Stoichiometric Homeostasis of N and P in the Leaves of Different-Aged Phyllostachys edulis after Bamboo Forest Expansion in Subtropical China
Abstract
:1. Introduction
2. Materials and Methods
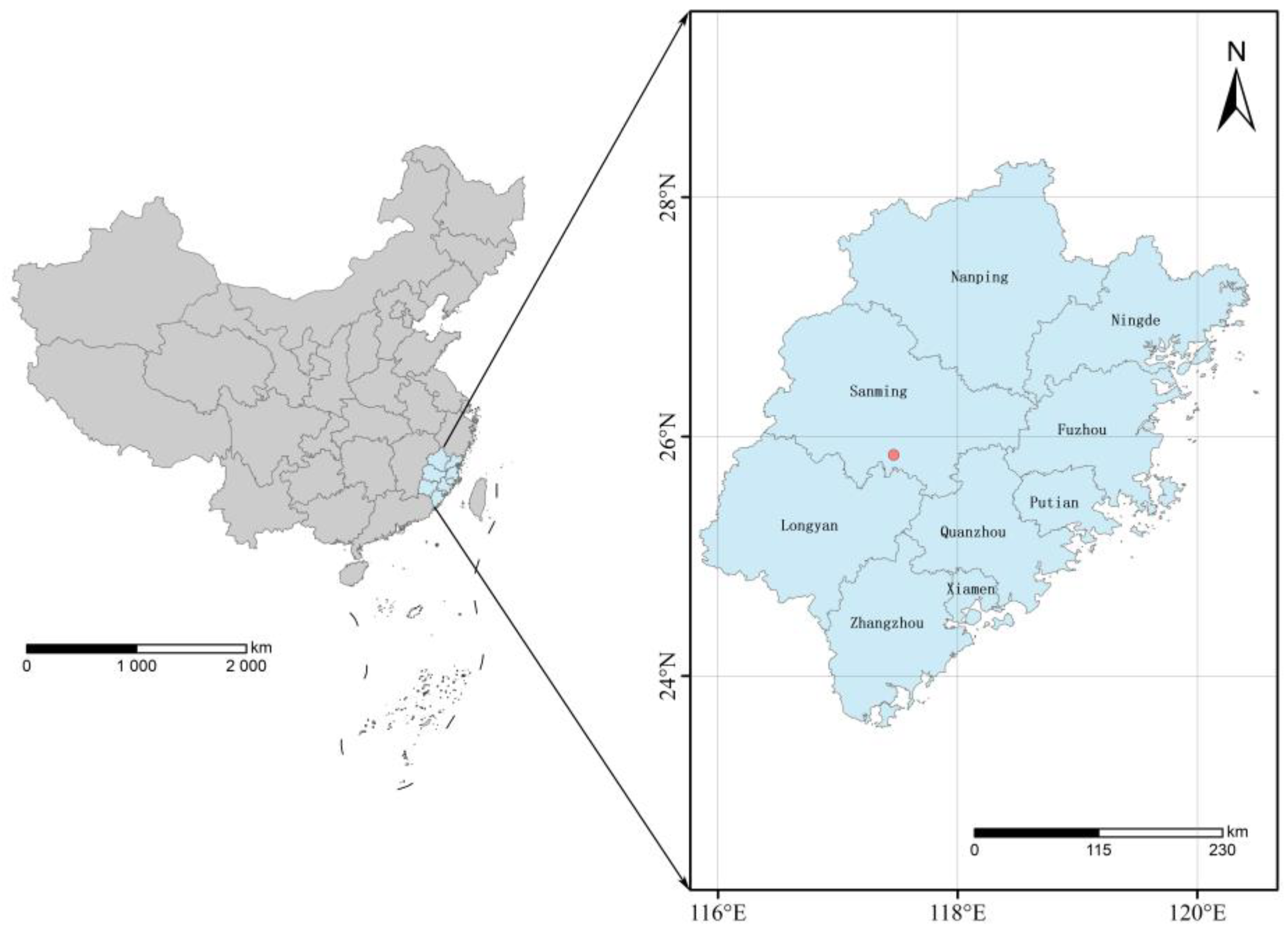
2.1. Site Description
2.2. Experimental Design
2.3. Plant Sampling and Analyses
2.4. Calculation Methods
2.5. Statistical Analysis
3. Results
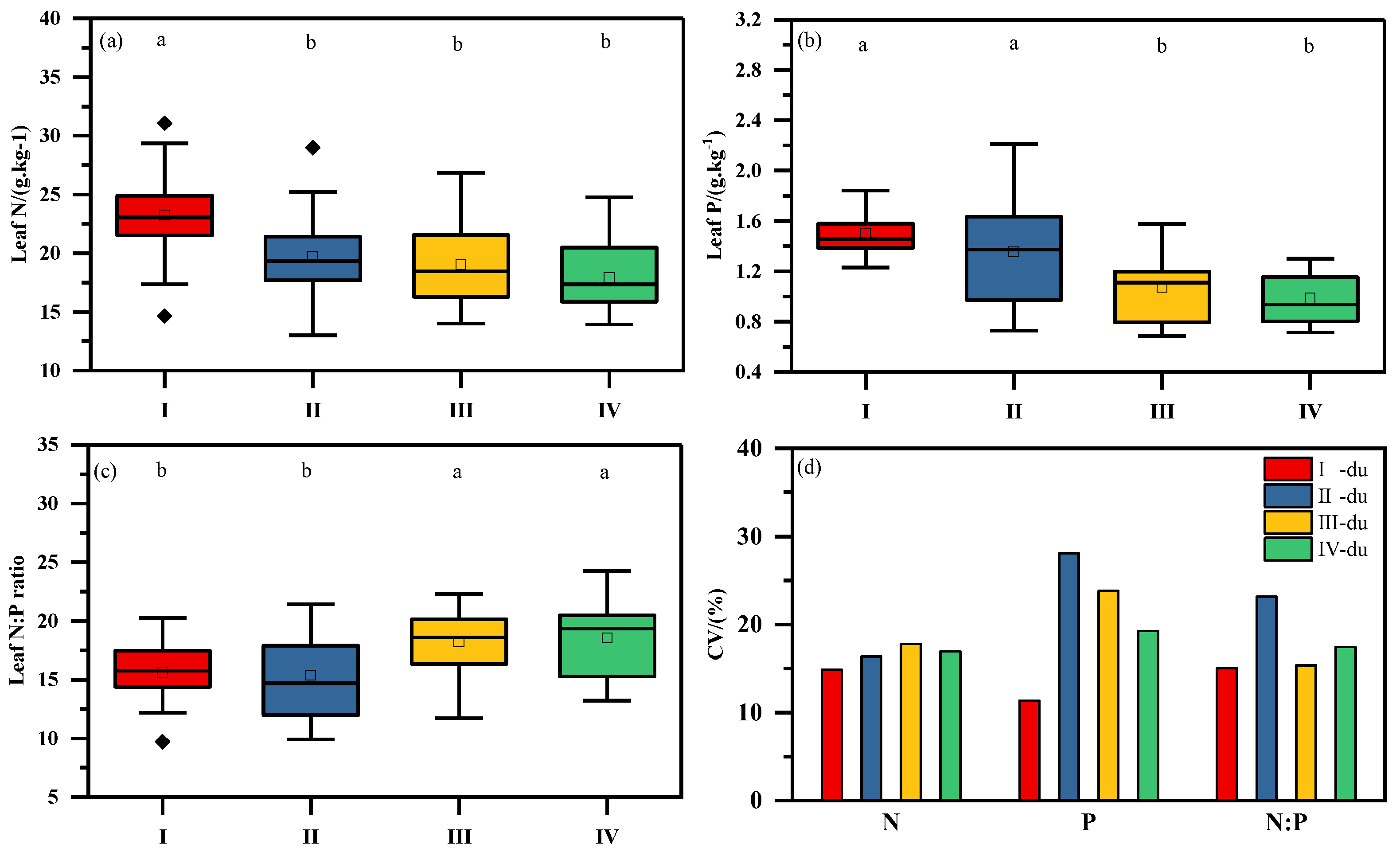
3.1. Stoichiometric Characteristics of N and P in Leaves of Moso Bamboo at Different Ages
3.2. Stoichiometric Characteristics of Soil C, N, and P in Phyllostachys pubescens Forests of Different Ages
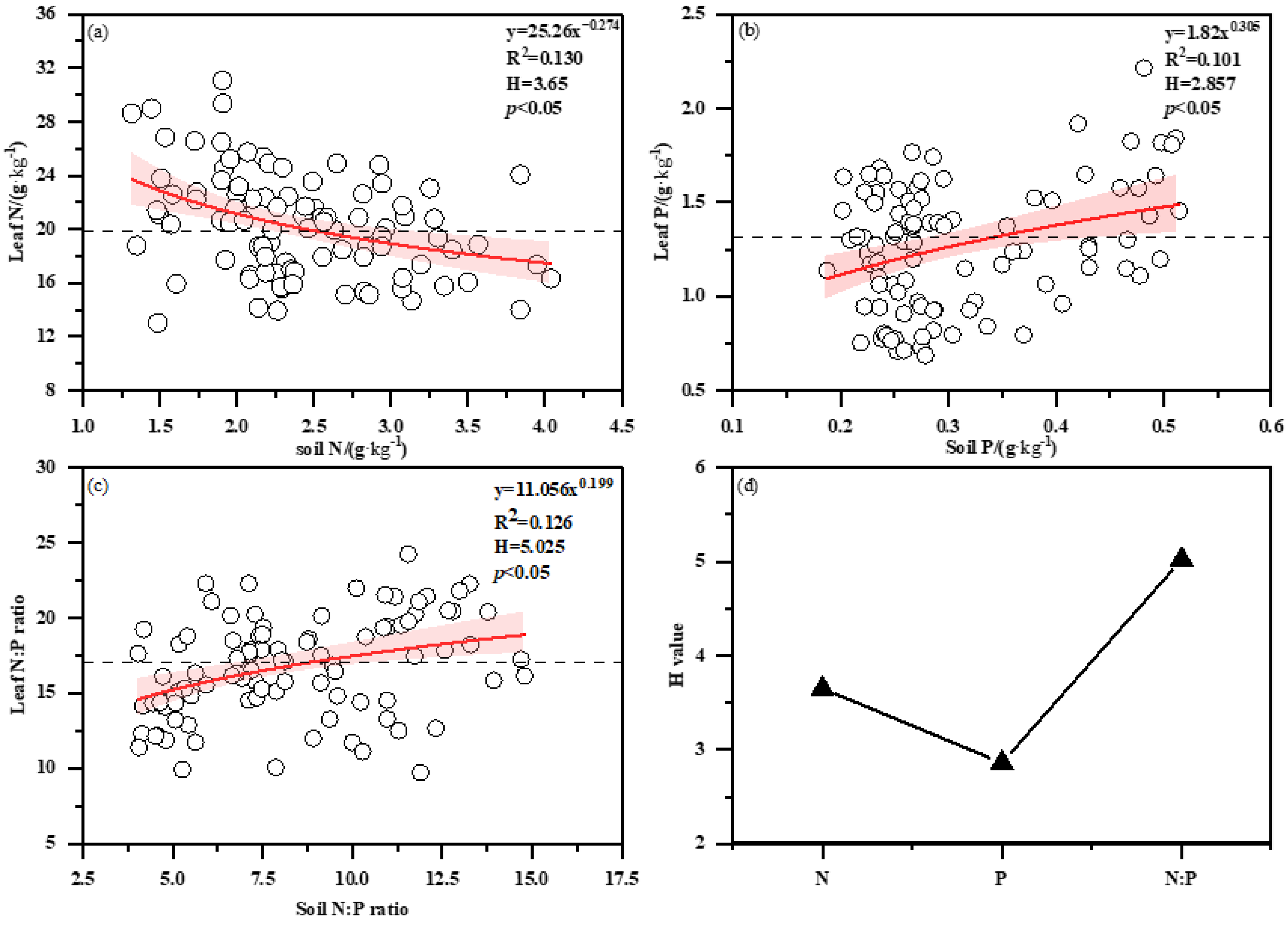
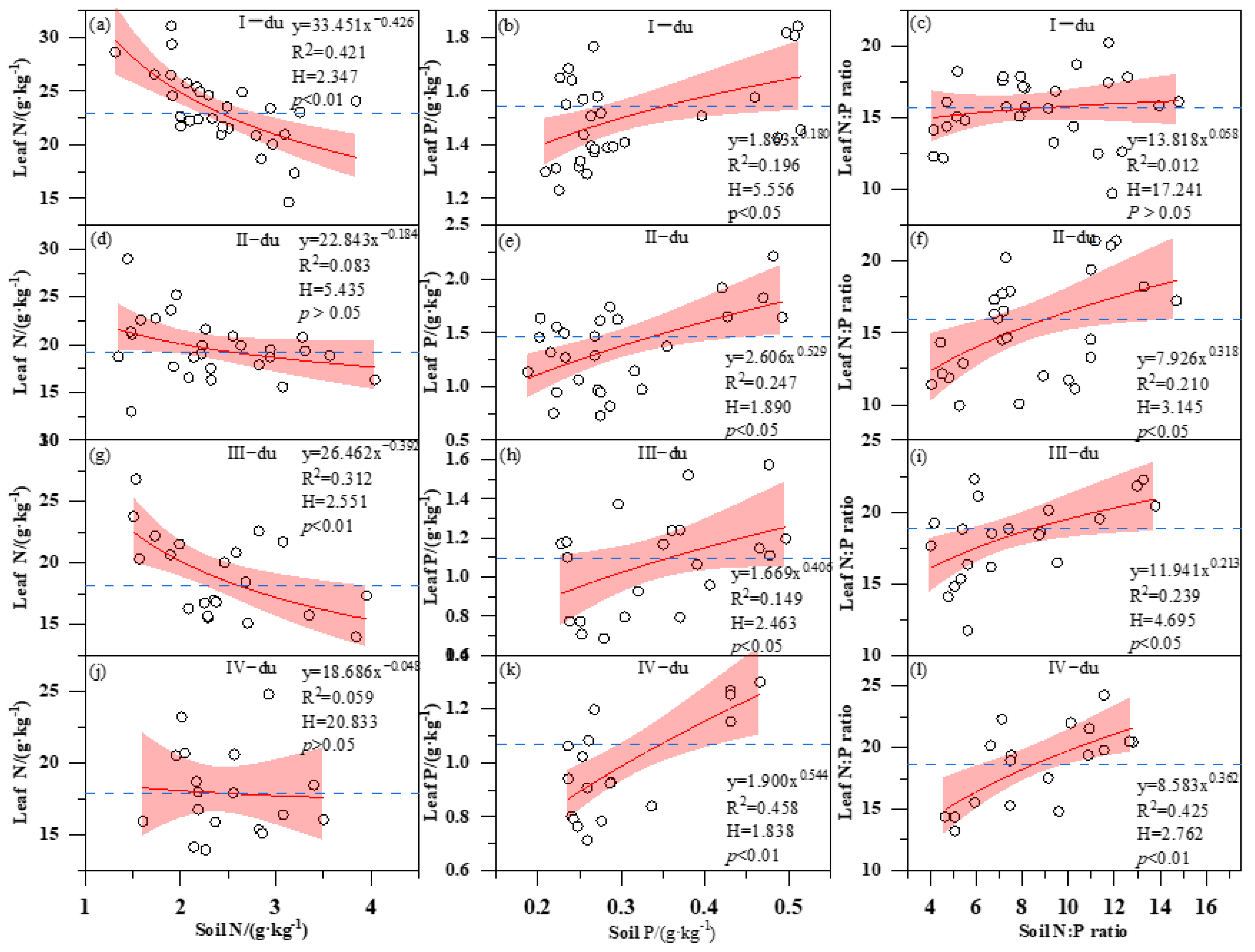
3.3. Correlation Analysis of Soil and Bamboo Plant Nutrients
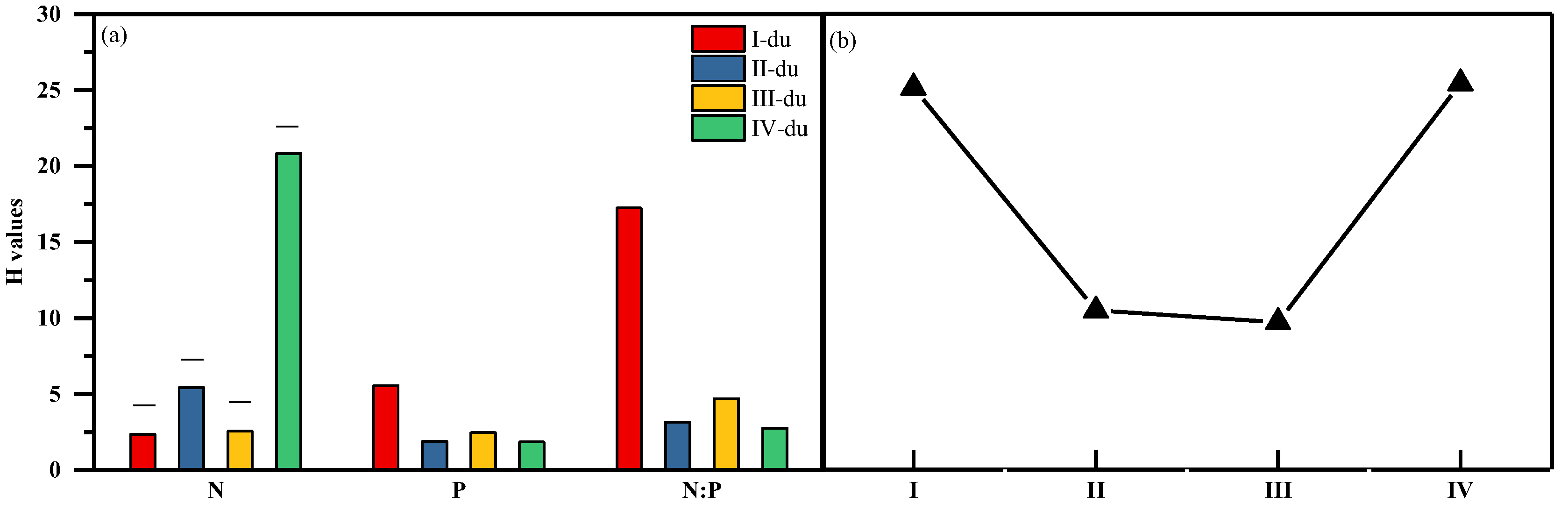
3.4. Variation of H with Bamboo Age
4. Discussion
4.1. Identification of N and P Ecological Stoichiometric Limiting Elements in the Leaves of Bamboo with Different Ages and Ecological Adaptation Strategies
4.2. Stoichiometric Homeostasis of Moso Bamboo Leaves
5. Conclusions
- (1)
- Under the condition of the same soil nutrients (N, P), the content of N and P in bamboo leaves decreased significantly with the increase in bamboo age, indicating that I-du bamboo accumulated more nutrients for rapid growth and development.
- (2)
- Under the condition of a lack of nitrogen and phosphorus in soil, the limiting effect of phosphorus on the growth and development of moso bamboo was greater than that of nitrogen, and the limiting effect of phosphorus on aged bamboo was greater than that of young bamboo.
