Biochar Application Improved Sludge-Amended Landscape Soil Fertility Index but with No Added Benefit in Plant Growth
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Pot Experiment Design
2.3. Plant Harvesting and Sample Collection and Analysis
2.4. Matrix Sample Collection and Testing
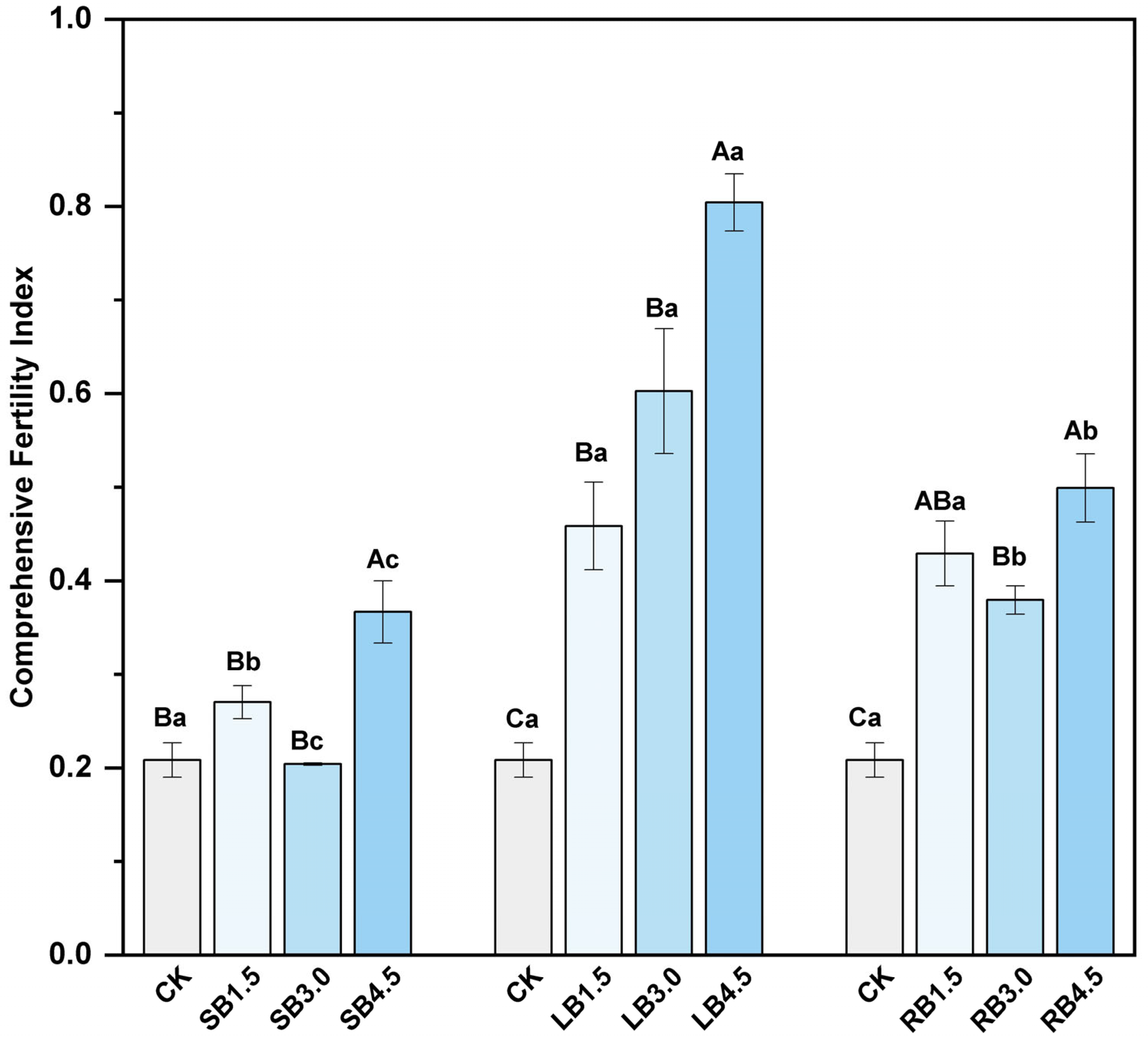
2.5. Comprehensive Evaluation of Soil Fertility
2.6. Comprehensive Evaluation of the Impact of Biochar on A. modestum Growth
2.7. Calculations and Statistical Analysis
3. Results
3.1. Soil Properties
3.1.1. Soil Physicochemical Properties
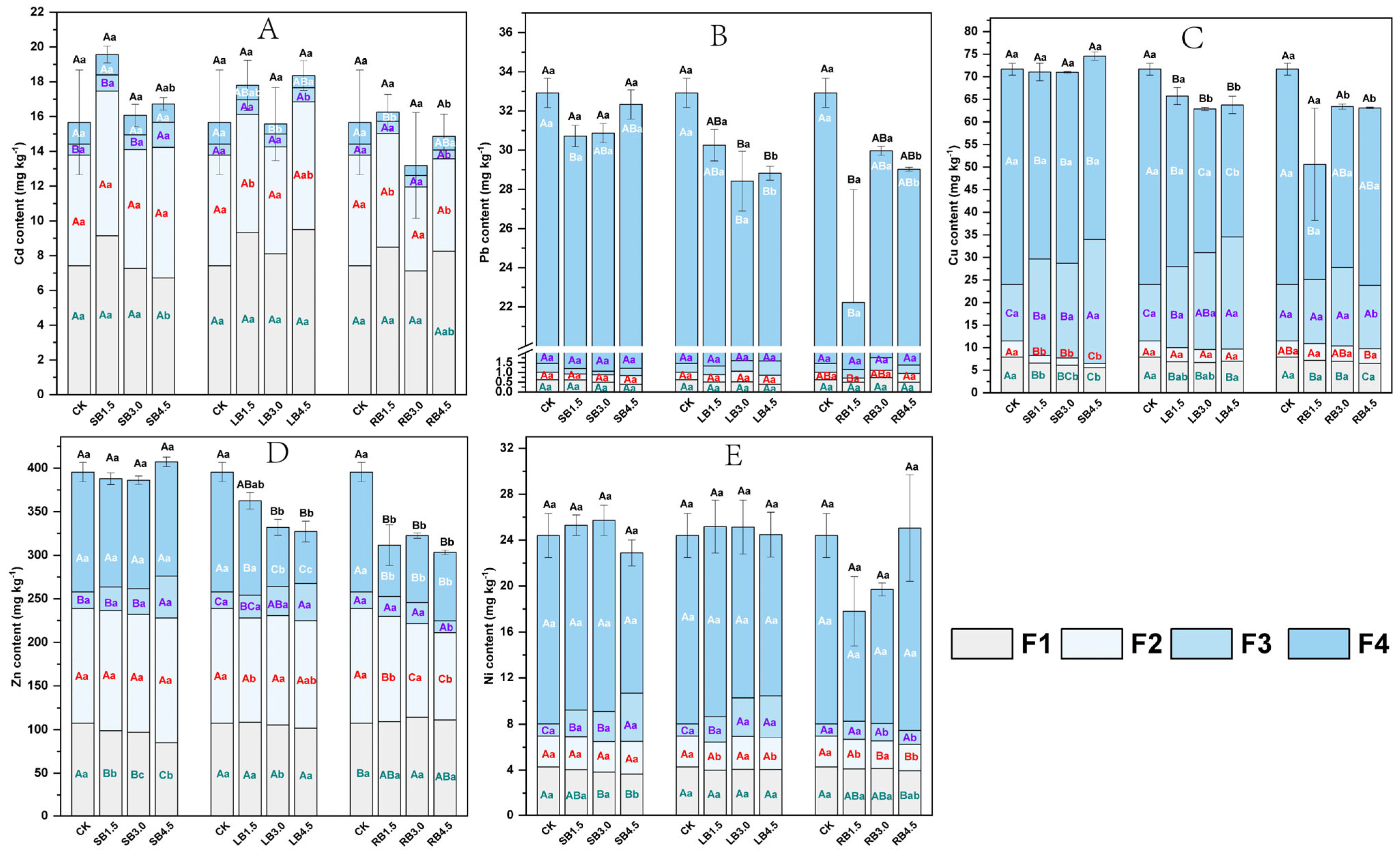
3.1.2. Potential Toxic Element Content and Fractionation
3.2. Growth and Survival Responses of A. modestum
3.2.1. Plant Height and Biomass
