Contrasting Regeneration Patterns in Abies alba-Dominated Stands: Insights from Structurally Diverse Mountain Forests across Europe
Abstract
:1. Introduction
2. Materials and Methods
2.1. Research Area
2.2. Data Collection
2.3. Tree Size Diversity and Admixture of Broadleaf Tree Species
- N is the number of classes;
- is the proportion of trees in the i-th class.
2.4. Developmental Stage of Fir Regeneration
- seedlings, small saplings, and tall saplings represent the per hectare density of silver fir regeneration in each respective developmental stage;
- density of all stages denotes the total density per hectare of silver fir regeneration across all developmental stages;
- 1, 3, 5—weights that signify the increasing importance of saplings with increasing developmental stage.
2.5. Climatic Data
- P—annual sum of precipitation in millimeters;
- T—average annual temperature in degrees.
2.6. Density of Ungulates
2.7. Statistical Analysis
2.7.1. Probability of the Regeneration
- denotes the probability of the regeneration (all species);
- coefficients , , and correspond to the intercept and the effects of the two predictors on the log odds of the event, respectively;
- Structure—structure type (even/uneven-aged);
- Site—study site.
- denotes the probability of the regeneration (silver fir);
- coefficients , , and correspond to the intercept and the effects of the two predictors on the log odds of the event, respectively;
- Structure—structure type (even/uneven-aged);
- Temp—mean annual temperature for the last 20 years;
- —random intercept for each site.
