Differential Responses of Bacterial and Fungal Community Structure in Soil to Nitrogen Deposition in Two Planted Forests in Southwest China in Relation to pH
Abstract
1. Introduction
2. Materials and Methods
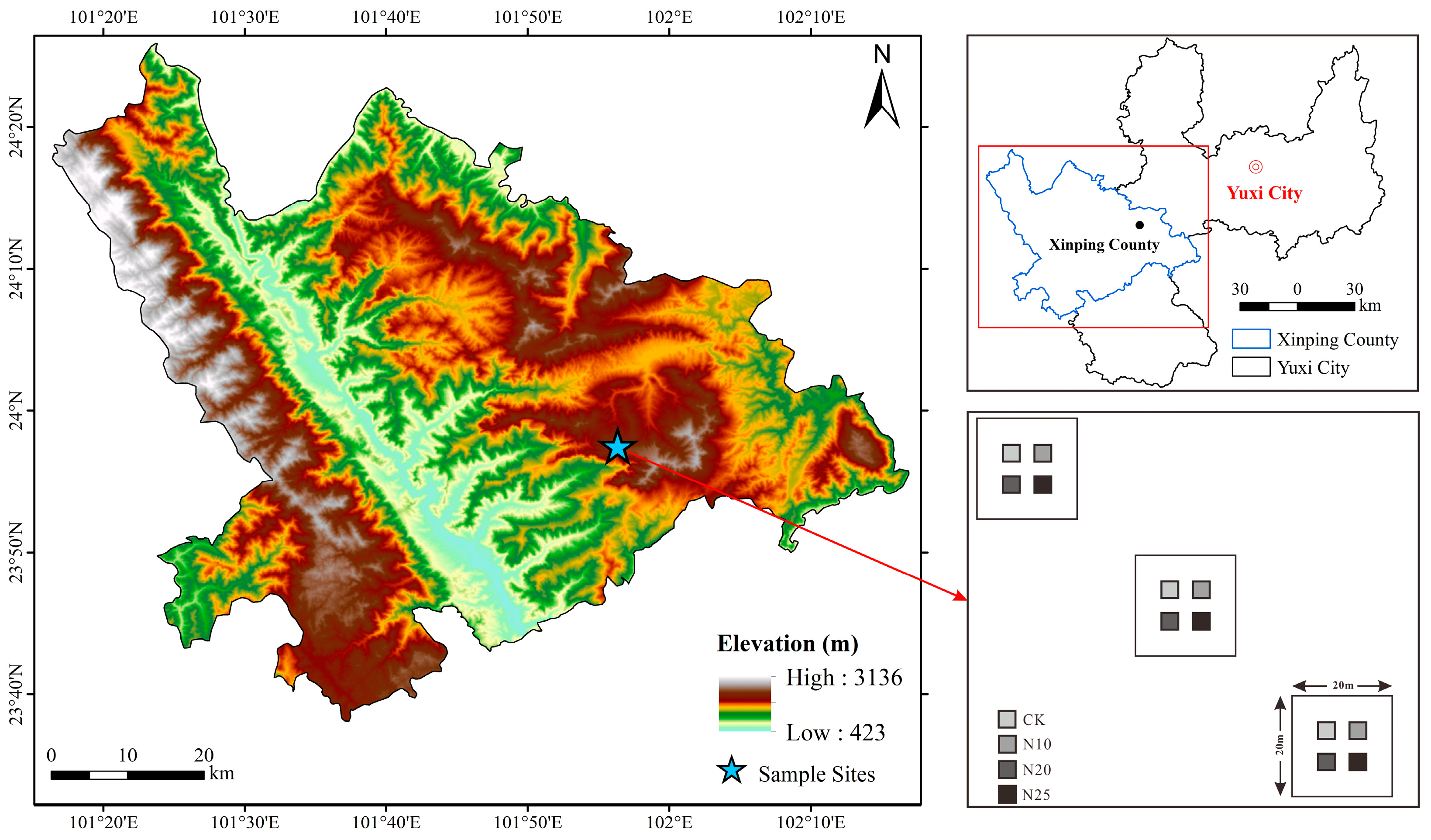
2.1. Site Description
2.2. Experimental Design
2.3. Soil Sampling
2.4. Measurement of Soil Chemical Properties
2.5. DNA Extraction and Illumina Sequencing
2.6. Microbial Data Analysis and Co-Occurrence Network Construction
2.7. Statistical Analysis
3. Results
3.1. Soil Properties in Response to N Addition
3.2. Soil Microbial Diversity in Response to N Addition
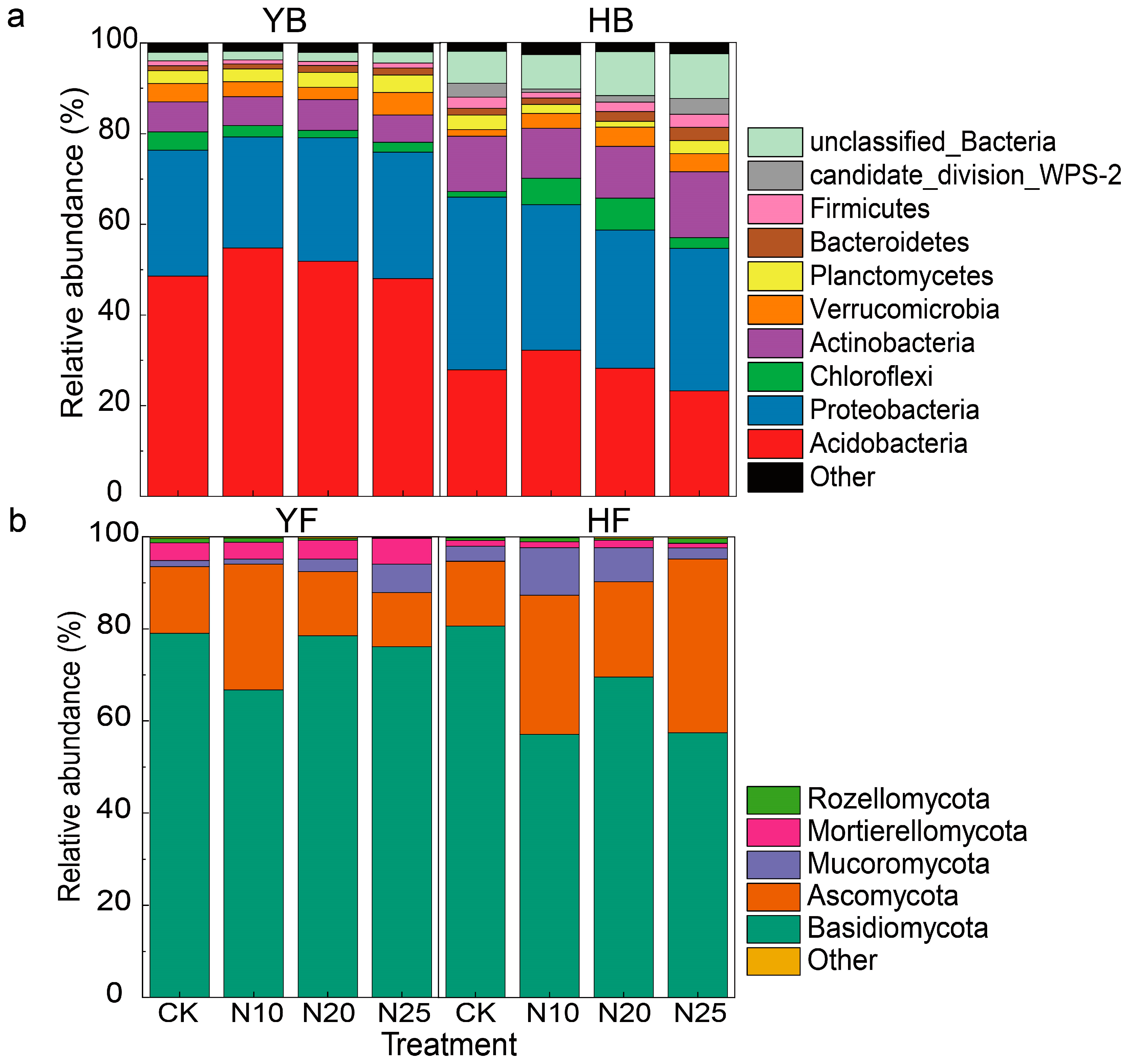
3.3. Effects of N Addition on Soil Microbial Community Composition
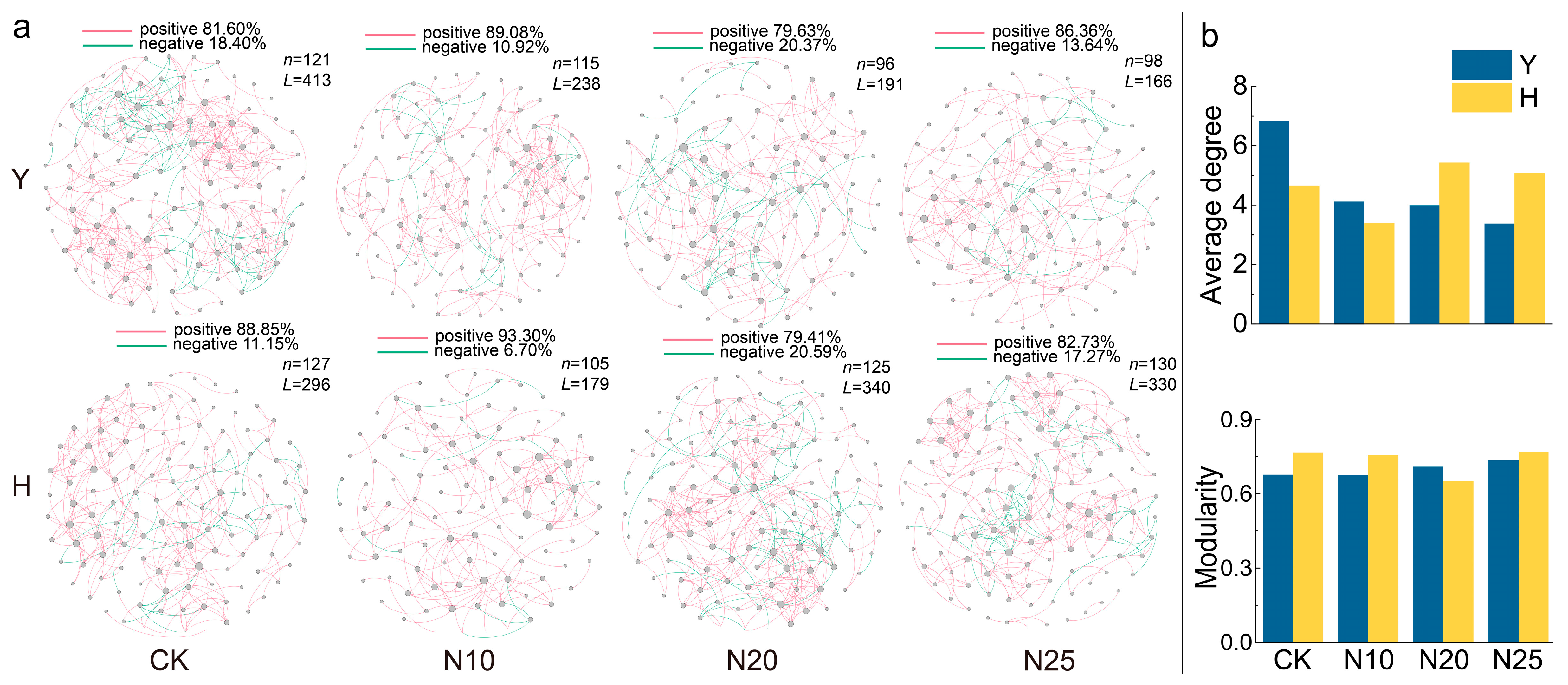
3.4. Soil Microbial Co-Occurrence Networks
3.5. Relationships between Microbial Community and Soil Chemical Properties
4. Discussion
4.1. Afforestation Tree Species Differences Lead to Distinct Responses of Soil Microbial Diversity to Nitrogen Addition
4.2. Nitrogen Addition Alters the Relative Abundance Composition of Microbial Communities
4.3. Drivers of Microbial Community Responses to Nitrogen Addition
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ackerman, D.; Millet, D.B.; Chen, X. Global estimates of inorganic nitrogen deposition across four decades. Glob. Biogeochem. Cycles 2019, 33, 100–107. [Google Scholar] [CrossRef]
- Feng, H.; Guo, J.; Peng, C.; Kneeshaw, D.; Roberge, G.; Pan, C.; Ma, X.; Zhou, D.; Wang, W. Nitrogen addition promotes terrestrial plants to allocate more biomass to aboveground organs: A global meta-analysis. Glob. Chang. Biol. 2023, 29, 3970–3989. [Google Scholar] [CrossRef]
- Chen, T.; Cheng, R.; Xiao, W.; Shen, Y.; Wang, L.; Sun, P.; Zhang, M.; Li, J. Nitrogen Addition Enhances Soil Nitrogen Mineralization through an Increase in Mineralizable Organic Nitrogen and the Abundance of Functional Genes. J. Soil Sci. Plant Nutr. 2024, 24, 975–987. [Google Scholar] [CrossRef]
- Galloway, J.N.; Townsend, A.R.; Erisman, J.W.; Bekunda, M.; Cai, Z.; Freney, J.R.; Martinelli, L.A.; Seitzinger, S.P.; Sutton, M.A. Transformation of the nitrogen cycle: Recent trends, questions, and potential solutions. Science 2008, 320, 889–892. [Google Scholar] [CrossRef]
