The Development of an Improved Medium for the In Vitro Germination of Corylus avellana L. Pollen
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Storage
2.2. In Vitro Pollen Germination
2.3. Analysis of the Microelements
2.4. Statistical Analysis
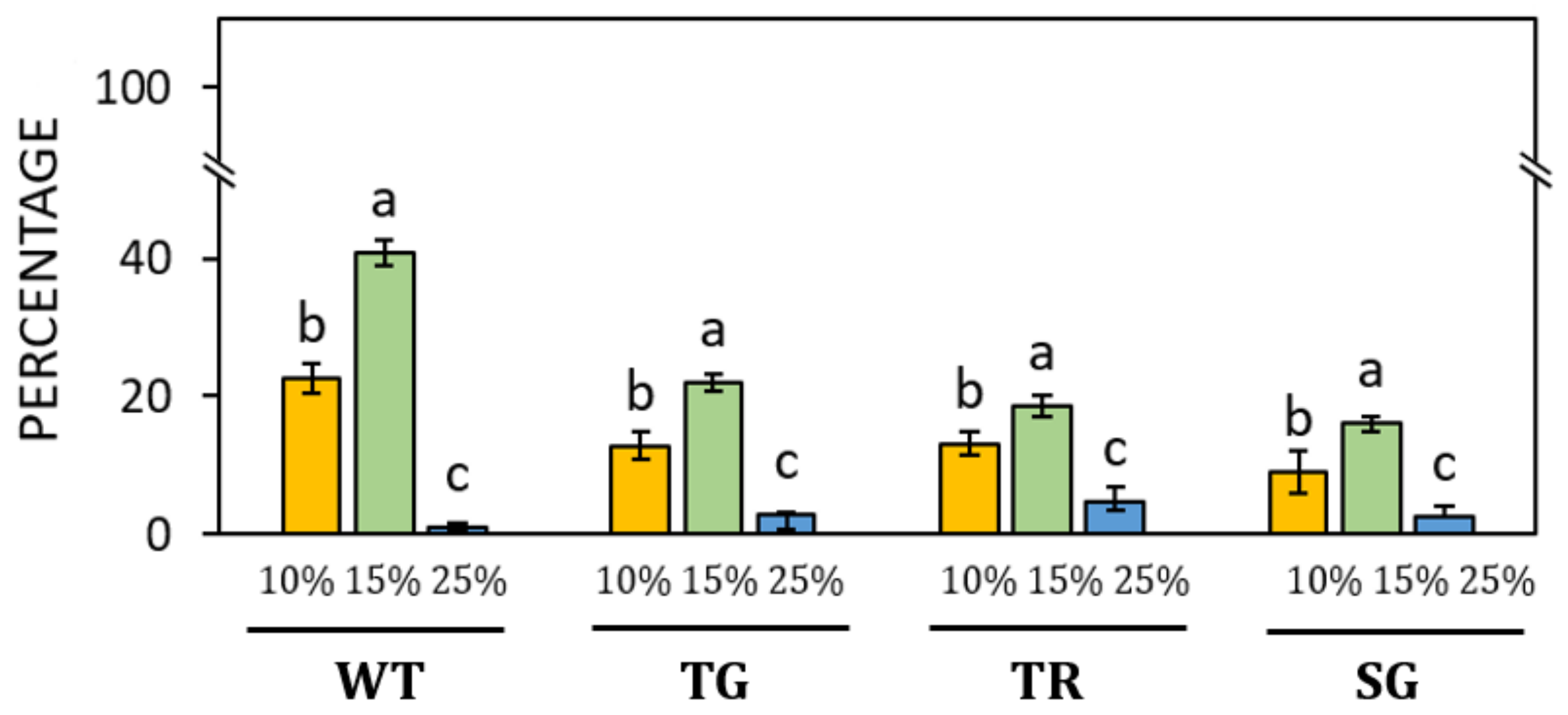
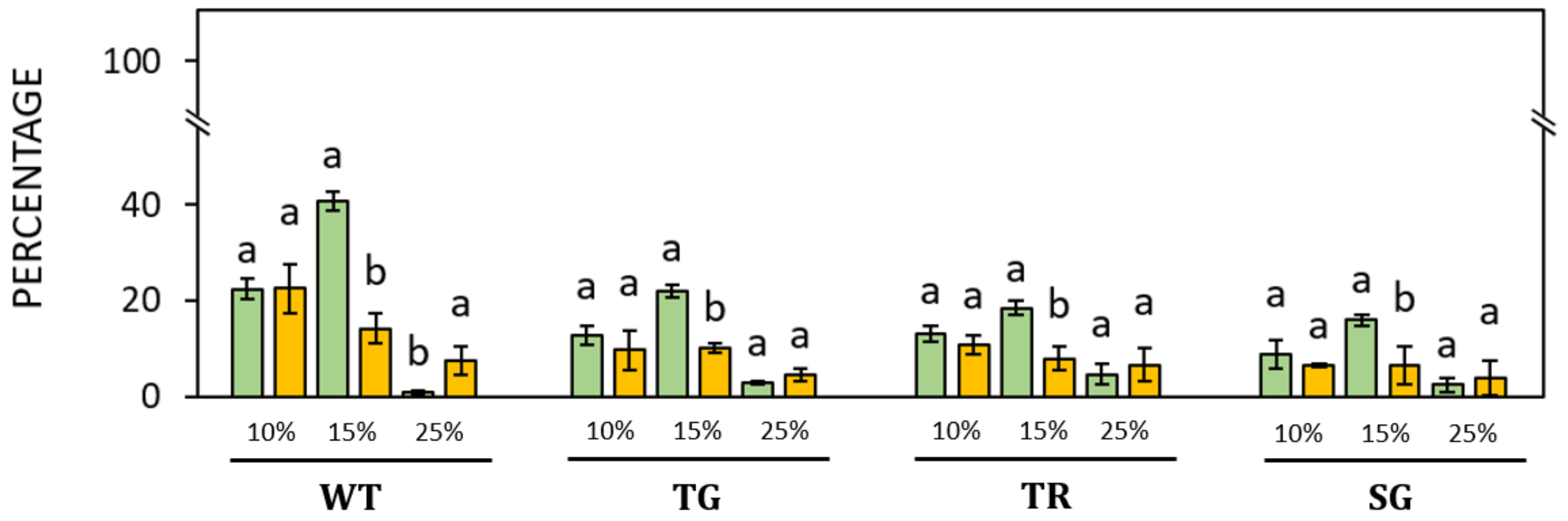
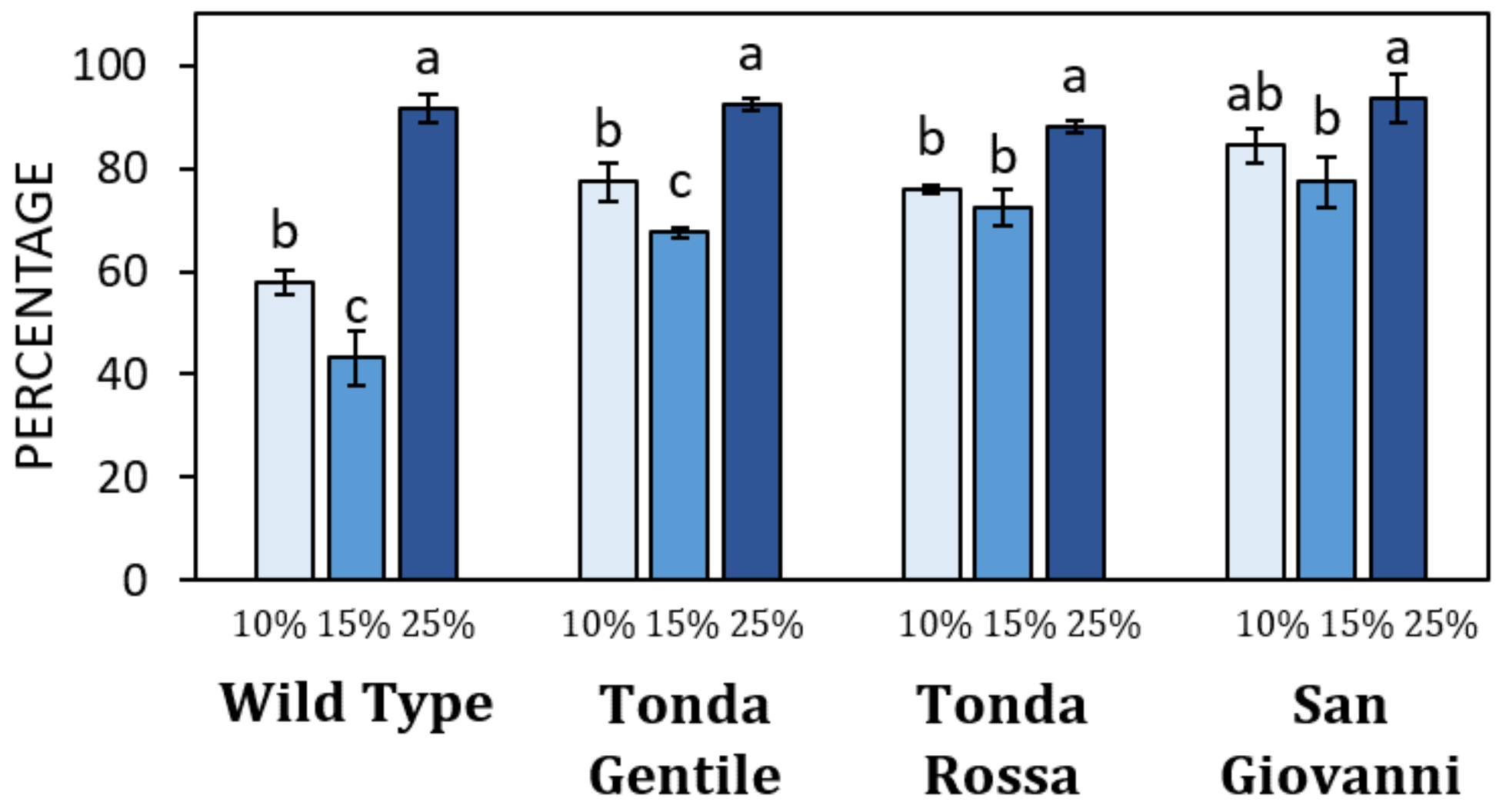
