Tree-Level Climate Sensitivity Reveals Size Effects and Impending Growth Decline in Silver Fir Affected by Dieback
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Species
2.2. Study Area
2.3. Field Sampling and Dendrochronological Methods
2.4. Climate Data and Drought Index
2.5. Statistical Analysis
3. Results
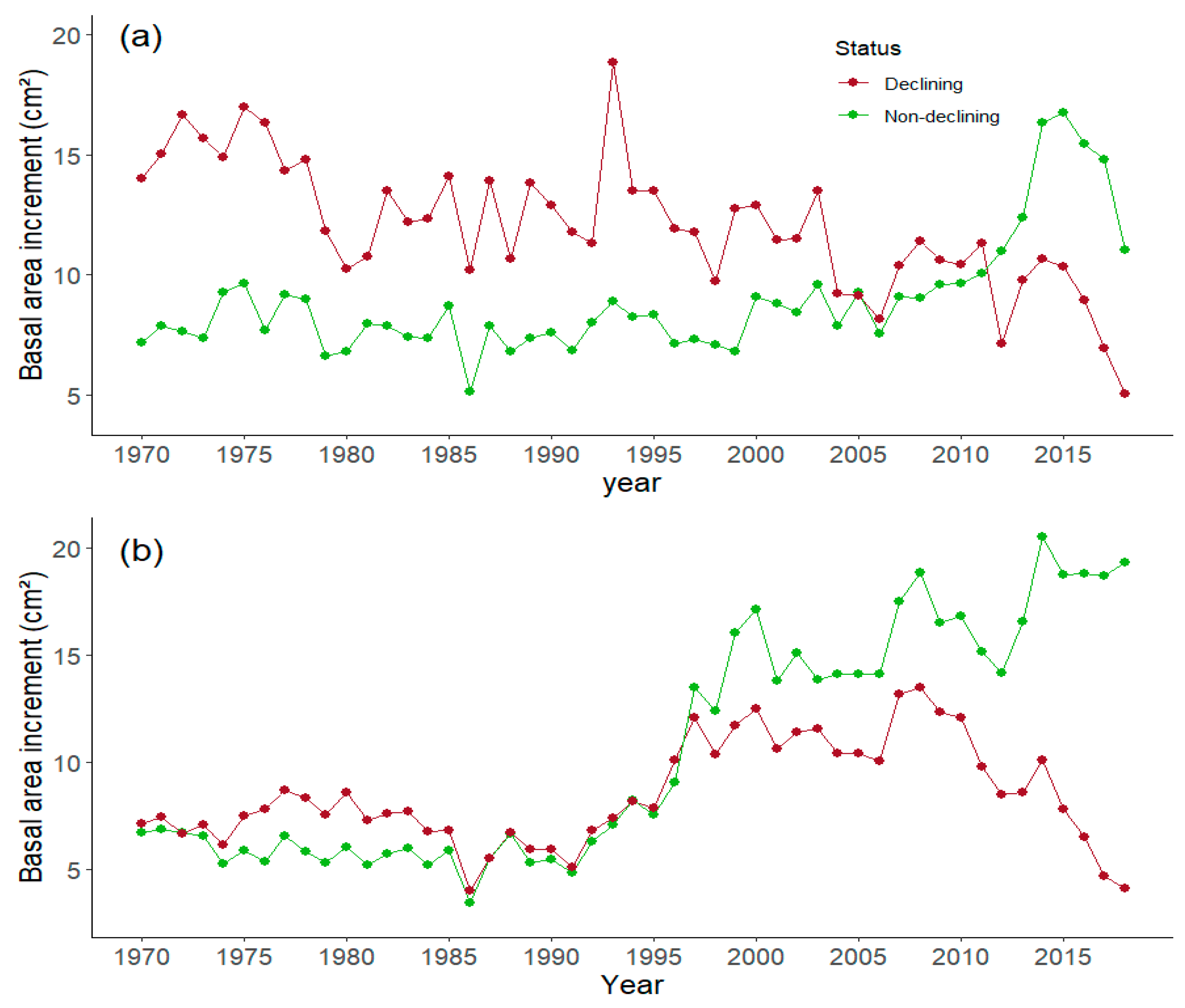
3.1. Dendrochronology
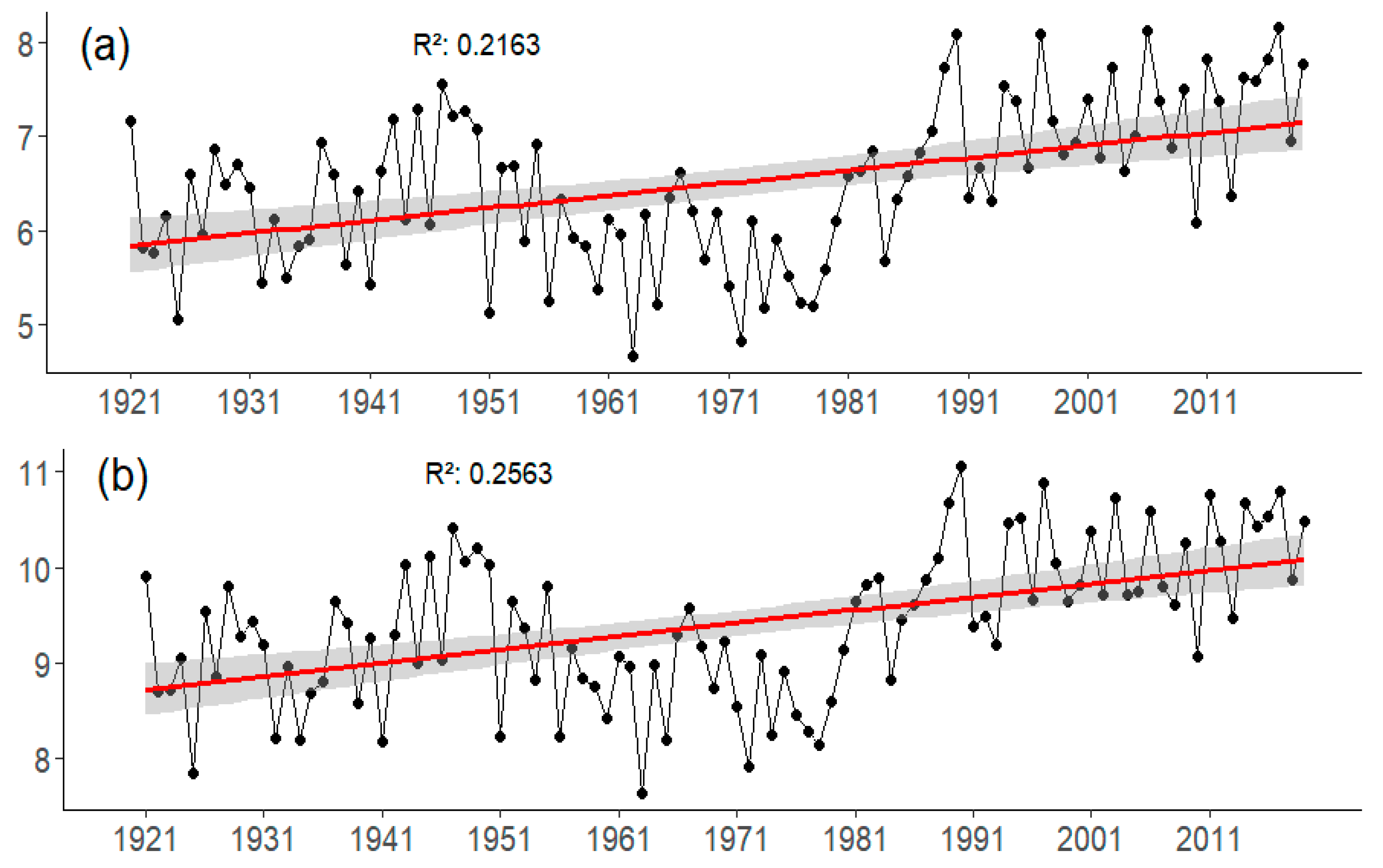
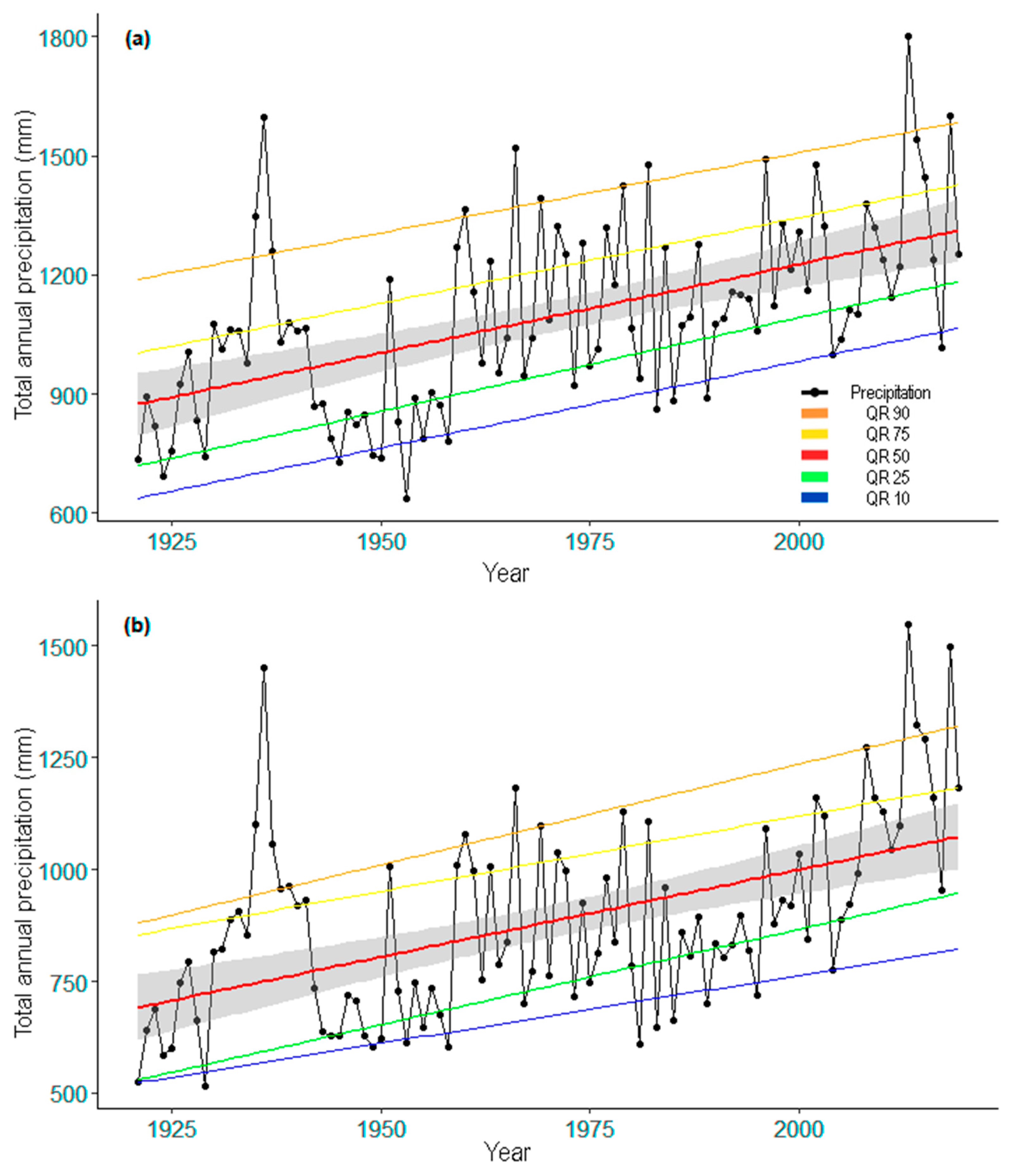
3.2. Temperature, Precipitation, and SPEI
3.2.1. Temperature Trend
3.2.2. Precipitation Trend
3.2.3. Drought Index (SPEI)
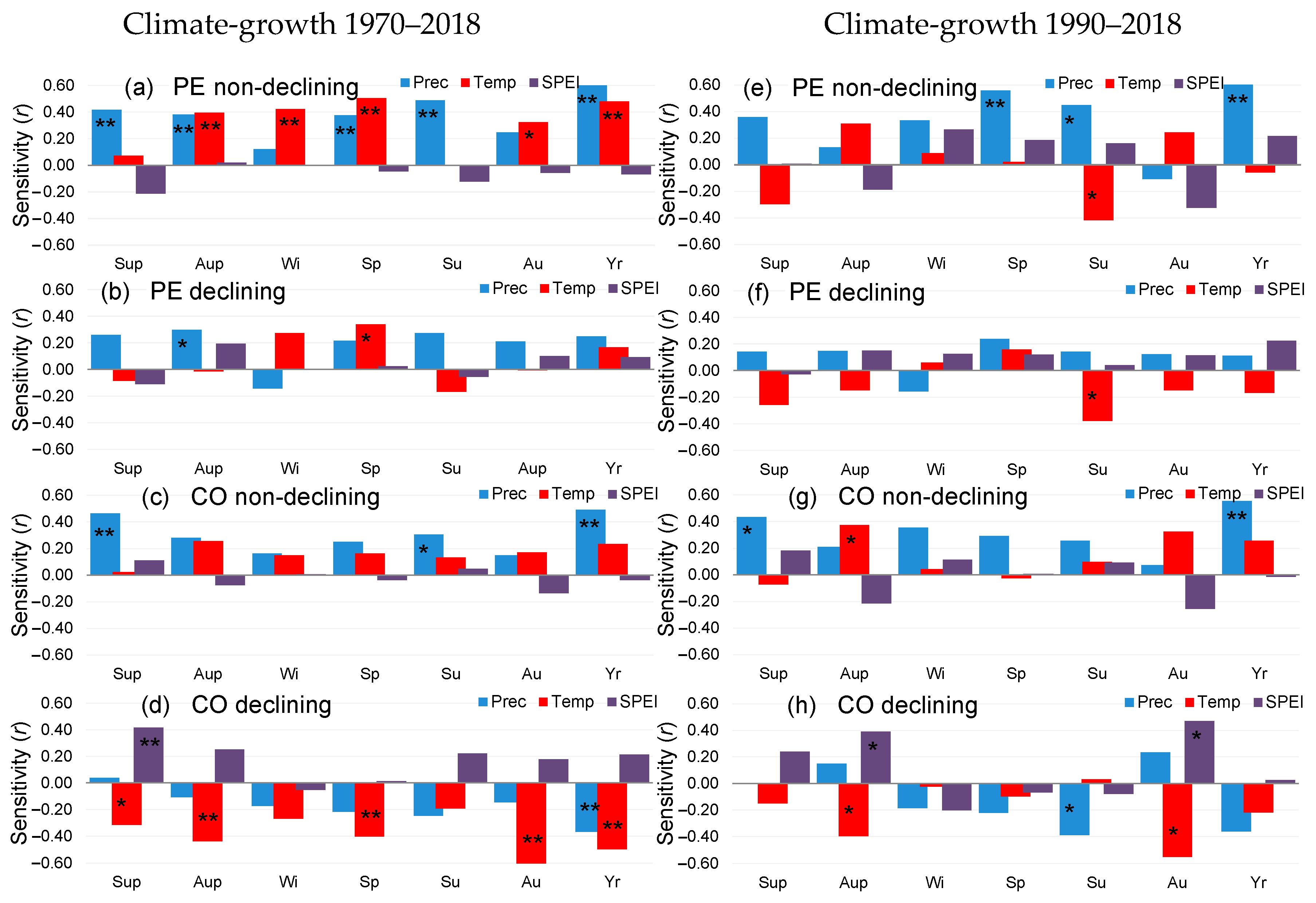
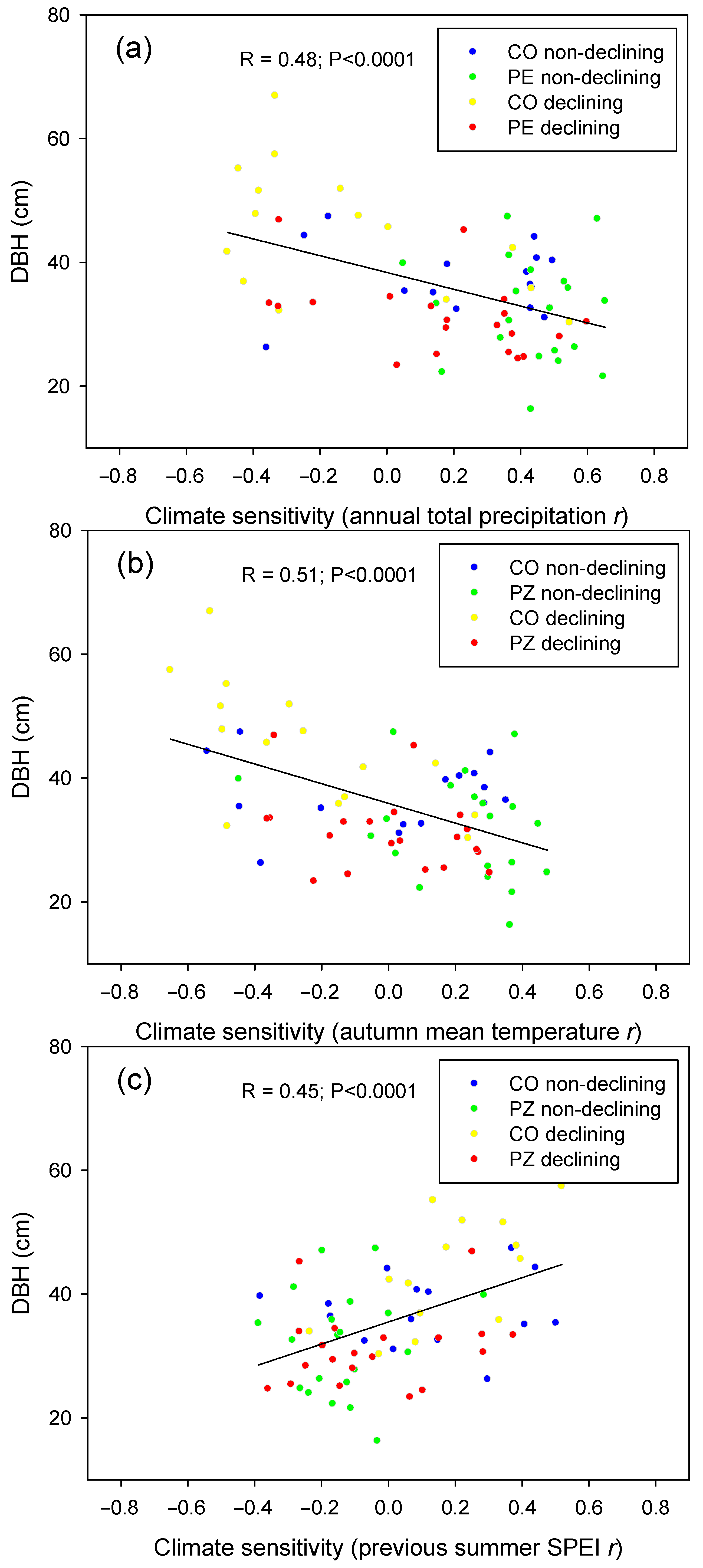
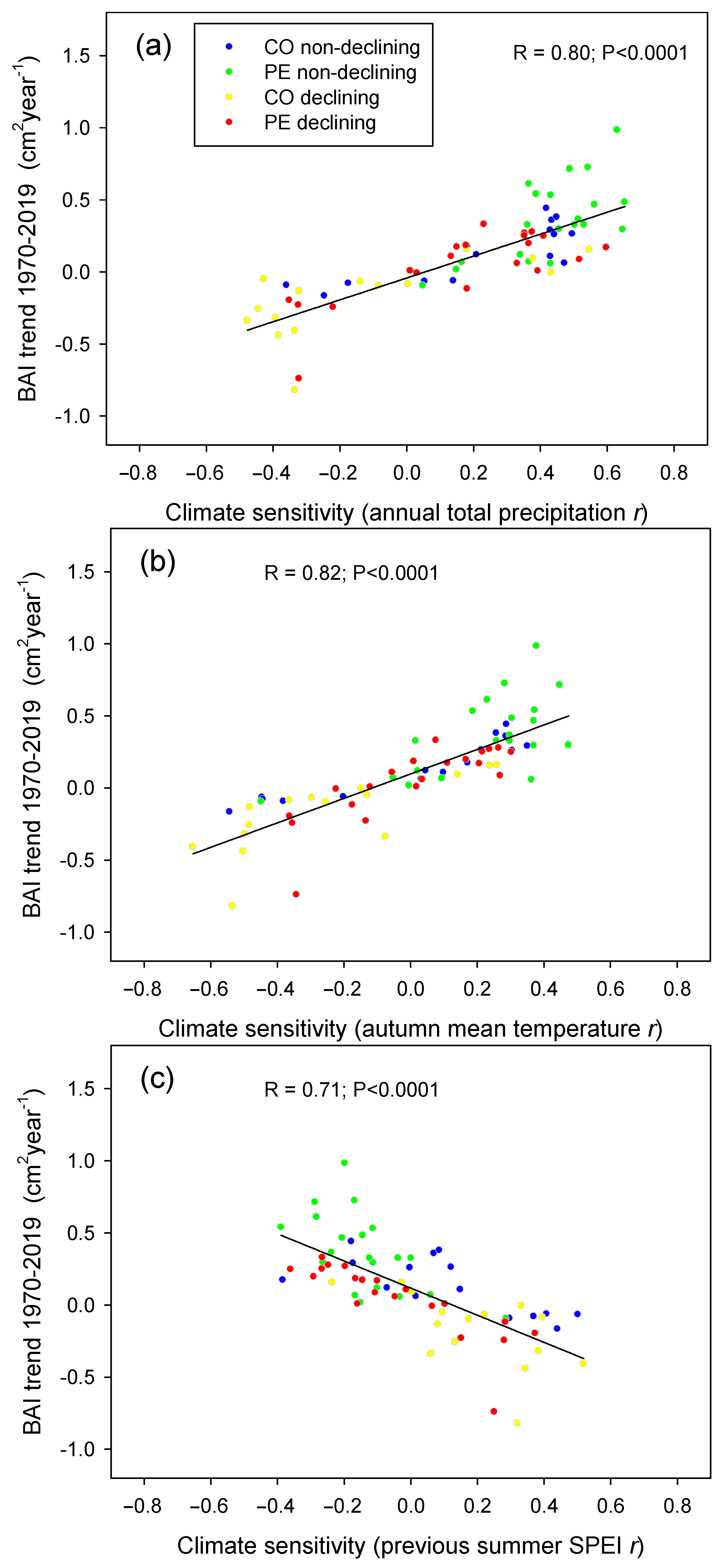
3.3. Climate Sensitivity
3.4. BAI Responsiveness to Drought
3.5. Age and Size Effect on Growth Patterns
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Allen, C.D.; Breshears, D.D.; McDowell, N.G. On Underestimation of Global Vulnerability to Tree Mortality and Forest Die-off from Hotter Drought in the Anthropocene. Ecosphere 2015, 6, 1–55. [Google Scholar] [CrossRef]