2.7.2. Regeneration Density
2.7.3. Dominant Developmental Stage of Silver Fir Regeneration
2.7.4. Presence of Large Overstory Trees
3. Results
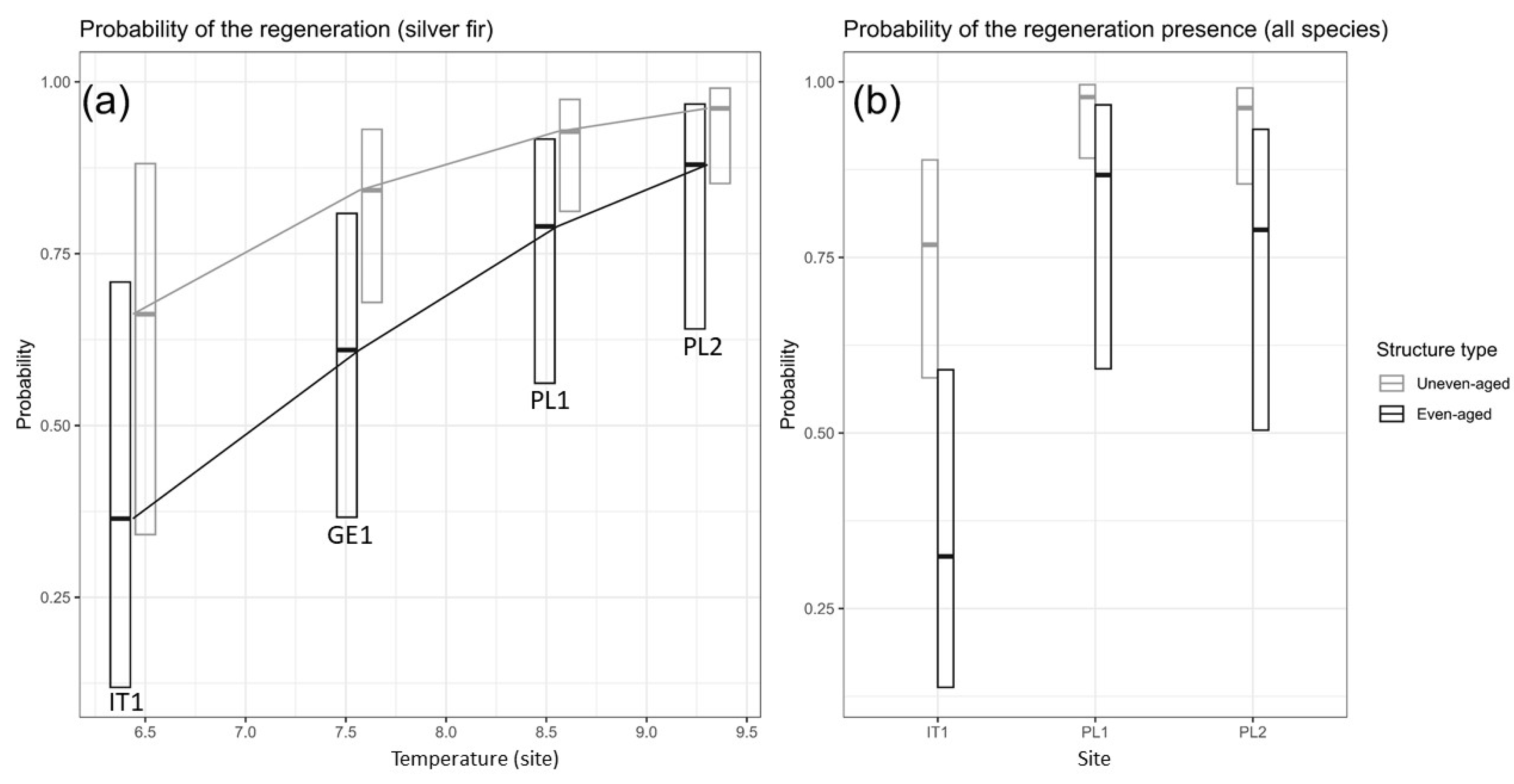
3.1. Probability of the Regeneration Appearance
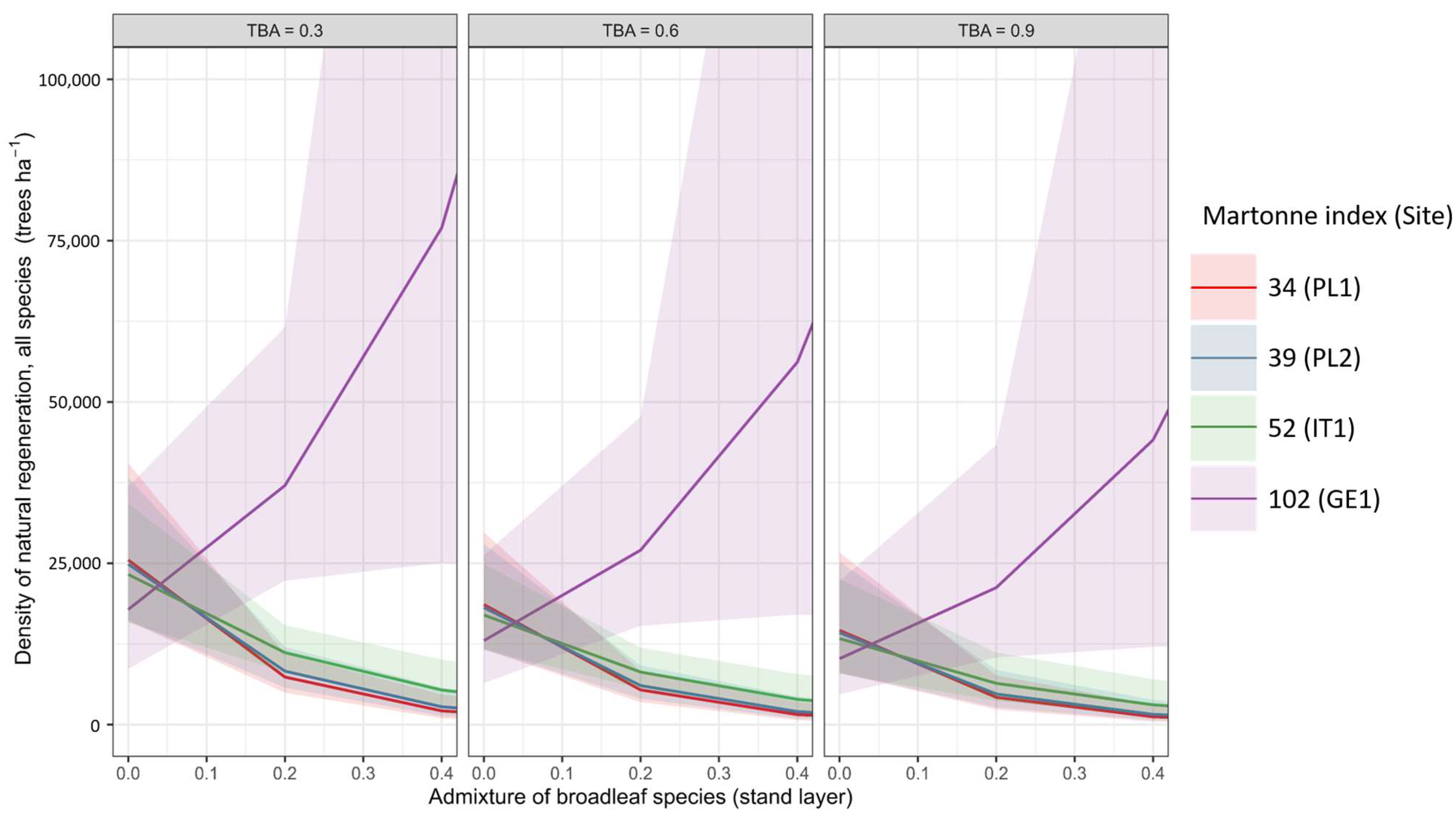
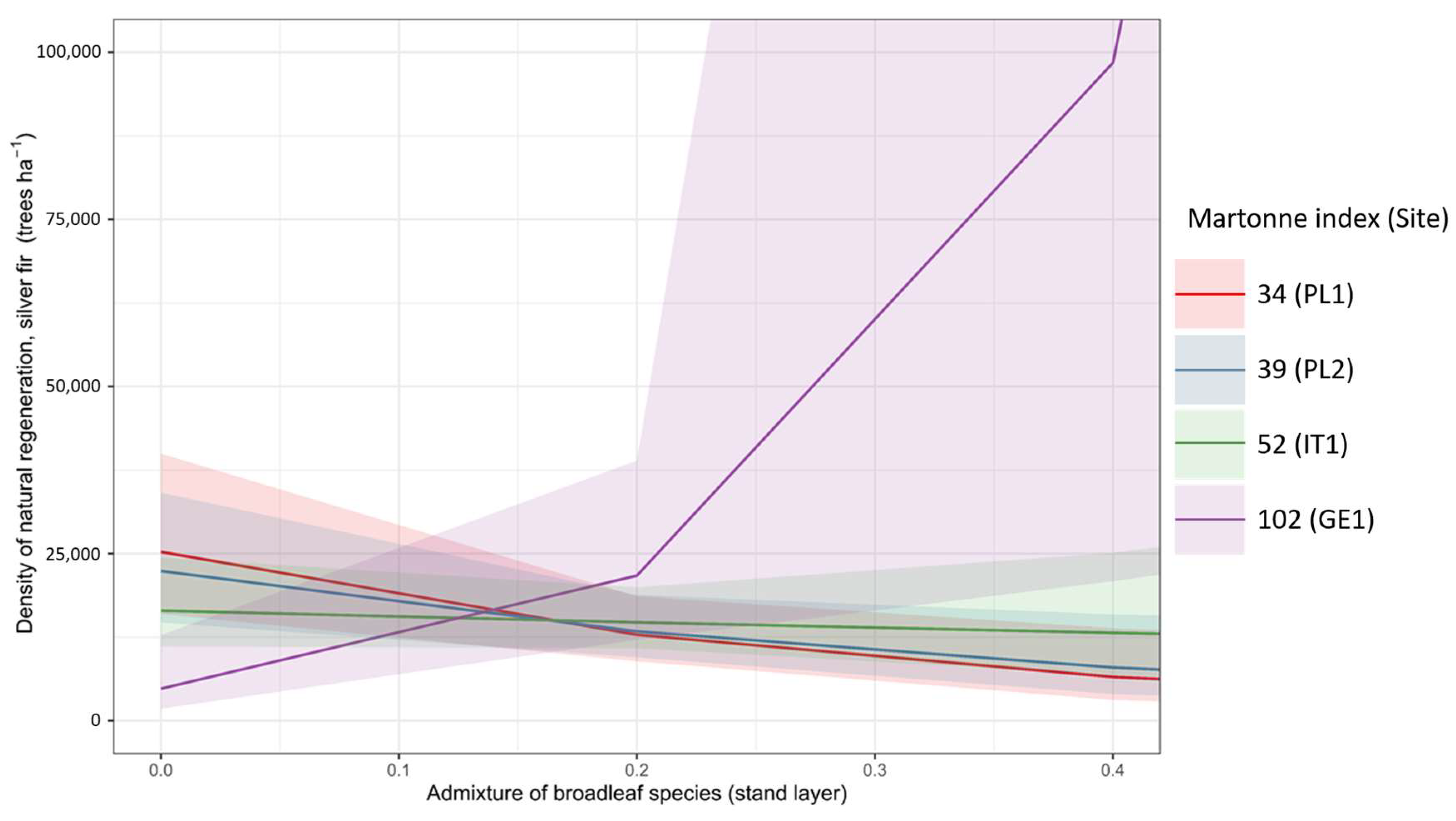
3.2. Regeneration Density
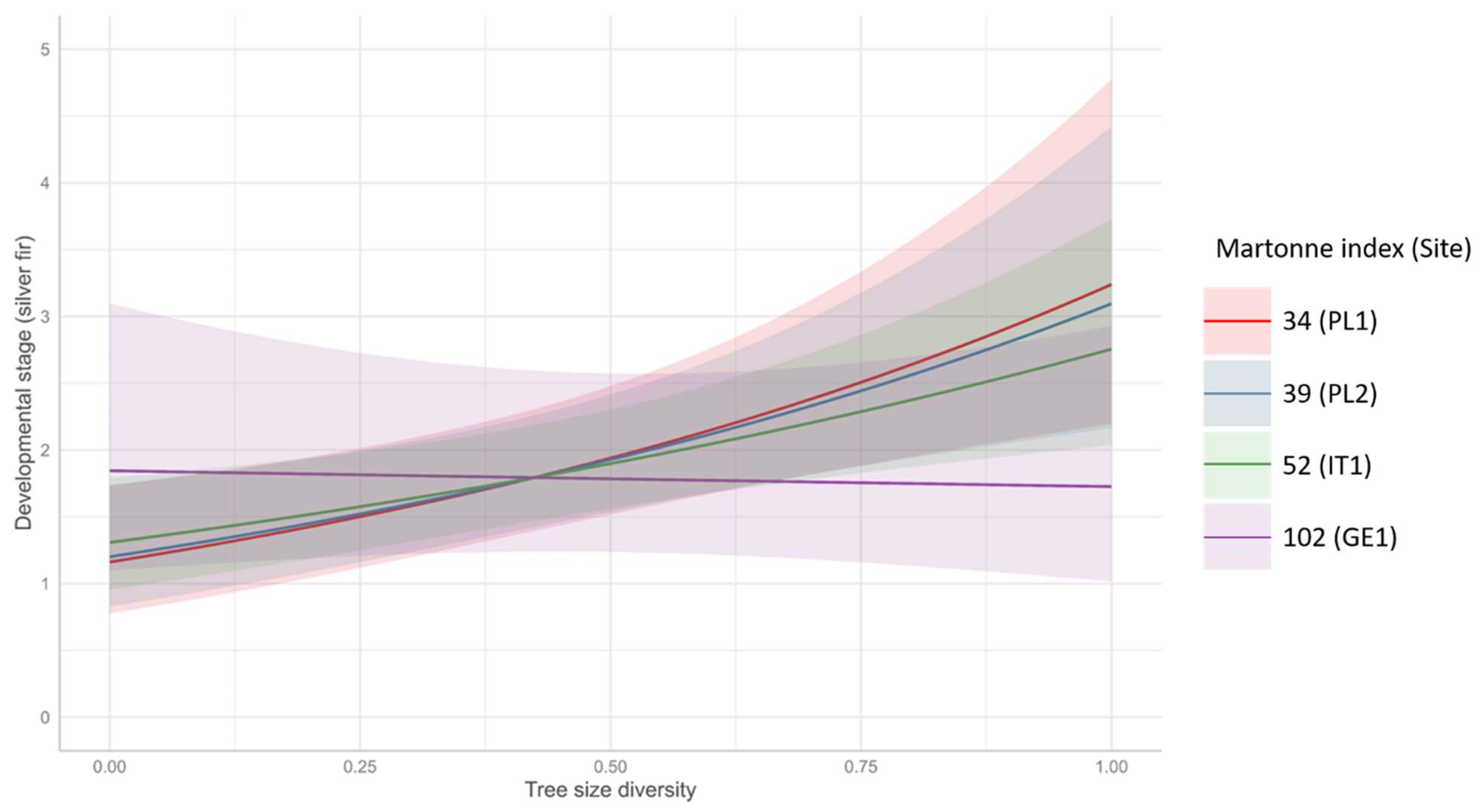
3.3. Dominant Developmental Stage of Silver Fir Regeneration
4. Discussion
4.1. The Likelihood of Regeneration Success—To Be or Not to Be?
4.2. The Complexity of Choice: The Impact of Stand Density and Tree Size Diversity on the Natural Regeneration
4.3. Influence of Broadleaf and Conifer Interplay on the Natural Regeneration
4.4. Influence of Ungulates on the Regeneration of Silver Fir
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smith, D.M.; Larson, B.C.; Kelty, M.J.; Ashton, P.M.S. The Practice of Silviculture: Applied Forest Ecology. In The Practice of Silviculture: Applied Forest Ecology; Wiley: Hoboken, NJ, USA, 1997. [Google Scholar]
- Tappeiner, U.; Borsdorf, A.; Bahn, M. Long-Term Socio-Ecological Research in Mountain Regions: Perspectives from the Tyrolean Alps. In Long Term Socio-Ecological Research: Studies in Society-Nature Interactions across Spatial and Temporal Scales; Singh, S.J., Haberl, H., Chertow, M., Mirtl, M., Schmid, M., Eds.; Human-Environment Interactions; Springer: Dordrecht, The Netherlands, 2013; pp. 505–525. ISBN 978-94-007-1177-8. [Google Scholar]
- Hamilton, L.S. The Protective Role of Mountain Forests. GeoJournal 1992, 27, 13–22. [Google Scholar] [CrossRef]
- Nolet, P.; Kneeshaw, D.; Messier, C.; Béland, M. Comparing the Effects of Even- and Uneven-aged Silviculture on Ecological Diversity and Processes: A Review. Ecol. Evol. 2017, 8, 1217–1226. [Google Scholar] [CrossRef]
- Diaci, J.; Rozenbergar, D.; Fidej, G.; Nagel, T.A. Challenges for Uneven-Aged Silviculture in Restoration of Post-Disturbance Forests in Central Europe: A Synthesis. Forests 2017, 8, 378. [Google Scholar] [CrossRef]