- Widdig, M.; Heintz-Buschart, A.; Schleuss, P.-M.; Guhr, A.; Borer, E.T.; Seabloom, E.W.; Spohn, M. Effects of nitrogen and phosphorus addition on microbial community composition and element cycling in a grassland soil. Soil Biol. Biochem. 2020, 151, 108041. [Google Scholar] [CrossRef]
- Wang, J.; Shi, X.; Zheng, C.; Suter, H.; Huang, Z. Different responses of soil bacterial and fungal communities to nitrogen deposition in a subtropical forest. Sci. Total Environ. 2021, 755, 142449. [Google Scholar] [CrossRef] [PubMed]
- Ke, Y.; Yu, Q.; Wang, H.; Zhao, Y.; Jia, X.; Yang, Y.; Zhang, Y.; Zhou, W.; Wu, H.; Xu, C. The potential bias of nitrogen deposition effects on primary productivity and biodiversity. Glob. Chang. Biol. 2023, 29, 1054–1061. [Google Scholar] [CrossRef]
- Hu, J.; Huang, C.; Zhou, S.; Kuzyakov, Y. Nitrogen addition to soil affects microbial carbon use efficiency: Meta-analysis of similarities and differences in 13C and 18O approaches. Glob. Chang. Biol. 2022, 28, 4977–4988. [Google Scholar] [CrossRef]
- Cao, M.; Zheng, X.; Cui, L.; Wu, F.; Gao, H.; Jiang, J. Soil bacterial communities are more sensitive to short-term nitrogen deposition than fungal communities in subtropical Chinese fir forests. For. Ecol. Manag. 2023, 549, 121490. [Google Scholar] [CrossRef]
- Chen, W.; Su, F.; Nie, Y.; Zhong, B.; Zheng, Y.; Mo, J.; Xiong, B.; Lu, X. Divergent responses of soil microbial functional groups to long-term high nitrogen presence in the tropical forests. Sci. Total Environ. 2022, 821, 153251. [Google Scholar] [CrossRef]
- He, J.; Jiao, S.; Tan, X.; Wei, H.; Ma, X.; Nie, Y.; Liu, J.; Lu, X.; Mo, J.; Shen, W. Adaptation of soil fungal community structure and assembly to long-versus short-term nitrogen addition in a tropical forest. Front. Microbiol. 2021, 12, 689674. [Google Scholar] [CrossRef]
- Ma, S.; Verheyen, K.; Props, R.; Wasof, S.; Vanhellemont, M.; Boeckx, P.; Boon, N.; De Frenne, P. Plant and soil microbe responses to light, warming and nitrogen addition in a temperate forest. Funct. Ecol. 2018, 32, 1293–1303. [Google Scholar] [CrossRef]
- Wang, C.; Liu, D.; Bai, E. Decreasing soil microbial diversity is associated with decreasing microbial biomass under nitrogen addition. Soil Biol. Biochem. 2018, 120, 126–133. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, C.; Zheng, M.; Jiang, L.; Luo, Y. Patterns and mechanisms of responses by soil microbial communities to nitrogen addition. Soil Biol. Biochem. 2017, 115, 433–441. [Google Scholar] [CrossRef]
