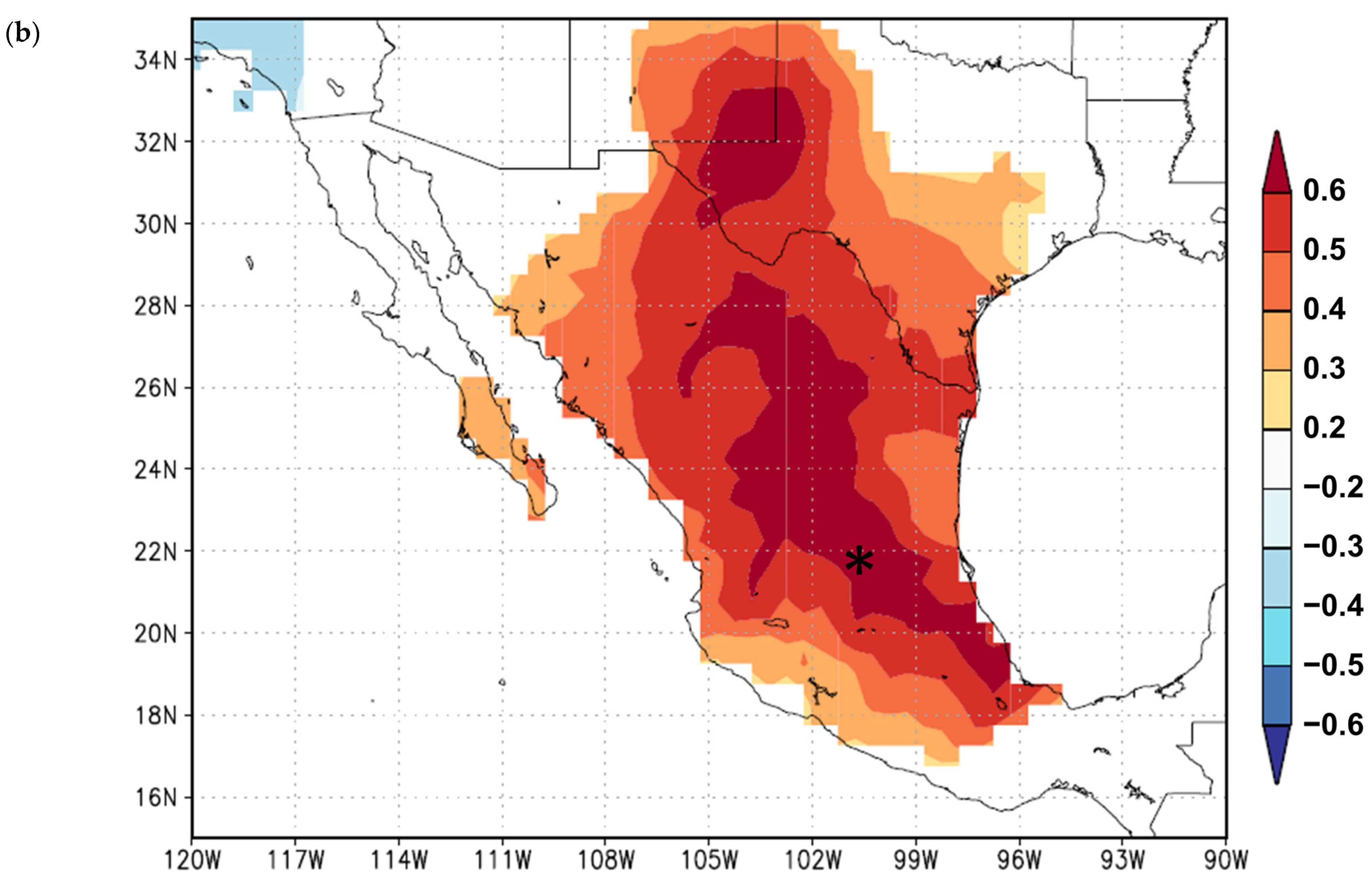
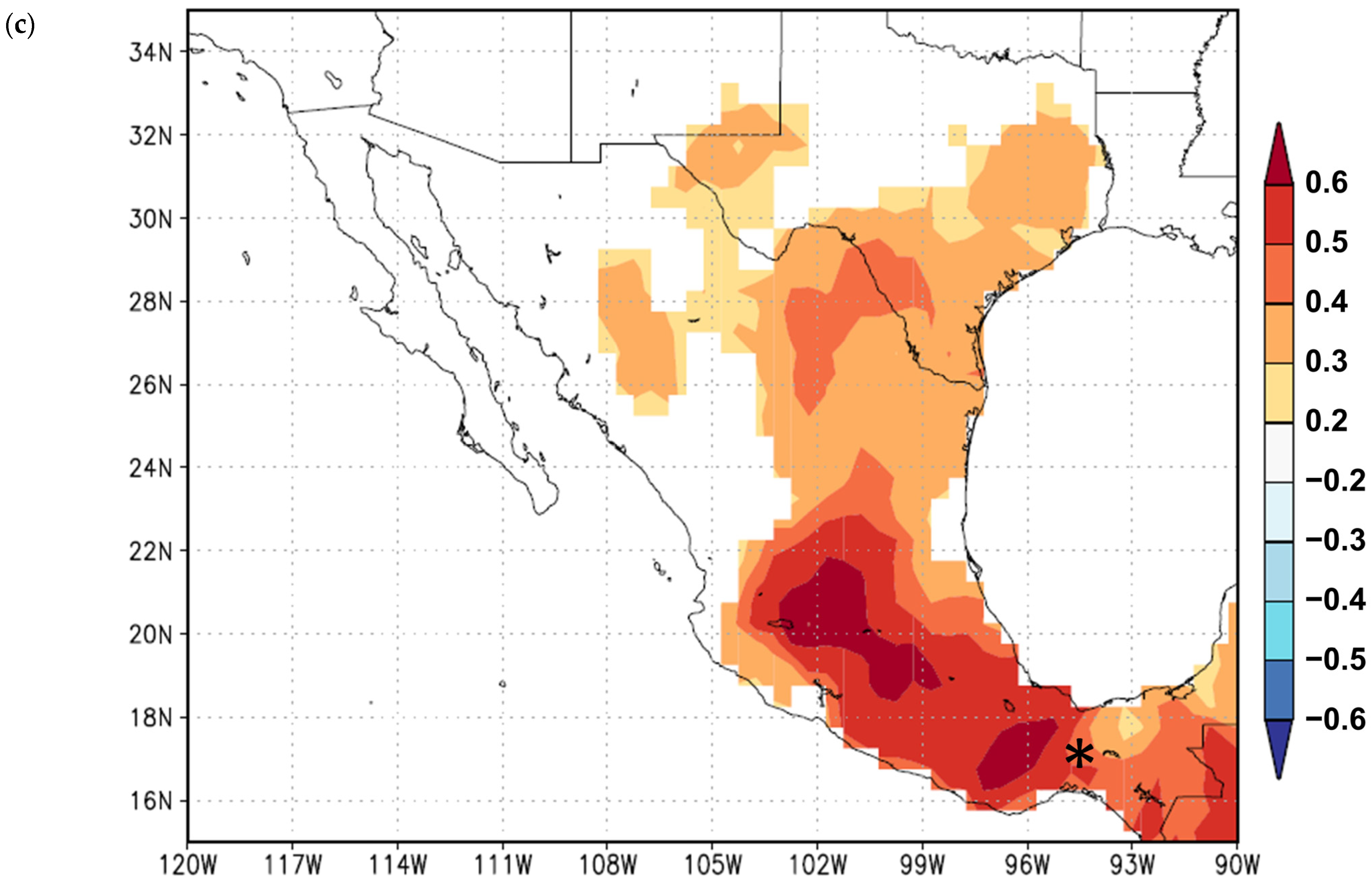
3. Results
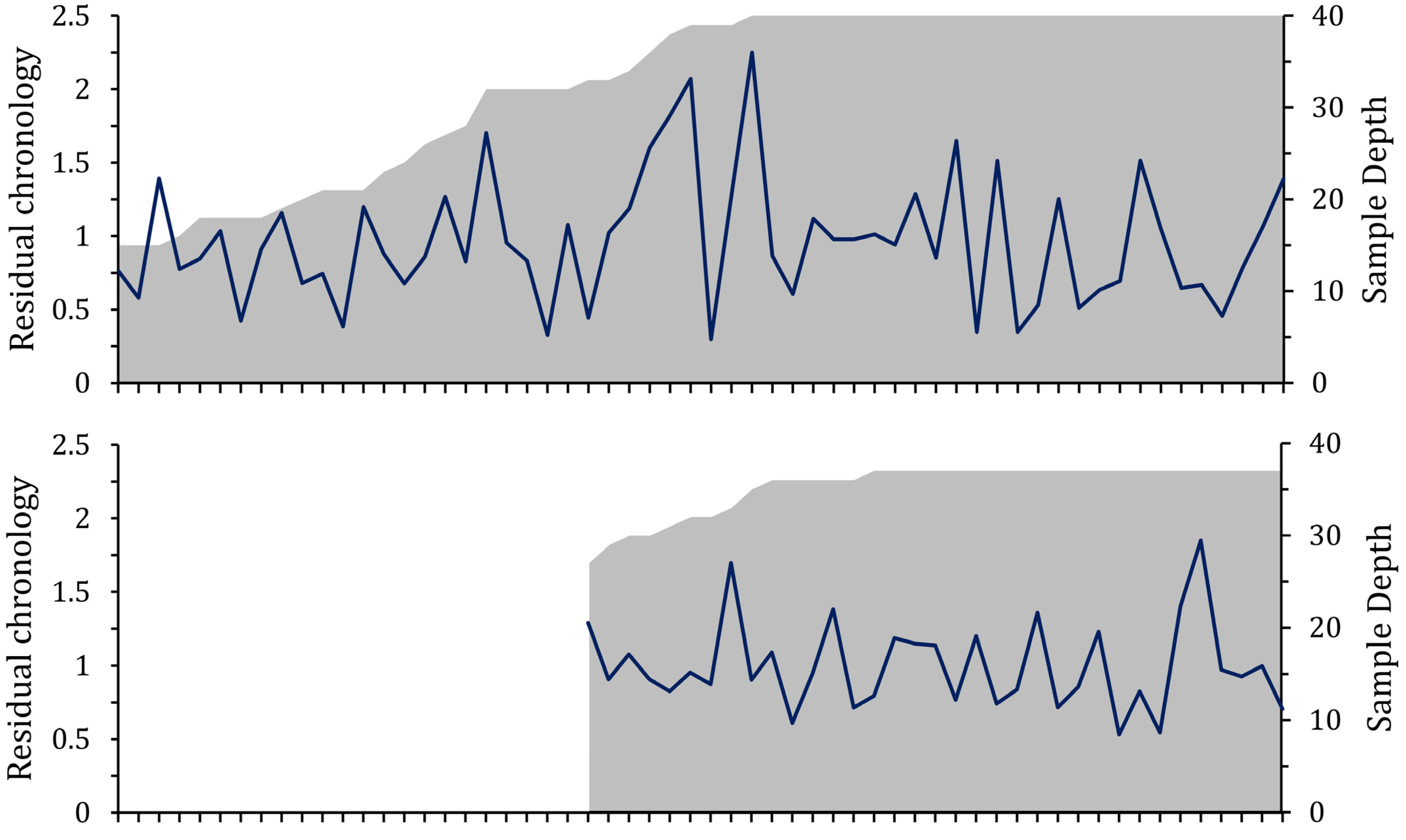
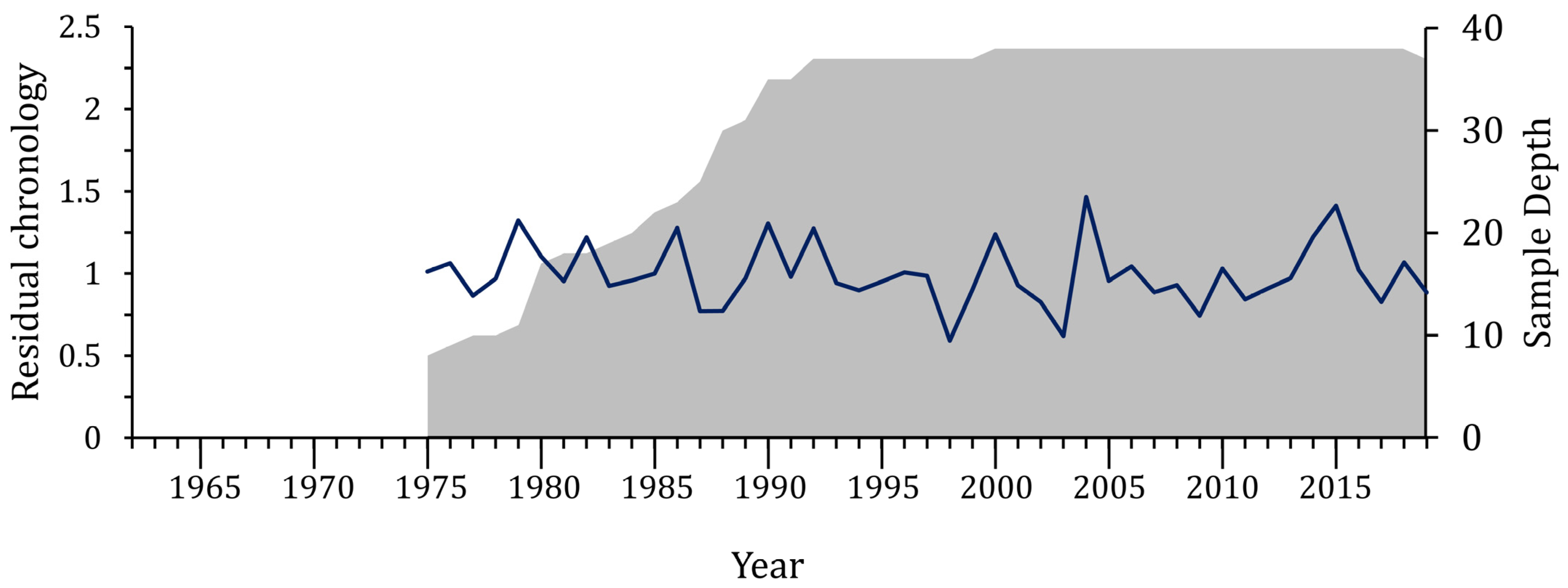
3.1. Growth Variables
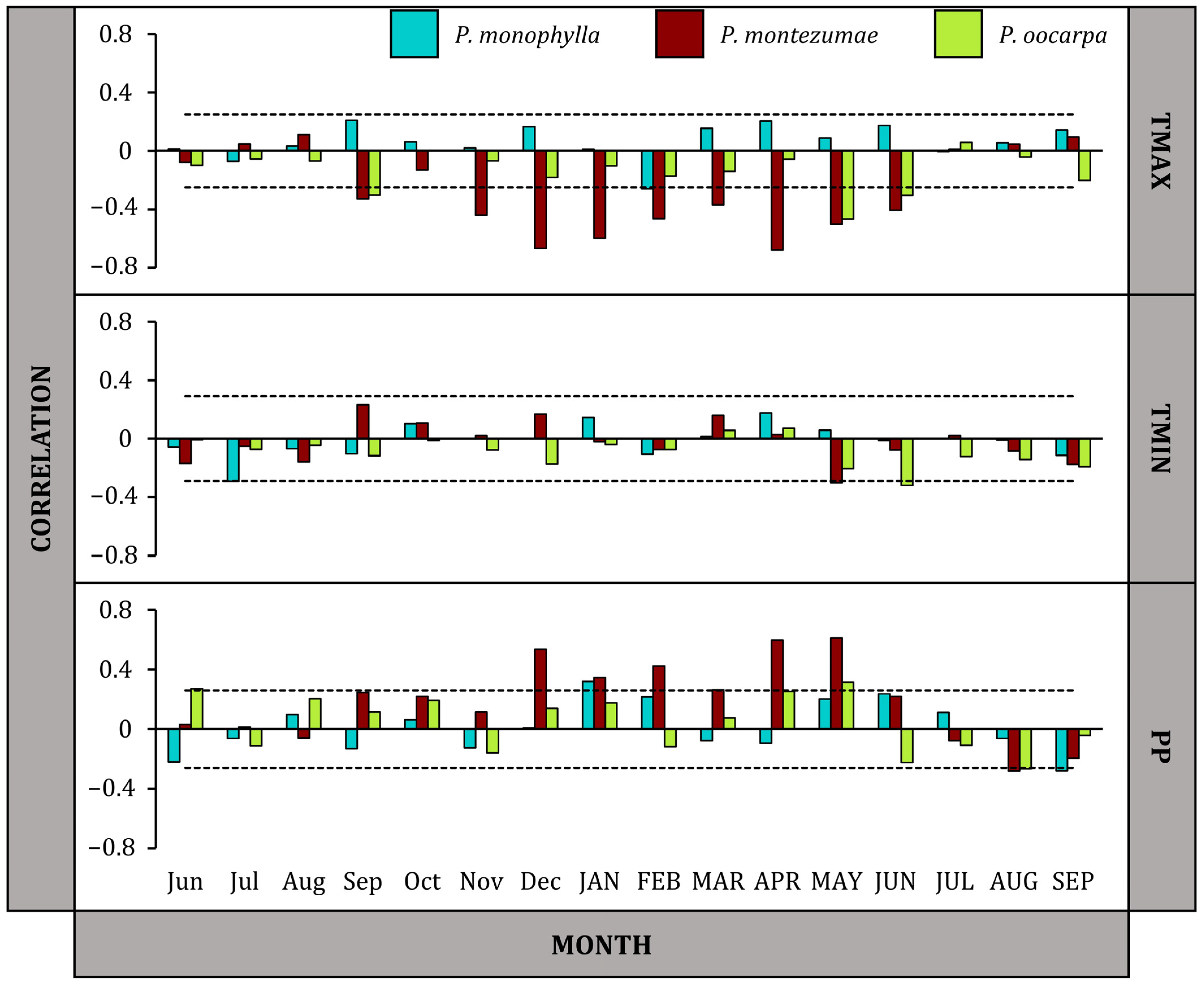
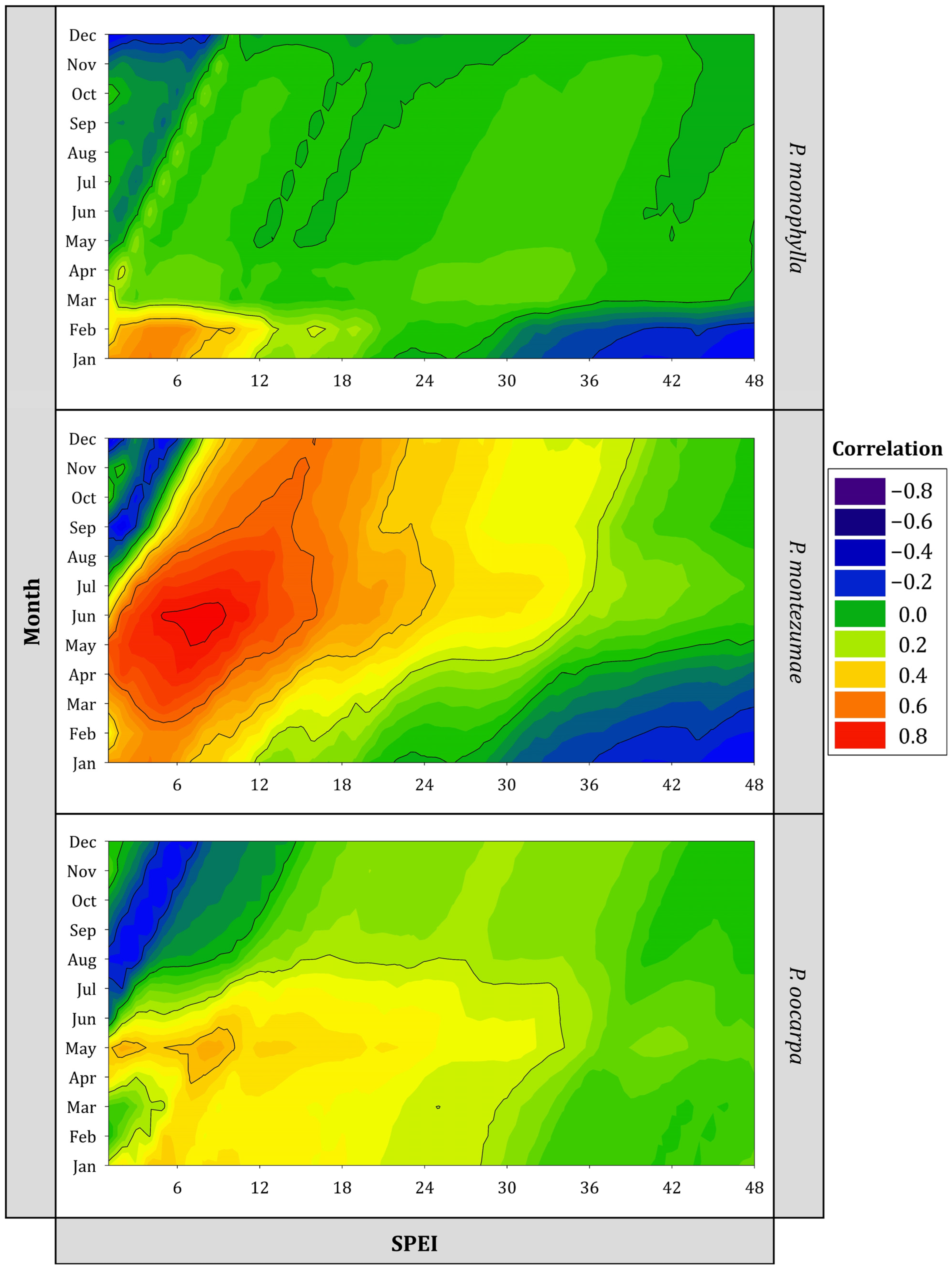
3.2. Growth Responses to Climate
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Rathgeber, C.B.K.; Pérez-de-Lis, G.; Fenández-de-Uña, L.; Fonti, P.; Rossi, S.; Treydte, K.; Gessler, A.; Deslauriers, A.; Fonti, M.V.; Ponton, S. Anatomical, Developmental and Physiological Bases of Tree-Ring Formation in Relation to Environmental Factors. In Stable Isotopes in Tree Rings, 1st ed.; Siegwolf, R.T.F., Brooks, J.R., Roden, J., Saurer, M., Eds.; Springer: Cham, Switzerland, 2022; Volume 8, pp. 61–99. [Google Scholar] [CrossRef]
- Jiao, L.; Wang, S.; Chen, K.; Liu, X. Dynamic response to climate change in the radial growth of Picea schrenkiana in western Tien Shan, China. J. For. Res. 2022, 33, 147–157. [Google Scholar] [CrossRef]
- Williams, A.P.; Cook, E.R.; Smerdon, J.E.; Cook, B.I.; Abatzoglou, J.T.; Bolles, K.; Baek, S.H.; Badger, A.M.; Livneh, B. Large contribution from anthropogenic warming to an emerging North American megadrought. Science 2020, 368, 314–318. [Google Scholar] [CrossRef] [PubMed]
- Szejner, P.; Belmecheri, S.; Ehleringer, J.R.; Monson, R.K. Recent increases in drought frequency cause observed multi-year drought legacies in the tree rings of semi-arid forests. Oecologia 2020, 192, 241–259. [Google Scholar] [CrossRef] [PubMed]
- Camarero, J.J.; Gazol, A.; Sangüesa-Barreda, G.; Cantero, A.; Sánchez-Salguero, R.; Sánchez-Miranda, A.; Granda, E.; Serra-Maluquer, X.; Ibáñez, R. Forest Growth Responses to Drought at Short- and Long-Term Scales in Spain: Squeezing the Stress Memory from Tree Rings. Front. Ecol. Evol. 2018, 6, 9. [Google Scholar] [CrossRef]
- Karelin, D.V.; Zamolodchikov, D.G.; Shilkin, A.V.; Popov, S.Y.; Kumanyaev, A.S.; de Gerenyu, V.O.L.; Tel’nova, N.O.; Gitarskiy, M.L. The effect of tree mortality on CO2 fluxes in an old-growth spruce forest. Eur. J. For. Res. 2021, 140, 287–305. [Google Scholar] [CrossRef]
- Pompa-García, M.; Vivar-Vivar, E.D.; Sigala-Rodríguez, J.A.; Padilla-Martínez, J.R. What Are Contemporary Mexican Conifers Telling Us? A Perspective Offered from Tree Rings Linked to Climate and the NDVI along a Spatial Gradient. Remote Sens. 2022, 14, 4506. [Google Scholar] [CrossRef]