3. Results
3.1. Preliminary Results on the WT Accession
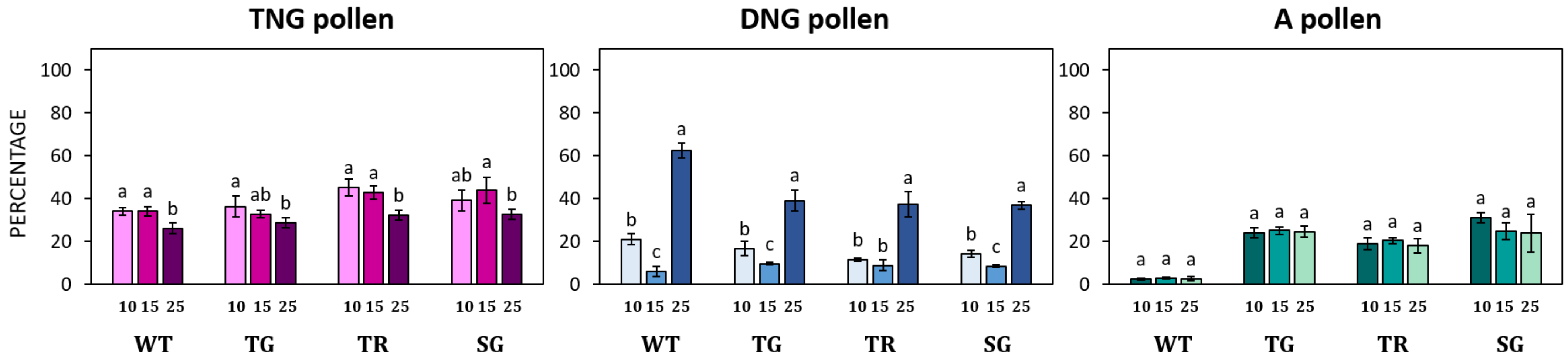
3.2. Germination of Pollen of Different Hazelnut Cultivars
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAOSTAT. Food and Agricultural Commodities Production. Available online: https://www.fao.org/faostat/en/#data (accessed on 16 January 2024).