- Batllori, E.; Lloret, F.; Aakala, T.; Anderegg, W.R.L.; Aynekulu, E.; Bendixsen, D.P.; Bentouati, A.; Bigler, C.; Burk, C.J.; Camarero, J.J.; et al. Forest and Woodland Replacement Patterns Following Drought-Related Mortality. Proc. Natl. Acad. Sci. USA 2020, 117, 29720–29729. [Google Scholar] [CrossRef] [PubMed]
- McDowell, N.G.; Allen, C.D.; Anderson-Teixeira, K.; Aukema, B.H.; Bond-Lamberty, B.; Chini, L.; Clark, J.S.; Dietze, M.; Grossiord, C.; Hanbury-Brown, A.; et al. Pervasive Shifts in Forest Dynamics in a Changing World. Science 2020, 368, eaaz9463. [Google Scholar] [CrossRef] [PubMed]
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; Hogg, E.T.; et al. A Global Overview of Drought and Heat-Induced Tree Mortality Reveals Emerging Climate Change Risks for Forests. For. Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef]
- Senf, C.; Buras, A.; Zang, C.S.; Rammig, A.; Seidl, R. Excess Forest Mortality Is Consistently Linked to Drought across Europe. Nat. Commun. 2020, 11, 6200. [Google Scholar] [CrossRef] [PubMed]
- Bennett, A.C.; McDowell, N.G.; Allen, C.D.; Anderson-Teixeira, K.J. Larger Trees Suffer Most during Drought in Forests Worldwide. Nat. Plants 2015, 1, 15139. [Google Scholar] [CrossRef] [PubMed]