- Bradford, J.B.; Shriver, R.K.; Robles, M.D.; McCauley, L.A.; Woolley, T.J.; Andrews, C.A.; Crimmins, M.; Bell, D.M. Tree mortality response to drought-density interactions suggests opportunities to enhance drought resistance. J. Appl. Ecol. 2022, 59, 549–559. [Google Scholar] [CrossRef]
- Redmond, M.D.; Forcella, F.; Barger, N.N. Declines in pinyon pine cone production associated with regional warming. Ecosphere 2012, 3, 1–14. [Google Scholar] [CrossRef]
- Herrera-Soto, G.; González-Cásares, M.; Pompa-García, M.; Camarero, J.J.; Solís-Moreno, R. Growth of Pinus cembroides Zucc. in Response to Hydroclimatic Variability in Four Sites Forming the Species Latitudinal and Longitudinal Distribution Limits. Forests 2018, 9, 440. [Google Scholar] [CrossRef]
- Vieira, J.; Carvalho, A.; Campelo, F. Tree Growth Under Climate Change: Evidence from Xylogenesis Timings and Kinetics. Front. Plant Sci. 2020, 11, 90. [Google Scholar] [CrossRef]
- Juárez-Barrera, F.; Bueno-Hernández, A.; Morrone, J.J.; Barahona-Echeverría, A.; Espinosa, D. Recognizing spatial patterns of biodiversity during the nineteenth century: The roots of contemporary biogeography. J. Biogeogr. 2018, 5, 995–1002. [Google Scholar] [CrossRef]
- Villanueva-Díaz, J.; Stahle, D.W.; Poulos, H.M.; Therrell, M.D.; Howard, I.; Martínez-Sifuentes, A.R.; Hermosillo-Rojas, D.; Cerano-Paredes, J.; Estrada-Ávalos, J. Climate and the Radial Growth of Conifers in Borderland Natural Areas of Texas and Northern Mexico. Atmosphere 2022, 13, 1326. [Google Scholar] [CrossRef]
- Forrester, D.I.; Ammer, C.; Annighöfer, P.J.; Barbeito, I.; Bielak, K.; Bravo-Oviedo, A.; Coll, L.; del Río, M.; Drössler, L.; Heym, M.; et al. Effects of crown architecture and stand structure on light absorption in mixed and monospecific Fagus sylvatica and Pinus sylvestris forests along a productivity and climate gradient through Europe. J. Ecol. 2017, 106, 746–760. [Google Scholar] [CrossRef]
- McDowell, N.G.; Allen, C.D.; Anderson-Teixeira, K.; Aukema, B.H.; Bond-Lamberty, B.; Chini, L.; Clark, J.S.; Dietze, M.; Grossiord, C.; Hanbury-Brown, A.; et al. Pervasive shifts in forest dynamics in a changing world. Science 2020, 368, eaaz9463. [Google Scholar] [CrossRef] [PubMed]