- Mohr Fuchslocher, J.V.; Navarro Gaete, S.A. Hazelnut Production Areas in Chile, Performance of Cultivars from Oregon State University, and an Equation to Predict Performance. Acta Hortic. 2023, 21–26. [Google Scholar] [CrossRef]
- Ascari, L.; Guastella, D.; Sigwebela, M.; Engelbrecht, G.; Stubbs, O.; Hills, D.; De Gregorio, T.; Siniscalco, C. Artificial Pollination on Hazelnut in South Africa: Preliminary Data and Perspectives. Acta Hortic. 2018, 141–148. [Google Scholar] [CrossRef]
- Baldwin, B.; Guisard, Y. The Status and Future Challenges for the Australian Hazelnut Industry. Acta Hortic. 2014, 321–327. [Google Scholar] [CrossRef]
- Jha, P.K.; Materia, S.; Zizzi, G.; Costa-Saura, J.M.; Trabucco, A.; Evans, J.; Bregaglio, S. Climate Change Impacts on Phenology and Yield of Hazelnut in Australia. Agric. Syst. 2021, 186, 102982. [Google Scholar] [CrossRef]
- Boccacci, P.; Aramini, M.; Valentini, N.; Bacchetta, L.; Rovira, M.; Drogoudi, P.; Silva, A.P.; Solar, A.; Calizzano, F.; Erdoğan, V.; et al. Molecular and Morphological Diversity of On-Farm Hazelnut (Corylus avellana L.) Landraces from Southern Europe and Their Role in the Origin and Diffusion of Cultivated Germplasm. Tree Genet. Genomes 2013, 9, 1465–1480. [Google Scholar] [CrossRef]
- Goldschmidt, E.E. The Evolution of Fruit Tree Productivity: A Review. Econ. Bot. 2013, 67, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Azarenko, A.N.; McCluskey, R.L.; Chambers, W.C. Does Canopy Management Help to Alleviate Biennial Bearing in ’ennis’ and ’montebello’ Hazel-Nut Trees in Oregon? Acta Hortic. 2005, 237–242. [Google Scholar] [CrossRef]
- Pacini, E.; Nepi, M. Effect of Pistil Age on Pollen Tube Growth, Fruit Development and Seed Set in Cucurbita pepo L. Acta Soc. Bot. Pol. 2014, 70, 165–172. [Google Scholar] [CrossRef][Green Version]
- Abu-Zahra, T.R.; Al-Abbadi, A.A. Effects of Artificial Pollination on Pistachio (Pistacia vera L.) Fruit Cropping. J. Plant Sci. 2007, 2, 228–232. [Google Scholar] [CrossRef]
- Mazinani, M.; Zarafshan, P.; Dehghani, M.; Vahdati, K.; Etezadi, H. Design and Analysis of an Aerial Pollination System for Walnut Trees. Biosyst. Eng. 2023, 225, 83–98. [Google Scholar] [CrossRef]
- Ascari, L.; Siniscalco, C.; Palestini, G.; Lisperguer, M.J.; Suarez Huerta, E.; De Gregorio, T.; Bregaglio, S. Relationships between Yield and Pollen Concentrations in Chilean Hazelnut Orchards. Eur. J. Agron. 2020, 115, 126036. [Google Scholar] [CrossRef]
- Germain, E. The Reproduction of Hazelnut (Corylus avellana L.): A Review. Acta Hortic. 1994, 195–210. [Google Scholar] [CrossRef]
- Cristofori, V.; Ferramondo, S.; Bertazza, G.; Bignami, C. Phenology and Yield Evaluation of Hazelnut Cultivars in Latium Region. Acta Hortic. 2009, 657–664. [Google Scholar] [CrossRef]
- Črepinšek, Z.; Štampar, F.; Kajfež-Bogataj, L.; Solar, A. The Response of Corylus avellana L. Phenology to Rising Temperature in North-Eastern Slovenia. Int. J. Biometeorol. 2012, 56, 681–694. [Google Scholar] [CrossRef] [PubMed]
- Capik, J.M.; Molnar, T.J. Flowering Phenology of Eastern Filbert Blight-Resistant Hazelnut Accessions in New Jersey. HortTechnology 2014, 24, 196–208. [Google Scholar] [CrossRef]
- Brandoli, C.; Sgarbi, E.; Cristofori, V.; Politi, M.; Todeschini, C.; Valentini, N.; Pagani, E.; Bevilacqua, F.; Siniscalco, C. Analysis of Pollen Viability in European Hazelnut Cultivars and in the Wild Type: Preliminary Data and Perspectives. Acta Hortic. 2023, 113–120. [Google Scholar] [CrossRef]
- Shivanna, K.R.; Linskens, H.F.; Cresti, M. Pollen Viability and Pollen Vigor. Theor. Appl. Genet. 1991, 81, 38–42. [Google Scholar] [CrossRef]