3.2.2. Nutrient Contents in A. modestum
3.2.3. Root Morphology and Physiological Index
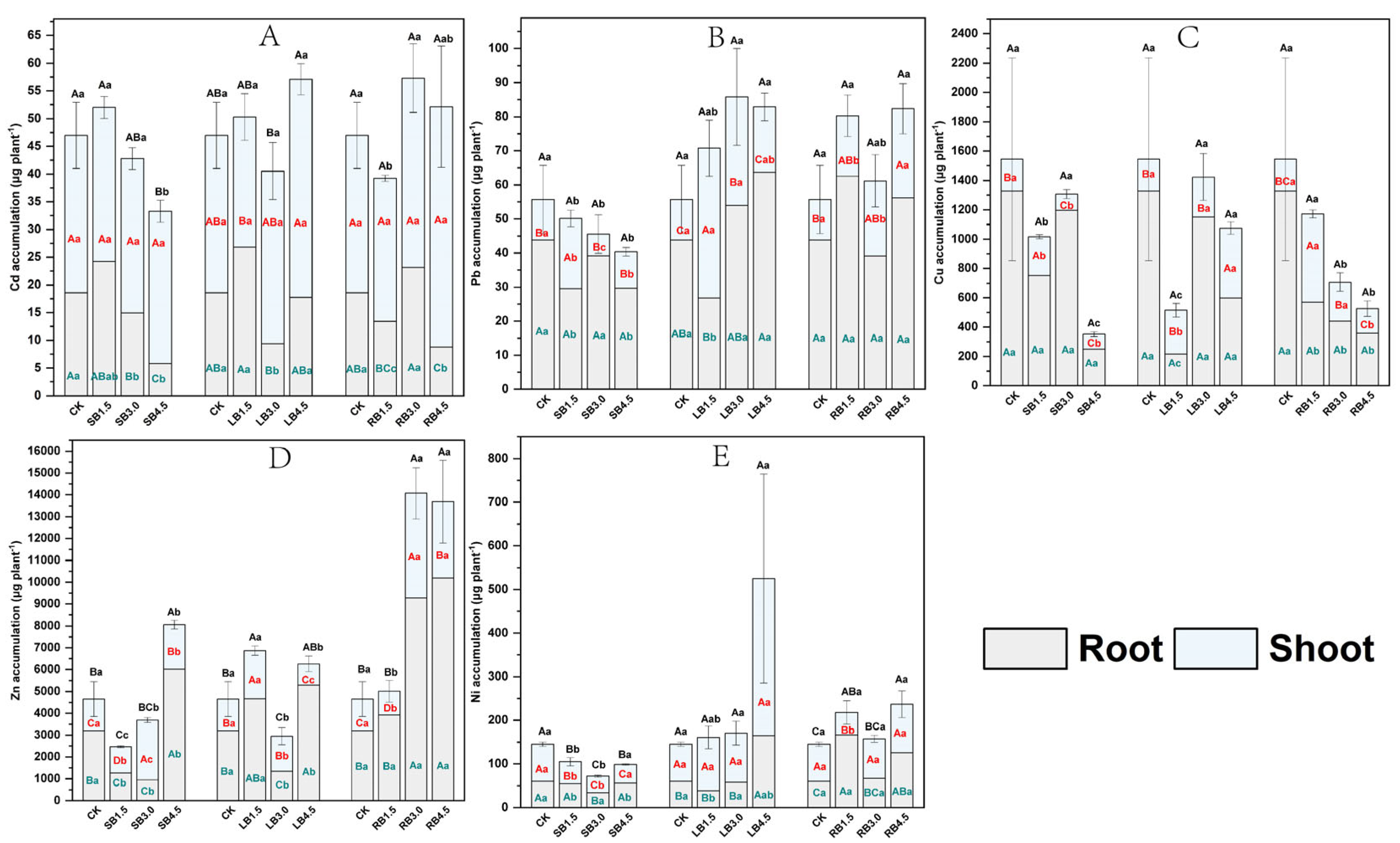
3.3. Plant Absorption and Accumulation of PTEs
3.3.1. PTEs Content in A. modestum Different Tissues
3.3.2. Accumulation of PTEs
3.4. Comprehensive Evaluation of A. modestum Growth Based on TOPSIS
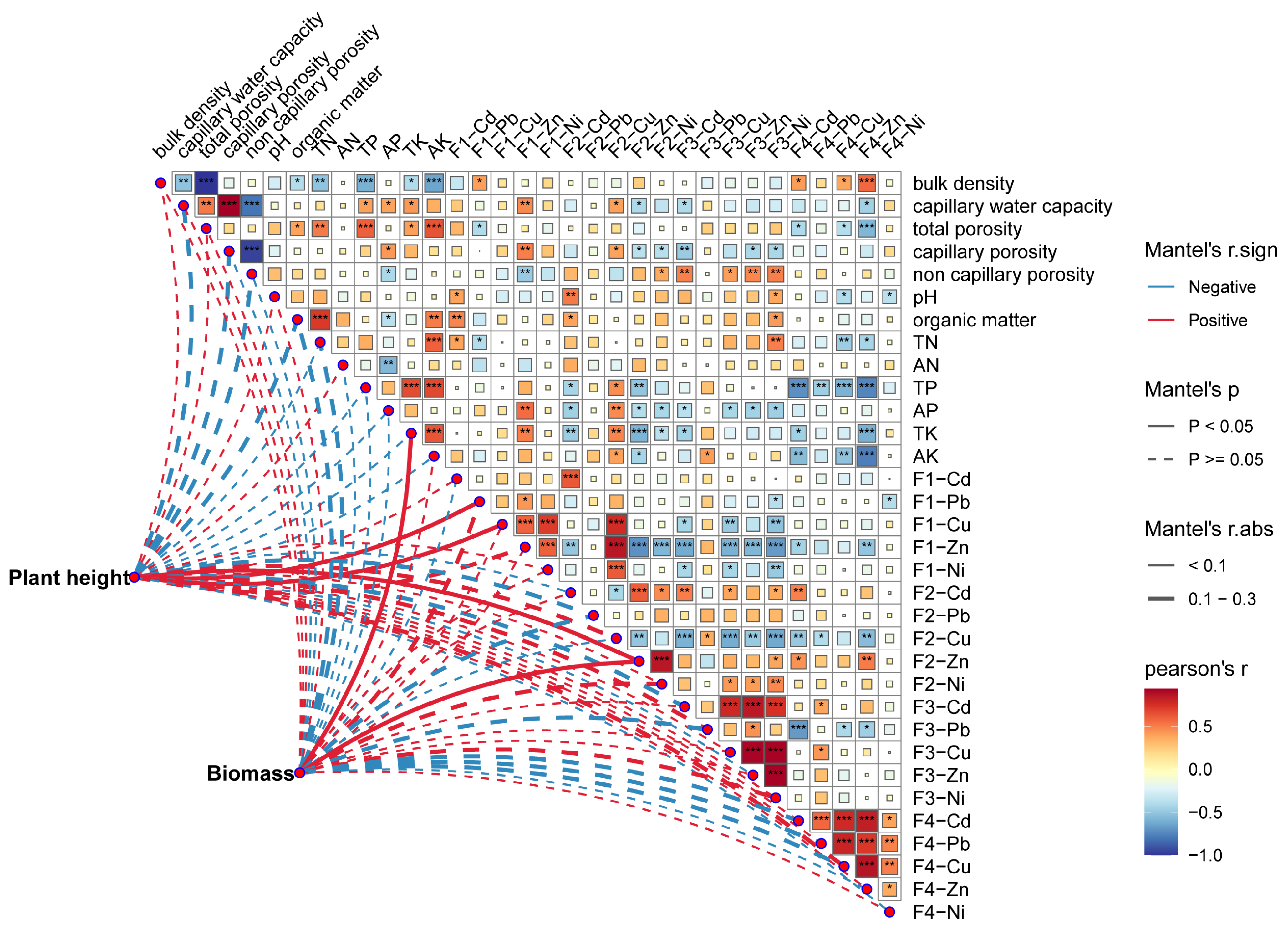
3.5. Factors Affecting the Growth of A. modestum
4. Discussion
4.1. Physicochemical Properties of Sludge-Amended Soil Improved with Biochar Addition
4.2. Biochar Application to SS-Amended Soil Affects A. modestum Growth and Nutrient Element Absorption
4.3. Effect of Adding Biochar in SS-Amended Soil on the Absorption and Accumulation of PTEs in A. modestum
4.4. Challenges and Research Needs in the Application of Biochar Combined with Sludge in Urban Landscaping/Forestry
- (i)
- Attention should be given to different plant types when considering the alleviation of phytotoxicity in SS-amended landscape soil through biochar addition. The response of plants to biochar addition may vary significantly depending on their adaptability. For instance, a study conducted by Gale et al. [23] observed contrasting growth responses in two different landscape plants in the SS-amended soil with the same biochar addition. Therefore, future research should explore the generalizability of the effects of biochar on plant growth.
- (ii)
- Attention needs to be paid to factors other than abiotic conditions. This study found that plant growth and the effectiveness of nutrients in soil physical properties induced by biochar are not the only mechanisms of biochar interactions. A reasonable assumption is that the changes in biological conditions induced by biochar may have a greater impact on plant growth compared to the changes in abiotic conditions, as previous reports indicated that biochar-initiated changes in microbial communities could continue beyond the short-lived abiotic changes due to density-dependent processes [50,51]. Therefore, it is imperative to study the effects of the effects of biochar amendments on soil physicochemical properties, pollutants, and microorganisms, as well as their interactions with multiple environmental factors prior to their widespread application.
