The Effects of Wild Boar Rooting on Epigeic Arthropods in Oak Forests
Abstract
:1. Introduction
2. Materials and Methods
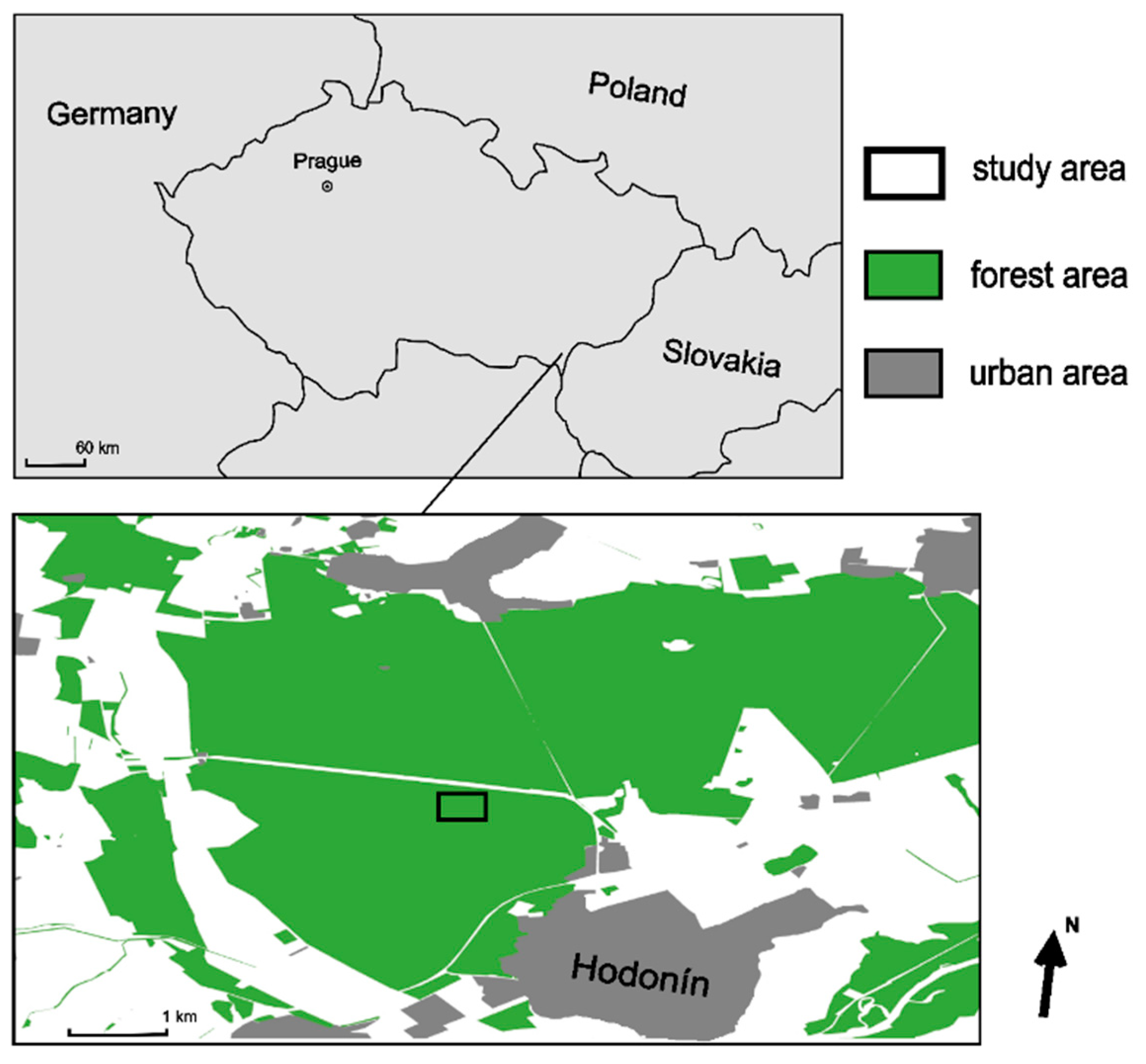
2.1. Study Sites
2.2. Pitfall Traps
2.3. Data Analysis Statistical Approach for Epigeic Community Assemblages
2.4. Evaluation of Carabid Assemblage
3. Results
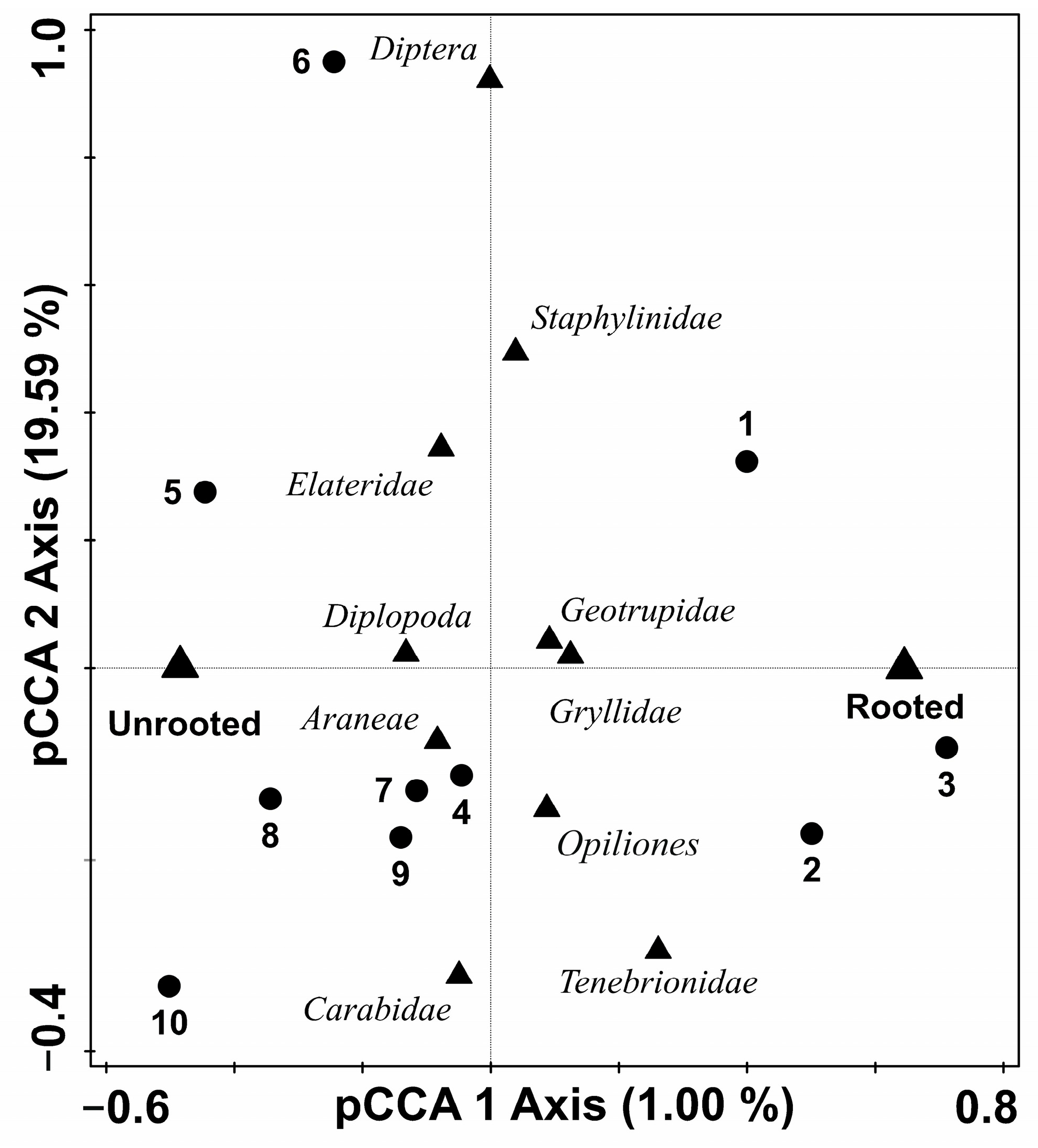
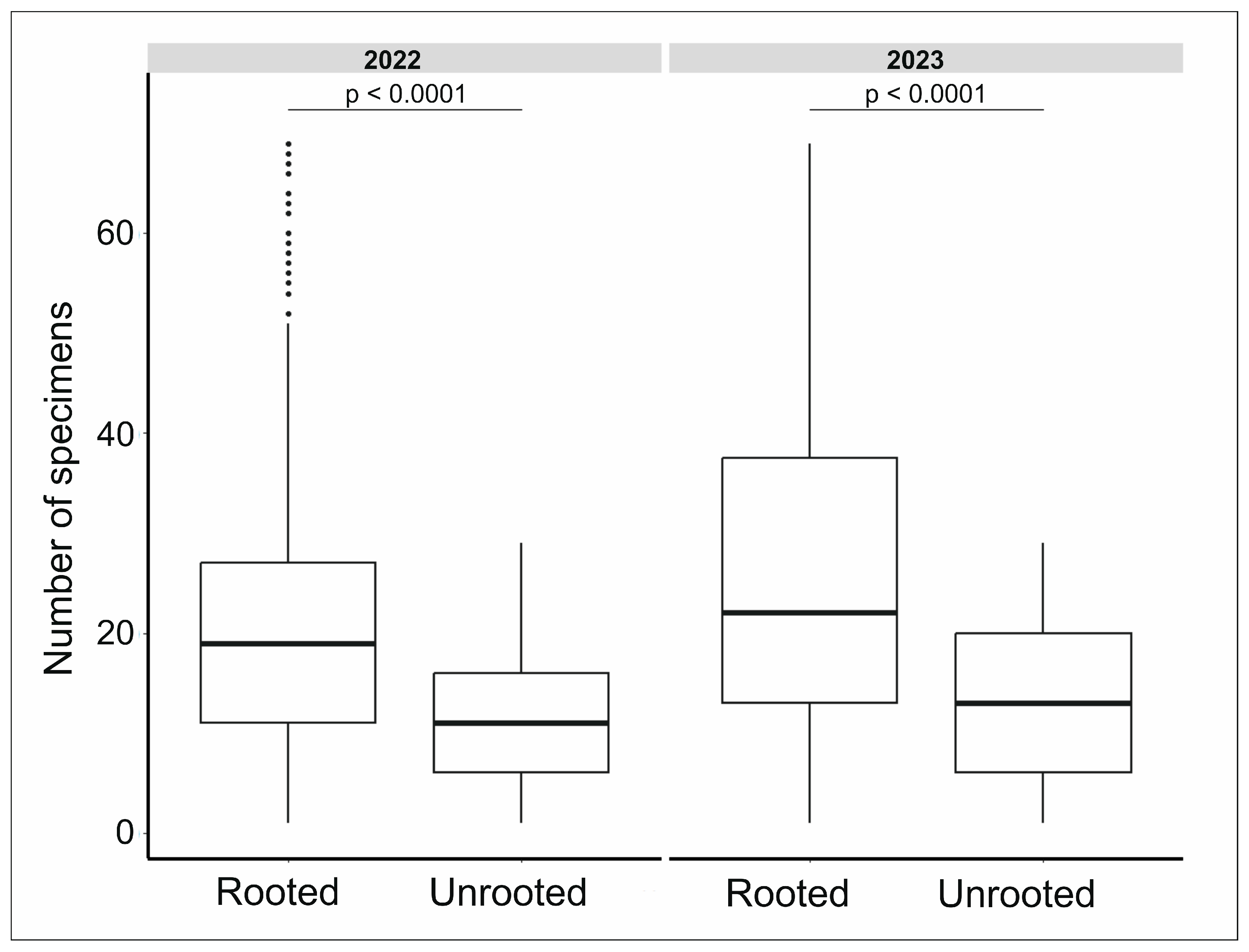
3.1. Wild Boar Disturbance Shapes Epigeic Assemblages
3.2. General Regeneration Path
3.3. Effect of Rooting on Carabid Assemblages
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Apollonio, M.; Andersen, R.; Putman, R. European Ungulates and Their Management in the 21st Century; Cambridge University Press: Cambridge, UK, 2010; 604p. [Google Scholar]
- Long, J.L. Introduced Mammals of the World: Their History, Distribution and Abundance. J. Mammal. 2003, 85, 363. [Google Scholar] [CrossRef]
- Barrios-Garcia, M.N.; Ballari, S.A. Impact of wild boar (Sus scrofa) in its introduced and native range: A review. Biol. Invasions 2012, 14, 2283–2300. [Google Scholar] [CrossRef]
