Soil Chemical Quality in Integrated Production Systems with the Presence of Native and Exotic Tree Components in the Brazilian Eastern Amazon
Abstract
1. Introduction
2. Materials and Methods
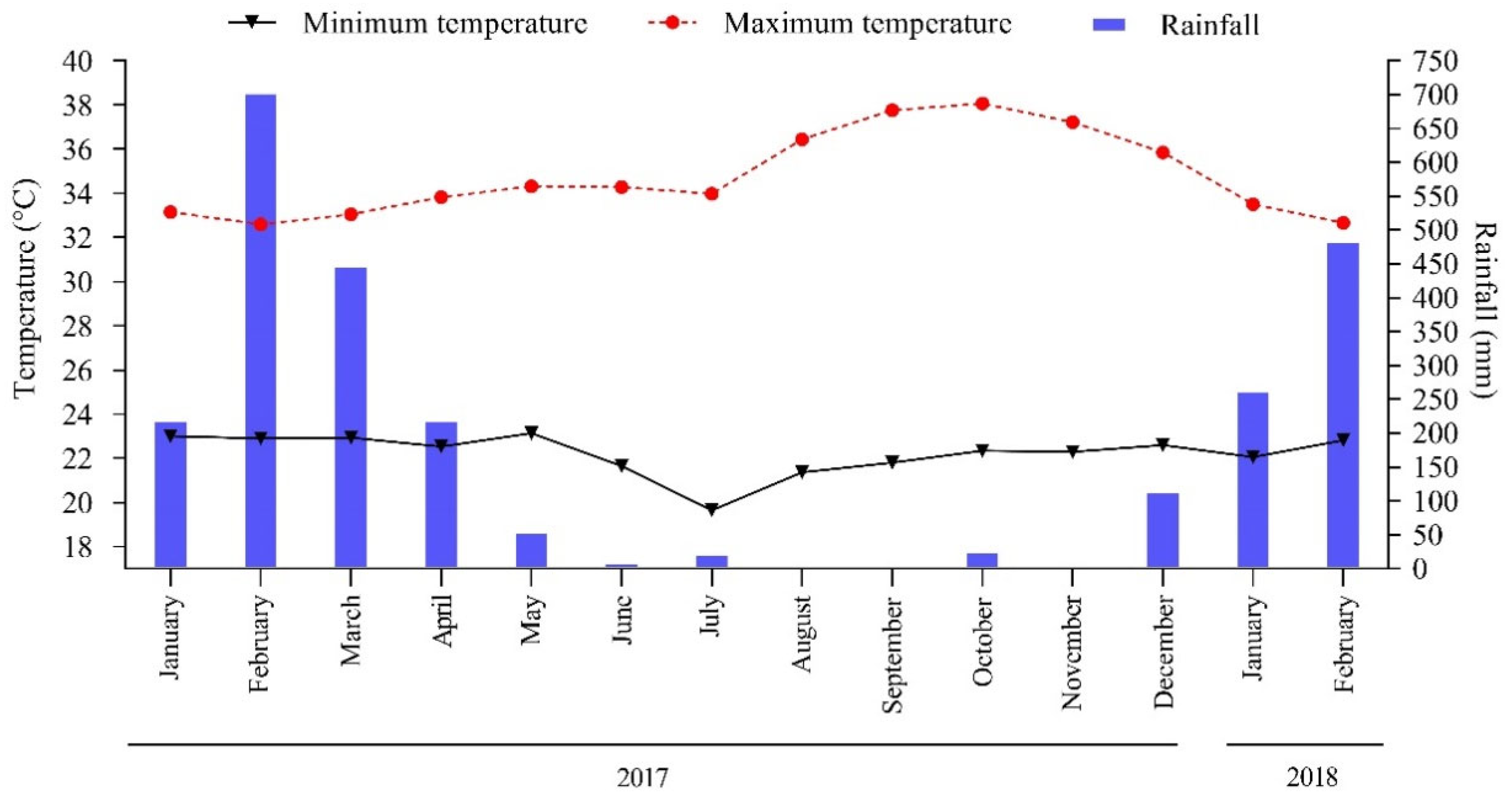
2.1. Climate and Soil of the Experimental Area
2.2. History of the Areas and Description of the Systems
2.3. Soil Sampling and Analysis
2.4. Statistical Analyses
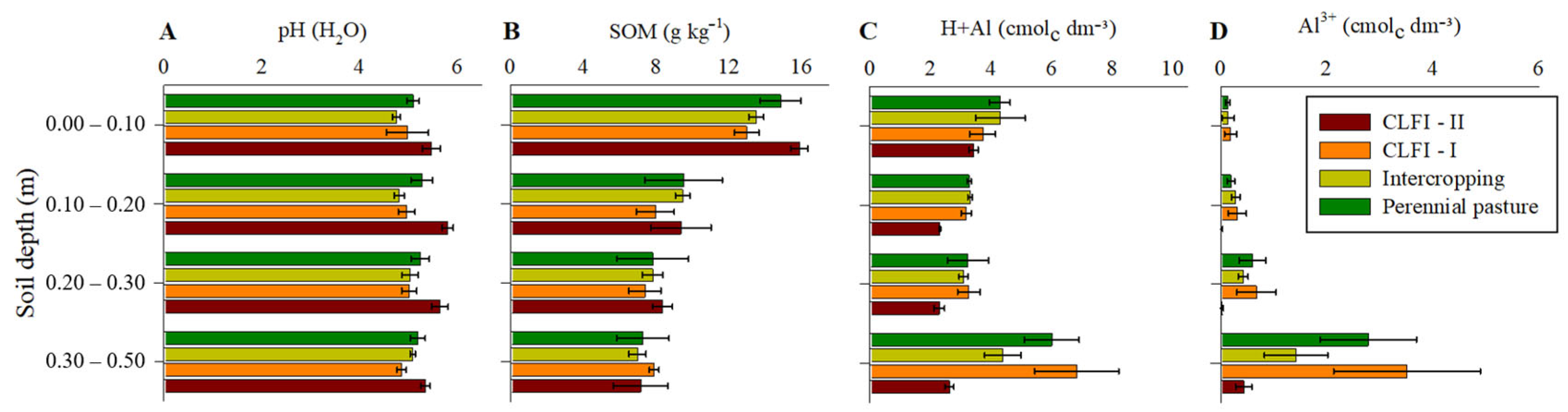
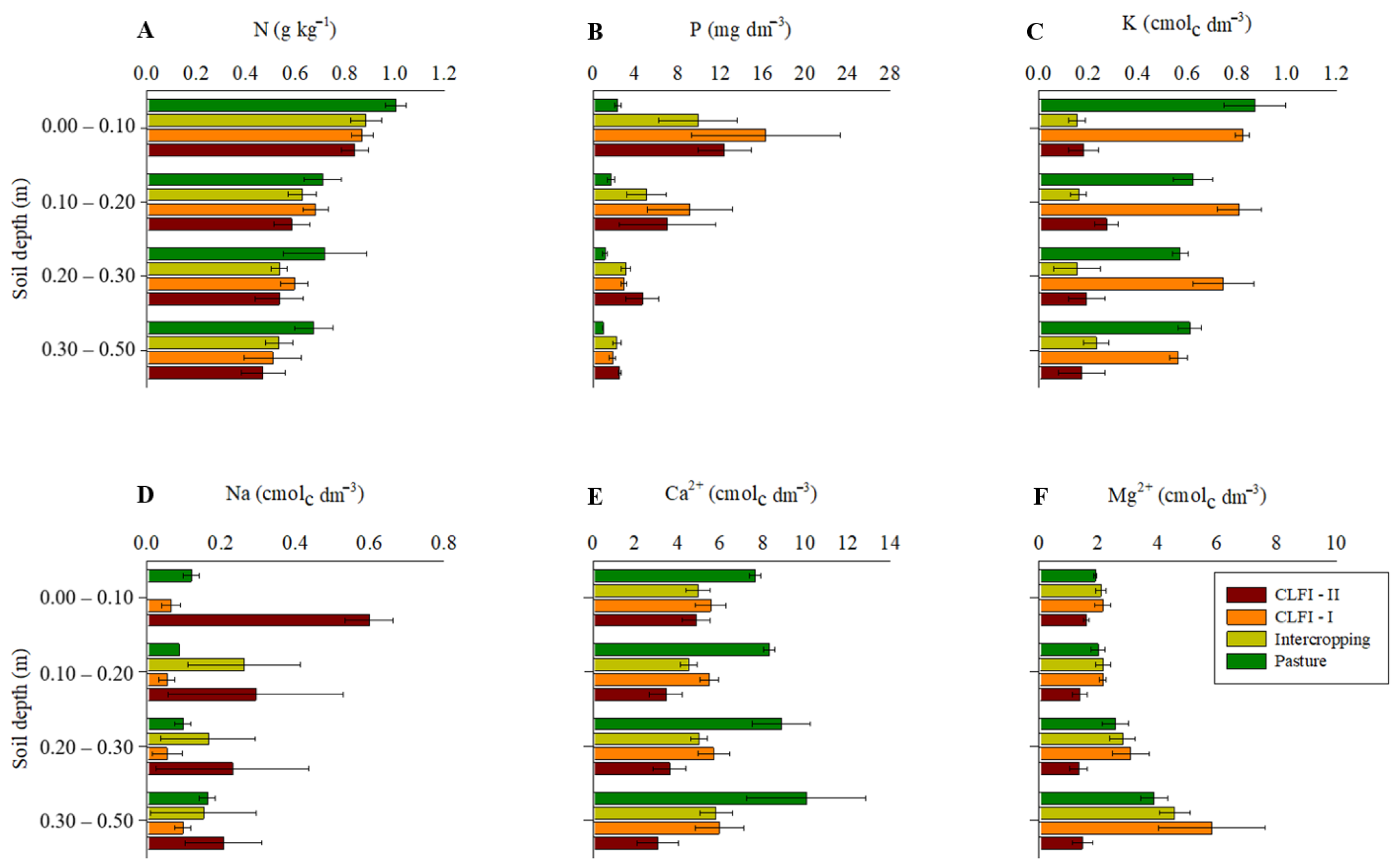