- Álvarez-Yépiz, J.C. Restoration Ecology in the Anthropocene: Learning from Responses of Tropical Forests to Extreme Disturbance Events. Restor. Ecol. 2020, 28, 271–276. [Google Scholar] [CrossRef]
- Dollinger, C.; Rammer, W.; Seidl, R. Climate Change Accelerates Ecosystem Restoration in the Mountain Forests of Central Europe. J. Appl. Ecol. 2023, 60, 2665–2675. [Google Scholar] [CrossRef]
- Vitali, V.; Büntgen, U.; Bauhus, J. Silver Fir and Douglas Fir Are More Tolerant to Extreme Droughts than Norway Spruce in South-Western Germany. Glob. Chang. Biol. 2017, 23, 5108–5119. [Google Scholar] [CrossRef]
- Dobrowolska, D.; Bončina, A.; Klumpp, R. Ecology and Silviculture of Silver Fir (Abies alba Mill.): A Review. J. For. Res. 2017, 22, 326–335. [Google Scholar] [CrossRef]
- Prokůpková, A.; Brichta, J.; Vacek, Z.; Bielak, K.; Andrzejczyk, T.; Vacek, S.; Štefančík, I.; Bílek, L.; Fuchs, Z. Effect of vegetation on natural regeneration of mixed silver fir forests in lowlands: Case study from the Rogów region. Sylwan 2021, 165, 779–795. [Google Scholar]
- Brzeziecki, B.; Kienast, F. Classifying the Life-History Strategies of Trees on the Basis of the Grimian Model. For. Ecol. Manag. 1994, 69, 167–187. [Google Scholar] [CrossRef]
- Bragg, D.C. The Development of Uneven-Aged Southern Pine Silviculture before the Crossett Experimental Forest (Arkansas, USA). For. Int. J. For. Res. 2017, 90, 332–342. [Google Scholar] [CrossRef]
- Adamic, M.; Diaci, J.; Rozman, A.; Hladnik, D. Long-Term Use of Uneven-Aged Silviculture in Mixed Mountain Dinaric Forests: A Comparison of Old-Growth and Managed Stands. For. Int. J. For. Res. 2017, 90, 279–291. [Google Scholar] [CrossRef]
- Paluch, J.G.; Jastrzębski, R. Natural Regeneration of Shade-Tolerant Abies alba Mill. in Gradients of Stand Species Compositions: Limitation by Seed Availability or Safe Microsites? For. Ecol. Manag. 2013, 307, 322–332. [Google Scholar] [CrossRef]