- Luo, L.; Meng, H.; Wu, R.-N.; Gu, J.-D. Impact of nitrogen pollution/deposition on extracellular enzyme activity, microbial abundance and carbon storage in coastal mangrove sediment. Chemosphere 2017, 177, 275–283. [Google Scholar] [CrossRef]
- Rousk, J.; Bååth, E.; Brookes, P.C.; Lauber, C.L.; Lozupone, C.; Caporaso, J.G.; Knight, R.; Fierer, N. Soil bacterial and fungal communities across a pH gradient in an arable soil. ISME J. 2010, 4, 1340–1351. [Google Scholar] [CrossRef]
- Mao, Q.; Lu, X.; Zhou, K.; Chen, H.; Zhu, X.; Mori, T.; Mo, J. Effects of long-term nitrogen and phosphorus additions on soil acidification in an N-rich tropical forest. Geoderma 2017, 285, 57–63. [Google Scholar] [CrossRef]
- Lu, F.; Hu, H.; Sun, W.; Zhu, J.; Liu, G.; Zhou, W.; Zhang, Q.; Shi, P.; Liu, X.; Wu, X. Effects of national ecological restoration projects on carbon sequestration in China from 2001 to 2010. Proc. Natl. Acad. Sci. USA 2018, 115, 4039–4044. [Google Scholar] [CrossRef]
- Zhang, L.; Shen, Y.; Hu, Y.; Li, J.; Liu, Y.; Chen, S.; Wang, L.; Liu, S.; Li, H.; You, C. Response of soil phosphorus fractions to litter removal in subalpine coniferous forest. Sci. Total Environ. 2023, 898, 166383. [Google Scholar] [CrossRef]
- Tian, Y.; Lu, H.; Wang, J.; Lin, Y.; Campbell, D.E.; Jian, S. Effects of canopy and understory nitrogen addition on the structure and eco-exergy of a subtropical forest community. Ecol. Indic. 2019, 106, 105459. [Google Scholar] [CrossRef]
- Liu, J.; Le, T.H.; Zhu, H.; Yao, Y.; Zhu, H.; Cao, Y.; Zhao, Z. Afforestation of cropland fundamentally alters the soil fungal community. Plant Soil 2020, 457, 279–292. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Q.; Ku, Y.; Zhang, W.; Zhu, H.; Zhao, Z. Precipitation and soil pH drive the soil microbial spatial patterns in the Robinia pseudoacacia forests at the regional scale. Catena 2022, 212, 106120. [Google Scholar] [CrossRef]
- Kang, E.; Li, Y.; Zhang, X.; Yan, Z.; Wu, H.; Li, M.; Yan, L.; Zhang, K.; Wang, J.; Kang, X. Soil pH and nutrients shape the vertical distribution of microbial communities in an alpine wetland. Sci. Total Environ. 2021, 774, 145780. [Google Scholar] [CrossRef]