- Colangelo, M.; Camarero, J.J.; Gazol, A.; Piovesan, G.; Borghetti, M.; Baliva, M.; Gentilesca, T.; Rita, A.; Schettino, A.; Ripullone, F. Mediterranean old-growth forests exhibit resistance to climate warming. Sci. Total Environ. 2021, 801, 149684. [Google Scholar] [CrossRef]
- Ruiz-Benito, P.; Madrigal-González, J.; Ratcliffe, S.; Coomes, D.A.; Kändler, G.; Lehtonen, A.; Wirth, C.; Zavala, M.A. Stand Structure and Recent Climate Change Constrain Stand Basal Area Change in European Forests: A Comparison Across Boreal, Temperate, and Mediterranean Biomes. Ecosystems 2014, 17, 1439–1454. [Google Scholar] [CrossRef]
- Szeicz, J.M.; MacDonald, G.M. Age-dependent tree-ring growth responses of subarctic white spruce to climate. Can. J. For. Res. 1994, 24, 120–132. [Google Scholar] [CrossRef]
- Trouillier, M.; van der Maaten-Theunissen, M.; Scharnweber, T.; Würth, D.; Burger, A.; Schnittler, M.; Wilmking, M. Size matters—A comparison of three methods to assess age- and size-dependent climate sensitivity of trees. Trees 2019, 33, 183–192. [Google Scholar] [CrossRef]
- Tellez, O.; Mattana, E.; Diazgranados, M.; Kühn, N.; Castillo-Lorenzo, E.; Lira, R.; Montes-Leyva, L.; Rodriguez, I.; Ortiz, C.M.F.; Way, M.; et al. Native trees of Mexico: Diversity, distribution, uses and conservation. PeerJ 2020, 8, e9898. [Google Scholar] [CrossRef] [PubMed]
- Stahle, D.W.; Cook, E.R.; Burnette, D.J.; Villanueva, J.; Cerano, J.; Burns, J.N.; Griffin, D.; Cook, B.I.; Acuña, R.; Toberson, M.C.A.; et al. The Mexican Drought Atlas: Tree-ring reconstructions of the soil moisture balance during the late pre-Hispanic, colonial, and modern eras. Quat. Sci. Rev. 2016, 149, 34–60. [Google Scholar] [CrossRef]
- Seager, R.; Ting, M.; Davis, M.; Cane, M.; Naik, N.; Nakamura, J.; Li, C.; Cook, E.; Stahle, D.W. Mexican drought: An observational modeling and tree ring study of variability and climate change. Atmósfera 2009, 22, 1–31. [Google Scholar]
- Lanner, R.M. Conifers of California; Cachuma Press: Los Olivos, CA, USA, 1999; p. 274. [Google Scholar]
- Meeuwig, R.O.; Budy, J.D.; Everett, R.L. Pius monophylla Torr. & Frem. Singleleaf Pinyon. In Silvics of North America: Conifers, 1st ed.; Unites States Department of Agriculture: Washington, DC, USA, 1990; Volume 1, pp. 380–384. [Google Scholar]