- Camarero, J.J. The Multiple Factors Explaining Decline in Mountain Forests: Historical Logging and Warming-Related Drought Stress Is Causing Silver-Fir Dieback in the Aragón Pyrenees. In High Mountain Conservation in a Changing World; Catalan, J., Ninot, J.M., Aniz, M.M., Eds.; Advances in Global Change Research; Springer International Publishing: Cham, Switzerland, 2017; Volume 62, pp. 131–154. [Google Scholar] [CrossRef]
- European Commission. Joint Research Centre. In European Atlas of Forest Tree Species; Publications Office: Luxembourg, 2016. [Google Scholar]
- Bigler, C.; Gričar, J.; Bugmann, H.; Čufar, K. Growth Patterns as Indicators of Impending Tree Death in Silver Fir. For. Ecol. Manag. 2004, 199, 183–190. [Google Scholar] [CrossRef]
- Dobrowolska, D.; Bončina, A.; Klumpp, R. Ecology and Silviculture of Silver Fir (Abies alba Mill.): A Review. J. For. Res. 2017, 22, 326–335. [Google Scholar] [CrossRef]
- Gazol, A.; González De Andrés, E.; Colangelo, M.; Valeriano, C.; Camarero, J.J. Pyrenean Silver Fir Forests Retain Legacies of Past Disturbances and Climate Change in Their Growth, Structure and Composition. Forests 2023, 14, 713. [Google Scholar] [CrossRef]