- (iii)
- It is important to extend the test cycle. Similar to this study, most research on the effects of biochar on plant and soil characteristics has been conducted in the short term, while long-term studies are limited. Previous studies have shown that the increase in production caused by biochar becomes more complex due to occasional delayed reactions, initially producing negative or no effects in the first year but increasing production to varying degrees in subsequent years [52,53]. Another connected hypothesis is that as biochar ages or factors that hinder plant growth diminish, new patterns of plant growth may emerge. For instance, the decomposition of OM previously added in SS could lead to significant changes in the chemical forms of PTEs once the addition of SS ceases. This is because PTEs may precipitate as inorganic compounds as unstable substances degrade rapidly, subsequently forming stable compounds with inorganic salts [54], which could reduce the phytotoxicity of PTEs in SS. However, these patterns need to be verified through long-term experiments.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chu, S.; Jacobs, D.F.; Liao, D.; Liang, L.L.; Wu, D.; Chen, P.; Lai, C.; Zhong, F.; Zeng, S. Effects of landscape plant species and concentration of sewage sludge compost on plant growth, nutrient uptake, and heavy metal removal. Environ. Sci. Pollut. Res. 2018, 25, 35184–35199. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Peng, W.; Bao, L.; Yu, X.; Dong, X.; Lai, M.; Liang, Z.; Xie, S.; Jacobs, D.F.; Zeng, S. Biochar alleviating heavy metals phytotoxicity in sludge-amended soil varies with plant adaptability. Environ. Res. 2022, 215, 114248. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xie, H.; Liu, X.; Kong, D.; Zhang, S.; Wang, C. A novel green substrate made by sludge digestate and its biochar: Plant growth and greenhouse emission. Sci. Total Environ. 2021, 797, 149194. [Google Scholar] [CrossRef] [PubMed]
- Chu, S.; Yang, W.; Xia, D.; Liang, Z.; Su, S.; Zhao, N.; Wang, J.; Long, M.; Ouyang, J.; Liao, D.; et al. Changes in potentially toxic element concentration and potential ecological risk in topsoil caused by sewage sludge application on forestland: A 3-year field trial. For. Ecol. Manag. 2021, 500, 119657. [Google Scholar] [CrossRef]
- Chu, S.; Xian, L.; Lai, C.; Yang, W.; Wang, J.; Long, M.; Ouyang, J.; Liao, D.; Zeng, S. Migration and risks of potentially toxic elements from sewage sludge applied to acid forest soil. J. For. Res. 2023, 34, 2011–2026. [Google Scholar] [CrossRef]
- Chu, S.; Xian, L.; Zhao, N.; Lai, C.; Yang, W.; Wang, J.; Long, M.; Liao, D.; Ouyang, J.; Wang, Z.; et al. Combined addition of bagasse and zeolite stabilizes potentially toxic elements in sewage sludge compost and improves Eucalyptus urophylla seedling growth. For. Ecol. Manag. 2023, 539, 121003. [Google Scholar] [CrossRef]
- Chu, S.; Wu, D.; Liang, L.L.; Zhong, F.; Hu, Y.; Hu, X.; Lai, C.; Zeng, S. Municipal sewage sludge compost promotes Mangifera persiciforma tree growth with no risk of heavy metal contamination of soil. Sci. Rep. 2017, 7, 13408. [Google Scholar] [CrossRef] [PubMed]
- Urbaniak, M.; Wyrwicka, A.; Toloczko, W.; Serwecinska, L.; Zielinski, M. The effect of sewage sludge application on soil properties and willow (Salix sp.) cultivation. Sci. Total Environ. 2017, 586, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Mansoor, S.; Kour, N.; Manhas, S.; Zahid, S.; Wani, O.A.; Sharma, V.; Wijaya, L.; Alyemeni, M.N.; Alsahli, A.A.; El-Serehy, H.A.; et al. Biochar as a tool for effective management of drought and heavy metal toxicity. Chemosphere 2021, 271, 129458. [Google Scholar] [CrossRef]
- Bogusz, A.; Oleszczuk, P. Sequential extraction of nickel and zinc in sewage sludge- or biochar/sewage sludge-amended soil. Sci. Total Environ. 2018, 636, 927–935. [Google Scholar] [CrossRef]