- Nepi, M.; Franchi, G.G.; Padni, E. Pollen Hydration Status at Dispersal: Cytophysiological Features and Strategies. Protoplasma 2001, 216, 171–180. [Google Scholar] [CrossRef]
- Pacini, E.; Guarnieri, M.; Nepi, M. Pollen Carbohydrates and Water Content during Development, Presentation, and Dispersal: A Short Review. Protoplasma 2006, 228, 73–77. [Google Scholar] [CrossRef]
- Zielinski, Q.B. Techniques for Collecting, Handling Germinating, and Storing of Pollen of the Filbert (Corylus Spp.). Euphytica 1968, 17, 121–125. [Google Scholar] [CrossRef]
- Franchi, G.G.; Piotto, B.; Nepi, M.; Baskin, C.C.; Baskin, J.M.; Pacini, E. Pollen and Seed Desiccation Tolerance in Relation to Degree of Developmental Arrest, Dispersal, and Survival. J. Exp. Bot. 2011, 62, 5267–5281. [Google Scholar] [CrossRef] [PubMed]
- Rimoldi, J. A Study of Pollination and Fertilization in the Filbert (Corylus avellana). Master’s of Thesis, Oregon Agricultural College, Corvallis, OR, USA, 1921. [Google Scholar]
- Schuster, C. Filberts: 2. Experi mental Data on Filbert Pollination. Org. Agric. Exp. Stn. Bull. 1924, 208, 548–555. [Google Scholar]
- Arikan, F. The Possibilities of Im-Proving Filbert Growing; Guzel Sanatlar Matbaasi: Esenyurt, Turkey, 1963. [Google Scholar]
- Bodmer, H.S. Zur Physiologie Der Pollenkeimung Bei Covylus Mellana. Protoplasma 1936, 25, 337–371. [Google Scholar]
- Visser, T. Germination and Storage of Pollen. Ph.D. Thesis, Veenman, Wageningen, The Netherlands, 1955. [Google Scholar]
- Trotter, A. Characteristics and Phenomena of Reproduction in the Filbert, Coryh Avellana: Pollination. Accad. Naz. Dei Lincei 1947, 8, 745–749. [Google Scholar]
- Riera, F.J. La pollinization et la ficundation chez le noisetier. In Proceedings of the 89th Congress of Pomology, Florence, Italy, 1958; pp. 157–170. [Google Scholar]
- Cox, L.G. Preliminary Studies on Catkin Forcing and Pollen Storage of Corylus and Juglans. In Proceedings of the 34th Report of the Northern Nut Growers’ Association; 1943; pp. 58–60. [Google Scholar]
- Kim, S.K.; Lagerstedt, H.B.; Daley, L.S. Germination Responses of Filbert Pollen to pH, Temperature, Glucose, Fructose, and Sucrose. HortScience 1985, 20, 944–946. [Google Scholar] [CrossRef]
- Lagerstedt, H.B. Filberts; Janick, J., Moore, J.N., Eds.; Purdue University press: West Lafayette, IN, USA, 1975; pp. 456–489. [Google Scholar]
- Manusěv, B. Germination of Pollen and Size of Pollen Grain with Tubes of Some Filbert Varieties. Rev. Res. Works 1972, 1972, 107–118. [Google Scholar]
- Brewbaker, J.L.; Kwack, B.H. The Essential Role of Calcium Ion in Pollen Germination and Pollen Tube Growth. Am. J. Bot. 1963, 50, 859–865. [Google Scholar] [CrossRef]
- Thompson, M.M. Genetics of Incompatibility in Corylus avellana L. Theor. Appl. Genet. 1979, 54, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Heslop-Harrison, Y.; Heslop-Harrison, J.S.; Heslop-Harrison, J. Germination of Corylus avellana L. (Hazel) Pollen: Hydration and the Function of the Oncus. Acta Bot. Neerl. 1986, 35, 265–284. [Google Scholar] [CrossRef]
- Novara, C.; Ascari, L.; La Morgia, V.; Reale, L.; Genre, A.; Siniscalco, C. Viability and Germinability in Long Term Storage of Corylus Avellana Pollen. Sci. Hortic. 2017, 214, 295–303. [Google Scholar] [CrossRef]
- Regione Lazio Rural Development Plan 2014–2020. Reg. CE n. 1305/2013; European Union: Maastricht, The Netherlands, 2020. [Google Scholar]