- Sangüesa-Barreda, G.; Camarero, J.J.; Oliva, J.; Montes, F.; Gazol, A. Past Logging, Drought and Pathogens Interact and Contribute to Forest Dieback. Agric. For. Meteorol. 2015, 208, 85–94. [Google Scholar] [CrossRef]
- Valeriano, C.; Tumajer, J.; Gazol, A.; González De Andrés, E.; Sánchez-Salguero, R.; Colangelo, M.; Linares, J.C.; Valor, T.; Sangüesa-Barreda, G.; Julio Camarero, J. Delineating Vulnerability to Drought Using a Process-Based Growth Model in Pyrenean Silver Fir Forests. For. Ecol. Manag. 2023, 541, 121069. [Google Scholar] [CrossRef]
- Vejpustková, M.; Čihák, T.; Fišer, P. The Increasing Drought Sensitivity of Silver Fir (Abies alba Mill.) Is Evident in the Last Two Decades. J. For. Sci. 2023, 69, 67–79. [Google Scholar] [CrossRef]
- Fritts, H.C. Tree Rings and Climate; Academic Press: London, UK, 1976. [Google Scholar]
- Housset, J.M.; Tóth, E.G.; Girardin, M.P.; Tremblay, F.; Motta, R.; Bergeron, Y.; Carcaillet, C. Tree-Rings, Genetics and the Environment: Complex Interactions at the Rear Edge of Species Distribution Range. Dendrochronologia 2021, 69, 125863. [Google Scholar] [CrossRef]