- Wang, H.; Liu, S.; Zhang, X.; Mao, Q.; Li, X.; You, Y.; Wang, J.; Zheng, M.; Zhang, W.; Lu, X. Nitrogen addition reduces soil bacterial richness, while phosphorus addition alters community composition in an old-growth N-rich tropical forest in southern China. Soil Biol. Biochem. 2018, 127, 22–30. [Google Scholar] [CrossRef]
- Lauber, C.L.; Strickland, M.S.; Bradford, M.A.; Fierer, N. The influence of soil properties on the structure of bacterial and fungal communities across land-use types. Soil Biol. Biochem. 2008, 40, 2407–2415. [Google Scholar] [CrossRef]
- Wang, L.; Macko, S.A. Constrained preferences in nitrogen uptake across plant species and environments. Plant Cell Environ. 2011, 34, 525–534. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, W.; Li, Y.; Wang, S.; Mao, J.; Mo, J.; Zheng, M. Long-term nitrogen deposition does not exacerbate soil acidification in tropical broadleaf plantations. Environ. Res. Lett. 2021, 16, 114042. [Google Scholar] [CrossRef]
- Urbanová, M.; Šnajdr, J.; Baldrian, P. Composition of fungal and bacterial communities in forest litter and soil is largely determined by dominant trees. Soil Biol. Biochem. 2015, 84, 53–64. [Google Scholar] [CrossRef]
- Zhou, Q.; Li, F.; Cai, X.-a.; Rao, X.; Zhou, L.; Liu, Z.; Lin, Y.; Fu, S. Survivorship of plant species from soil seedbank after translocation from subtropical natural forests to plantation forests. For. Ecol. Manag. 2019, 432, 741–747. [Google Scholar] [CrossRef]
- Lu, X.; Mao, Q.; Mo, J.; Gilliam, F.S.; Zhou, G.; Luo, Y.; Zhang, W.; Huang, J. Divergent responses of soil buffering capacity to long-term N deposition in three typical tropical forests with different land-use history. Environ. Sci. Technol. 2015, 49, 4072–4080. [Google Scholar] [CrossRef]
- Tian, D.; Niu, S. A global analysis of soil acidification caused by nitrogen addition. Environ. Res. Lett. 2015, 10, 024019. [Google Scholar] [CrossRef]
- Xing, J.; Hu, C.; Song, C.; Wang, K.; Song, Y. Nitrogen Deposition Modulates Litter Decomposition and Enhances Water Retention in Subtropical Forests. Forests 2024, 15, 522. [Google Scholar] [CrossRef]