- Unkule, M.; Piedallu, C.; Balandier, P.; Courbaud, B. Climate and Ungulate Browsing Impair Regeneration Dynamics in Spruce-Fir-Beech Forests in the French Alps. Ann. For. Sci. 2022, 79, 11. [Google Scholar] [CrossRef]
- Kupferschmid, A.D.; Bütikofer, L.; Hothorn, T.; Schwyzer, A.; Brang, P. Ungulate Species and Abundance as Well as Environmental Factors Determine the Probability of Terminal Shoot Browsing on Temperate Forest Trees. Forests 2020, 11, 764. [Google Scholar] [CrossRef]
- Dobrowolska, D. Structure of Silver Fir (Abies alba Mill.) Natural Regeneration in the Jata’reserve in Poland. For. Ecol. Manag. 1998, 110, 237–247. [Google Scholar] [CrossRef]
- Becker, M.; Drapier, J.M. Rôle de l’allélopathie Dans Les Difficultés de Régénération Du Sapin (Abies alba Mill.). I. Propriétés Phytotoxiques Des Hydrosolubles d’aiguilles de Sapin. Acta Oecol. 1984, 5, 347–356. [Google Scholar]
- O’Hara, K. Multiaged Silviculture: Managing for Complex Forest Stand Structures; Oxford University Press: Oxford, UK, 2014; ISBN 978-0-19-870306-8. [Google Scholar]
- Polish State Forest Enterprise. Principles of Silviculture [Zasady Hodowli Lasu]. 2023. Available online: https://www.lasy.gov.pl/pl/publikacje/copy_of_gospodarka-lesna/hodowla/zasady-hodowli-lasu-dokument-w-opracowaniu/@@download/file/Zasady_hodowli_lasu%20_1_stycznia_2024.pdf (accessed on 23 May 2024).
- Cornes, R.C.; Van Der Schrier, G.; Van Den Besselaar, E.J.M.; Jones, P.D. An Ensemble Version of the E-OBS Temperature and Precipitation Data Sets. J. Geophys. Res. Atmos. 2018, 123, 9391–9409. [Google Scholar] [CrossRef]
- Bronisz, A.; Bijak, S.; Bronisz, K. Dendroclimatological Characteristics of Silver Fir (Abies alba Mill.) in the Swietokrzyskie Mountains. Sylwan 2010, 154, 463–470. [Google Scholar]
- Bruchwald, A.; Dmyterko, E.; Niemczyk, M.; Łukaszewicz, J. The Characteristics of Selected Fir Stands in Beskid Niski and the Method of Their Management. Sylwan 2015, 159, 722–731. [Google Scholar]
- Institute of Forest Ecosystem Research. Field-Map. 2024. Available online: https://www.fieldmap.cz/ (accessed on 23 May 2024).