- Camarero, J.J.; Bigler, C.; Linares, J.C.; Gil-Pelegrín, E. Synergistic Effects of Past Historical Logging and Drought on the Decline of Pyrenean Silver Fir Forests. For. Ecol. Manag. 2011, 262, 759–769. [Google Scholar] [CrossRef]
- Holmes, R.L. Computer-Assisted Quality Control in Tree-Ring Dating and Measurement. Tree-Ring Bull. 1983, 43, 69–78. [Google Scholar]
- Larsson, L.; Larsson, P. CooRecorder/CDendro Package. Available online: http://www.cybis.se/forfun/dendro (accessed on 10 May 2024).
- Biondi, F.; Qeadan, F. A Theory-Driven Approach to Tree-Ring Standardization: Defining the Biological Trend from Expected Basal Area Increment. Tree-Ring Res. 2008, 64, 81–96. [Google Scholar] [CrossRef]
- Linares, J.C.; Camarero, J.J. Growth Patterns and Sensitivity to Climate Predict Silver Fir Decline in the Spanish Pyrenees. Eur. J. For. Res. 2012, 131, 1001–1012. [Google Scholar] [CrossRef]
- Cornes, R.C.; Van Der Schrier, G.; Van Den Besselaar, E.J.M.; Jones, P.D. An Ensemble Version of the E-OBS Temperature and Precipitation Data Sets. JGR Atmos. 2018, 123, 9391–9409. [Google Scholar] [CrossRef]