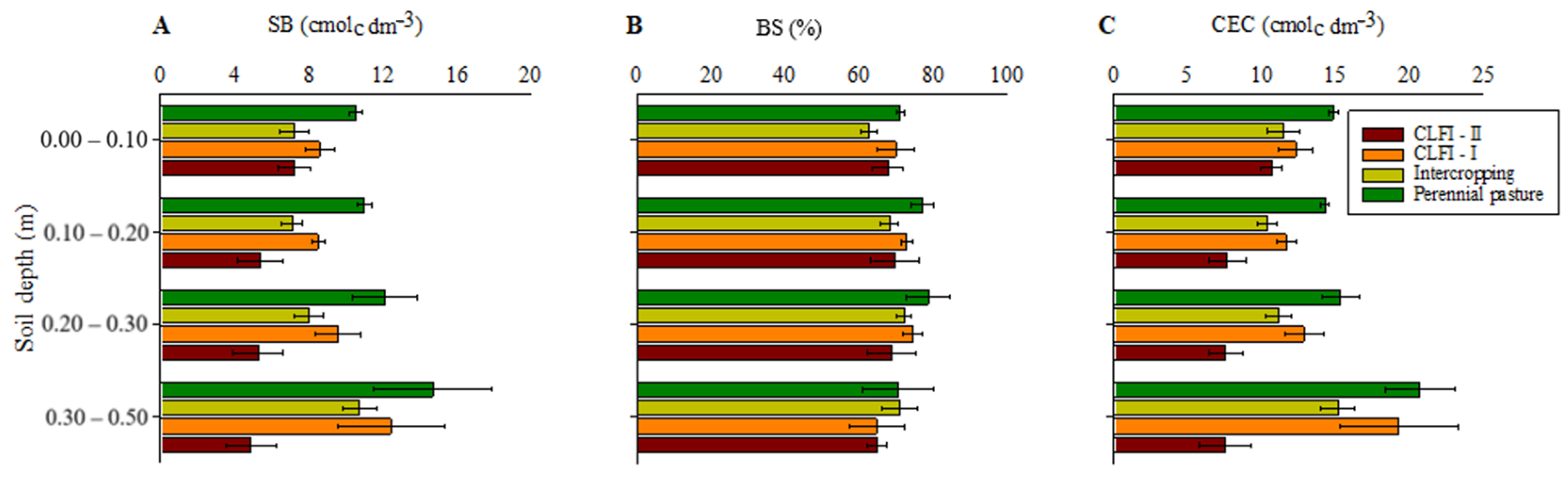
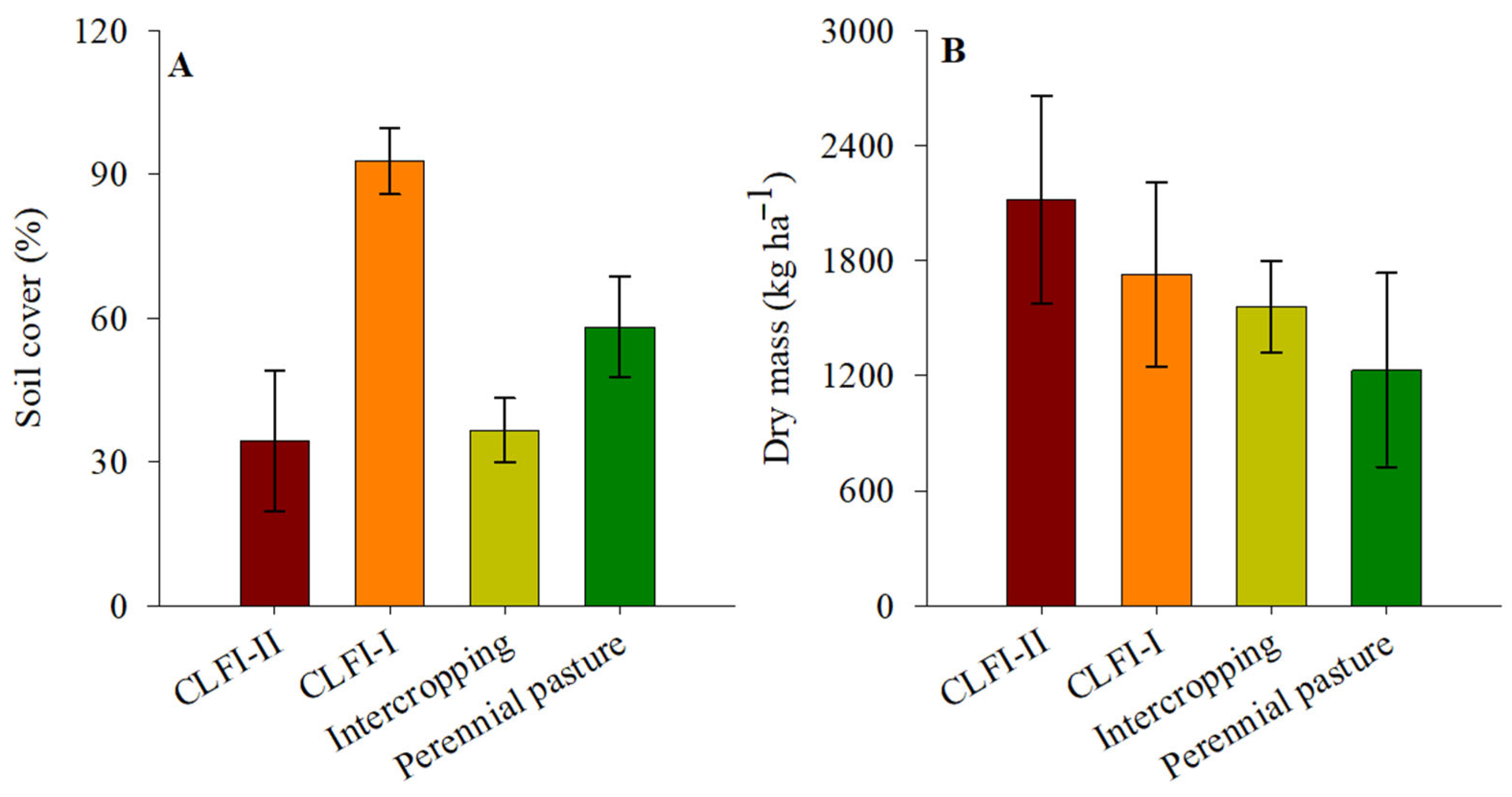
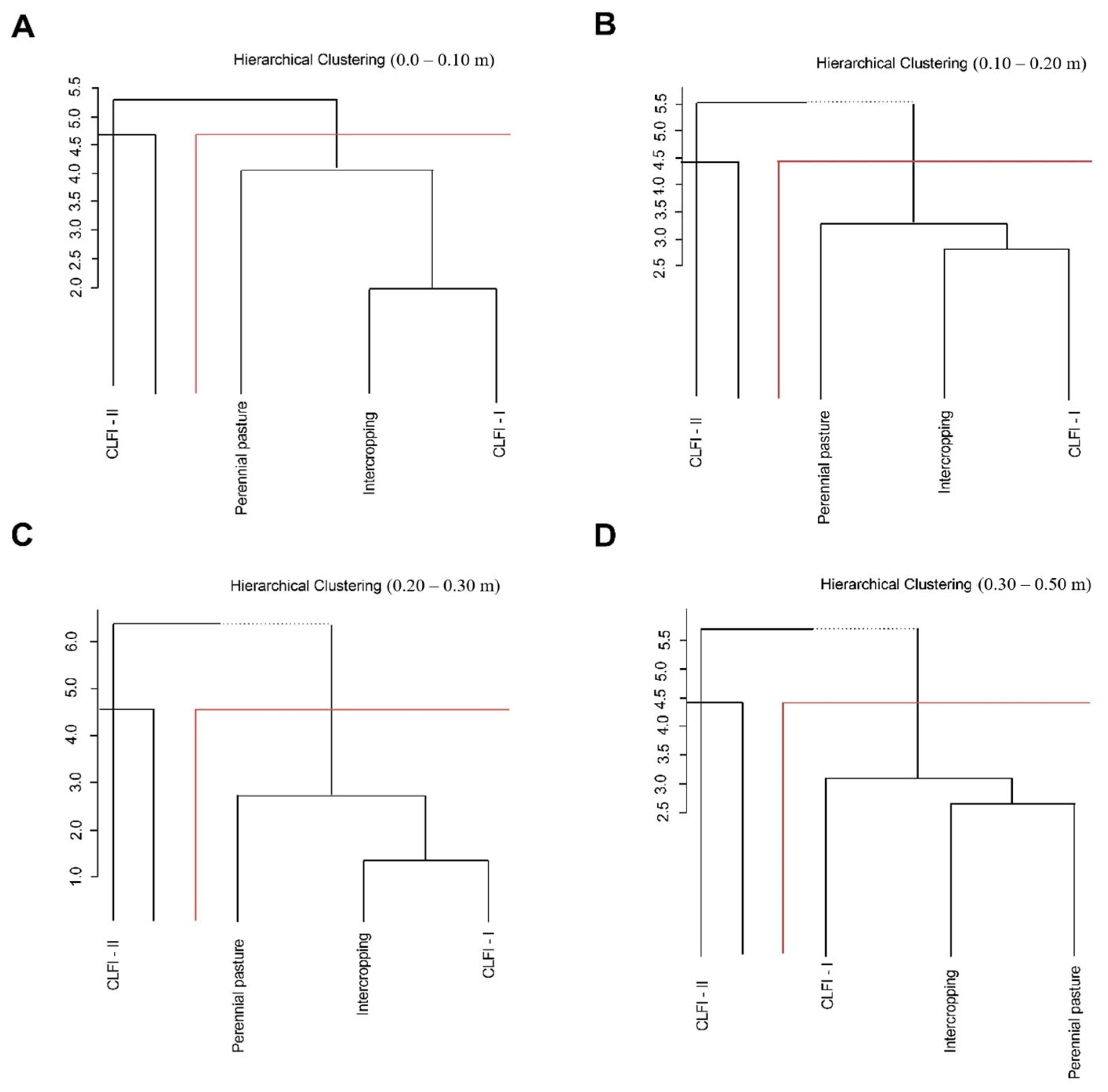
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Koch, N.; Ermgassen, E.H.J.Z.; Wehkamp, J.; Oliveira Filho, F.J.B.; Schwerhoff, G. Agricultural productivity and forest conservation: Evidence from the Brazilian Amazon. Amer. J. Agr. Econ. 2019, 101, 919–940. [Google Scholar] [CrossRef]
- Ribeiro, T.M.; Mendonça, B.A.F.; Oliveira-Júnior, J.F.; Fernandes-Filho, E.I. Fire foci assessment in the Western Amazon (2000–2015). Environ. Dev. Sustain. 2021, 23, 1485–1498. [Google Scholar] [CrossRef]
- Gatti, L.V.; Basso, L.S.; Miller, J.B.; Gloor, M.; Domingues, L.G.; Cassol, H.L.G.; Tejada, G.; Aragão, L.E.O.C.; Nobre, C.; Peters, W.; et al. Amazonia as a carbon source linked to deforestation and climate change. Nature 2021, 595, 388–393. [Google Scholar] [CrossRef] [PubMed]
- Faria, B.L.; Staal, A.; Silva, C.A.; Martin, P.A.; Panday, P.K.; Dantas, V.L. Climate change and deforestation increase the vulnerability of Amazonian forests to post-fire grass invasion. Glob. Ecol. Biogeogr. 2021, 30, 2368–2381. [Google Scholar] [CrossRef]
- Instituto Nacional de Pesquisas Espaciais—INPE. TerraBrasilis/PRODES. Monitoramento do Desmatamento da Floresta Amazônica Brasileira por Satélite. 2024. Available online: http://terrabrasilis.dpi.inpe.br/app/dashboard/deforestation/biomes/amazon/increments (accessed on 18 April 2024).