- Pacchiarelli, A.; Lupo, M.; Ferrucci, A.; Giovanelli, F.; Priori, S.; Pica, A.L.; Silvestri, C.; Cristofori, V. Phenology, Yield and Nut Traits Evaluation of Twelve European Hazelnut Cultivars Grown in Central Italy. Forests 2024, 15, 833. [Google Scholar] [CrossRef]
- Delgado, A.; Quinet, M.; Dapena, E. Analysis of the Variability of Floral and Pollen Traits in Apple Cultivars—Selecting Suitable Pollen Donors for Cider Apple Orchards. Agronomy 2021, 11, 1717. [Google Scholar] [CrossRef]
- Toillon, J.; Hamidi, R.; Paradinas, A.; Ramade, L.; Thomas, M.; Suarez Huerta, E. Consolidated BBCH Scale for Hazelnut Phenotyping. Acta Hortic. 2023, 1379, 159–168. [Google Scholar] [CrossRef]
- Ascari, L.; Cristofori, V.; Macrì, F.; Botta, R.; Silvestri, C.; De Gregorio, T.; Huerta, E.S.; Di Berardino, M.; Kaufmann, S.; Siniscalco, C. Hazelnut Pollen Phenotyping Using Label-Free Impedance Flow Cytometry. Front. Plant Sci. 2020, 11, 615922. [Google Scholar] [CrossRef]
- Ertas, A.; Anderson, E.E.; Kiris, I. Properties of a New Liquid Desiccant Solution—Lithium Chloride and Calcium Chloride Mixture. Sol. Energy 1992, 49, 205–212. [Google Scholar] [CrossRef]
- Sun, T.; Morgan, H. Single-Cell Microfluidic Impedance Cytometry: A Review. Microfluid. Nanofluid. 2010, 8, 423–443. [Google Scholar] [CrossRef]
- Cheung, K.; Gawad, S.; Renaud, P. Impedance Spectroscopy Flow Cytometry: On-Chip Label-Free Cell Differentiation. Cytom. A 2005, 65A, 124–132. [Google Scholar] [CrossRef]
- Conner, P.J. Optimization of In Vitro Pecan Pollen Germination. HortScience 2011, 46, 571–576. [Google Scholar] [CrossRef]
- Sakhanokho, H.F.; Rajasekaran, K. Pollen Biology of Ornamental Ginger (Hedychium Spp. J. Koenig). Sci. Hortic. 2010, 125, 129–135. [Google Scholar] [CrossRef]
- Shivanna, K.R.; Sawhney, V.K. Polyethylene Glycol Improves the In Vitro Growth of Brassica Pollen Tubes without Loss in Germination. J. Exp. Bot. 1995, 46, 1771–1774. [Google Scholar] [CrossRef]
- Jayaprakash, P.; Sarla, N. Development of an Improved Medium for Germination of Cajanus cajan (L.) Millsp. Pollen In Vitro. J. Exp. Bot. 2001, 52, 851–855. [Google Scholar] [CrossRef] [PubMed]
- Read, S.M.; Clarke, A.E.; Bacic, A. Stimulation of Growth of Cultured Nicotiana Tabacum W 38 Pollen Tubes by Poly(Ethylene Glycol) and Cu(II) Salts. Protoplasma 1993, 177, 1–14. [Google Scholar] [CrossRef]
- Subbaiah, C.C. A Polyethylene Glycol Based Medium for In Vitro Germination of Cashew Pollen. Can. J. Bot. 1984, 62, 2473–2475. [Google Scholar] [CrossRef]
- Pérez, V.; Herrero, M.; Hormaza, J.I. Pollen Performance in Mango (Mangifera indica L., Anacardiaceae): Andromonoecy and Effect of Temperature. Sci. Hortic. 2019, 253, 439–446. [Google Scholar] [CrossRef]
- Weng, Z.; Deng, Y.; Tang, F.; Zhao, L.; Zhao, L.; Wang, Y.; Dai, X.; Zhou, Z.; Cao, Q. Screening and Optimisation of In Vitro Pollen Germination Medium for Sweetpotato (Ipomoea batatas). Plant Methods 2023, 19, 93. [Google Scholar] [CrossRef]
- Hamilton, E.S.; Haswell, E.S. The Tension-Sensitive Ion Transport Activity of MSL8 Is Critical for Its Function in Pollen Hydration and Germination. Plant Cell Physiol. 2017, 58, 1222–1237. [Google Scholar] [CrossRef] [PubMed]
- Mulcahy, G.; Mulcahy, D.L. The Effect of Supplemented Media on the Growth In Vitro of Bi- and Trinucleate Pollen. Plant Sci. 1988, 55, 213–216. [Google Scholar] [CrossRef]
- Pacini, E. Pollen Viability Related to Type of Pollination in Six Angiosperm Species. Ann. Bot. 1997, 80, 83–87. [Google Scholar] [CrossRef]
- Shivanna, K.R.; Heslop-Harrison, J. Membrane State and Pollen Viability. Ann. Bot. 1981, 47, 759–770. [Google Scholar] [CrossRef]