- (3)
- In this study area, the stoichiometric homeostasis index(H) of N and P of moso bamboo leaves is HN:P > HN > HP, which means that the homeostasis of bamboo is closely related to the limiting elements.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sundareshwar, P.V.; Morris, J.T.; Koepfler, E.K.; Fornwalt, B. Phosphorus limitation of coastal ecosystem processes. Science 2003, 299, 563. [Google Scholar] [CrossRef]
- Andersen, T.; Elser, J.J.; Hessen, D.O. Stoichiometry and population dynamics. Ecol. Lett. 2004, 7, 884–900. [Google Scholar] [CrossRef]
- Sterner, R.W.; Elser, J.J. Ecological Stoichiometry: The Biology of Elements from Molecules to the Biosphere; Princeton University Press: Princeton, NJ, USA, 2002; pp. 225–226. [Google Scholar]
- Kooijman, S.A.L.M. The stoichiometry of animal energetics. J. Theor. Biol. 1998, 177, 139–149. [Google Scholar] [CrossRef]
- Hessen, D.O.; Agren, G.I.; Anderson, T.R.; Elser, J.J.; De Ruiter, P.C. Carbon, sequestration in ecosystems: The role of stoichiometry. Ecology 2004, 85, 1179–1192. [Google Scholar] [CrossRef]
- Elser, J.J.; Fagan, W.F.; Kerkhoff, A.J.; Swenson, N.G.; Enquist, B.J. Biological stoichiometry of plant production: Metabolism, scaling and ecological response to global change. New Phytol. 2010, 186, 593–608. [Google Scholar] [CrossRef]
- Elser, J.J.; Bracken, M.E.S.; Cleland, E.E.; Gruner, D.S.; Harpole, W.S.; Hillebrand, H.; Ngai, J.T.; Seabloom, E.W.; Shurin, J.B.; Smith, J.E. Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecol. Lett. 2007, 10, 1135–1142. [Google Scholar] [CrossRef]