- Gatz, D.F.; Smith, L. The Standard Error of a Weighted Mean Concentration—I. Bootstrapping vs. Other Methods. Atmos. Environ. 1995, 29, 1185–1193. [Google Scholar] [CrossRef]
- Pellicone, G.; Caloiero, T.; Guagliardi, I. The De Martonne Aridity Index in Calabria (Southern Italy). J. Maps 2019, 15, 788–796. [Google Scholar] [CrossRef]
- Mattioli, S.; Meneguz, P.G.; Brugnoli, A.; Nicoloso, S. Red Deer in Italy: Recent Changes in Range and Numbers. Hystrix Ital. J. Mammal. 2001, 12, 27–35. [Google Scholar] [CrossRef]
- Kinser, V.A.; Koop, K.; Freiherr von Munchhausen, H. Die Rotwildverbreitung in Deutschland. AFZ Wald. 2010, 5, 32–34. [Google Scholar]
- Żmudzki, J.; Jablonski, A.; Arent, Z.; Zębek, S.; Nowak, A.; Stolarek, A.; Parzeniecka-Jaworska, M. First Report of Leptospira Infections in Red Deer, Roe Deer, and Fallow Deer in Poland. J. Vet. Res. 2016, 60, 257–260. [Google Scholar] [CrossRef]
- van Beeck Calkoen, S.T.S.; Kuijper, D.P.J.; Apollonio, M.; Blondel, L.; Dormann, C.F.; Storch, I.; Heurich, M. Numerical Top-down Effects on Red Deer (Cervus Elaphus) Are Mainly Shaped by Humans Rather than Large Carnivores across Europe. J. Appl. Ecol. 2023, 60, 2625–2635. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Brooks, M.E.; Kristensen, K.; van Benthem, K.J.; Magnusson, A.; Berg, C.W.; Nielsen, A.; Skaug, H.J.; Mächler, M.; Bolker, B.M. glmmTMB Balances Speed and Flexibility Among Packages for Zero-Inflated Generalized Linear Mixed Modeling. R J. 2017, 9, 378–400. [Google Scholar] [CrossRef]
- Strassburg, B.B.N.; Barros, F.S.M.; Crouzeilles, R.; Iribarrem, Á.; dos Santos, J.S.; Silva, D.S.; Sansevero, J.B.B.; Alves-Pinto, H.; Feltran-Barbieri, R.; Latawiec, A.E. The Role of Natural Regeneration to Ecosystem Services Provision and Habitat Availability: A Case Study in the Brazilian Atlantic Forest. Biotropica 2016, 8, 890–899. [Google Scholar] [CrossRef]
- Khaine, I.; Woo, S.Y.; Kwak, M.J.; Lee, S.H.; Je, S.M.; You, H.; Lee, T.; Jang, J.; Lee, H.K.; Cheng, H.C.; et al. Factors Affecting Natural Regeneration of Tropical Forests across a Precipitation Gradient in Myanmar. Forests 2018, 9, 143. [Google Scholar] [CrossRef]
- Chazdon, R.L.; Guariguata, M.R. Natural Regeneration as a Tool for Large-scale Forest Restoration in the Tropics: Prospects and Challenges. Biotropica 2016, 48, 716–730. [Google Scholar] [CrossRef]