- Oviedo, E.H.C.; Goicochea, J.A.P.; Arreola, O.M.; Manzo, S.V.; Celestino, F.L. Índice de sitio para Pinus montezumae Lamb. en la región de Cd. Hidalgo, Michoacán. Rev. Fitotec. Mex. 2005, 28, 213–219. [Google Scholar]
- Flores-Garnica, J.G.; Reyes-Cárdenas, O. Distribución espacial de Pinus oocarpa Schiede ex Schltdl. mediante la estimación de la densidad Kernel. Rev. Mex. Cienc. For. 2019, 10, 21–40. [Google Scholar] [CrossRef]
- Holmes, R.L. Computer-Assisted Quality Control in Tree-Ring Dating and Measurement. Tree-Ring Bull. 1983, 43, 69–78. [Google Scholar]
- Pompa-García, M.; Dávalos-Sotelo, R.; Rodríguez-Téllez, E.; Aguirre-Calderón, O.A.; Treviño-Garza, E.J. Sensibilidad climática de tres versiones dendrocronológicas para una conífera mexicana. Madera Bosques 2014, 20, 139–151. [Google Scholar] [CrossRef]
- Bunn, A.G. Statistical and visual crossdating in R using the dplR library. Dendrochronologia 2010, 28, 251–258. [Google Scholar] [CrossRef]
- Wigley, T.M.L.; Briffa, K.R.; Jones, P.D. On the Average Value of Correlated Time Series, with Applications in Dendroclimatology and Hydrometeorology. J. Appl. Meteorol. Climatol. 1984, 23, 201–213. [Google Scholar] [CrossRef]
- Harris, I.; Osborn, T.J.; Jones, P.; Lister, D. Version 4 of the CRU TS monthly high-resolution gridded multivariate climate dataset. Sci. Data 2020, 7, 109. [Google Scholar] [CrossRef] [PubMed]
- Trouet, V.; Van Oldenborgh, G.J. KNMI Climate Explorer: A Web-Based Research Tool for High-Resolution Paleoclimatology. Tree-Ring Res. 2013, 69, 3–13. [Google Scholar] [CrossRef]
- Zang, C.; Biondi, F. treeclim: An R package for the numerical calibration of proxy-climate relationships. Ecography 2015, 38, 431–436. [Google Scholar] [CrossRef]
- Vicente-Serrano, S.M.; Beguería, S.; López-Moreno, J.I. A Multiscalar Drought Index Sensitive to Global Warming: The Standardized Precipitation Evapotranspiration Index. J. Clim. 2010, 23, 1696–1718. [Google Scholar] [CrossRef]
- Gazol, A.; Camarero, J.J.; Anderegg, W.R.L.; Vicente-Serrano, S.M. Impacts of droughts on the growth resilience of Northern Hemisphere forests. Glob. Ecol. Biogeogr. 2017, 26, 166–176. [Google Scholar] [CrossRef]
- Carrer, M.; Urbinati, C. Age-dependent tree-ring growth responses to climate in Larix decidua and Pinus cembra. Ecology 2004, 85, 730–740. [Google Scholar] [CrossRef]