- Wang, K.; Li, Y.H.; Jiang, T.; Yao, H.J.; Zhang, S.T. Effects of drought stress on N and P stoichiometry and allocation of poplar seedlings. Chin. J. Ecol. 2017, 36, 3116–3122. [Google Scholar]
- Koerselman, W.; Meuleman, A.M.F. The vegetation N:P ratio: A new tool to detect the nature of nutrient limitation. J. Appl. Ecol. 1996, 33, 1441–1450. [Google Scholar] [CrossRef]
- Güsewell, S. N: P ratios in terrestrial plants: Variation and functional significance. New Phytol. 2004, 164, 243–266. [Google Scholar] [CrossRef]
- Zhang, L.X.; Bai, Y.F.; Han, X.G. Differential responses of N:P stoichiometry of Leymus chinensis and Carex korshinskyi to N additions in a steppe ecosystem in Nei Mongol. Acta Bot. Sin. 2004, 46, 259–270. [Google Scholar]
- Han, W.X.; Fang, J.Y.; Guo, D.L.; Zhang, Y. Leaf nitrogen and phosphorus stoichiometry across 753 terrestrial plantspecies in China. New Phytol. 2005, 168, 377–385. [Google Scholar] [CrossRef]
- Persson, J.; Kato, S. To be or not to be what you eat: Regulation of stoichiometric homeostasis among autotrophsand heterotrophs. Oikos 2010, 119, 741–751. [Google Scholar] [CrossRef]
- Rhee, G.Y. Effects of N: P atomic ratios and nitrate limitation on algal growth, cell composition, and nitrate uptake. Limnol. Oceanogr. 1978, 23, 10–25. [Google Scholar] [CrossRef]
- Makino, W.; Cotner, J.B.; Sterner, R.W.; Elser, J.J. Are bacteria more like plants or animals? Growth rate and resource dependence of bacterial C:N:P stoichiometry. Funct. Ecol. 2003, 17, 121–130. [Google Scholar] [CrossRef]