- Bogusz, A.; Oleszczuk, P. Effect of biochar addition to sewage sludge on cadmium, copper and lead speciation in sewage sludge-amended soil. Chemosphere 2020, 239, 124719. [Google Scholar] [CrossRef] [PubMed]
- Kończak, M.; Oleszczuk, P. Application of biochar to sewage sludge reduces toxicity and improve organisms growth in sewage sludge-amended soil in long term field experiment. Sci. Total Environ. 2018, 625, 8–15. [Google Scholar] [CrossRef]
- Stefaniuk, M.; Tsang DC, W.; Ok, Y.S.; Oleszczuk, P. A field study of bioavailable polycyclic aromatic hydrocarbons (PAHs) in sewage sludge and biochar amended soils. J. Hazard. Mater. 2018, 349, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Khan, Z.; Fan, X.; Khan, M.N.; Khan, M.A.; Zhang, K.; Fu, Y.; Shen, H. The toxicity of heavy metals and plant signaling facilitated by biochar application: Implications for stress mitigation and crop production. Chemosphere 2022, 308, 136466. [Google Scholar] [CrossRef] [PubMed]
- Biederman, L.A.; Harpole, W.S. Biochar and its effects on plant productivity and nutrient cycling: A meta-analysis. GCB Bioenergy 2013, 5, 202–214. [Google Scholar] [CrossRef]
- Jeffery, S.; Verheijen FG, A.; van der Velde, M.; Bastos, A.C. A quantitative review of the effects of biochar application to soils on crop productivity using meta-analysis. Agric. Ecosyst. Environ. 2011, 144, 175–187. [Google Scholar] [CrossRef]
- Spokas, K.A.; Novak, J.M.; Stewart, C.E.; Cantrell, K.B.; Uchimiy, M.; DuSaire, M.G.; Ro, K.S. Qualitative analysis of volatile organic compounds on biochar. Chemosphere 2011, 85, 869–882. [Google Scholar] [CrossRef]
- Qiu, B.; Tao, X.; Wang, H.; Li, W.; Ding, X.; Chu, H. Biochar as a low-cost adsorbent for aqueous heavy metal removal: A review. J. Anal. Appl. Pyrolysis 2021, 155, 105081. [Google Scholar] [CrossRef]
- Cely, P.; Gascó, G.; Paz-Ferreiro, J.; Méndez, A. Agronomic properties of biochars from different manure wastes. J. Anal. Appl. Pyrolysis 2015, 111, 173–182. [Google Scholar] [CrossRef]
- Rigane, H.; Chtourou, M.; Ben Mahmoud, I.; Medhioub, K.; Ammar, E. Polyphenolic compounds progress during olive mill wastewater sludge and poultry manure co-composting, and humic substances building (Southeastern Tunisia). Waste Manag. Res. J. A Sustain. Circ. Econ. 2015, 33, 73–80. [Google Scholar] [CrossRef]
- Kammann, C.I.; Linsel, S.; Gößling, J.W.; Koyro, H. Influence of biochar on drought tolerance of Chenopodium quinoa Willd and on soil–plant relations. Plant Soil 2011, 345, 195–210. [Google Scholar] [CrossRef]
- Rajkovich, S.; Enders, A.; Hanley, K.; Hyland, C.; Zimmerman, A.R.; Lehmann, J. Corn growth and nitrogen nutrition after additions of biochars with varying properties to a temperate soil. Biol. Fertil. Soils 2012, 48, 271–284. [Google Scholar] [CrossRef]
- Gale, N.V.; Sackett, T.E.; Thomas, S.C. Thermal treatment and leaching of biochar alleviates plant growth inhibition from mobile organic compounds. PeerJ 2016, 4, e2385. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, C.; Mai, Z.; Lin, J.; Nie, L.; Maharachchikumbura, S.S.N.; You, C.; Xiang, M.; Hyde, K.D.; Manawasinghe, I.S. Co-infection of Fusarium aglaonematis sp. nov. and Fusarium elaeidis Causing Stem Rot in Aglaonema modestum in China. Front. Microbiol. 2022, 13, 930790. [Google Scholar] [CrossRef] [PubMed]
- Bao, S. Soil and Agro-Chemistry Analysis, 3rd ed.; China Agricultural Press: Beijing, China, 2000. [Google Scholar]
- Qu, F.; Zhang, Q.; Jiang, Z.; Zhang, C.; Zhang, Z.; Hu, X. Optimizing irrigation and fertilization frequency for greenhouse cucumber grown at different air temperatures using a comprehensive evaluation model. Agric. Water Manag. 2022, 273, 107876. [Google Scholar] [CrossRef]
- Omondi, M.O.; Xia, X.; Nahayo, A.; Liu, X.; Korai, P.K.; Pan, G. Quantification of biochar effects on soil hydrological properties using meta-analysis of literature data. Geoderma 2016, 274, 28–34. [Google Scholar] [CrossRef]