- Haylock, M.R.; Hofstra, N.; Klein Tank, A.M.G.; Klok, E.J.; Jones, P.D.; New, M. A European Daily High-resolution Gridded Data Set of Surface Temperature and Precipitation for 1950–2006. J. Geophys. Res. 2008, 113, 2008JD010201. [Google Scholar] [CrossRef]
- Beguería, S.; Vicente-Serrano, S.M.; Angulo-Martínez, M. A Multiscalar Global Drought Dataset: The SPEIbase: A New Gridded Product for the Analysis of Drought Variability and Impacts. Bull. Am. Meteor. Soc. 2010, 91, 1351–1356. [Google Scholar] [CrossRef]
- Vicente-Serrano, S.M.; Beguería, S.; López-Moreno, J.I. A Multiscalar Drought Index Sensitive to Global Warming: The Standardized Precipitation Evapotranspiration Index. J. Clim. 2010, 23, 1696–1718. [Google Scholar] [CrossRef]
- Beguería, S.; Vicente-Serrano, S.M.; Reig, F.; Latorre, B. Standardized Precipitation Evapotranspiration Index (SPEI) Revisited: Parameter Fitting, Evapotranspiration Models, Tools, Datasets and Drought Monitoring. Int. J. Climatol. 2014, 34, 3001–3023. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. 2024. Available online: https://www.R-project.org/ (accessed on 10 May 2024).