- Anderson, A.; Slootmaker, C.; Harper, E.; Holderieath, J.; Shwiff, S.A. Economic estimates of feral swine damage and control in 11 US states. Crop. Prot. 2016, 89, 89–94. [Google Scholar] [CrossRef]
- Fernández-Llario, P.; Mateos-Quesada, P.; Rodríguez-Pastor, R. Factors influencing the increase of wild boar damage to crops in an agricultural landscape. Agric. Ecosyst. Environ. 2019, 270, 67–74. [Google Scholar] [CrossRef]
- Skoták, V.; Drimaj, J.; Kamler, J. Evaluation of Damage to Forest Tree Plantations by Wild Boar in the Czech Republic. Hum. Wildl. Interact. 2021, 15, 13. [Google Scholar] [CrossRef]
- Drimaj, J.; Skoták, V.; Kamler, J.; Plhal, R.; Adamec, Z.; Mikulka, O.; Janata, P. Comparison of Methods for Estimating Damage by Wild Ungulates on Field Crops. Agriculture 2023, 13, 1184. [Google Scholar] [CrossRef]
- Fern, M.P.; Armstrong, J.B.; Barlow, R.J.; Kush, J.S. Ecological factors influencing wild pig damage to planted pine and hardwood seedlings. Hum. Wildl. Interact. 2020, 14, 228–238. [Google Scholar] [CrossRef]
- Burrascano, S.; Copiz, R.; Del Vico, E.; Fagiani, S.; Giarrizzo, E.; Mei, M.; Mortellilti, A.; Sabatini, F.M.; Blasi, C. Wild boar rooting intensity determines shifts in understorey composition and functional traits. Community Ecol. 2015, 16, 244–253. [Google Scholar] [CrossRef]