- Edlund, A.F.; Swanson, R.; Preuss, D. Pollen and Stigma Structure and Function: The Role of Diversity in Pollination. Plant Cell Online 2004, 16, S84–S97. [Google Scholar] [CrossRef]
- Stanley, R.G.; Linskens, H.F. Pollen; Springer: Berlin/Heidelberg, Germany, 1974; ISBN 978-3-642-65907-2. [Google Scholar]
- Shivanna, K.R.; Rangaswamy, N.S. Pollen Biology; Springer: Berlin/Heidelberg, Germany, 1992; ISBN 978-3-540-55170-6. [Google Scholar]
- Deng, Z.; Harbaugh, B.K. Technique for In Vitro Pollen Germination and Short-Term Pollen Storage in Caladium. HortScience 2004, 39, 365–367. [Google Scholar] [CrossRef]
- Çetinbaş-Genç, A.; Cai, G.; Vardar, F.; Ünal, M. Differential Effects of Low and High Temperature Stress on Pollen Germination and Tube Length of Hazelnut (Corylus avellana L.) Genotypes. Sci. Hortic. 2019, 255, 61–69. [Google Scholar] [CrossRef]
- Van Aelst, A.C.; Van Went, J.L. Effects of Anoxia on Pollen Tube Growth and Tube Wall Formation of Impatiens Glandulifera. Sex. Plant Reprod. 1989, 2, 85–89. [Google Scholar] [CrossRef]
- Connor, K.F.; Towill, L.E. Pollen-Handling Protocol and Hydration/Dehydration Characteristics of Pollen for Application to Long-Term Storage. Euphytica 1993, 68, 77–84. [Google Scholar] [CrossRef]
- Dickinson, D.B. Influence of Borate and Pentaerythritol Concentrations on Germination and Tube Growth of Lilium Longiflorum Pollen1. J. Am. Soc. Hortic. Sci. 1978, 103, 413–416. [Google Scholar] [CrossRef]
- Potts, B.; Marsden-Smedley, J. In Vitro Germination of Eucalyptus Pollen: Response to Variation in Boric Acid and Sucrose. Aust. J. Bot. 1989, 37, 429. [Google Scholar] [CrossRef]
- Wang, Q.; Lu, L.; Wu, X.; Li, Y.; Lin, J. Boron Influences Pollen Germination and Pollen Tube Growth in Picea meyeri. Tree Physiol. 2003, 23, 345–351. [Google Scholar] [CrossRef]
- Muengkaew, R.; Chaiprasart, P.; Wongsawad, P. Calcium-Boron Addition Promotes Pollen Germination and Fruit Set of Mango. Int. J. Fruit Sci. 2017, 17, 147–158. [Google Scholar] [CrossRef]
- Hepler, P.K.; Winship, L.J. Calcium at the Cell Wall-Cytoplast Interface. J. Integr. Plant Biol. 2010, 52, 147–160. [Google Scholar] [CrossRef]
- Peaucelle, A.; Braybrook, S.; Höfte, H. Cell Wall Mechanics and Growth Control in Plants: The Role of Pectins Revisited. Front. Plant Sci. 2012, 3, 121. [Google Scholar] [CrossRef]
- O’Neill, M.A.; Ishii, T.; Albersheim, P.; Darvill, A.G. Rhamnogalacturonan II: Structure and Function of a Borate Cross-Linked Cell Wall Pectic Polysaccharide. Annu. Rev. Plant Biol. 2004, 55, 109–139. [Google Scholar] [CrossRef] [PubMed]
- Fang, K.; Zhang, W.; Xing, Y.; Zhang, Q.; Yang, L.; Cao, Q.; Qin, L. Boron Toxicity Causes Multiple Effects on Malus Domestica Pollen Tube Growth. Front. Plant Sci. 2016, 7, 208. [Google Scholar] [CrossRef] [PubMed]
- Lau, T.; Stephenson, A.G. Effects of Soil Nitrogen on Pollen Production, Pollen Grain Size, and Pollen Performance in Cucurbita pepo (Cucurbitaceae). Am. J. Bot. 1993, 80, 763–768. [Google Scholar] [CrossRef]
- Zhang, H.Q.; Croes, A.F. Protection of Pollen Germination from Adverse Temperatures: A Possible Role for Proline [Lily]. Plant Cell Environ. 1983, 6, 6. [Google Scholar]
- Chiang, H.-H.; Dandekar, A.M. Regulation of Proline Accumulation in Arabidopsis Thaliana (L.) Heynh during Development and in Response to Desiccation. Plant Cell Environ. 1995, 18, 1280–1290. [Google Scholar] [CrossRef]
- Mattioli, R.; Biancucci, M.; El Shall, A.; Mosca, L.; Costantino, P.; Funck, D.; Trovato, M. Proline Synthesis in Developing Microspores Is Required for Pollen Development and Fertility. BMC Plant Biol. 2018, 18, 356. [Google Scholar] [CrossRef] [PubMed]