- Cavalcante, R.B.L.; Nunes, S.; Viademonte, S.; Rodrigues, C.M.F.; Gomes, W.C.; Ferreira, J.S., Jr.; Pontes, P.R.M.; Giannini, T.C.; Awade, M.; Miranda, L.S.; et al. Multicriteria approach to prioritize forest restoration areas for biodiversity conservation in the eastern Amazon. J Environ. Manage. 2022, 318, 115590. [Google Scholar] [CrossRef] [PubMed]
- Brandão, D.O.; Barata, L.E.S.; Nobre, C.A. The effects of environmental changes on plant species and forest dependent communities in the Amazon Region. Forests 2022, 13, 466. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, X.; Riley, W.J.; Xue, Y.; Nobre, C.A.; Lovejoy, T.E.; Jia, G. Deforestation triggering irreversible transition in Amazon hydrological cycle. Environ. Res. Lett. 2022, 17, 034037. [Google Scholar] [CrossRef]
- Kastens, J.H.; Brown, J.C.; Coutinho, A.C.; Bishop, C.R.; Esquerdo, J.C.D.M. Soy moratorium impacts on soybean and deforestation dynamics in Mato Grosso, Brazil. PLoS ONE 2017, 12, e0176168. [Google Scholar] [CrossRef] [PubMed]
- Stürmer, S.L.; Siqueira, J.O. Species richness and spore abundance of arbuscular mycorrhizal fungi across distinct land uses in Western Brazilian Amazon. Mycorrhiza 2011, 21, 255–267. [Google Scholar] [CrossRef]
- Rueda, B.L.; McRoberts, K.C.; Blake, R.W.; Nicholson, C.F.; Valentim, J.F.; Fernandes, E.C.M.; Moral, M.T. Nutrient status of cattle grazing systems in the western Brazilian Amazon. Cogent Food Agric. 2020, 6, 1722350. [Google Scholar] [CrossRef]
- Rocha, G.P.E.; Vieira, D.L.M.; Simon, M.F. Fast natural regeneration in abandoned pastures in southern Amazonia. For. Ecol. Manag. 2016, 370, 93–101. [Google Scholar] [CrossRef]
- Schielein, J.; Börner, J. Recent transformations of land-use and land-cover dynamics across different deforestation frontiers in the Brazilian Amazon. Land Use Policy 2018, 76, 81–94. [Google Scholar] [CrossRef]
- Molossi, L.; Hoshide, A.K.; Abreu, D.C.; Oliveira, R.A. Agricultural Support and Public Policies Improving Sustainability in Brazil’s Beef Industry. Sustainability 2023, 15, 4801. [Google Scholar] [CrossRef]
- Szymczak, L.S.; Carvalho, P.C.F.; Lurette, A.; Moraes, A.; Nunes, P.A.A.; Martins, A.P.; Moulin, C.-H. System diversification and grazing management as resilience-enhancing agricultural practices: The case of crop-livestock integration. Agric. Syst. 2020, 184, 102904. [Google Scholar] [CrossRef]
- Kisaka, M.O.; Shisanya, C.; Cournac, L.; Manlay, J.R.; Gitari, H.; Muriuki, J. Integrating no-tillage with agroforestry augments soil quality indicators in Kenya’s dry-land agroecosystems. Soil Tillage Res. 2023, 227, 105586. [Google Scholar] [CrossRef]
- Koutika, L.S.; Taba, K.; Ndongo, M.; Kaonga, M. Nitrogen-fixing trees increase organic carbon sequestration in forest and agroforestry ecosystems in the Congo basin. Reg. Environ. Chang. 2021, 21, 109. [Google Scholar] [CrossRef]
- Brewer, K.M.; Gaudin, A.C.M. Potential of crop-livestock integration to enhance carbon sequestration and agroecosystem functioning in semi-arid croplands. Soil Biol. Biochem. 2020, 149, 107936. [Google Scholar] [CrossRef]
- Kruchelski, S.; Trautenmüller, J.W.; Orso, G.A.; Triches, G.P.; Silva, V.P.; Moraes, A. Growth and productivity of Eucalyptus benthamii in integrated crop-livestock systems in southern Brazil. Agrofor. Syst. 2023, 97, 45–57. [Google Scholar] [CrossRef]
- Sarto, M.V.M.; Borges, W.L.B.; Sarto, J.R.W.; Pires, C.A.B.; Rice, C.W.; Rosolem, C.A. Soil microbial community and activity in a tropical integrated crop-livestock system. Appl. Soil Ecol. 2020, 145, 103350. [Google Scholar] [CrossRef]
- Pezzopane, J.R.M.; Bosi, C.; Bernardi, A.C.C.; Muller, M.D.; Oliveira, P.P.A. Managing eucalyptus trees in agroforestry systems: Productivity parameters and PAR transmittance. Agr. Ecosyst. Environ. 2021, 312, 107350. [Google Scholar] [CrossRef]
- Behling, M.; Martínez, G.B.; Silva, A.R.; Oliveira, T.K.; Cipriani, H.N. O eucalipto em sistemas de integração lavoura-pecuária-floresta (ILPF) na Amazônia. In O Eucalipto e a Embrapa: Quatro Décadas de Pesquisa e Desenvolvimento; Oliveira, E.B., Pinto, J.E., Jr., Eds.; Embrapa: Brasília, Brazil, 2021; pp. 1041–1053. Available online: https://www.embrapa.br/busca-de-publicacoes/-/publicacao/1131985/o-eucalipto-em-sistemas-de-integracao-lavoura-pecuaria-floresta-ilpf-na-amazonia (accessed on 18 April 2024).