- Cernusak, L.A.; Winter, K.; Turner, B.L. Leaf nitrogen to phosphorus ratios of tropical trees: Experimental assessment of physiological and environmental controls. New Phytol. 2009, 185, 770–779. [Google Scholar] [CrossRef]
- Matzek, V.; Vitousek, P.M. N:P stoichiometry and protein:RNA ratios in vascular plants: An evaluation of the growth-rate hypothesis. Ecol. Lett. 2009, 12, 765–771. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Elser, J.J.; He, N.P.; Wu, H.H.; Chen, Q.S.; Zhang, G.M.; Han, X.G. Stoichiometric homeostasis of vascular plants in the Inner Mongolia grassland. Oecologia 2011, 166, 1–10. [Google Scholar] [CrossRef]
- Yu, Q.; Chen, Q.S.; Elser, J.J.; Cease, A.; He, N.P.; Wu, H.H.; Zhang, G.M.; Wu, J.G.; Bai, Y.F.; Han, X.G. Linking stoichiometric homeostasis with ecosystem structure, functioning, and stability. Ecol. Lett. 2010, 13, 1390. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.L.; Zeng, C.S.; Shao, J.J.; Zhou, X.H. Plant nutrient dynamics and stoichiometric homeostasis of invasive species Spartina alterniflora and native Cyperus malaccensis var.brevifolius in the Minjiang River estuarine wetlands. Chin. J. PlantEcol. 2017, 41, 450–460. [Google Scholar]
- Frost, P.C.; Elser, J.J. Growth responses of littoral mayflies to the phosphorus content of their food. Ecol. Lett. 2002, 5, 232–240. [Google Scholar] [CrossRef]
- Yen, T.M.; Lee, J.S. Comparing Aboveground Carbon Sequestration between Moso Bamboo (Phyllostachys heterocycla) and China Fir (Cunninghamia lanceolata) Forests Based on the Allometric Model. For. Ecol. Manag. 2011, 261, 995–1002. [Google Scholar] [CrossRef]