- Camarero, J.J.; Gazol, A.; Sangüesa-Barreda, G.; Oliva, J.; Vicente-Serrano, S.M. To die or not to die: Early warnings of tree dieback in response to a severe drought. J. Ecol. 2015, 103, 44–57. [Google Scholar] [CrossRef]
- Gutierrez-Garcia, G.; Leavitt, S.W.; Trouet, V.; Carriquiry, J.D. Tree Ring-Based Historic Hydroclimatic Variability of the Baja California Peninsula. J. Geophys. Res. 2020, 125, e2020JD032675. [Google Scholar] [CrossRef]
- Biondi, F.; Hay, M.; Strachan, S. The tree-ring interpolation model (TRIM) and its application to Pinus monophylla chronologies in the Great Basin of North America. Forestry 2014, 87, 582–597. [Google Scholar] [CrossRef][Green Version]
- Escalante-Sandoval, C.; Nuñez-Garcia, P. Meteorological drought features in northern and northwestern parts of Mexico under different climate change scenarios. J. Arid Land 2017, 9, 65–75. [Google Scholar] [CrossRef]
- Cortés-Cortés, O.; Cornejo-Oviedo, E.H.; Cerano-Paredes, J.; Cervantes-Martínez, R.; Flores-López, C.; Valencia-Manzo, S. Relationship between climate variability and radial growth of Pinus montezumae Lamb. in Coyuca de Catalán, Guerrero. Rev. Chapingo Ser. Cienc. For. Ambiente 2021, 27, 109–126. [Google Scholar] [CrossRef]
- Huang, J.; Tardif, J.C.; Bergeron, Y.; Denneler, B.; Berninger, F.; Girardin, M.P. Radial growth response of four dominant boreal tree species to climate along a latitudinal gradient in the eastern Canadian boreal forest. Glob. Chang. Biol. 2010, 16, 711–731. [Google Scholar] [CrossRef]
- Lian, X.; Jiao, L.; Liu, Z.; Jia, Q.; Zhong, J.; Fang, M.; Wang, W. Multi-spatiotemporal heterogeneous legacy effects of climate on terrestrial vegetation dynamics in China. GISci. Remote Sens. 2022, 59, 164–183. [Google Scholar] [CrossRef]
- Castagneri, D.; Nola, P.; Motta, R.; Carrer, M. Summer climate variability over the last 250 years differently affected tree species radial growth in a mesic Fagus–Abies–Picea old-growth forest. For. Ecol. Manag. 2014, 320, 21–29. [Google Scholar] [CrossRef]
- Brodribb, T.J.; Bowman, D.J.M.S.; Nichols, S.; Delzon, S.; Burlett, R. Xylem function and growth rate interact to determine recovery rates after exposure to extreme water deficit. New Phytol. 2010, 188, 533–542. [Google Scholar] [CrossRef]