- Barrios-Garcia, M.N.; Simberloff, D. Linking the pattern to the mechanism: How an introduced mammal facilitates plant invasions. Austral Ecol. 2013, 38, 884–890. [Google Scholar] [CrossRef]
- de Schaetzen, F.; van Langevelde, F.; WallisDeVries, M.F. The influence of wild boar (Sus scrofa) on microhabitat quality for the endangered butterfly Pyrgus malvae in the Netherlands. J. Insect Conserv. 2018, 22, 51–59. [Google Scholar] [CrossRef]
- Cuevas, M.F.; Novillo, A.; Campos, C.; Dacar, M.A.; Ojeda, R.A. Food habits and impact of rooting behaviour of the invasive wild boar, Sus scrofa, in a protected area of the Monte Desert, Argentina. J. Arid Environ. 2010, 74, 1582–1585. [Google Scholar] [CrossRef]
- Kenyeres, Z.; Szabo, S.; Bauer, N. Conservation possibilities of the rare grasshopper Stenobothrus eurasius Zubovski, 1898 are hampered by wild game in its fragmented western outposts. J. Insect Conserv. 2020, 24, 115–124. [Google Scholar] [CrossRef]
- Herrero, J.; García-Serrano, A.; Couto, S.; Ortuño, V.M.; García-González, R. Diet of wild boar Sus scrofa L. and crop damage in an intensive agroecosystem. Eur. J. Wildl. Res. 2006, 52, 245–250. [Google Scholar] [CrossRef]
- Labadessa, R.; Ancillotto, L. Beauty and the beast: Multiple effects of wild boar rooting on butterfly microhabitat. Biodivers. Conserv. 2023, 32, 1189–1204. [Google Scholar] [CrossRef]
- Cabon, V.; Bùi, M.; Kühne, H.; Seitz, B.; Kowarik, I.; von der Lippe, M.; Buchholz, S. Endangered animals and plants are positively or neutrally related to wild boar (Sus scrofa) soil disturbance in urban grasslands. Sci. Rep. 2022, 12, 16649. [Google Scholar] [CrossRef] [PubMed]
- Ghannem, S.; Bejaoui, M.; Gahdab, C.; Boumaiza, M. Biodiversity of Ground Beetles (Coleoptera: Carabidae) from Northern Tunisia. J. Kansas Entomol. Soc. 2017, 90, 31–43. [Google Scholar] [CrossRef]
- Rainio, J.; Niemelä, J. Ground beetles (Coleoptera: Carabidae) as bioindicators. Biodivers. Conserv. 2003, 12, 487–506. [Google Scholar] [CrossRef]
- Kassambara, A. R Package, Version 0.6.0; ggpubr: ‘ggplot2’ Based Publication Ready Plots. 2023. Available online: https://rpkgs.datanovia.com/ggpubr/ (accessed on 1 May 2024).