- Liu, G.L.; Fan, S.H.; Cai, C.J.; Zhang, C.S.; Guan, F.Y. Soil Anti-erodibility Under Moso Bamboo with Different Mixture Patterns. Bull. Soil Water Conserv. 2015, 35, 12–17. [Google Scholar]
- Feng, P.F.; Li, Y.M. China’s Bamboo Resources in 2021. World Bamboo Ratt. 2023, 21, 100–103. [Google Scholar]
- Zhao, J.; Wang, B.; Li, Q.; Yang, H.; Xu, K. Analysis of soil degradation causes in Phyllostachys edulis forests with different mulching years. Forests 2018, 9, 149. [Google Scholar] [CrossRef]
- Sun, H.Y.; Wang, J.F.; Hui, L.; Li, T.K.; Gao, Z.M. Advancements and challenges in bamboo breeding for sustainable development. Tree Physiol. 2023, 43, 1705–1717. [Google Scholar] [CrossRef]
- Guan, F.; Xia, M.; Tang, X.; Fan, S. Spatial variability of soil nitrogen, phosphorus and potassium contents in moso bamboo forests in Yong’an City, China. Catena 2017, 150, 161–172. [Google Scholar] [CrossRef]
- Su, W.; Liu, B.; Liu, X.; Li, X.; Ren, T.; Cong, R.; Lu, J. Effect of depth of fertilizer banded-placement on growth, nutrient uptake and yield of oil seed rap (Brassicanapuw L.). Eur. J. Agron. 2015, 62, 38–45. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, H.; Liu, X.; Liu, Y.; Gao, S.; Zhou, J. The effect of N fertilizer placement on the fate of urea-15N and yield of winter wheat in southeast China. PLoS ONE 2016, 11, e0153701. [Google Scholar] [CrossRef] [PubMed]