- Leng, Q.; Cui, J.; Zhou, F.; Du, K.; Zhang, L.; Fu, C.; Liu, Y.; Wang, H.; Shi, G.; Gao, M. Wet-only deposition of atmospheric inorganic nitrogen and associated isotopic characteristics in a typical mountain area, southwestern China. Sci. Total Environ. 2018, 616, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Xing, J.; Hu, C.; Song, C.; Wang, Q.; Wang, S. Decomposition and Carbon and Nitrogen Releases of Twig and Leaf Litter Were Inhibited by Increased Level of Nitrogen Deposition in a Subtropical Evergreen Broad-Leaved Forest in Southwest China. Forests 2024, 15, 492. [Google Scholar] [CrossRef]
- Bao, S.D. Soil and Agricultural Chemistry Analysis; China Agricultural Press: Beijing, China, 2000. [Google Scholar]
- Logue, J.B.; Stedmon, C.A.; Kellerman, A.M.; Nielsen, N.J.; Andersson, A.F.; Laudon, H.; Lindström, E.S.; Kritzberg, E.S. Experimental insights into the importance of aquatic bacterial community composition to the degradation of dissolved organic matter. ISME J. 2016, 10, 533–545. [Google Scholar] [CrossRef] [PubMed]
- Gardes, M.; Bruns, T.D. ITS primers with enhanced specificity for basidiomycetes-application to the identification of mycorrhizae and rusts. Mol. Ecol. 1993, 2, 113–118. [Google Scholar] [CrossRef] [PubMed]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Academic Press: London, UK, 1990; pp. 315–322. [Google Scholar]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef] [PubMed]
- Shannon, C.E. A mathematical theory of communication. Bell Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef]
- Chao, A. Nonparametric estimation of the number of classes in a population. Scand. J. Stat. 1984, 11, 265–270. [Google Scholar]
- Feng, K.; Peng, X.; Zhang, Z.; Gu, S.; He, Q.; Shen, W.; Wang, Z.; Wang, D.; Hu, Q.; Li, Y. iNAP: An Integrated Network Analysis Pipeline for Microbiome Studies. iMeta 2022, 1, e13. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’hara, R.; Simpson, G.L.; Solymos, P.; Stevens, M.H.H.; Wagner, H. R Package Version 2.2-0. Package ‘Vegan’. Community Ecology Package. 2013. Available online: https://cran.r-project.org/web/packages/vegan/index.html (accessed on 22 May 2024).
- Wickham, H.; Chang, W.; Wickham, M.H. Version 3.5.1. Package ‘ggplot2’. Create Elegant Data Visualisations Using the Grammar of Graphics. 2016. Available online: https://search.r-project.org/CRAN/refmans/ggplot2/html/ggplot2-package.html (accessed on 22 May 2024).