- Blumstein, M.J.; Furze, M.E. Interannual dynamics of stemwood nonstructural carbohydrates in temperate forest trees surrounding drought. J. For. Res. 2023, 34, 77–86. [Google Scholar] [CrossRef]
- Cailleret, M.; Jansen, S.; Robert, E.M.R.; Desoto, L.; Aakala, T.; Antos, J.A.; Beikircher, B.; Bigler, C.; Bugmann, H.; Caccianiga, M.; et al. A synthesis of radial growth patterns preceding tree mortality. Glob. Chang. Biol. 2017, 23, 1675–1690. [Google Scholar] [CrossRef]
- Sáenz-Romero, C.; Mendoza-Maya, E.; Gómez-Pineda, E.; Blanco-García, A.; Endara-Agramont, A.R.; Lindig-Cisneros, R.; López-Upton, J.; Trejo-Ramírez, O.; Wehenkel, C.; Cibrián-Tovar, D.; et al. Recent evidence of Mexican temperate forest decline and the need for ex situ conservation, assisted migration, and translocation of species ensembles as adaptive management to face projected climatic change impacts in a megadiverse country. Can. J. For. Res. 2020, 50, 843–854. [Google Scholar] [CrossRef]
- Lloret, F.; Keeling, E.G.; Sala, A. Components of tree resilience: Effects of successive low-growth episodes in old ponderosa pine forests. Oikos 2011, 120, 1909–1920. [Google Scholar] [CrossRef]
- Sun, S.; Lei, S.; Jia, H.; Li, C.; Zhang, J.; Meng, P. Tree-Ring Analysis Reveals Density-Dependent Vulnerability to Drought in Planted Mongolian Pines. Forests 2020, 11, 98. [Google Scholar] [CrossRef]
- Anderegg, W.R.L. Spatial and temporal variation in plant hydraulic traits and their relevance for climate change impacts on vegetation. New Phytol. 2015, 205, 1008–1014. [Google Scholar] [CrossRef]
- Iqbal, A.; Fahad, S.; Iqbal, M.; Alamzeb, M.; Ahmad, A.; Anwar, S.; Khan, A.A.; Amanullah, M.A.; Inamullah, S.; Saeed, M.; et al. Special Adaptive Features of Plant Species in Response to Drought. In Salt and Drought Stress Tolerance in Plants, 1st ed.; Hasanuzzaman, M., Tanveer, M., Eds.; Springer: Cham, Switzerland, 2020; Volume 1, pp. 77–118. [Google Scholar] [CrossRef]
- Stuart-Haëntjens, E.; De Boeck, H.J.; Lemoine, N.P.; Mänd, P.; Kröel-Dulay, G.; Schmidt, I.K.; Jentsch, A.; Stampfli, A.; Anderegg, W.R.L.; Bahn, M.; et al. Mean annual precipitation predicts primary production resistance and resilience to extreme drought. Sci. Total Environ. 2018, 636, 360–366. [Google Scholar] [CrossRef]