- Hlásny, T.; König, L.; Krokene, P.; Lindner, M.; Montagné-Huck, C.; Müller, J.; Qin, H.; Raffa, K.F.; Schelhaas, M.-J.; Svoboda, M.; et al. Bark Beetle Outbreaks in Europe: State of Knowledge and Ways Forward for Management. Curr. For. Rep. 2021, 7, 138–165. [Google Scholar] [CrossRef]
- O’Connor, J.C.; Dekker, S.C.; Staal, A.; Tuinenburg, O.A.; Rebel, K.T.; Santos, M.J. Forests Buffer against Variations in Precipitation. Glob. Chang. Biol. 2021, 27, 4686–4696. [Google Scholar] [CrossRef] [PubMed]
- Verbiest, W.W.M.; Smith, G.R.; Mirzagholi, L.; Lauber, T.; Zohner, C.M.; Maynard, D.S.; Schemm, S.; Crowther, T.W. Enhanced Local Cooling Effects of Forests across the Globe. bioRxiv 2023. [Google Scholar] [CrossRef]
- Nyland, R.D. Even- to Uneven-Aged: The Challenges of Conversion. For. Ecol. Manag. 2003, 172, 291–300. [Google Scholar] [CrossRef]
- Reventlow, D.O.J.; Nord-Larsen, T.; Biber, P.; Hilmers, T.; Pretzsch, H. Simulating Conversion of Even-Aged Norway Spruce into Uneven-Aged Mixed Forest: Effects of Different Scenarios on Production, Economy and Heterogeneity. Eur. J. For. Res. 2021, 140, 1005–1027. [Google Scholar] [CrossRef]
- Sinha, A.; Rämö, J.; Malo, P.; Kallio, M.; Tahvonen, O. Optimal Management of Naturally Regenerating Uneven-Aged Forests. Eur. J. Oper. Res. 2017, 256, 886–900. [Google Scholar] [CrossRef]
- Mayer, A.M.; Poljakoff-Mayber, A. The Germination of Seeds; Elsevier: Amsterdam, The Netherlands, 2014; ISBN 978-1-4831-3804-6. [Google Scholar]
- Martiník, A.; Sendecký, M.; Urban, J. Survival and Early Growth of Silver Fir and Pioneer Species on Two Sites in Nurse Crop Regeneration Systems in the Czech Republic. Dendrobiology 2018, 80, 81–90. [Google Scholar] [CrossRef]
- Tateno, R.; Takeda, H. Forest Structure and Tree Species Distribution in Relation to Topography-mediated Heterogeneity of Soil Nitrogen and Light at the Forest Floor. Ecol. Res. 2003, 18, 559–571. [Google Scholar] [CrossRef]
- Keram, A.; Halik, Ü.; Aishan, T.; Keyimu, M.; Jiapaer, K.; Li, G. Tree Mortality and Regeneration of Euphrates Poplar Riparian Forests along the Tarim River, Northwest China. For. Ecosyst. 2021, 8, 49. [Google Scholar] [CrossRef]
- Paluch, J. Spatial Distribution of Regeneration in West-Carpathian Uneven-Aged Silver Fir Forests. Eur. J. For. Res. 2005, 124, 47–54. [Google Scholar] [CrossRef]
- Scheller, R.M.; Mladenoff, D.J. Understory Species Patterns and Diversity in Old-Growth and Managed Northern Hardwood Forests. Ecol. Appl. 2002, 12, 1329–1343. [Google Scholar] [CrossRef]