- Koivula, M.J. Useful model organisms, indicators, or both? Ground beetles (Coleoptera, Carabidae) reflecting environmental conditions. ZooKeys 2011, 100, 287–317. [Google Scholar] [CrossRef]
- Kosewska, A.; Kędzior, R.; Nietupski, M.; Borkowski, J. Epigeic Carabids (Coleoptera, Carabidae) as Bioindicators in Different Variants of Scots Pine Regeneration: Implication for Forest Landscape Management. Sustainability 2023, 15, 13322. [Google Scholar] [CrossRef]
- Fagiani, S.; Fipaldini, D.; Santarelli, L.; Burrascano, S.; Del Vico, E.; Giarrizzo, E.; Mei, M.; Vigna Taglianti, A.; Boitani, L.; Mortelliti, A. Monitoring protocols for the evaluation of the impact of wild boar (Sus scrofa) rooting on plants and animals in forest ecosystems. Hystrix 2014, 25, 31–38. [Google Scholar] [CrossRef]
- Marshall, J.C.; Blessing, J.; Clifford, S.; Negus, P.; Steward, A. Epigeic invertebrates of pig-damaged, exposed wetland sediments are rooted: An ecological response to feral pigs (Sus scrofa). Aquat. Conserv. 2020, 30, 2207–2220. [Google Scholar] [CrossRef]
- AOPK. Hodonínská Dúbrava. 2024. Available online: https://drusop.nature.cz/ost/chrobjekty/zchru/index.php?SHOW_ONE=1&ID=14276 (accessed on 20 April 2024).
- ČHMÚ. 2024. Available online: https://www.chmi.cz/historicka-data/pocasi/mesicni-data/mesicni-data-dle-z.-123-1998-Sb (accessed on 20 April 2024).
- Kotze, D.J.; Brandmayr, P.; Casale, A.; Dauffy-Richard, E.; Dekoninck, W.; Koivula, M.J.; Lövei, G.L.; Mossakowski, D.; Noordijk, J.; Paarmann, W.; et al. Forty years of carabid beetle research in Europe—From taxonomy, biology, ecology and population studies to bioindication, habitat assessment and conservation. ZooKeys 2011, 100, 55–148. [Google Scholar] [CrossRef] [PubMed]
- Digweed, S.C.; Currie, C.R.; Carcamo, H.A.; Spence, J.R. Dig-ging out the ‘digging-in effect’ of pitfall traps: Influences deple-tion and disturbance on catches of ground beetles (Coleoptera: Carabidae). Pedobiologia 1995, 39, 561–576. [Google Scholar] [CrossRef]
- Jiménez-CarmonaSoledad, F.; Carpintero, S.; Reyes-Lópezm, J.C. The digging-in effect on ant studies with pitfall traps: Influence of type of habitat and sampling time. Entomol. Exp. Appl. 2019, 167, 906–914. [Google Scholar] [CrossRef]
- Ter Braak, C.; Šmilauer, P. Canoco Reference Manual and User’s Guide: Software of Ordination (Version 5.0); Microcomputer Power: Ithaca, NY, USA, 2012; 496p. [Google Scholar]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2009; 213p. [Google Scholar]
- Chao, A.; Jost, L. Coverage-based rarefaction and extrapolation: Standardizing samples by completeness rather than size. Ecology 2012, 93, 2533–2547. [Google Scholar] [CrossRef] [PubMed]
- Bürkner, P. Bayesian Item Response Modeling in R with brms and Stan. J. Stat. Softw. 2021, 100, 1–54. [Google Scholar] [CrossRef]