- Ahmad, A.; Chowdhary, P.; Khan, N.; Chaurasia, D.; Varjani, S.; Pandey, A.; Chaturvedi, P. Effect of sewage sludge biochar on the soil nutrient, microbial abundance, and plant biomass: A sustainable approach towards mitigation of solid waste. Chemosphere 2022, 287, 132112. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.Z.; Bahar, M.M.; Sarkar, B.; Donne, S.W.; Ok, Y.S.; Palansooriya, K.N.; Kirkham, M.B.; Chowdhury, S.; Bolan, N. Biochar and its importance on nutrient dynamics in soil and plant. Biochar 2020, 2, 379–420. [Google Scholar] [CrossRef]
- Karim, A.A.; Kumar, M.; Singh, E.; Kumar, A.; Kumar, S.; Ray, A.; Dhal, N.K. Enrichment of primary macronutrients in biochar for sustainable agriculture: A review. Crit. Rev. Environ. Sci. Technol. 2022, 52, 1449–1490. [Google Scholar] [CrossRef]
- Blanco-Canqui, H. Biochar and Soil Physical Properties. Soil Sci. Soc. Am. J. 2017, 81, 687–711. [Google Scholar] [CrossRef]
- Singh, H.; Northup, B.K.; Rice, C.W.; Prasad, P.V.V. Biochar applications influence soil physical and chemical properties, microbial diversity, and crop productivity: A meta-analysis. Biochar 2022, 4, 8. [Google Scholar] [CrossRef]
- Haider, F.U.; Wang, X.; Farooq, M.; Hussain, S.; Cheema, S.A.; Ain, N.U.; Virk, A.L.; Ejaz, M.; Janyshova, U.; Liqun, C. Biochar application for the remediation of trace metals in contaminated soils: Implications for stress tolerance and crop production. Ecotoxicol. Environ. Saf. 2022, 230, 113165. [Google Scholar] [CrossRef] [PubMed]
- Qian, S.; Zhou, X.; Fu, Y.; Song, B.; Yan, H.; Chen, Z.; Sun, Q.; Ye, H.; Qin, L.; Lai, C. Biochar-compost as a new option for soil improvement: Application in various problem soils. Sci. Total Environ. 2023, 870, 162024. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, B.A.; Ellis, N.; Kim, C.S.; Bi, X.; Chen, W. Engineered biochars from catalytic microwave pyrolysis for reducing heavy metals phytotoxicity and increasing plant growth. Chemosphere 2021, 271, 129808. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.C.; Gale, N. Biochar and forest restoration: A review and meta-analysis of tree growth responses. New For. 2015, 46, 931–946. [Google Scholar] [CrossRef]
- Zou, Z.; Fan, L.; Li, X.; Dong, C.; Zhang, L.; Zhang, L.; Fu, J.; Han, W.; Yan, P. Response of Plant Root Growth to Biochar Amendment: A Meta-Analysis. Agronomy 2021, 11, 2442. [Google Scholar] [CrossRef]
- Hale, S.E.; Lehmann, J.; Rutherford, D.; Zimmerman, A.R.; Bachmann, R.T.; Shitumbanuma, V.; OToole, A.; Sundqvist, K.L.; Arp HP, H.; Cornelissen, G. Quantifying the Total and Bioavailable Polycyclic Aromatic Hydrocarbons and Dioxins in Biochars. Environ. Sci. Technol. 2012, 46, 2830–2838. [Google Scholar] [CrossRef] [PubMed]
- Dignam, B.E.A.; O’Callaghan, M.; Condron, L.M.; Raaijmakersc, J.M.; Kowalchukd, G.A.; Wakelin, S.A. Impacts of long-term plant residue management on soil organic matter quality, Pseudomonas community structure and disease suppressiveness. Soil Biol. Biochem. 2019, 135, 396–406. [Google Scholar] [CrossRef]
- Huang, X.; Liu, S.; Liu, X.; Zhang, S.; Li, L.; Zhao, H.; Zhao, J.; Zhang, J.; Cai, Z. Plant pathological condition is associated with fungal community succession triggered by root exudates in the plant-soil system. Soil Biol. Biochem. 2020, 151, 108046. [Google Scholar] [CrossRef]
- Hol, W.H.G.; Vestergård, M.; Ten Hooven, F.; Duyts, H.; van de Voorde, T.H.J.; Bezemer, T.M. Transient negative biochar effects on plant growth are strongest after microbial species loss. Soil Biol. Biochem. 2017, 115, 442–451. [Google Scholar] [CrossRef]
- Prendergast Miller, M.T.; Duvall, M.; Sohi, S.P. Biochar-root interactions are mediated by biochar nutrient content and impacts on soil nutrient availability. Eur. J. Soil Sci. 2014, 65, 173–185. [Google Scholar] [CrossRef]
- Rafique, M.; Ortas, I.; Rizwan, M.; Chaudhary, H.J.; Gurmani, A.R.; Munism, U.F.H. Residual effects of biochar and phosphorus on growth and nutrient accumulation by maize (Zea mays L.) amended with microbes in texturally different soils. Chemosphere 2020, 238, 124710. [Google Scholar]