- Paluch, J.; Bartkowicz, L.; Moser, W.K. Interspecific Effects between Overstorey and Regeneration in Small-Scale Mixtures of Three Late-Successional Species in the Western Carpathians (Southern Poland). Eur. J. For. Res. 2019, 138, 889–905. [Google Scholar] [CrossRef]
- Mauri, A.; De Rigo, D.; Caudullo, G. Abies alba in Europe: Distribution, Habitat, Usage and Threats. In European Atlas of Forest Tree Species; Publication Office of the European Union: Luxembourg, 2016; pp. 48–49. ISBN 978-92-79-36740-3. [Google Scholar]
- Grassi, G.; Minotta, G.; Tonon, G.; Bagnaresi, U. Dynamics of Norway Spruce and Silver Fir Natural Regeneration in a Mixed Stand under Uneven-Aged Management. Can. J. For. Res. 2004, 34, 141–149. [Google Scholar] [CrossRef]
- Klopčić, M.; Jerina, K.; Bončina, A. Long-Term Changes of Structure and Tree Species Composition in Dinaric Uneven-Aged Forests: Are Red Deer an Important Factor? Eur. J. For. Res. 2009, 129, 277–288. [Google Scholar] [CrossRef]
- Buongiorno, J. Quantifying the Implications of Transformation from Even to Uneven-Aged Forest Stands. For. Ecol. Manag. 2001, 151, 121–132. [Google Scholar] [CrossRef]
- Haight, R.G. Evaluating the Efficiency of Even-Aged and Uneven-Aged Stand Management. For. Sci. 1987, 33, 116–134. [Google Scholar] [CrossRef]
- Hanewinkel, M. Economic Aspects of the Transformation from Even-Aged Pure Stands of Norway Spruce to Uneven-Aged Mixed Stands of Norway Spruce and Beech. For. Ecol. Manag. 2001, 151, 181–193. [Google Scholar] [CrossRef]
- Pukkala, T.; Lähde, E.; Laiho, O.; Salo, K.; Hotanen, J.-P. A Multifunctional Comparison of Even-Aged and Uneven-Aged Forest Management in a Boreal Region. Can. J. For. Res. 2011, 41, 851–862. [Google Scholar] [CrossRef]
- Alexander, R.R.; Edminster, C.B. Uneven-Aged Management of Old Growth Spruce-Fir Forests: Cutting Methods and Stand Structure Goals for the Initial Entry; Department of Agriculture, Forest Service, Rocky Mountain Forest and Range Experiment Station: Washington, DC, USA, 1977. [Google Scholar]
- Hanewinkel, M.; Frutig, F.; Lemm, R. Economic Performance of Uneven-Aged Forests Analysed with Annuities. Forestry 2013, 87, 49–60. [Google Scholar] [CrossRef]
- Kuuluvainen, T.; Tahvonen, O.; Aakala, T. Even-Aged and Uneven-Aged Forest Management in Boreal Fennoscandia: A Review. Ambio 2012, 41, 720–737. [Google Scholar] [CrossRef]
- Pukkala, T. Which Type of Forest Management Provides Most Ecosystem Services? For. Ecosyst. 2016, 3, 1. [Google Scholar] [CrossRef]