- Porro, R.; Sousa, R.C. Anatomy of babassu-nut value chain for policy guidance in support of traditional agroextractive communities in the Mearim Valley, Maranhão, Brazil. Rev. Econ. Sociol. Rural 2023, 61, e263743. [Google Scholar] [CrossRef]
- Luz, R.L.; Leite, M.F.A.; Zelarayán, M.C.; Boddey, R.M.; Gehring, C. Litter decomposition and nutrient release dynamics of leaves and roots of the babassu palm in eastern Amazonia. Acta Amazonica 2020, 50, 213–222. [Google Scholar] [CrossRef]
- Alvares, C.A.; Stape, J.L.; Sentelhas, P.C.; Gonçalves, J.L.M.; Sparovek, G. Köppen’s climate classification map for Brazil. Meteorol Z. 2013, 22, 711–728. [Google Scholar] [CrossRef]
- Santos, H.G.; Jacomine, P.K.T.; Anjos, L.H.C.; Oliveira, V.A.; Lumbreras, J.F.; Coelho, M.R.; Almeida, J.A.; Araujo Filho, J.C.; Oliveira, J.B.; Cunha, T.J.F. Sistema Brasileiro de Classificação de Solos, 5th ed.; Embrapa Solos: Rio de Janeiro, Brazil, 2018; 356p. [Google Scholar]
- IUSS Working Group WRB. World Reference Base for Soil Resources 2014, Update 2015: International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; World Soil Resources Reports, 106; Food and Agriculture Organization of the United Nations: Rome, Italy, 2015. [Google Scholar]
- Sousa, D.M.G.; Lobato, E. Cerrado: Soil Correction and Fertilization; Embrapa Cerrados Publishing: Planaltina, Brazil, 2004; 416p. [Google Scholar]
- Teixeira, P.C.; Donagemma, G.K.; Fontana, A.; Teixeira, W.G. Manual de Métodos de Análise de Solo, 3rd ed.; Embrapa: Brasília, Brazil, 2017; 574p. [Google Scholar]
- Pires, W. Manual de Pastagem: Formação, Manejo e Recuperação; Aprenda Fácil: Viçosa, Brazil, 2006; 302p. [Google Scholar]
- Gazziero, D.L.P.; Velini, E.D.; Osipe, R. Procedimentos Para Instalação, Avaliação e Análise de Experimentos com Herbicidas; Sociedade Brasileira da Ciência das Plantas Daninhas: Londrina, Brazil, 1995; 42p. [Google Scholar]
- Payton, M.E.; Miller, A.E.; Raun, W.R. Testing statistical hypothesis using standard error bars and confidence intervals. Commun. Soil Sci. PLAN. 2000, 31, 547–551. [Google Scholar] [CrossRef]
- Manly, B.J.F. Multivariate Statistical Methods: An Introduction; Bookman: Porto Alegre, Brazil, 2008; 229p. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: https://www.R-project.org/ (accessed on 12 February 2024).
- Oliveira, H.M.R.; Santos, A.C.; Leite, R.C.; Oliveira, L.B.T.; Silva, I.R.; Oliveira, T.S. Repercussion of pastoral systems in C and N fractions stock in northeast Amazonia. Catena 2022, 208, 105742. [Google Scholar] [CrossRef]
- Teixeira, M.; Carvalho, M.G. Regulatory mechanism for biomass renewable energy in Brazil, a case study of the Brazilian babassu oil extraction industry. Energy 2007, 32, 999–1005. [Google Scholar] [CrossRef]
- Seneviratne, G. Litter quality and nitrogen release in tropical agriculture: A synthesis. Biol. Fertil. Soils 2000, 31, 60–64. [Google Scholar] [CrossRef]
- Hättenschwiler, S.; Vitousek, P.M. The role of polyphenols in terrestrial ecosystem nutrient cycling. Trends Ecol. Evol. 2000, 15, 238–243. [Google Scholar] [CrossRef]
- Gehring, C.; Zelarayán, M.C.; Luz, R.L.; Almeida, R.B.; Boddey, R.M.; Leite, M.F.A. Babassu palm (Attalea speciosa Mart.) super-dominance shapes its surroundings via multiple biotic, soil chemical, and physical interactions and accumulates soil carbon: A case study in eastern Amazonia. Plant Soil 2020, 454, 447–460. [Google Scholar] [CrossRef]
- Lajtha, K.; Bowden, R.D.; Nadelhoffer, K. Litter and root manipulations provide insights into soil organic matter dynamics and stability. Soil Sci. Soc. Am. J. 2014, 78, S261–S269. [Google Scholar] [CrossRef]
- Six, J.; Conant, R.T.; Paul, E.A.; Paustian, K. Stabilization mechanisms of soil organic matter: Implications for C-saturation of soils. Plant and Soil 2002, 241, 155–176. [Google Scholar] [CrossRef]
- Cotrufo, M.F.; Ranalli, M.G.; Haddix, M.L.; Six, J.; Lugato, E. Soil carbon storage informed by particulate and mineral-associated organic matter. Nat. Geosci. 2019, 12, 989–994. [Google Scholar] [CrossRef]
- Sousa, J.T.R.; Moraes, F.H.R.; Gehring, C. Root biomass in a shifting cultivation system in the eastern periphery of Amazonia, and contribution of the babassu palm. Agrofor Syst 2016, 90, 351–360. [Google Scholar] [CrossRef]
- Redin, M.; Guénon, R.; Recous, S.; Schmatz, R.; de Freitas, L.L.; Aita, C.; Giacomini, S.J. Carbon mineralization in soil of roots from twenty crop species, as affected by their chemical composition and botanical family. Plant Soil 2014, 378, 205–214. [Google Scholar] [CrossRef]
- Camiré, C.; Côté, B.; Brulotte, S. Decomposition of roots of black alder and hybrid poplar in short-rotation plantings: Nitrogen and lignin control. Plant Soil 1991, 138, 123–132. [Google Scholar] [CrossRef]