- Chu, H.; Su, W.; Zhou, Y.; Wang, Z.; Long, Y.; Sun, Y.; Fan, S. Enzyme Activity Stoichiometry Suggests That Fertilization, Especially Nitrogen Fertilization, Alleviates Nutrient Limitation of Soil Microorganisms in Moso Bamboo Forests. Forests 2024, 15, 1040. [Google Scholar] [CrossRef]
- Zhao, J.C.; Su, W.H.; Fan, S.H.; Cai, C.J.; Su, H.R.; Zeng, X.L. Ammonia volatilization and nitrogen runoff losses from moso bamboo forests under different fertilization practices. Can. J. Forest Res. 2019, 49, 213–220. [Google Scholar] [CrossRef]
- Lu, Q.F.; Lin, S.P.; Liu, S.H.; Zheng, X.; Bi, Y.F.; Xiao, Z.Z.; Jiang, J.; Wang, A.K.; Du, X.H. Meta-analysis of the effect of fertilization on the yield of Phyllostachys edulis forest. J. Nanjing For. Univ. Nat. Sci. Ed. 2024, 48, 88–96. [Google Scholar]
- Tang, X.; Fan, S.; Qi, L.; Guan, F.; Du, M.; Zhang, H. Soil respiration and net ecosystem production in relation to intensive management in moso bamboo forests. Catena 2016, 137, 219–228. [Google Scholar] [CrossRef]
- Sparks, D.L.; Page, A.L.; Helmke, P.A.; Loeppert, R.H. (Eds.) Methods of Soil Analysis. Part 3: Chemical Methods; Soil Science Society of America and American Society of Agronomy: Madison, WI, USA, 1996; pp. 1085–1121. [Google Scholar]
- Kuo, S. Phosphorus. In Methods of Soil Analysis. Part 3: Chemical Methods; Sparks, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Eds.; Soil Science Society of America and American Society of Agronomy: Madison, WI, USA, 1996; pp. 891–893. [Google Scholar]
- Bao, S.D. Soil Agricultural Chemistry Analysis; Agriculture Press: Beijing, China, 2000. [Google Scholar]