- Soliveres, S.; Van Der Plas, F.; Manning, P.; Prati, D.; Gossner, M.M.; Renner, S.C.; Alt, F.; Arndt, H.; Baumgartner, V.; Binkenstein, J.; et al. Biodiversity at multiple trophic levels is needed for ecosystem multifunctionality. Nature 2016, 536, 456–459. [Google Scholar] [CrossRef] [PubMed]
- Sommaggio, D.; Peretti, E.; Burgio, G. The effect of cover plants management on soil invertebrate fauna in vineyard in Northern Italy. BioControl 2018, 63, 795–806. [Google Scholar] [CrossRef]
- Cruz-Miralles, J.; Guzzo, M.; Ibáñez-Gual, M.V.; Dembilio, Ó.; Jaques, J.A. Ground-covers affect the activity density of ground-dwelling predators and their impact on the Mediterranean fruit fly, Ceratitis capitata. BioControl 2022, 67, 583–592. [Google Scholar] [CrossRef]
- Barrios-Garcia, M.N.; Gonzalez-Polo, M.; Simberloff, D.; Classen, A.T. Wild boar rooting impacts soil function differently in different plant community types. Biol. Invasions 2023, 25, 583–592. [Google Scholar] [CrossRef]
- Hamřík, T.; Košulič, O. Impact of small-scale conservation management methods on spider assemblages in xeric grassland. Agric. Ecosyst. Environ. 2021, 307, 107225. [Google Scholar] [CrossRef]
- Kittawornrat, A.; Zimmerman, J.J. Toward a better understanding of pig behavior and pig welfare. Anim. Health Res. Rev. 2011, 12, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Mori, E.; Ferretti, F.; Lagrotteria, A.; La Greca, L.; Solano, E.; Fattorini, N. Impact of wild boar rooting on small forest-dwelling rodents. Ecol. Res. 2020, 35, 675–681. [Google Scholar] [CrossRef]
- Calosi, M.; Gabbrielli, C.; Lazzeri, L.; Fattorini, N.; Cesaretti, G.; Burrini, L.; Petrillo, O.; Ferretti, F. Seasonal and Ecological Determinants of Wild Boar Rooting on Priority Protected Grasslands. Environ. Manag. 2024. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.; Gallandt, E.R. Behavioural Studies of Harpalus rufipes De Geer: An Important Weed Seed Predator in Northeastern US Agroecosystems. Int. J. Ecol. 2012, 2012, 1–6. [Google Scholar] [CrossRef]
- Baur, B.; Gilgado, J.D.; Coray, A. Prey handling and feeding habits of the snail predator Licinus depressus (Coleoptera, Carabidae). Alp. Entomol. 2023, 7, 63–68. [Google Scholar] [CrossRef]
- Hejda, R.; Farkač, J.; Chobot, K. Red List of Threatened Species of Czech Republic: Invertebrates; Nature Conservation Agency of the Czech Republic: Prague, Czech Republic, 2017; 760p. [Google Scholar]
- Wilman, H.; Belmaker, J.; Simpson, J.; Rosa, C.; Rivadeneira, M.; Jetz, W. EltonTraits 1.0: Species-level foraging attributes of the world’s birds and mammals. Ecology 2014, 95, 2027. [Google Scholar] [CrossRef]
- Massei, G.; Kindberg, J.; Licoppe, A.; Gačić, D.; Šprem, N.; Kamler, J.; Baubet, E.; Hohmann, U.; Monaco, A.; Ozoliņš, J.; et al. Wild boar populations up, numbers of hunters down? A review of trends and implications for Europe. Pest Manag. Sci. 2015, 71, 492–500. [Google Scholar] [CrossRef]