- Brooks, M.H. Comparative Analyses of Some of the Free Amino Acids in Anthers of Fertile and Genetic Cytoplasmic Male Sterile Sorghum. Genetics 1962, 47, 1629–1638. [Google Scholar] [CrossRef]
- Fang, X.; Fu, H.-F.; Gong, Z.-H.; Chai, W.-G. Involvement of a Universal Amino Acid Synthesis Impediment in Cytoplasmic Male Sterility in Pepper. Sci. Rep. 2016, 6, 23357. [Google Scholar] [CrossRef] [PubMed]
- Mattioli, R.; Palombi, N.; Funck, D.; Trovato, M. Proline Accumulation in Pollen Grains as Potential Target for Improved Yield Stability Under Salt Stress. Front. Plant Sci. 2020, 11, 582877. [Google Scholar] [CrossRef]
- Mondo, J.M.; Agre, P.A.; Asiedu, R.; Akoroda, M.O.; Asfaw, A. Optimized Protocol for In Vitro Pollen Germination in Yam (Dioscorea Spp.). Plants 2021, 10, 795. [Google Scholar] [CrossRef]
- Vasil, I. Physiology and Culture of Pollen. In International Review of Cytology; Elsevier: Amsterdam, The Netherlands, 1987; Volume 107, pp. 127–174. ISBN 978-0-12-364507-4. [Google Scholar]
- Soroka, A.; Lyakh, V. How Ensure In Vitro Pollen Germination of Cultivated Flax and Its Wild Relatives. J. Agric. For. 2022, 68, 285–292. [Google Scholar] [CrossRef]
- Hong-Qi, Z.; Croes, A.F. A New Medium for Pollen Germination In Vitro. Acta Bot. Neerl. 1982, 31, 113–119. [Google Scholar] [CrossRef]
- Kumari, M.; Prasad, A.; Rahman, L.U.; Mathur, A.K.; Mathur, A. In Vitro Germination, Storage and Microscopic Studies of Pollen Grains of Four Ocimum Species. Ind. Crops Prod. 2022, 177, 114445. [Google Scholar] [CrossRef]
- Pacini, E. Types and Meaning of Pollen Carbohydrate Reserves. Sex. Plant Reprod. 1996, 9, 362–366. [Google Scholar] [CrossRef]
- Pacini, E.; Hesse, M. Cytophysiology of Pollen Presentation and Dispersal. Flora Morphol. Distrib. Funct. Ecol. Plants 2004, 199, 273–285. [Google Scholar] [CrossRef]
- Firon, N.; Nepi, M.; Pacini, E. Water Status and Associated Processes Mark Critical Stages in Pollen Development and Functioning. Ann. Bot. 2012, 109, 1201–1214. [Google Scholar] [CrossRef] [PubMed]
- Hoekstra, F.A.; Van Roekel, T. Desiccation Tolerance of Papaver dubium L. Pollen during Its Development in the Anther: Possible Role of Phospholipid Composition and Sucrose Content. Plant Physiol. 1988, 88, 626–632. [Google Scholar] [CrossRef] [PubMed]
- Ilgin, M.; Ergenoglu, F.; Caglar, S. Viability, Germination and Amount of Pollen in Selected Caprifig Types. University of Karachi: Karachi, Pakistan, 2007. [Google Scholar]
- Bolat, İ.; Pirlak, L. An Investigation on Pollen Viability, Germination and Tube Growth in Some Stone Fruits. Turk. J. Agric. For. 1999, 23, 4. [Google Scholar]
- Okusaka, K.; Hiratsuka, S. Fructose Inhibits Pear Pollen Germination on Agar Medium without Loss of Viability. Sci. Hortic. 2009, 122, 51–55. [Google Scholar] [CrossRef]
- Hirsche, J.; Garcïa Fernàndez, J.M.; Stabentheiner, E.; Großkinsky, D.K.; Roitsch, T. Differential Effects of Carbohydrates on Arabidopsis Pollen Germination. Plant Cell Physiol. 2017, 58, 691–701. [Google Scholar] [CrossRef]
- Portnoi, L.; Horovitz, A. Sugars in Natural and Artificial Pollen Germination Substrates. Ann. Bot. 1977, 41, 21–27. [Google Scholar] [CrossRef]
- Mehlenbacher, S.A. Revised Dominance Hierarchy for S-Alleles in Corylus avellana L. Theor. Appl. Genet. 1997, 94, 360–366. [Google Scholar] [CrossRef]
- Mehlenbacher, S.A. Geographic Distribution of Incompatibility Alleles in Cultivars and Selections of European Hazelnut. J. Am. Soc. Hortic. Sci. 2014, 139, 191–212. [Google Scholar] [CrossRef]