- Zheng, J.Y.; Shao, M.A.; Zhang, X.C. Spatial Variation of Surface Soil’s Bulk Density and Saturated Hydraulic Conductivity on Slope in Loess Region. J. Soil Water Conserv. 2004, 3, 53–56. [Google Scholar]
- Garnier, E. Interspecific Variation in Plasticity of Grasses in Response to Nitrogen Supply; Cambridge University Press: Cambridge, UK, 1998; pp. 155–181. [Google Scholar]
- Luo, X.; Keenan, T.F.; Chen, J.M.; Croft, F.; Prentice, I.C.; Smith, N.G.; Walker, A.P.; Wang, H.; Wang, R.; Xu, C.G.; et al. Global variation in the fraction of leaf nitrogen allocated to photosynthesis. Nat. Commun. 2021, 12, 4866. [Google Scholar] [CrossRef] [PubMed]
- Güsewell, S.; Koerselman, W. Variation in nitrogen and phosphorus concentrations of wetland plants. Perspect. Plant Ecol. Evol. Syst. 2002, 5, 37–61. [Google Scholar] [CrossRef]
- Cao, J.; Yan, W.; Farooq, T.H.; Chen, X.; Wang, J.; Yuan, C.; Qi, Y.; Khan, K.A. Ecological Stoichiometry of N and P across a Chronosequence of Chinese Fir Plantation Forests. Forests 2023, 14, 1685. [Google Scholar] [CrossRef]
- Elser, J.J.; Sterner, R.; Gorokhova, E.; Fagan, W.F.; Markow, T.A.; Cotner, J.B.; Weider, L.W. Biological stoichiometry from genes to ecosystems. Ecol. Lett. 2000, 3, 540–550. [Google Scholar] [CrossRef]
- Fan, X.Y. Spatial Variation in Nutrient of Dominant Plant and Ecological Stoichiometry from Laohu Gou of Qilian Mountain. Master Thesis, Lanzhou University, Lanzhou, China, 2012. [Google Scholar]
- Vitousek, P.M. Nutrient limitation to nitrogen fixation in young volcanic sites. Ecosystems 1999, 2, 505–510. [Google Scholar] [CrossRef]