- Berthrong, S.T.; Jobbágy, E.G.; Jackson, R.B. A global meta-analysis of soil exchangeable cations, pH, carbon, and nitrogen with afforestation. Ecol. Appl. 2009, 19, 2228–2241. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Mao, Q.; Gilliam, F.S.; Luo, Y.; Mo, J. Nitrogen deposition contributes to soil acidification in tropical ecosystems. Glob. Chang. Biol. 2014, 20, 3790–3801. [Google Scholar] [CrossRef] [PubMed]
- Philippot, L.; Chenu, C.; Kappler, A.; Rillig, M.C.; Fierer, N. The interplay between microbial communities and soil properties. Nat. Rev. Microbiol. 2024, 22, 226–239. [Google Scholar] [CrossRef]
- Wild, B.; Alaei, S.; Bengtson, P.; Bodé, S.; Boeckx, P.; Schnecker, J.; Mayerhofer, W.; Rütting, T. Short-term carbon input increases microbial nitrogen demand, but not microbial nitrogen mining, in a set of boreal forest soils. Biogeochemistry 2017, 136, 261–278. [Google Scholar] [CrossRef]
- Wang, X.; Feng, J.; Ao, G.; Qin, W.; Han, M.; Shen, Y.; Liu, M.; Chen, Y.; Zhu, B. Globally nitrogen addition alters soil microbial community structure, but has minor effects on soil microbial diversity and richness. Soil Biol. Biochem. 2023, 179, 108982. [Google Scholar] [CrossRef]
- Strickland, M.S.; Rousk, J. Considering fungal: Bacterial dominance in soils–methods, controls, and ecosystem implications. Soil Biol. Biochem. 2010, 42, 1385–1395. [Google Scholar] [CrossRef]
- Müller, K.; Marhan, S.; Kandeler, E.; Poll, C. Carbon flow from litter through soil microorganisms: From incorporation rates to mean residence times in bacteria and fungi. Soil Biol. Biochem. 2017, 115, 187–196. [Google Scholar] [CrossRef]
- Lu, X.; Vitousek, P.M.; Mao, Q.; Gilliam, F.S.; Luo, Y.; Turner, B.L.; Zhou, G.; Mo, J. Nitrogen deposition accelerates soil carbon sequestration in tropical forests. Proc. Natl. Acad. Sci. USA 2021, 118, e2020790118. [Google Scholar] [CrossRef]
- Cao, J.; Wang, H.; Holden, N.M.; Adamowski, J.F.; Biswas, A.; Zhang, X.; Feng, Q. Soil properties and microbiome of annual and perennial cultivated grasslands on the Qinghai–Tibetan Plateau. Land Degrad. Dev. 2021, 32, 5306–5321. [Google Scholar] [CrossRef]
- Kaspari, M.; Bujan, J.; Weiser, M.D.; Ning, D.; Michaletz, S.T.; Zhili, H.; Enquist, B.J.; Waide, R.B.; Zhou, J.; Turner, B.L. Biogeochemistry drives diversity in the prokaryotes, fungi, and invertebrates of a Panama forest. Ecology 2017, 98, 2019–2028. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, X.; Quan, X.; Liang, H.; Wang, L.; Yan, X. Effects of nitrogen stress and nitrogen form ratios on the bacterial community and diversity in the root surface and rhizosphere of Cunninghamia lanceolata and Schima superba. Front. Plant Sci. 2023, 14, 1240675. [Google Scholar] [CrossRef]
- Chen, X.; Chen, H.Y.; Searle, E.B.; Chen, C.; Reich, P.B. Negative to positive shifts in diversity effects on soil nitrogen over time. Nat. Sustain. 2021, 4, 225–232. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, C.; Chen, X.; Liu, S.; Lu, X.; Chen, H.Y.; Ruan, H. Phosphorus additions imbalance terrestrial ecosystem C: N: P stoichiometry. Glob. Chang. Biol. 2022, 28, 7353–7365. [Google Scholar] [CrossRef]
- Yang, L.; Wang, X.; Mao, Z.; Jiang, Z.; Gao, Y.; Chen, X.; Aubrey, D.P. Root exudation rates decrease with increasing latitude in some tree species. Forests 2020, 11, 1045. [Google Scholar] [CrossRef]
- Zhang, Y.; Gu, M.; Xia, X.; Shi, K.; Zhou, Y.; Yu, J. Effects of phenylcarboxylic acids on mitosis, endoreduplication and expression of cell cycle-related genes in roots of cucumber (Cucumis sativus L.). J. Chem. Ecol. 2009, 35, 679–688. [Google Scholar] [CrossRef] [PubMed]
- Haichar, F.e.Z.; Marol, C.; Berge, O.; Rangel-Castro, J.I.; Prosser, J.I.; Balesdent, J.; Heulin, T.; Achouak, W. Plant host habitat and root exudates shape soil bacterial community structure. ISME J. 2008, 2, 1221–1230. [Google Scholar] [CrossRef] [PubMed]
- Fierer, N.; Bradford, M.A.; Jackson, R.B. Toward an ecological classification of soil bacteria. Ecology 2007, 88, 1354–1364. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Wang, J.; Ge, Y.; Lei, Y.; Wei, X.; Xu, Y.; Zheng, X. Partial substitution of nitrogen fertilizers by organic products of rural waste co-composting impacts on farmland soil quality. Environ. Technol. Innov. 2023, 33, 103470. [Google Scholar] [CrossRef]