- Park, A.D. Environmental influences on post-harvest natural regeneration in Mexican pine–oak forests. For. Ecol. Manag. 2001, 144, 213–228. [Google Scholar] [CrossRef]
- Weerasekara, C.S.; Udawatta, R.P.; Gantzer, C.J.; Kremer, R.J.; Jose, S.; Veum, K.S. Effects of Cover Crops on Soil Quality: Selected Chemical and Biological Parameters. Commun. Soil Sci. Plant Anal. 2017, 48, 2074–2082. [Google Scholar] [CrossRef]
- Kwon, H.; Law, B.E.; Thomas, C.K.; Johnson, B.G. The influence of hydrological variability on inherent water use efficiency in forests of contrasting composition, age, and precipitation regimes in the Pacific Northwest. Agric. For. Meteorol. 2018, 249, 488–500. [Google Scholar] [CrossRef]
- Bartholomew, D.C.; Bittencourt, P.R.L.; da Costa, A.C.L.; Banin, L.F.; Costa, P.B.; Coughlin, S.I.; Domingues, T.F.; Ferreira, L.V.; Giles, A.; Mencuccini, M.; et al. Small tropical forest trees have a greater capacity to adjust carbon metabolism to long-term drought than large canopy trees. Plant Cell Environ. 2020, 43, 2380–2393. [Google Scholar] [CrossRef] [PubMed]
- Anderegg, W.R.L.; Meinzer, F.C. Wood Anatomy and Plant Hydraulics in a Changing Climate. In Functional and Ecological Xylem Anatomy, 1st ed.; Hacke, U., Ed.; Springer: Cham, Switzerland, 2015; Volume 1, pp. 235–253. [Google Scholar] [CrossRef]
- Jactel, H.; Bauhus, J.; Boberg, J.; Bonal, D.; Castagneyrol, B.; Gardiner, B.; Gonzalez-Olabarria, J.R.; Koricheva, J.; Meurisse, N.; Brockerhoff, E.G. Tree Diversity Drives Forest Stand Resistance to Natural Disturbances. Curr. For. Rep. 2017, 3, 223–243. [Google Scholar] [CrossRef]
- Bye, R.; Linares, E. Ethnobotany in the Sierra Tarahumara, Mexico: Mountains as Barriers, Conduits, and Generators of Plant-People Interactions and Relationships. In Ethnobotany of the Mountain Regions of Mexico, 1st ed.; Casas, A., Vázquez, J.J.B., Eds.; Springer: Cham, Switzerland, 2022; Volume 1, pp. 1–20. [Google Scholar] [CrossRef]
- Wang, J.; Rich, P.M.; Price, K.P.; Kettle, W.D. Relations between NDVI and tree productivity in the central Great Plains. Int. J. Remote Sens. 2004, 25, 3127–3138. [Google Scholar] [CrossRef]
- Acosta-Hernández, A.C.; Pompa-García, M.; Camarero, J.J. An Updated Review of Dendrochronological Investigations in Mexico, a Megadiverse Country with a High Potential for Tree-Ring Sciences. Forests 2017, 8, 160. [Google Scholar] [CrossRef]