- Alderotti, F.; Sillo, F.; Brilli, L.; Bussotti, F.; Centritto, M.; Ferrini, F.; Gori, A.; Inghes, R.; Pasquini, D.; Pollastrini, M.; et al. Quercus ilex L. Dieback Is Genetically Determined: Evidence Provided by Dendrochronology, δ13C and SSR Genotyping. Sci. Total Environ. 2023, 904, 166809. [Google Scholar] [CrossRef] [PubMed]
- Van Mantgem, P.J.; Stephenson, N.L.; Byrne, J.C.; Daniels, L.D.; Franklin, J.F.; Fulé, P.Z.; Harmon, M.E.; Larson, A.J.; Smith, J.M.; Taylor, A.H.; et al. Widespread Increase of Tree Mortality Rates in the Western United States. Science 2009, 323, 521–524. [Google Scholar] [CrossRef] [PubMed]
- Adams, H.D.; Guardiola-Claramonte, M.; Barron-Gafford, G.A.; Villegas, J.C.; Breshears, D.D.; Zou, C.B.; Troch, P.A.; Huxman, T.E. Temperature Sensitivity of Drought-Induced Tree Mortality Portends Increased Regional Die-off under Global-Change-Type Drought. Proc. Natl. Acad. Sci. USA 2009, 106, 7063–7066. [Google Scholar] [CrossRef] [PubMed]
- McDowell, N.; Pockman, W.T.; Allen, C.D.; Breshears, D.D.; Cobb, N.; Kolb, T.; Plaut, J.; Sperry, J.; West, A.; Williams, D.G.; et al. Mechanisms of Plant Survival and Mortality during Drought: Why Do Some Plants Survive While Others Succumb to Drought? New Phytol. 2008, 178, 719–739. [Google Scholar] [CrossRef] [PubMed]
- Intergovernmental Panel on Climate Change. Climate Change 2021—The Physical Science Basis: Working Group I Contribution to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change, 1st ed.; Cambridge University Press: Cambridge, UK, 2023. [Google Scholar] [CrossRef]
- Carrer, M.; Nola, P.; Motta, R.; Urbinati, C. Contrasting Tree-Ring Growth to Climate Responses of Abies alba toward the Southern Limit of Its Distribution Area. Oikos 2010, 119, 1515–1525. [Google Scholar] [CrossRef]
- Hammond, W.M.; Williams, A.P.; Abatzoglou, J.T.; Adams, H.D.; Klein, T.; López, R.; Sáenz-Romero, C.; Hartmann, H.; Breshears, D.D.; Allen, C.D. Global Field Observations of Tree Die-off Reveal Hotter-Drought Fingerprint for Earth’s Forests. Nat. Commun. 2022, 13, 1761. [Google Scholar] [CrossRef] [PubMed]
- Konnert, M.; Bergmann, F. The Geographical Distribution of Genetic Variation of Silver Fir (Abies alba, Pinaceae) in Relation to Its Migration History. Pl. Syst. Evol. 1995, 196, 19–30. [Google Scholar] [CrossRef]
- Liepelt, S.; Cheddadi, R.; De Beaulieu, J.-L.; Fady, B.; Gömöry, D.; Hussendörfer, E.; Konnert, M.; Litt, T.; Longauer, R.; Terhürne-Berson, R.; et al. Postglacial Range Expansion and Its Genetic Imprints in Abies alba (Mill.)—A Synthesis from Palaeobotanic and Genetic Data. Rev. Palaeobot. Palynol. 2009, 153, 139–149. [Google Scholar] [CrossRef]
- Bosela, M.; Popa, I.; Gömöry, D.; Longauer, R.; Tobin, B.; Kyncl, J.; Kyncl, T.; Nechita, C.; Petráš, R.; Sidor, C.G.; et al. Effects of Post-glacial Phylogeny and Genetic Diversity on the Growth Variability and Climate Sensitivity of European Silver Fir. J. Ecol. 2016, 104, 716–724. [Google Scholar] [CrossRef]
- Dobbertin, M. Tree Growth as Indicator of Tree Vitality and of Tree Reaction to Environmental Stress: A Review. Eur. J. For. Res. 2005, 124, 319–333. [Google Scholar] [CrossRef]
- Wyckoff, P.H.; Clark, J.S. Predicting Tree Mortality from Diameter Growth: A Comparison of Maximum Likelihood and Bayesian Approaches. Can. J. For. Res. 2000, 30, 156–167. [Google Scholar] [CrossRef]