- Hao, C.; Du, P.; Ren, J.; Hu, L.; Zhang, Z. Halophyte Elymus dahuricus colonization regulates microbial community succession by mediating saline-alkaline and biogenic organic matter in bauxite residue. Sci. Total Environ. 2023, 905, 167140. [Google Scholar] [CrossRef]
- Yan, G.; Han, S.; Wang, Q.; Wang, X.; Hu, C.; Xing, Y. Variations of the effects of reduced precipitation and N addition on microbial diversity among different seasons in a temperate forest. Appl. Soil Ecol. 2021, 166, 103995. [Google Scholar] [CrossRef]
- Sorokin, D.Y.; Vejmelkova, D.; Lücker, S.; Streshinskaya, G.M.; Rijpstra, W.I.C.; Sinninghe Damsté, J.S.; Kleerbezem, R.; van Loosdrecht, M.; Muyzer, G.; Daims, H. Nitrolancea hollandica gen. nov., sp. nov., a chemolithoautotrophic nitrite-oxidizing bacterium isolated from a bioreactor belonging to the phylum Chloroflexi. Int. J. Syst. Evol. Microbiol. 2014, 64, 1859–1865. [Google Scholar] [CrossRef] [PubMed]
- Wendong, X.; Xiaotong, Z.; Wenjun, L. Research status and prospect on bacterial phylum Chloroflexi. Acta Microbiol. Sin. 2020, 60, 1801–1820. [Google Scholar]
- Choma, M.; Tahovska, K.; Kaštovská, E.; Bárta, J.; Růžek, M.; Oulehle, F. Bacteria but not fungi respond to soil acidification rapidly and consistently in both a spruce and beech forest. FEMS Microbiol. Ecol. 2020, 96, fiaa174. [Google Scholar] [CrossRef] [PubMed]
- Corsaro, D.; Walochnik, J.; Venditti, D.; Hauröder, B.; Michel, R. Solving an old enigma: Morellospora saccamoebae gen. nov., sp. nov. (Rozellomycota), a Sphaerita-like parasite of free-living amoebae. Parasitol. Res. 2020, 119, 925–934. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Tian, Y.; Guo, R.; Li, S.; Guo, J.; Zhang, T. Effects of warming and nitrogen addition on soil fungal and bacterial community structures in a temperate meadow. Front. Microbiol. 2023, 14, 1231442. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yang, H.; Zhou, L.; Jin, S.-S.; Xie, L.; Lin, C.; He, J.-Z.; Zheng, Y. Dynamic response of root-associated fungal community structure to nitrogen and phosphorus additions in a subtropical forest. Pedobiologia 2023, 101, 150909. [Google Scholar] [CrossRef]
- Allison, S.D.; LeBauer, D.S.; Ofrecio, M.R.; Reyes, R.; Ta, A.-M.; Tran, T.M. Low levels of nitrogen addition stimulate decomposition by boreal forest fungi. Soil Biol. Biochem. 2009, 41, 293–302. [Google Scholar] [CrossRef]
- Weber, C.F.; Vilgalys, R.; Kuske, C.R. Changes in fungal community composition in response to elevated atmospheric CO2 and nitrogen fertilization varies with soil horizon. Front. Microbiol. 2013, 4, 78. [Google Scholar] [CrossRef]
- de Vries, F.T.; Griffiths, R.I.; Bailey, M.; Craig, H.; Girlanda, M.; Gweon, H.S.; Hallin, S.; Kaisermann, A.; Keith, A.M.; Kretzschmar, M. Soil bacterial networks are less stable under drought than fungal networks. Nat. Commun. 2018, 9, 3033. [Google Scholar] [CrossRef]
- Gao, C.; Xu, L.; Montoya, L.; Madera, M.; Hollingsworth, J.; Chen, L.; Purdom, E.; Singan, V.; Vogel, J.; Hutmacher, R.B. Co-occurrence networks reveal more complexity than community composition in resistance and resilience of microbial communities. Nat. Commun. 2022, 13, 3867. [Google Scholar] [CrossRef] [PubMed]
- He, D.; Shen, W.; Eberwein, J.; Zhao, Q.; Ren, L.; Wu, Q.L. Diversity and co-occurrence network of soil fungi are more responsive than those of bacteria to shifts in precipitation seasonality in a subtropical forest. Soil Biol. Biochem. 2017, 115, 499–510. [Google Scholar] [CrossRef]
- Ma, X.; Wang, T.; Shi, Z.; Chiariello, N.R.; Docherty, K.; Field, C.B.; Gutknecht, J.; Gao, Q.; Gu, Y.; Guo, X. Long-term nitrogen deposition enhances microbial capacities in soil carbon stabilization but reduces network complexity. Microbiome 2022, 10, 112. [Google Scholar]
- Puri, G.; Ashman, M. Microbial immobilization of 15N-labelled ammonium and nitrate in a temperate woodland soil. Soil Biol. Biochem. 1999, 31, 929–932. [Google Scholar] [CrossRef]
- Bai, T.; Wang, P.; Ye, C.; Hu, S. Form of nitrogen input dominates N effects on root growth and soil aggregation: A meta-analysis. Soil Biol. Biochem. 2021, 157, 108251. [Google Scholar] [CrossRef]
- Dong, L.; Berg, B.; Sun, T.; Wang, Z.; Han, X. Response of fine root decomposition to different forms of N deposition in a temperate grassland. Soil Biol. Biochem. 2020, 147, 107845. [Google Scholar] [CrossRef]