- Mehlenbacher, S.A. Incompatibility Alleles of Hazelnut Cultivars. Acta Hortic. 2014, 10, 107–116. [Google Scholar] [CrossRef]
- Loguercio, L.L. Pollen Treatment in High Osmotic Potential: A Simple Tool for In Vitro Preservation and Manipulation of Viability in Gametophytic Populations. Braz. J. Plant Physiol. 2002, 14, 65–70. [Google Scholar] [CrossRef]
- Salesses, G. Cytological study of genus Corylus: A heterozygotic translocation in some low male fertile varieties of hazelnut (Corylus avellana). Ann. Amelior. Plantes 1973, 23, 59–66. [Google Scholar]
- Salesses, G.; Bonnet, A. Cytogenetic Studies of Hybrides among Corylus avellana Having Translocations in Heterozygotic States. Cytologia 1988, 53, 407–413. [Google Scholar] [CrossRef]
- Marinoni, D.; Valentini, N.; Portis, E.; Acquadro, A.; Beltramo, C.; Mehlenbacher, S.A.; Mockler, T.C.; Rowley, E.R.; Botta, R. High Density SNP Mapping and QTL Analysis for Time of Leaf Budburst in Corylus avellana L. PLoS ONE 2018, 13, e0195408. [Google Scholar] [CrossRef]

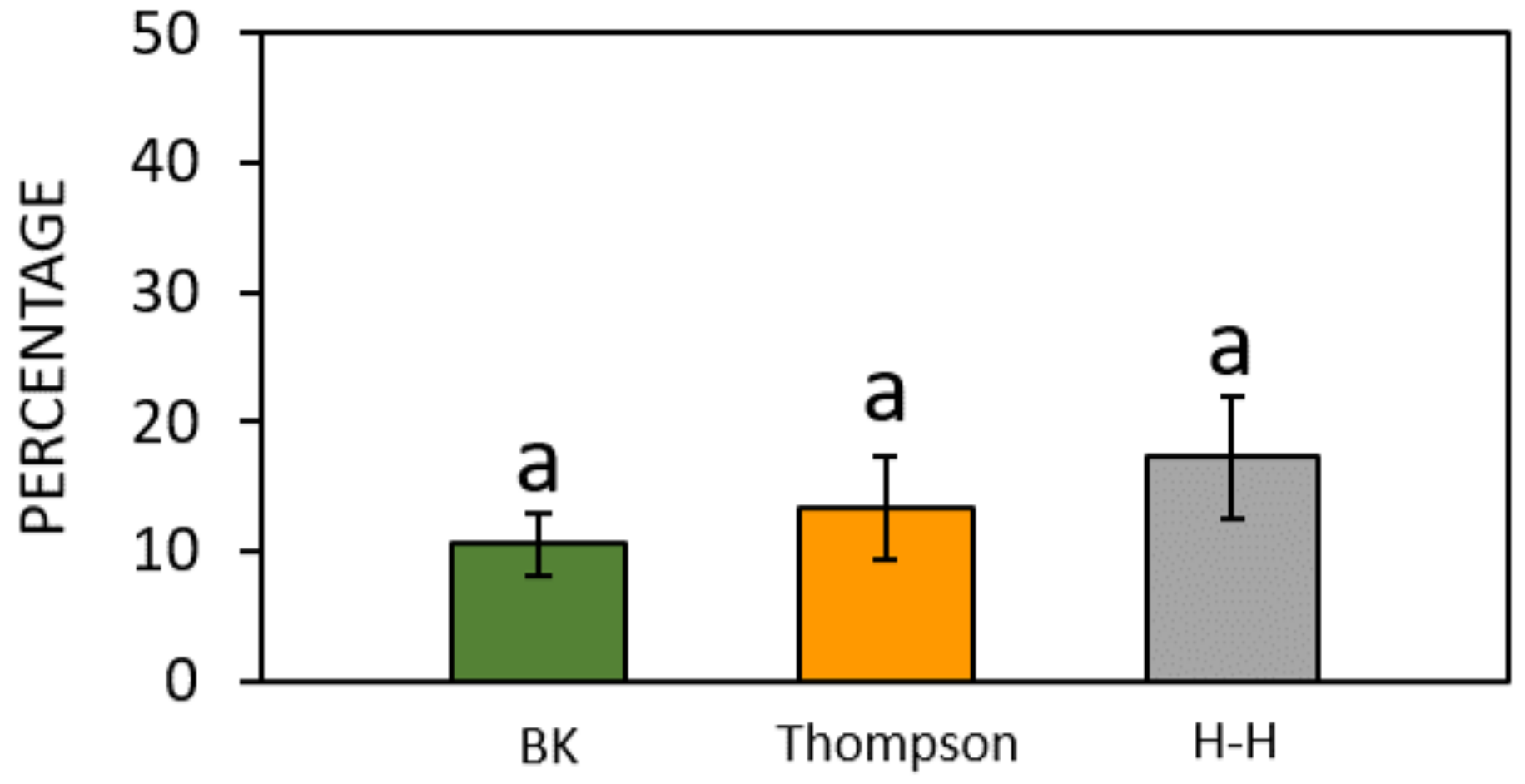
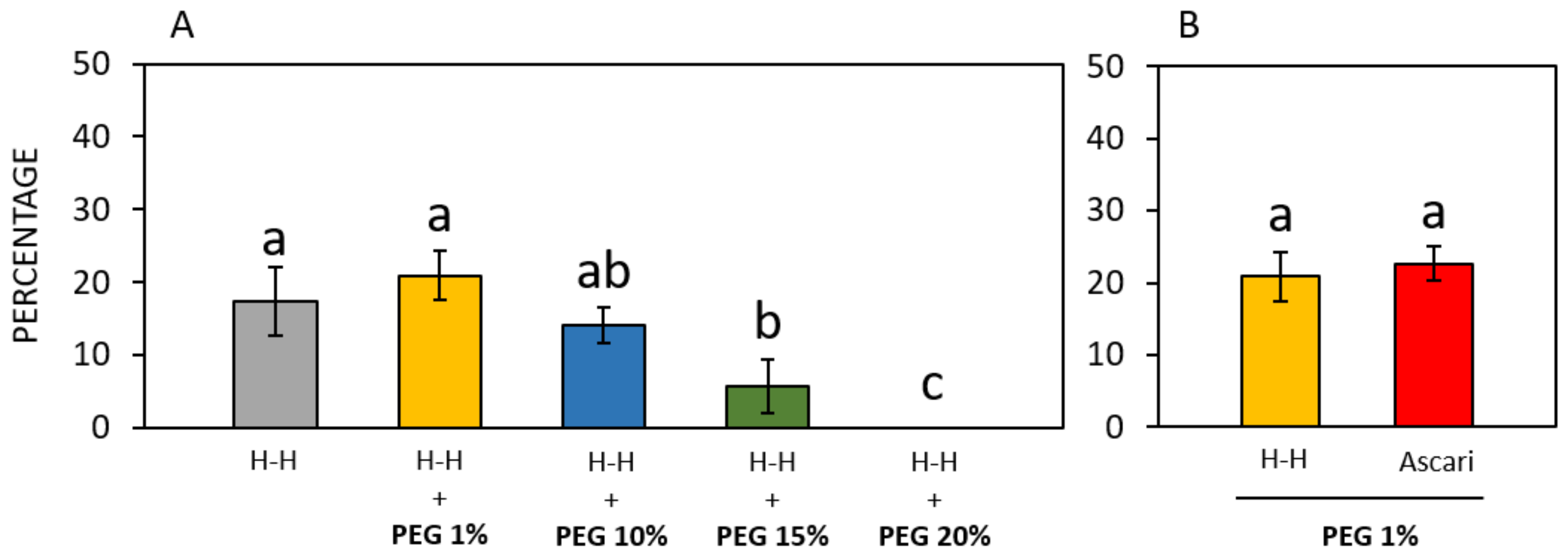
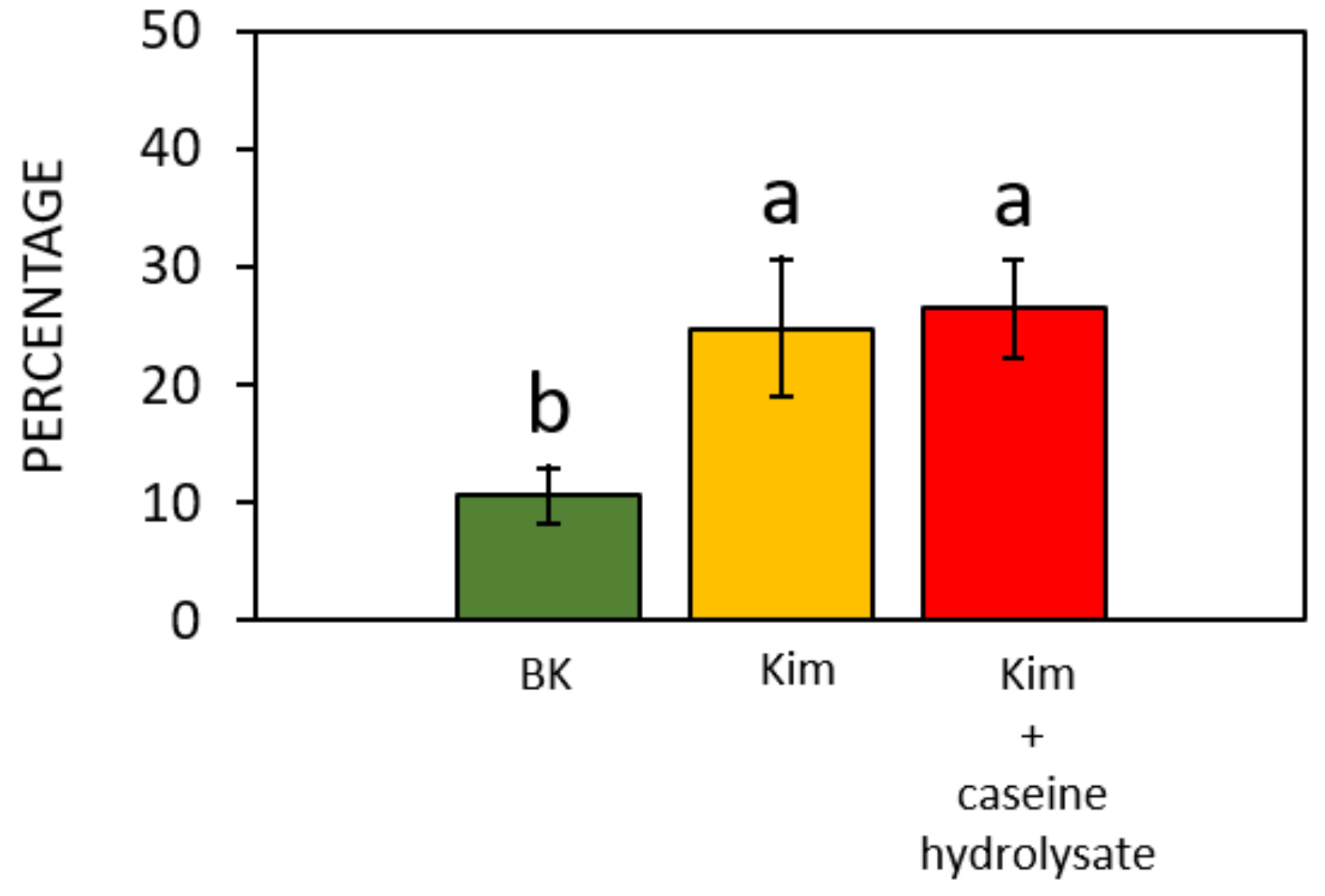
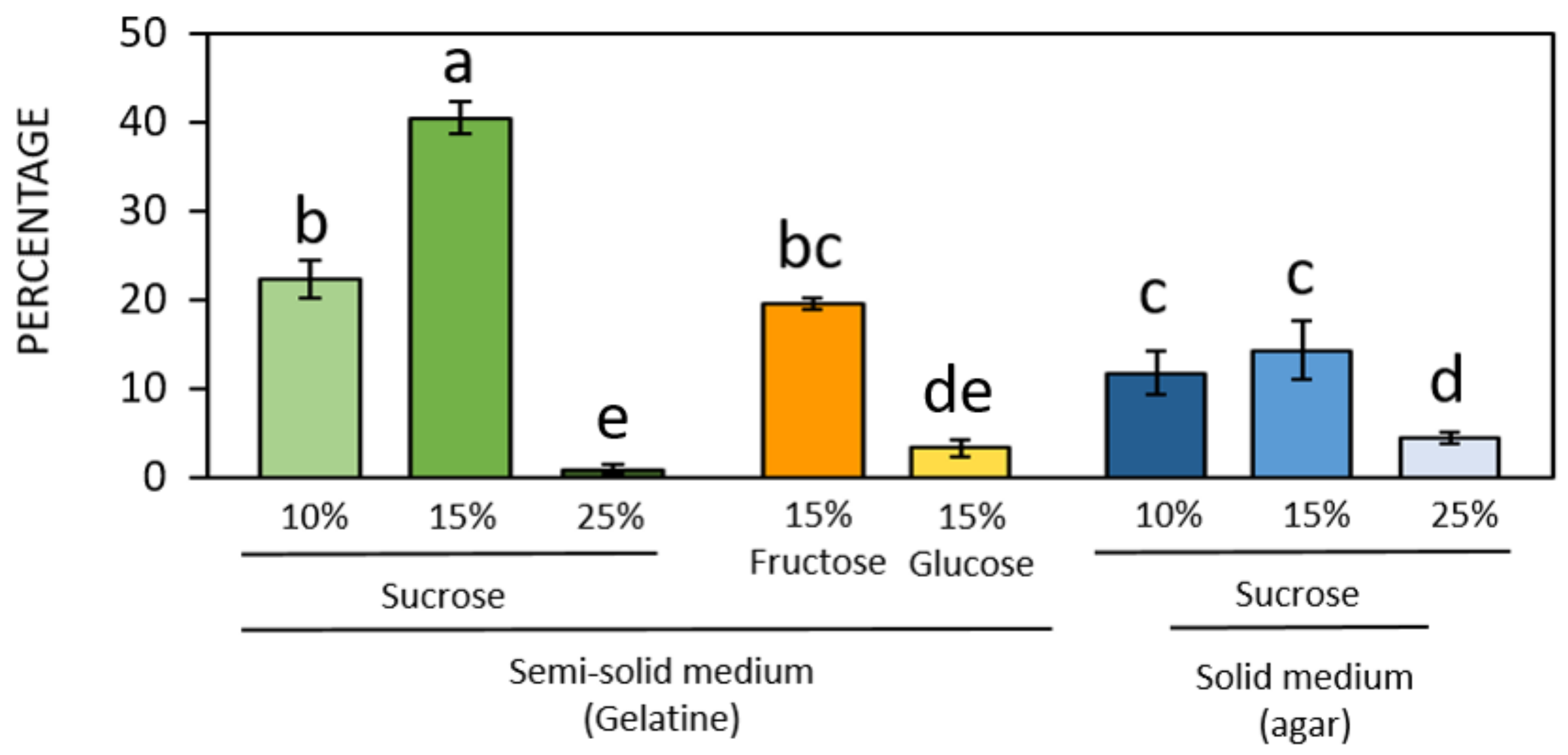
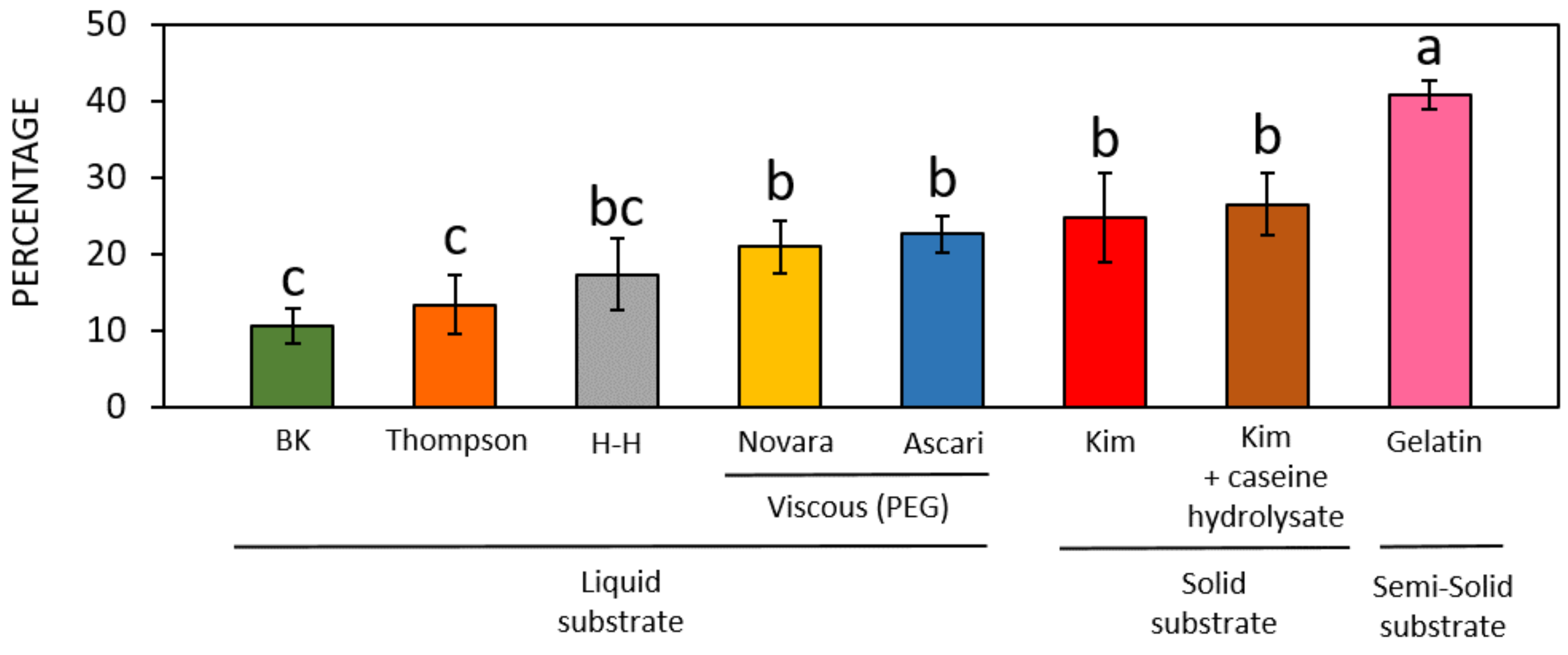
| Brewbaker and Kwack (1963) [34] | Thompson et al. (1978) [35] | Heslop- Harrison et al. (1986) [36] | Kim et al. (1985) [31] | Novara et al. (2017) [37] | Ascari et al. (2018) [3] | Collagen-Based Gelatin | |
|---|---|---|---|---|---|---|---|
| Abbreviations | (BK) | (Thompson) | (H-H) | (Kim) | (Novara) | (Ascari) | (Gelatin) |
| Liquid | Liquid | Liquid | Solid | Liquid (Viscous) | Liquid (Viscous) | Semi-Solid | |
| H3BO3 | 100 mg/L | 0.1% | 10−3 M | 100 mg/L | 10−3 M | 1.6 mM | - |
| Ca(NO3)2 | 300 mg/L | - | 0.5 × 10−3 M | 300 mg/L | 0.5 × 10−3 M | - | - |
| MgSO4 | 200 mg/L | - | - | 200 mg/L | - | 1.7 mM | - |
| KNO3 | 100 mg/L | - | - | 100 mg/L | - | - | - |
| CaCl2 | - | - | - | - | - | 1.8 mM | - |
| KCl | - | - | - | - | - | 1 mM | - |
| Sucrose | 10% | 0.7 M | 10% | 15% | 10% | 0.2 M | 15% |
| Gelatin | - | - | - | - | - | - | 1% |
| PEG | - | - | - | - | unreported | 0.04 M | - |
| Agar | - | - | - | 1% | - | - | - |
| B3+ | Ca2+ | Mg2+ | K+ | Nitrogen | ||
|---|---|---|---|---|---|---|
| Brewbaker and Kwack (1963) | [34] | 17.48 | 73.26 | 40.38 | 3.06 | 65.03 |
| Thompson et al. (1978) | [35] | 174.8 | - | - | - | 13.99 |
| Heslop-Harrison et al. (1986) | [36] | 10.82 | 20.04 | - | - | 14 |
| Ascari et al. (2018) | [3] | 17.31 | 88.55 | 59.96 | 21.74 | - |
| Collagen-based gelatin | 0.006 | 0.564 | <0.25 | 0.777 | 160 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brandoli, C.; Cristofori, V.; Silvestri, C.; Todeschini, C.; Sgarbi, E. The Development of an Improved Medium for the In Vitro Germination of Corylus avellana L. Pollen. Forests 2024, 15, 1095. https://doi.org/10.3390/f15071095
Brandoli C, Cristofori V, Silvestri C, Todeschini C, Sgarbi E. The Development of an Improved Medium for the In Vitro Germination of Corylus avellana L. Pollen. Forests. 2024; 15(7):1095. https://doi.org/10.3390/f15071095
Chicago/Turabian StyleBrandoli, Claudio, Valerio Cristofori, Cristian Silvestri, Claudio Todeschini, and Elisabetta Sgarbi. 2024. "The Development of an Improved Medium for the In Vitro Germination of Corylus avellana L. Pollen" Forests 15, no. 7: 1095. https://doi.org/10.3390/f15071095
APA StyleBrandoli, C., Cristofori, V., Silvestri, C., Todeschini, C., & Sgarbi, E. (2024). The Development of an Improved Medium for the In Vitro Germination of Corylus avellana L. Pollen. Forests, 15(7), 1095. https://doi.org/10.3390/f15071095