- Huang, L.; Gu, M. Effects of Biochar on Container Substrate Properties and Growth of Plants—A Review. Horticulturae 2019, 5, 14. [Google Scholar] [CrossRef]
- Somerville, P.D.; Farrell, C.; May, P.B.; Livesley, S.J. Biochar and compost equally improve urban soil physical and biological properties and tree growth, with no added benefit in combinatio. Sci. Total Environ. 2020, 706, 135736. [Google Scholar] [CrossRef]
- Yoo, S.Y.; Kim, Y.J.; Yoo, G. Understanding the role of biochar in mitigating soil water stress in simulated urban roadside soil. Sci. Total Environ. 2020, 738, 139798. [Google Scholar] [CrossRef] [PubMed]
- Teodoro, M.; Clemente, R.; Ferrer-Bustins, E.; Martínez-Fernández, D.; Pilar Bernal, M.; Vítková, M.; Vítek, P.; Komárek, M. Nanoscale Zero-Valent Iron Has Minimum Toxicological Risk on the Germination and Early Growth of Two Grass Species with Potential for Phytostabilization. Nanomaterials 2020, 10, 1537. [Google Scholar] [CrossRef]
- Sarmah, M.; Borgohain, A.; Gogoi, B.B.; Yeasin, M.; Paul, R.K.; Malakar, H.; Handique, J.G.; Saikia, J.; Deka, D.; Khare, P.; et al. Insights into the effects of tea pruning litter biochar on major micronutrients (Cu, Mn, and Zn) pathway from soil to tea plant: An environmental armour. J. Hazard. Mater. 2023, 442, 129970. [Google Scholar] [CrossRef]
- Stefaniuk, M.; Oleszczuk, P. Addition of biochar to sewage sludge decreases freely dissolved PAHs content and toxicity of sewage sludge-amended soil. Environ. Pollut. 2016, 218, 242–251. [Google Scholar] [CrossRef]
- Mukherjee, A.; Zimmerman, A.R.; Hamdan, R.; Cooper, W.T. Physicochemical changes in pyrogenic organic matter (biochar) after 15 months of field aging. Solid Earth 2014, 5, 693–704. [Google Scholar] [CrossRef]
- Oram, N.J.; van de Voorde, T.F.J.; Ouwehand, G.; Bezemer, T.M.; Mommer, L.; Jeffery, S.; Groenigen, J.W.V. Soil amendment with biochar increases the competitive ability of legumes via increased potassium availability. Agric. Ecosyst. Environ. 2014, 191, 92–98. [Google Scholar] [CrossRef]
- Gaskin, J.W.; Speir, R.A.; Harris, K.; Das, K.C.; Lee, R.D.; Morris, L.A.; Fisher, D.S. Effect of Peanut Hull and Pine Chip Biochar on Soil Nutrients, Corn Nutrient Status, and Yield. Agron. J. 2010, 102, 623–633. [Google Scholar] [CrossRef]
- Major, J.; Lehmann, J.; Rondon, M.; Goodale, C. Fate of soil-applied black carbon: Downward migration, leaching and soil respiration. Glob. Change Biol. 2010, 16, 1366–1379. [Google Scholar] [CrossRef]
- Torri, S.I.; Lavado, R.S. Dynamics of Cd, Cu and Pb added to soil through different kinds of sewage sludge. Waste Manag. 2008, 28, 821–832. [Google Scholar] [CrossRef] [PubMed]




| Treatment | Ci | Ranking Number | ||
|---|---|---|---|---|
| CK | 43.13 | 66.80 | 0.61 | 2 |
| SB1.5 | 74.42 | 22.41 | 0.23 | 7 |
| SB3.0 | 64.99 | 35.39 | 0.35 | 4 |
| SB4.5 | 67.88 | 28.46 | 0.30 | 6 |
| LB1.5 | 85.21 | 8.80 | 0.09 | 10 |
| LB3.0 | 66.98 | 35.65 | 0.35 | 5 |
| LB4.5 | 33.31 | 71.41 | 0.68 | 1 |
| RB1.5 | 80.00 | 16.41 | 0.17 | 8 |
| RB3.0 | 49.46 | 41.63 | 0.46 | 3 |
| RB4.5 | 85.31 | 9.96 | 0.10 | 9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chu, S.; Xiao, M.; Peng, W.; Long, F.; Wu, D.; Hu, D.; Zeng, S. Biochar Application Improved Sludge-Amended Landscape Soil Fertility Index but with No Added Benefit in Plant Growth. Forests 2024, 15, 1128. https://doi.org/10.3390/f15071128
Chu S, Xiao M, Peng W, Long F, Wu D, Hu D, Zeng S. Biochar Application Improved Sludge-Amended Landscape Soil Fertility Index but with No Added Benefit in Plant Growth. Forests. 2024; 15(7):1128. https://doi.org/10.3390/f15071128
Chicago/Turabian StyleChu, Shuangshuang, Mengrui Xiao, Weixin Peng, Fengling Long, Daoming Wu, Dongnan Hu, and Shucai Zeng. 2024. "Biochar Application Improved Sludge-Amended Landscape Soil Fertility Index but with No Added Benefit in Plant Growth" Forests 15, no. 7: 1128. https://doi.org/10.3390/f15071128