- Sun, K.; Jiang, H.-J.; Pan, Y.-T.; Lu, F.; Zhu, Q.; Ma, C.-Y.; Zhang, A.-Y.; Zhou, J.-Y.; Zhang, W.; Dai, C.-C. Hyphosphere microorganisms facilitate hyphal spreading and root colonization of plant symbiotic fungus in ammonium-enriched soil. ISME J. 2023, 17, 1626–1638. [Google Scholar] [CrossRef] [PubMed]
- Xiang, X.; Liu, J.; Zhang, J.; Li, D.; Xu, C.; Kuzyakov, Y. Divergence in fungal abundance and community structure between soils under long-term mineral and organic fertilization. Soil Tillage Res. 2020, 196, 104491. [Google Scholar] [CrossRef]



| Forest Types | Treatments | Soil Physicochemical Properties | ||||||
|---|---|---|---|---|---|---|---|---|
| pH | SOM | NH4+-N | NO3−-N | AP | K+ | TN | ||
| (g·kg−1) | (mg·kg−1) | (mg·kg−1) | (mg·kg−1) | (mg·kg−1) | (g·kg−1) | |||
| P. yunnanensis Franch. Forest | CK | 4.17(0.04) c | 64.67(4.13) a | 11.62(0.34) c | 1.09(0.18) c | 0.19(0.01) ab | 29.19(7.02) b | 0.28(0.01) b |
| N10 | 4.25(0.02) b | 70.73(11.27) a | 14.92(0.28) b | 1.64(0.08) a | 0.20(0.01) ab | 19.81(1.23) c | 0.30(0.00) a | |
| N20 | 4.32(0.04) ab | 64.67(4.59) a | 16.14(0.34) a | 1.36(0.13) b | 0.17(0.04) b | 40.97(2.41) a | 0.29(0.01) ab | |
| N25 | 4.35(0.04) a | 64.27(3.69) a | 15.08(0.20) b | 1.04(0.06) d | 0.22(0.02) a | 21.42(1.23) b | 0.31(0.02) a | |
| P armandii Franch. Forest | CK | 4.71(0.02) a | 47.18(4.91) b | 14.20(0.98) c | 1.60(0.22) d | 0.13(0.02) b | 25.17(2.32) a | 0.27(0.03) b |
| N10 | 4.53(0.05) b | 58.88(2.21) a | 18.53(0.29) a | 3.64(0.49) c | 0.19(0.02) a | 22.49(1.39) ab | 0.32(0.02) a | |
| N20 | 4.42(0.04) c | 48.16(2.44) b | 15.39(0.16) b | 8.80(0.24) b | 0.21(0.03) a | 19.28(1.61) b | 0.26(0.03) b | |
| N25 | 4.18(0.04) d | 62.00(3.84) a | 11.40(0.57) d | 9.71(0.21) a | 0.18(0.02) ab | 21.42(1.23) b | 0.33(0.02) a | |
| Total | N | 0.000 *** | 0.020 * | 0.000 *** | 0.000 *** | 0.041 * | 0.000 *** | 0.001 ** |
| Forest types | 0.000 *** | 0.000 *** | 0.035 * | 0.000 *** | 0.103 | 0.000 *** | 0.864 | |
| N× Forest types | 0.000 *** | 0.093 | 0.000 *** | 0.000 *** | 0.009 ** | 0.000 *** | 0.17 | |
| Forest Types | Bacteria | Fungi | |||||
|---|---|---|---|---|---|---|---|
| Treatments | OTUs | Shannon | Chao | OTUs | Shannon | Chao | |
| P. yunnanensis Franch. Forest | CK | 3416(189) a | 5.92(0.05) a | 4068(201) a | 481(28) c | 2.69(0.11) c | 487(34) c |
| N10 | 2787(17) bc | 5.85(0.04) ab | 3524(112) b | 884(4) a | 3.21(0.07) b | 1087(34) ab | |
| N20 | 2578(95) c | 5.82(0.05) b | 3200(190) b | 886(33) a | 3.83(0.22) a | 1128(58) a | |
| N25 | 2819(118) b | 5.78(0.03) b | 3322(231) b | 793(16) b | 3.07(0.02) b | 1028(10) b | |
| P. armandii Franch. Forest | CK | 2388(153) c | 5.63(0.06) c | 2778(59) c | 741(37) a | 2.76(0.06) a | 973(57) a |
| N10 | 2979(103) a | 6.00(0.05) a | 3758(87) a | 287(51) c | 3.20(0.07) b | 305(34) c | |
| N20 | 2689(65) b | 5.99(0.02) a | 3161(176) b | 363(15) bc | 2.80(0.02) a | 456(44) b | |
| N25 | 2217(148) cd | 5.83(0.10) b | 2683(65) c | 434(57) b | 3.93(0.14) c | 444(60) b | |
| Total | N | 0.000 *** | 0.000 *** | 0.000 *** | 0.291 | 0.000 *** | 0.014 * |
| Forest types | 0.000 *** | 0.307 | 0.000 *** | 0.000 *** | 0.51 | 0.000 *** | |
| N×Forest types | 0.000 *** | 0.000 *** | 0.000 *** | 0.000 *** | 0.000 *** | 0.000 *** | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, Z.; Zhang, X.; Chen, W.; Liang, Z.; Wang, K.; Zhang, Y.; Song, Y. Differential Responses of Bacterial and Fungal Community Structure in Soil to Nitrogen Deposition in Two Planted Forests in Southwest China in Relation to pH. Forests 2024, 15, 1112. https://doi.org/10.3390/f15071112
Hou Z, Zhang X, Chen W, Liang Z, Wang K, Zhang Y, Song Y. Differential Responses of Bacterial and Fungal Community Structure in Soil to Nitrogen Deposition in Two Planted Forests in Southwest China in Relation to pH. Forests. 2024; 15(7):1112. https://doi.org/10.3390/f15071112
Chicago/Turabian StyleHou, Zheng, Xiaohua Zhang, Wen Chen, Ziqi Liang, Keqin Wang, Ya Zhang, and Yali Song. 2024. "Differential Responses of Bacterial and Fungal Community Structure in Soil to Nitrogen Deposition in Two Planted Forests in Southwest China in Relation to pH" Forests 15, no. 7: 1112. https://doi.org/10.3390/f15071112
APA StyleHou, Z., Zhang, X., Chen, W., Liang, Z., Wang, K., Zhang, Y., & Song, Y. (2024). Differential Responses of Bacterial and Fungal Community Structure in Soil to Nitrogen Deposition in Two Planted Forests in Southwest China in Relation to pH. Forests, 15(7), 1112. https://doi.org/10.3390/f15071112