- Chen, Y.H.; Han, W.X.; Tang, L.Y.; Tang, Z.Y.; Fang, J.Y. Leaf nitrogen and phosphorus concentrations of woody plants differ in responses to climate, soil and plant growth form. Ecography 2013, 36, 178–184. [Google Scholar] [CrossRef]
- Zeng, Y.; Fang, Y.; Wang, X.; Tang, Y. Stoichiometric and nutrient resorption characteristics of dominant tree species in subtropical Chinese forests. Ecol. Evol. 2017, 7, 11033–11043. [Google Scholar] [CrossRef]


| Transect No. | Density (Trees·hm−2) | Mean DBH (cm) | Slope (°) | Aspect |
|---|---|---|---|---|
| 1 | 1880 | 10.30 | 11 | NE |
| 2 | 2707 | 9.20 | 10 | NE |
| 3 | 1569 | 8.60 | 11 | NE |
| 4 | 900 | 11.89 | 20 | SW |
| 5 | 960 | 10.70 | 15 | SW |
| 6 | 1100 | 10.20 | 25 | SW |
| 7 | 2142 | 9.18 | 25 | NE |
| 8 | 2365 | 9.95 | 15 | NW |
| 9 | 2597 | 10.71 | 15 | SE |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, J.; Fan, S.; Zhang, J.; Liu, G. Stoichiometric Homeostasis of N and P in the Leaves of Different-Aged Phyllostachys edulis after Bamboo Forest Expansion in Subtropical China. Forests 2024, 15, 1181. https://doi.org/10.3390/f15071181
Shen J, Fan S, Zhang J, Liu G. Stoichiometric Homeostasis of N and P in the Leaves of Different-Aged Phyllostachys edulis after Bamboo Forest Expansion in Subtropical China. Forests. 2024; 15(7):1181. https://doi.org/10.3390/f15071181
Chicago/Turabian StyleShen, Jingxin, Shaohui Fan, Jiapeng Zhang, and Guanglu Liu. 2024. "Stoichiometric Homeostasis of N and P in the Leaves of Different-Aged Phyllostachys edulis after Bamboo Forest Expansion in Subtropical China" Forests 15, no. 7: 1181. https://doi.org/10.3390/f15071181