- Drimaj, J.; Kamler, J.; Plhal, R.; Janata, P.; Adamec, Z.; Homolka, M. Intensive Hunting Pressure Changes Local Distribution of Wild Boar. Hum. Wildl. Interact. 2021, 15, 9. [Google Scholar] [CrossRef]


| 2022 | 2023 | |||
|---|---|---|---|---|
| Taxon | Rooted | Unrooted | Rooted | Unrooted |
| Araneae | 24 ± 15 | 11 ± 7 | 26 ± 16 | 13 ± 8 |
| Carabidae | 30 ± 17 | 14 ± 8 | 32 ± 17 | 16 ± 9 |
| Diplopoda | 20 ± 18 | 11 ± 7 | 23 ± 18 | 14 ± 9 |
| Diptera | 20 ± 23 | 12 ± 5 | 22 ± 17 | 15 ± 9 |
| Elateridae | 29 ± 10 | 14 ± 10 | 26 ± 14 | 13 ± 8 |
| Geotrupidae | 20 ± 15 | 11 ± 7 | 27 ± 17 | 13 ± 8 |
| Gryllidae | 21 ± 15 | 13 ± 7 | 25 ± 16 | 15 ± 9 |
| Opiliones | 20 ± 16 | 13 ± 7 | 24 ± 18 | 13 ± 7 |
| Staphylinidae | 18 ± 13 | 9 ± 6 | 23 ± 14 | 10 ± 7 |
| Tenebrionidae | 34 ± 22 | 10 ± 8 | 31 ± 23 | 11 ± 8 |
| Average | 22 ± 16 | 12 ± 7 | 26 ± 17 | 14 ± 8 |
| Model Parameters | Explained Variation (%) | Pseudo-F | p | p (adj) |
|---|---|---|---|---|
| Unrooted areas | 1.0 | 4.6 | 0.046 | 0.046 |
| Rooted areas | 1.0 | 4.6 | 0.044 | 0.046 |
| Taxon | N | Factor of Variation | SS | df | MS | F | p-Value |
|---|---|---|---|---|---|---|---|
| Araneae | 7441 | Rooting | 7.85 | 1 | 7.853 | 84.445 | 0.01 |
| Year | 0.21 | 1 | 0.206 | 2.217 | 0.137 | ||
| Rooting:Year | 0.05 | 1 | 0.05 | 0.542 | 0.462 | ||
| Residuals | 34.78 | 374 | 0.093 | ||||
| Carabidae | 5435 | Rooting | 5.774 | 1 | 5.774 | 52.902 | 0.01 |
| Year | 0.083 | 1 | 0.083 | 0.764 | 0.383 | ||
| Rooting:Year | 0.016 | 1 | 0.016 | 0.145 | 0.703 | ||
| Residuals | 24.341 | 223 | 0.109 | ||||
| Diplopoda | 4484 | Rooting | 2.36 | 1 | 2.364 | 11.922 | 0.01 |
| Year | 0.19 | 1 | 0.187 | 0.943 | 0.332 | ||
| Rooting:Year | 0.05 | 1 | 0.046 | 0.231 | 0.631 | ||
| Residuals | 46.8 | 236 | 0.198 | ||||
| Diptera | 1330 | Rooting | 0.464 | 1 | 0.464 | 2.938 | 0.09 |
| Year | 0.058 | 1 | 0.058 | 0.367 | 0.5469 | ||
| Rooting:Year | 0.004 | 1 | 0.004 | 0.023 | 0.8795 | ||
| Residuals | 10.736 | 68 | 0.158 | ||||
| Elateridae | 1938 | Rooting | 1.44 | 1 | 1.44 | 8.94 | 0.01 |
| Year | 0.099 | 1 | 0.099 | 0.613 | 0.436 | ||
| Rooting:Year | 0.027 | 1 | 0.027 | 0.169 | 0.682 | ||
| Residuals | 14.494 | 90 | 0.161 | ||||
| Geotrupidae | 6874 | Rooting | 6.3 | 1 | 6.3 | 33.853 | 0.01 |
| Year | 1.17 | 1 | 1.17 | 6.286 | 0.01 | ||
| Rooting:Year | 0.22 | 1 | 0.222 | 1.194 | 0.275 | ||
| Residuals | 69.6 | 374 | 0.186 | ||||
| Gryllidae | 4718 | Rooting | 3.182 | 1 | 3.182 | 30.337 | 0.01 |
| Year | 0.1 | 1 | 0.1 | 0.95 | 0.331 | ||
| Rooting:Year | 0.092 | 1 | 0.092 | 0.882 | 0.349 | ||
| Residuals | 25.805 | 246 | 0.105 | ||||
| Opiliones | 4823 | Rooting | 1.29 | 1 | 1.28 | 8.223 | 0.01 |
| Year | 0.1 | 1 | 0.166 | 1.064 | 0.303 | ||
| Rooting:Year | 0.23 | 1 | 0.229 | 1.46 | 0.228 | ||
| Residuals | 41.96 | 268 | 0.157 | ||||
| Staphylinidae | 1722 | Rooting | 4.058 | 1 | 4.058 | 25.246 | 0.01 |
| Year | 0.136 | 1 | 0.136 | 0.844 | 0.36 | ||