| Variables | Tree Species | ||
|---|---|---|---|
| Tree species | Pinus monophylla Torr. & Frém | Pinus montezumae Lamb. | Pinus oocarpa Schiede ex Schltdl |
| Longitude °W | 116.01295 | 100.34697 | 92.90631 |
| Latitude °N | 32.44812 | 21.86355 | 16.83479 |
| Elevation (m a.s.l.) | 1374 | 2349 | 1096 |
| Region | Semi-arid region of the Californian Mediterranean (NW Mexico) | Semi-cold and temperate sub-humid region of the Sierra Madre Oriental (central Mexico) | Tropical subhumid region of the Sierra Madre Centroamericana and Altos de Chiapas (SE Mexico) |
| Sampling date | 11 January 2022 | 10 August 2020 | 21 January 2020 |
| CRU grid cell | 32.0°, 32.5° −116.5°, −116.0° | 21.5°, 22.2° −100.5°, −100.0° | 16.5°, 17.0° −93.0°, −92.5° |
| SPEI grid cell coordinates | 32.5°, −116.25° | 21.75°, −100.25° | 16.75°, −92.75° |
| Variable | Tree Species | ||
|---|---|---|---|
| Species | P. monophylla | P. montezumae | P. oocarpa |
| No. of trees dated * | 20 | 18 | 19 |
| No. of cores dated * | 40 | 37 | 38 |
| Age estimated at 1.3 m (years) | 54 ± 3 b | 38 ± 1 a | 37 ± 1 a |
| Tree-ring width (mm) | 0.92 ± 0.05 a | 3.70 ± 0.14 c | 2.68 ± 0.15 b |
| AC | 0.34 ± 0.03 a | 0.35 ± 0.03 a | 0.58 ± 0.04 b |
| MS | 0.56 ± 0.01 c | 0.40 ± 0.01 b | 0.35 ± 0.01 a |
| RBT | 0.568 | 0.534 | 0.296 |
| EPS | 0.981 | 0.968 | 0.918 |
| Timespan | 1962–2021 | 1985–2019 | 1975–2019 |
| Length of chronology (years) | 60 | 35 | 45 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vivar-Vivar, E.D.; Pompa-García, M.; Camarero, J.J. Tree Rings Elucidate Differential Drought Responses in Stands of Three Mexican Pines. Forests 2024, 15, 994. https://doi.org/10.3390/f15060994
Vivar-Vivar ED, Pompa-García M, Camarero JJ. Tree Rings Elucidate Differential Drought Responses in Stands of Three Mexican Pines. Forests. 2024; 15(6):994. https://doi.org/10.3390/f15060994
Chicago/Turabian StyleVivar-Vivar, Eduardo Daniel, Marín Pompa-García, and Jesús Julio Camarero. 2024. "Tree Rings Elucidate Differential Drought Responses in Stands of Three Mexican Pines" Forests 15, no. 6: 994. https://doi.org/10.3390/f15060994
APA StyleVivar-Vivar, E. D., Pompa-García, M., & Camarero, J. J. (2024). Tree Rings Elucidate Differential Drought Responses in Stands of Three Mexican Pines. Forests, 15(6), 994. https://doi.org/10.3390/f15060994