- Ebeling, A.G.; dos Anjos, L.H.C.; Perez, D.V.; Pereira, M.G.; Valladares, G.S. Relação entre acidez e outros atributos químicos em solos com teores elevados de matéria orgânica. Bragantia 2008, 67, 429–439. [Google Scholar] [CrossRef]
- Santos, A.M.; Mitja, D.; Miranda, I.S.; Loisel, P.; Delaître, E.; Demagistri, L. What is the influence of anthropogenic impact on the population structure of Attalea speciosa Mart. ex Spreng. in the Brazilian Amazonian region? Acta Bot. Bras. 2022, 36, e2020abb0543. [Google Scholar] [CrossRef]
- Teixeira, H.M.; Bianchi, F.J.; Cardoso, I.M.; Tittonell, P.; Pena-Claros, M. Impact of agroecological management on plant diversity and soil-based ecosystem services in pasture and coffee systems in the Atlantic forest of Brazil. Agr. Ecosyst. Environ. 2021, 305, 107171. [Google Scholar] [CrossRef]
- Brito, L.C.R.; Souza, H.A.; Araújo Neto, R.B.; Azevedo, D.M.P.; Sagrilo, E.; Vogado, R.F.; Carvalho, S.P.; Ferreira, A.C.M.; Cavigelli, M.A. Improved soil fertility, plant nutrition and grain yield of soybean and millet following maize intercropped with forage grasses and crotalaria in the Brazilian savanna. Crop Pasture Sci. 2023, 75, 438–448. [Google Scholar] [CrossRef]
- Bieluczyk, W.; Piccolo, M.C.; Pereira, M.G.; de Moraes, M.T.; Soltangheisi, A.; Bernardi, A.C.C.; Pezzopane, J.R.M.; Oliveira, P.P.A.; Moreira, M.Z.; Camargo, P.B.; et al. RIntegrated farming systems influence soil organic matter dynamics in southeastern Brazil. Geoderma 2020, 371, 114368. [Google Scholar] [CrossRef]
- Haynes, R.J.; Williams, P.H. Nutrient cycling and soil fertility in the grazed pasture ecosystem. Adv. Agron. 1993, 49, 119–199. [Google Scholar]
- Oenema, O.; Wrage, N.; Velthof, G.L.; van Groeningen, J.W.; Dolfing, J.; Kuikman, P.J. Trends in global nitrous oxide emissions from animal production systems. Nutr. Cycling Agroecosyst. 2005, 72, 51–65. [Google Scholar] [CrossRef]
- Ferreira, A.C.M.; Souza, H.A.; Sagrilo, E.; Silva Júnior, G.B.; Natale, W.; Sobral, A.H.S.; Vera, G.S.; dos Santos, F.S.C.B. Absorption, partitioning, and export of nutrients by phenological stage in maize cultivated in Eastern Maranhão, Brazil. J. Plant Nutr. 2023, 47, 240–256. [Google Scholar] [CrossRef]
- Tiecher, T.; Fontoura, S.M.V.; Ambrosini, V.G.; Araújo, E.A.; Alves, L.A.; Bayer, C.; Gatiboni, L.C. Soil phosphorus forms and fertilizer use efficiency are affected by tillage and soil acidity management. Geoderma 2023, 435, 116495. [Google Scholar] [CrossRef]
- Fink, J.R.; Inda, A.V.; Tiecher, T.; Barrón, V. Iron oxides and organic matter on soil phosphorus availability. Ci. Agrotec. 2016, 40, 369–379. [Google Scholar] [CrossRef]
- Ferreira, J.L.S.; Calil, F.N.; Silva Neto, C.M. Estoque de nutrientes no componente florestal em um sistema de integração lavoura-pecuária-floresta no Brasil Central. Rev. Ibero-Am. Ciênc. Ambient. 2021, 12, 86–97. [Google Scholar]
- Reis, V.R.R.; Deon, D.S.; Muniz, L.C.; Garcia, U.S.; Cantanhêde, I.S.L.; Rego, C.A.R.M.; Costa, J.B.; Marques, E.O. Soil chemical attributes under crop-livestock-forest integration system and in different land uses in Mata dos Cocais region. J. Agric. Sci. 2018, 10, 370–380. [Google Scholar] [CrossRef]
- Corbeels, M.; Scopel, E.; Cardoso, A.; Bernoux, M.; Douzet, J.-M.; Siqueira Neto, M. Soil carbon storage potential of direct seeding mulch-based cropping systems in the cerrados of Brazil. Glob. Chang. Biol. 2006, 12, 1773–1787. [Google Scholar] [CrossRef]
- Blanchart, E.; Bernoux, M.; Sarda, X.; Siqueira Neto, M.; Cerri, C.C.; Piccolo, M.; Douzet, J.M.; Scopel, E. Effect of direct seeding mulch-based systems on soil carbon storage and macrofauna in Central Brazil. Agric. Conspec. Sci. 2007, 72, 81–87. [Google Scholar]
- Carvalho, J.L.N.; Cerri, C.E.P.; Feigl, B.J.; Píccolo, M.C.; Godinho, V.P.; Cerri, C.C. Carbon sequestration in agricultural soils in the Cerrado region of the Brazilian Amazon. Soil Till. Res. 2009, 103, 342–349. [Google Scholar] [CrossRef]
- Lal, R. Soil carbon sequestration to mitigate climate change. Geoderma 2004, 123, 1–22. [Google Scholar] [CrossRef]
- Bayer, C.; Martin-Neto, L.; Mielniczuk, J.; Pavinato, A.; Dieckow, J. Carbon sequestration in two Brazilian Cerrado soils under no-till. Soil Till. Res. 2006, 86, 237–245. [Google Scholar] [CrossRef]
- Gualberto, A.V.S.; Souza, H.A.; Sagrilo, E.; Araujo, A.S.F.; Mendes, L.W.; Medeiros, E.V.; Pereira, A.P.A.; Costa, D.P.; Vogado, R.F.; Cunha, J.R.; et al. Organic C Fractions in Topsoil under Different Management Systems in Northeastern Brazil. Soil Syst. 2023, 7, 11. [Google Scholar] [CrossRef]

| Land Use | Historic |
|---|---|