- Ogle, K.; Whitham, T.G.; Cobb, N.S. Tree-Ring Variation in Pinyon Predicts Likelihood of Death Following Severe Drought. Ecology 2000, 81, 3237–3243. [Google Scholar] [CrossRef]

| Paco Ezpela (PE) | Cotatuero (CO) | |
|---|---|---|
| Latitude (N) | 42.750000 | 42.653576 |
| Longitude (W) | −0.520000 | −0.050732 |
| Aspect | N-NE | S-SO |
| Elevation (m.a.s.l.) | 1232 | 1640 |
| Slope (°) | 27 | 34 |
| Basal area (m2ha−1) | 15.1 | 29.4 |
| Number of sampled trees | 40 (20 + 20) | 30 (15 + 15) |
| Declining trees 1999–2001 (%) | 40 | No Data |
| Declining trees 2011–2012 (%) | 64 | 56 |
| Declining trees 2017–2020 (%) | 28 | 15 |
| Site (Code) | 1970 to 2018 | 1990 to 2018 | ||
|---|---|---|---|---|
| Mean BAI (cm2) | BAI Trend (cm2 × Year−1) | Mean BAI (cm2) | BAI Trend (cm2 × Year−1) | |
| Cotatuero (CO) Declining | 14.3 ± 2.20 a | −0.17 ± 0.06 c | 13.0 ± 1.92 ab | −0.31 ± 0.16 b |
| Cotatuero (CO) Non-declining | 11.3 ± 0.93 a | 0.13 ± 0.06 b | 12.2 ± 1.23 ab | 0.36 ± 0.10 a |
| Paco Ezpela (PE) Declining | 9.92 ± 0.91 a | 0.04 ± 0.05 bc | 10.8 ± 1.08 b | −0.07 ± 0.05 b |
| Paco Ezpela (PE) Non-declining | 12.8 ± 1.32 a | 0.36 ± 0.06 a | 16.5 ± 1.70 a | 0.48 ± 0.10 a |
| Site (Code) | Annual | Winter | Spring | Summer | Autumn |
|---|---|---|---|---|---|
| Temperature (°C) | |||||
| Paco Ezpela (PE) | 9.40 | 2.30 | 8.00 | 17.10 | 10.20 |
| Cotatuero (CO) | 6.00 | −0.40 | 4.90 | 14.20 | 7.30 |
| Precipitation (mm) | |||||
| Paco Ezpela (PE) | 882 | 228 | 254 | 165 | 236 |
| Cotatuero (CO) | 1093 | 281 | 320 | 208 | 284 |
| Cotatuero (CO) | 1985 | 1995 | 2011 |
|---|---|---|---|
| Combined (Non-Declining + Declining) | |||
| Drought sensitivity (DS) | 12.81% | −4.74% | 5.54% |
| Drought recovery (DR) | −9.97% | −20.13% | 10.60% |
| Non-declining | |||
| Drought sensitivity (DS) | −4.73% | 4.29% | 4.26% |
| Drought recovery (DR) | −6.49% | −4.05% | 20.51% |
| Declining | |||
| Drought sensitivity (DS) | 1.72% | −3.44% | −3.49% |
| Drought recovery (DR) | 4.86% | −11.24% | −12.99% |
| Paco Ezpela (PE) | 1985 | 1995 | 2011 |
|---|---|---|---|
| Combined (Non-Declining + Declining) | |||
| Drought sensitivity (DS) | −2.13% | 4.82% | −16.93% |
| Drought recovery (DR) | −18.41% | 53.59% | −12.96% |
| Non-declining | |||
| Drought sensitivity (DS) | 4.59% | 4.51% | −12.79% |
| Drought recovery (DR) | −7.88% | 61.76% | −1.80% |
| Declining | |||
| Drought sensitivity (DS) | −7.28% | 5.13% | −22.62% |
| Drought recovery (DR) | −26.49% | 45.68% | −28.34% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crespo-Antia, J.P.; González de Andrés, E.; Gazol, A.; Camarero, J.J.; Linares, J.C. Tree-Level Climate Sensitivity Reveals Size Effects and Impending Growth Decline in Silver Fir Affected by Dieback. Forests 2024, 15, 999. https://doi.org/10.3390/f15060999
Crespo-Antia JP, González de Andrés E, Gazol A, Camarero JJ, Linares JC. Tree-Level Climate Sensitivity Reveals Size Effects and Impending Growth Decline in Silver Fir Affected by Dieback. Forests. 2024; 15(6):999. https://doi.org/10.3390/f15060999
Chicago/Turabian StyleCrespo-Antia, Juan Pablo, Ester González de Andrés, Antonio Gazol, Jesús Julio Camarero, and Juan Carlos Linares. 2024. "Tree-Level Climate Sensitivity Reveals Size Effects and Impending Growth Decline in Silver Fir Affected by Dieback" Forests 15, no. 6: 999. https://doi.org/10.3390/f15060999