| Rooting:Year | 0.05 | 1 | 0.05 | 0.313 | 0.577 | ||
| Residuals | 17.84 | 111 | 0.161 | ||||
| Tenebrionidae | 797 | Rooting | 2.34 | 1 | 2.4 | 15.538 | 0.01 |
| Year | 0.008 | 1 | 0.008 | 0.053 | 0.818 | ||
| Rooting:Year | 0.069 | 1 | 0.069 | 0.445 | 0.508 | ||
| Residuals | 6.64 | 43 | 0.154 |
| Species | Rooted | Unrooted | Sum |
|---|---|---|---|
| Badister lacertosus (Sturm, 1815) | 1 | 1 | |
| Calathus fuscipes (Goeze, 1777) | 2 | 2 | |
| Carabus granulatus (Linnaeus, 1758) | 1 | 1 | |
| Carabus hortensis (Linnaeus, 1758) | 6 | 5 | 11 |
| Carabus violaceus (Linnaeus, 1758) | 1 | 7 | 8 |
| Harpalus rubripes (Duftschmid, 1812) | 1 | 1 | |
| Harpalus rufipes (DeGeer, 1774) | 3853 | 1582 | 5435 |
| Leistus ferrugineus (Linnaeus, 1758) | 1 | 1 | |
| Licinus depressus (Paykull, 1790) | 1 | 1 | |
| Nebria brevicolis (Fabricius, 1792) | 2 | 2 | 4 |
| Poecilus cupreus (Linnaeus, 1758) | 1 | 1 | |
| Pterostichus niger (Schaller, 1783) | 5 | 10 | 15 |
| Pterostichus oblongopunctatus (Fabricius, 1789) | 1 | 1 | |
| TOTAL | 3873 | 1610 | 5482 |
| Population-Level Effects: | |||||
|---|---|---|---|---|---|
| Estimate | Estimated Error | l-95% CI | u-95% CI | Rhat | |
| Intercept | 1.08 | 0.18 | 0.74 | 1.50 | 1.01 |
| Rooting | 0.67 | 0.20 | 0.28 | 1.05 | 1.00 |
| Body | −0.11 | 0.21 | −0.51 | 0.29 | 1.00 |
| Rooting:Large body | −0.57 | 0.28 | −1.11 | −0.03 | 1.00 |
| Group-level effects: | |||||
| sd (Intercept) | 0.40 | 0.05 | 0.32 | 0.52 | 1.00 |
| Month | 0.21 | 0.20 | 0.01 | 0.77 | 1.00 |
| Trap | 0.07 | 0.06 | 0.00 | 0.21 | 1.00 |
| Population-Level Effects: | |||||
|---|---|---|---|---|---|
| Estimate | Estimated Error | l-95% CI | u-95% CI | Rhat | |
| Intercept | 1.08 | 0.19 | 0.69 | 1.46 | 1.00 |
| Rooting | 0.24 | 0.19 | −0.13 | 0.63 | 1.00 |
| Habitat preferences | −0.12 | 0.25 | −0.62 | 0.36 | 1.00 |
| Rooting:Habitat preferences | 0.15 | 0.40 | −0.61 | 0.95 | 1.00 |
| Group-level effects: | |||||
| sd (Intercept) | 0.46 | 0.07 | 0.34 | 0.61 | 1.00 |
| Month | 0.20 | 0.21 | 0.01 | 0.80 | 1.00 |
| Trap | 0.15 | 0.10 | 0.01 | 0.39 | 1.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Špoula, J.; Stočes, D.; Drimaj, J.; Mikulka, O. The Effects of Wild Boar Rooting on Epigeic Arthropods in Oak Forests. Forests 2024, 15, 1169. https://doi.org/10.3390/f15071169
Špoula J, Stočes D, Drimaj J, Mikulka O. The Effects of Wild Boar Rooting on Epigeic Arthropods in Oak Forests. Forests. 2024; 15(7):1169. https://doi.org/10.3390/f15071169
Chicago/Turabian StyleŠpoula, Jakub, Dominik Stočes, Jakub Drimaj, and Ondřej Mikulka. 2024. "The Effects of Wild Boar Rooting on Epigeic Arthropods in Oak Forests" Forests 15, no. 7: 1169. https://doi.org/10.3390/f15071169
APA StyleŠpoula, J., Stočes, D., Drimaj, J., & Mikulka, O. (2024). The Effects of Wild Boar Rooting on Epigeic Arthropods in Oak Forests. Forests, 15(7), 1169. https://doi.org/10.3390/f15071169