| Perennial Pasture | Previously (1970–1999), Jaraguá grass (Hyparrhenia rufa (Ness) Stapf) was cultivated. After pasture renewal (2000–2017), it consisted of an area of 3.0 ha cultivated with Urochloa brizantha cv. Marandú, with no soil correction or fertilization, used for continuous extensive grazing with a stocking rate of 0.5 animals ha−1. The absence of management practices allowed the natural regeneration of native vegetation. |
| ICLF-I | Area of 3.5 ha with 2 years of maize cultivation (hybrids KWS 9304 and AG1051) in a spacing of 0.6 m × 0.3 m (66,600 plants ha−1), intercropped with Urochloa brizantha cv. Marandú. The tree component (eucalyptus) was implemented in February 2017, in double rows with a spacing of 3 m × 2 m and 28 m between rows. |
| ICLF-II | Area of 3.0 ha, with native babassu palm vegetation (average of 35 palm trees ha−1). Maize (Dow Herculex) intercropped with Massai grass (Megathyrsus maximum) was implemented in February 2017. Maize was sown at a spacing of 0.6 m × 0.3 m (55,000 plants ha−1). A cultural value of 98% was considered for sowing the Massai seeds. Before the implementation of the intercropping, the area was cultivated with Urochloa brizantha cv. Marandú for 20 years. |
| Maize + Massai intercropping | Area of 3.0 ha, with maize (Dow Herculex) planted in January 2017 intercropped with Massai grass (Megathyrsus maximum). Maize was sown at a spacing of 0.5 m × 0.25 m (83,000 plants ha−1). A cultural value of 98% was considered for sowing the Massai seeds. Before the implementation of the intercropping, the area was cultivated with Urochloa brizantha cv. Marandú for 20 years. |
| Chemical Attributes | Soil Depths (m) | |||||||
|---|---|---|---|---|---|---|---|---|
| 0.00–0.10 | 0.10–0.20 | 0.20–0.30 | 0.30–0.50 | |||||
| PC1 | PC2 | PC1 | PC2 | PC1 | PC2 | PC1 | PC2 | |
| pH | −0.13 | 0.45 | −0.25 | 0.44 | −0.22 | 0.48 | −0.22 | 0.27 |
| SOM | −0.08 | 0.42 | 0.00 | −0.03 | −0.13 | −0.17 | 0.09 | −0.41 |
| N | 0.34 | 0.13 | 0.31 | 0.07 | 0.17 | −0.10 | 0.20 | −0.01 |
| P | −0.21 | −0.22 | −0.13 | −0.25 | −0.32 | −0.07 | −0.29 | −0.23 |
| K+ | 0.35 | 0.06 | 0.26 | 0.18 | 0.26 | 0.00 | 0.35 | 0.00 |
| Na+ | −0.26 | 0.35 | −0.15 | 0.08 | 0.03 | 0.45 | −0.08 | 0.14 |
| Ca2+ | 0.39 | 0.17 | 0.36 | 0.25 | 0.34 | 0.23 | 0.31 | 0.37 |
| Mg2+ | 0.20 | −0.19 | 0.30 | −0.28 | 0.31 | −0.11 | 0.31 | −0.07 |
| H+Al | 0.22 | −0.25 | 0.24 | −0.43 | 0.32 | −0.20 | 0.33 | −0.28 |
| Al3+ | 0.17 | −0.38 | 0.30 | −0.35 | 0.25 | −0.47 | 0.32 | −0.31 |
| SB | 0.39 | 0.18 | 0.39 | 0.20 | 0.37 | 0.16 | 0.36 | 0.23 |
| CEC | 0.41 | 0.07 | 0.40 | 0.10 | 0.38 | 0.07 | 0.39 | 0.06 |
| BS | 0.19 | 0.34 | 0.23 | 0.45 | 0.29 | 0.42 | 0.07 | 0.56 |
| Eigenvalues | 5.36 | 3.76 | 5.90 | 2.35 | 6.75 | 2.21 | 6.41 | 2.47 |
| Total variance (%) | 41.3 | 28.9 | 45.4 | 18.1 | 51.9 | 17.1 | 49.3 | 19.1 |
| Cumulative variance (%) | 41.3 | 70.2 | 45.4 | 63.5 | 51.9 | 69.0 | 49.3 | 68.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Souza, I.M.d.; Sagrilo, E.; de Oliveira Júnior, J.O.L.; Araújo, M.D.M.; Muniz, L.C.; Costa, J.B.; Pompeu, R.C.F.F.; de Sousa, D.C.; de Andrade, H.A.F.; de Oliveira Neto, E.D.; et al. Soil Chemical Quality in Integrated Production Systems with the Presence of Native and Exotic Tree Components in the Brazilian Eastern Amazon. Forests 2024, 15, 1078. https://doi.org/10.3390/f15071078
Souza IMd, Sagrilo E, de Oliveira Júnior JOL, Araújo MDM, Muniz LC, Costa JB, Pompeu RCFF, de Sousa DC, de Andrade HAF, de Oliveira Neto ED, et al. Soil Chemical Quality in Integrated Production Systems with the Presence of Native and Exotic Tree Components in the Brazilian Eastern Amazon. Forests. 2024; 15(7):1078. https://doi.org/10.3390/f15071078
Chicago/Turabian StyleSouza, Ivanderlete Marques de, Edvaldo Sagrilo, José Oscar Lustosa de Oliveira Júnior, Maria Diana Melo Araújo, Luciano Cavalcante Muniz, Joaquim Bezerra Costa, Roberto Cláudio Fernandes Franco Pompeu, Daiane Conceição de Sousa, Hosana Aguiar Freitas de Andrade, Edson Dias de Oliveira Neto, and et al. 2024. "Soil Chemical Quality in Integrated Production Systems with the Presence of Native and Exotic Tree Components in the Brazilian Eastern Amazon" Forests 15, no. 7: 1078. https://doi.org/10.3390/f15071078
APA StyleSouza, I. M. d., Sagrilo, E., de Oliveira Júnior, J. O. L., Araújo, M. D. M., Muniz, L. C., Costa, J. B., Pompeu, R. C. F. F., de Sousa, D. C., de Andrade, H. A. F., de Oliveira Neto, E. D., Leite, L. F. C., Blanco, F. F., Lima, P. S. d. C., & Souza, H. A. d. (2024). Soil Chemical Quality in Integrated Production Systems with the Presence of Native and Exotic Tree Components in the Brazilian Eastern Amazon. Forests, 15(7), 1078. https://doi.org/10.3390/f15071078