- Brang, P.; Spathelf, P.; Larsen, J.B.; Bauhus, J.; Boncčìna, A.; Chauvin, C.; Drössler, L.; García-Güemes, C.; Heiri, C.; Kerr, G.; et al. Suitability of Close-to-Nature Silviculture for Adapting Temperate European Forests to Climate Change. For. Int. J. For. Res. 2014, 87, 492–503. [Google Scholar] [CrossRef]
- Kupferschmid, A.D.; Bugmann, H. Effect of Microsites, Logs and Ungulate Browsing on Picea Abies Regeneration in a Mountain Forest. For. Ecol. Manag. 2005, 205, 251–265. [Google Scholar] [CrossRef]
- Paluch, J.G.; Kołodziej, Z.; Skrzyszewski, J.; Bartkowicz, L.; Gruba, P. Regeneration Patterns of the Late-Successional Abies alba Mill.: Inhibition in Monospecific Stands and Colonization in Mixed Stands. Ann. For. Sci. 2016, 73, 1015–1024. [Google Scholar] [CrossRef]
- Drozdowski, S. Modelowanie Procesów Odnowieniowych w Lesie Naturalnym; Rozprawy Naukowe i Monografie-Szkoła Główna Gospodarstwa Wiejskiego 441; Wydawnictwo SGGW: Warszawa, Poland, 2013; ISBN 978-83-7583-492-5. [Google Scholar]
- Ling, P.-Y.; Aguilar-Amuchastegui, N.; Baldwin-Cantello, W.; Rayden, T.; Gordon, J.; Dainton, S.; Bagwill, A.; Pacheco-Balanza, P. Mapping Global Forest Regeneration–an Untapped Potential to Mitigate Climate Change and Biodiversity Loss. Environ. Res. Lett. 2023, 18, 054025. [Google Scholar] [CrossRef]
- Meeussen, C.; Pauw, K.D.; Sanczuk, P.; Brunet, J.; Cousins, S.A.O.; Gasperini, C.; Hedwall, P.-O.; Iacopetti, G.; Lenoir, J.; Plue, J.; et al. Initial Oak Regeneration Responses to Experimental Warming along Microclimatic and Macroclimatic Gradients. Plant Biol. 2022, 24, 745–757. [Google Scholar] [CrossRef] [PubMed]
- Petrie, M.D.; Bradford, J.B.; Hubbard, R.M.; Lauenroth, W.K.; Andrews, C.M.; Schlaepfer, D.R. Climate Change May Restrict Dryland Forest Regeneration in the 21st Century. Ecology 2017, 98, 1548–1559. [Google Scholar] [CrossRef]
- Kucbel, S.; Jaloviar, P.; Saniga, M.; Vencurik, J.; Klimaš, V. Canopy Gaps in an Old-Growth Fir-Beech Forest Remnant of Western Carpathians. Eur. J. For. Res. 2010, 129, 249–259. [Google Scholar] [CrossRef]
- Senn, J.; Suter, W. Ungulate Browsing on Silver Fir (Abies alba) in the Swiss Alps: Beliefs in Search of Supporting Data. For. Ecol. Manag. 2003, 181, 151–164. [Google Scholar] [CrossRef]

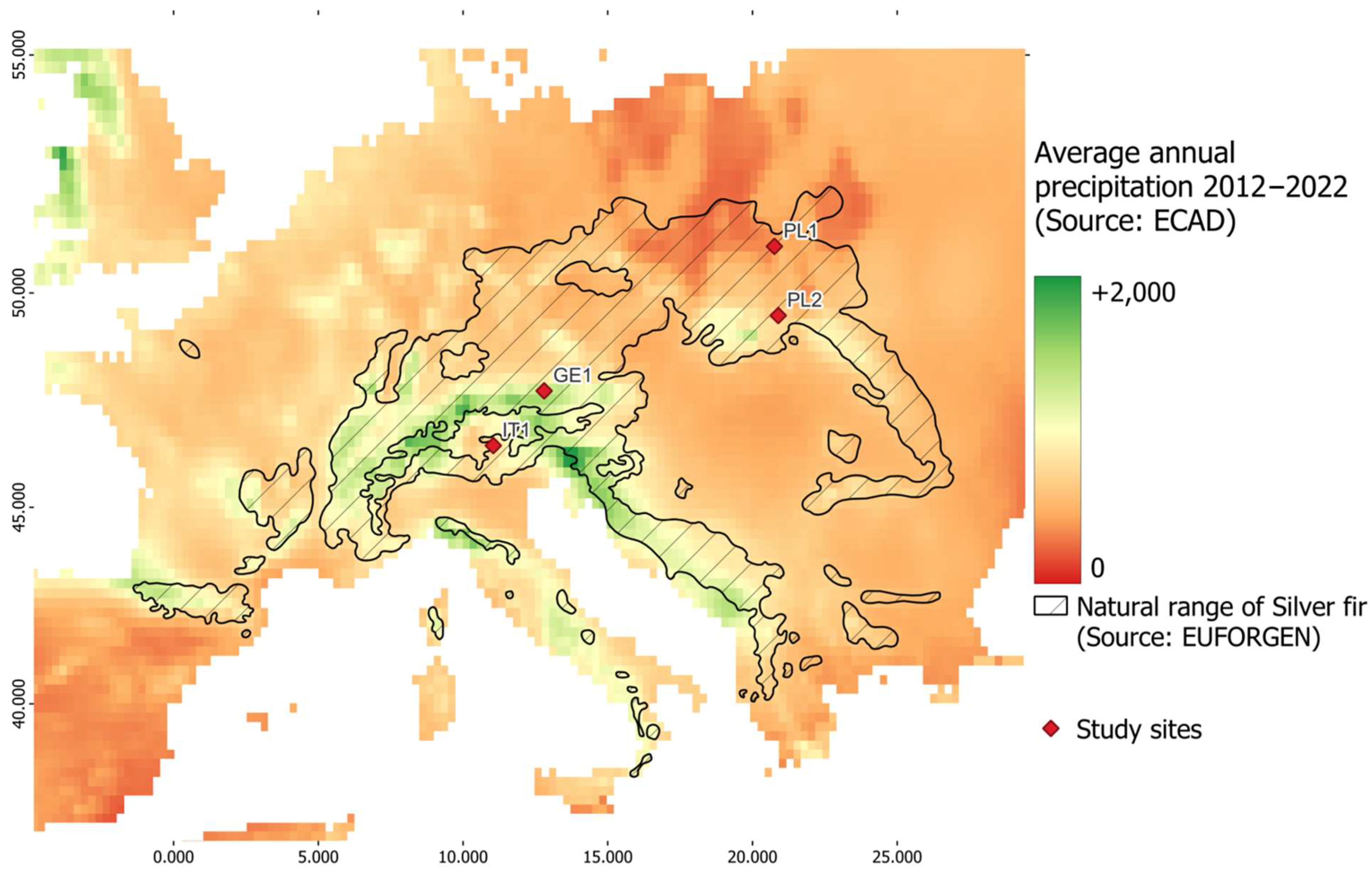
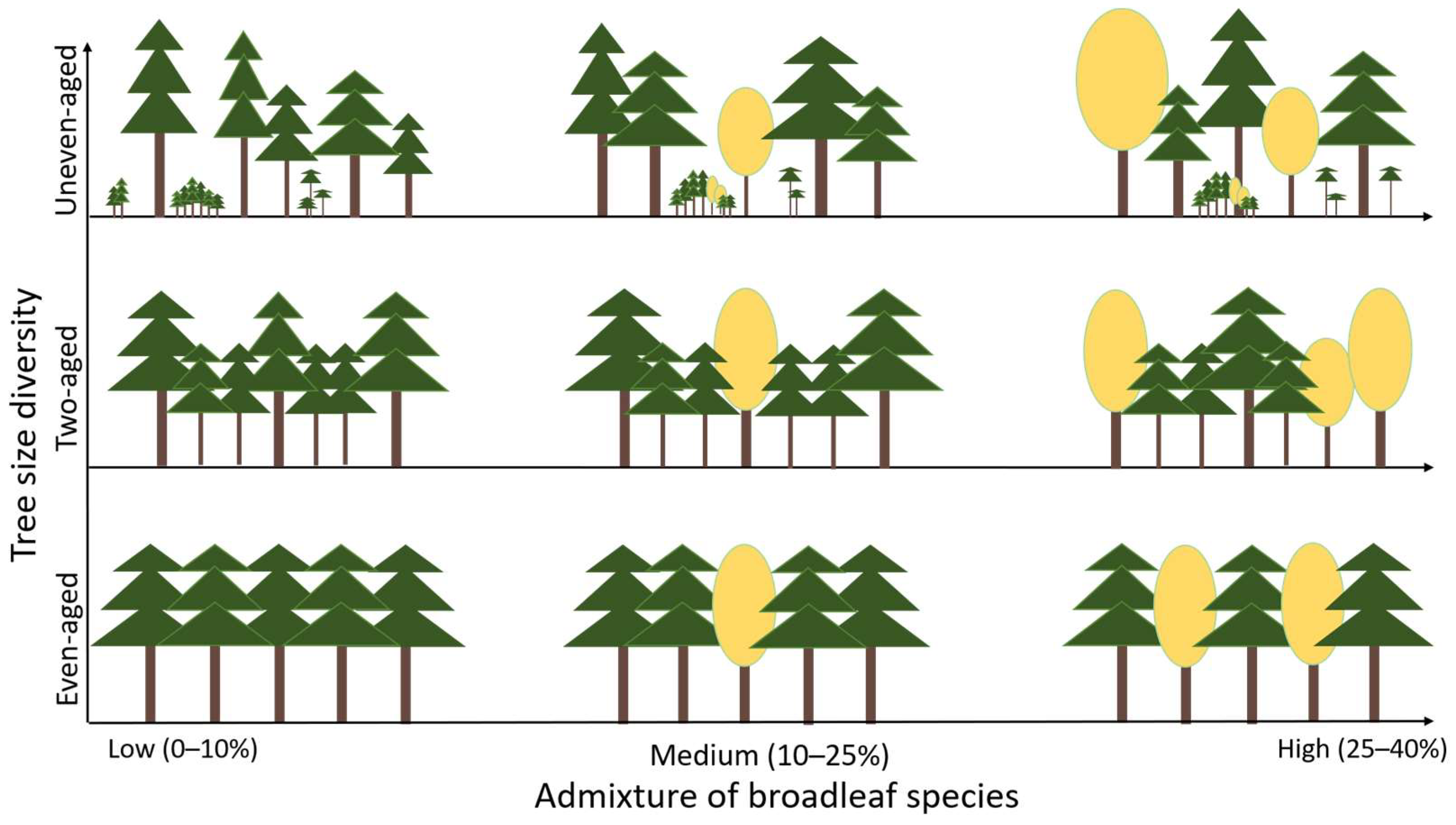
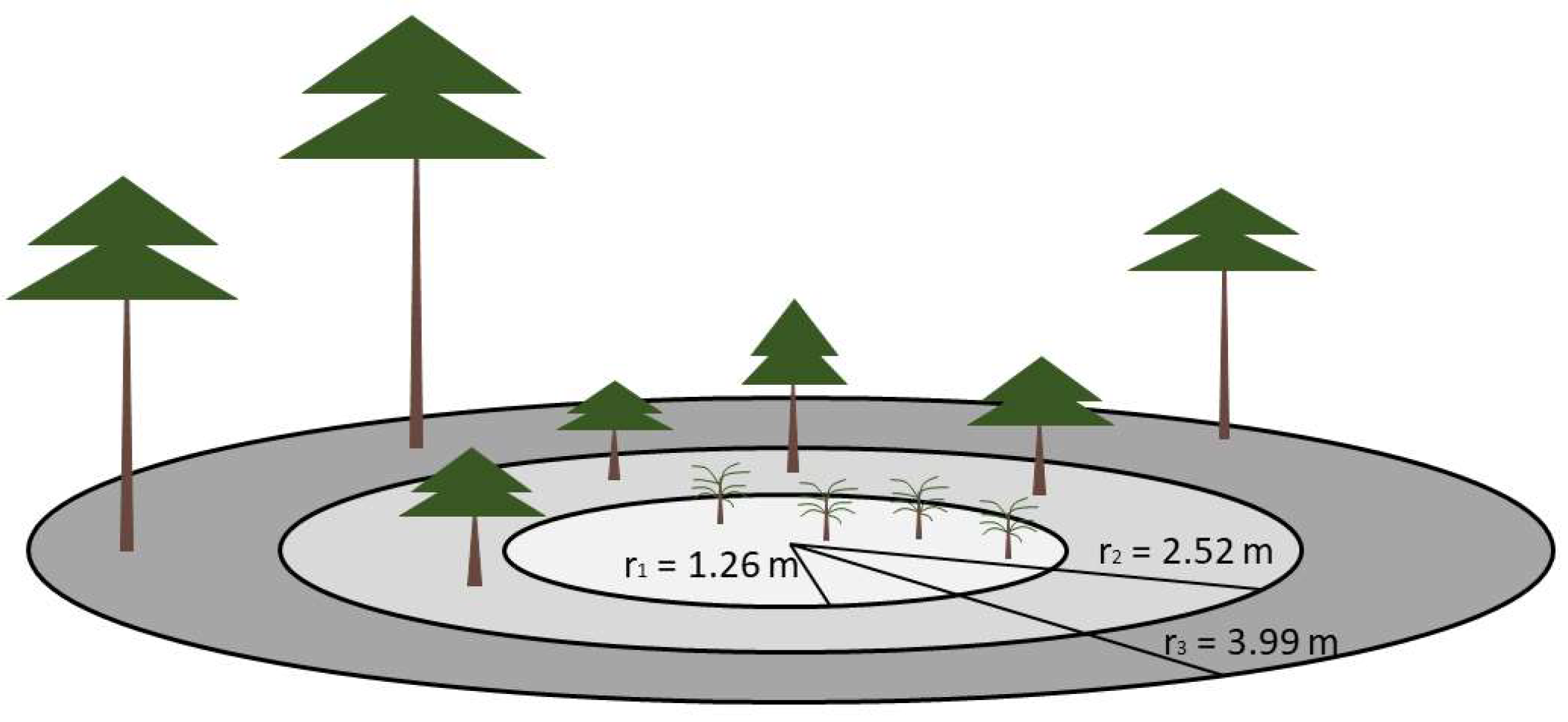
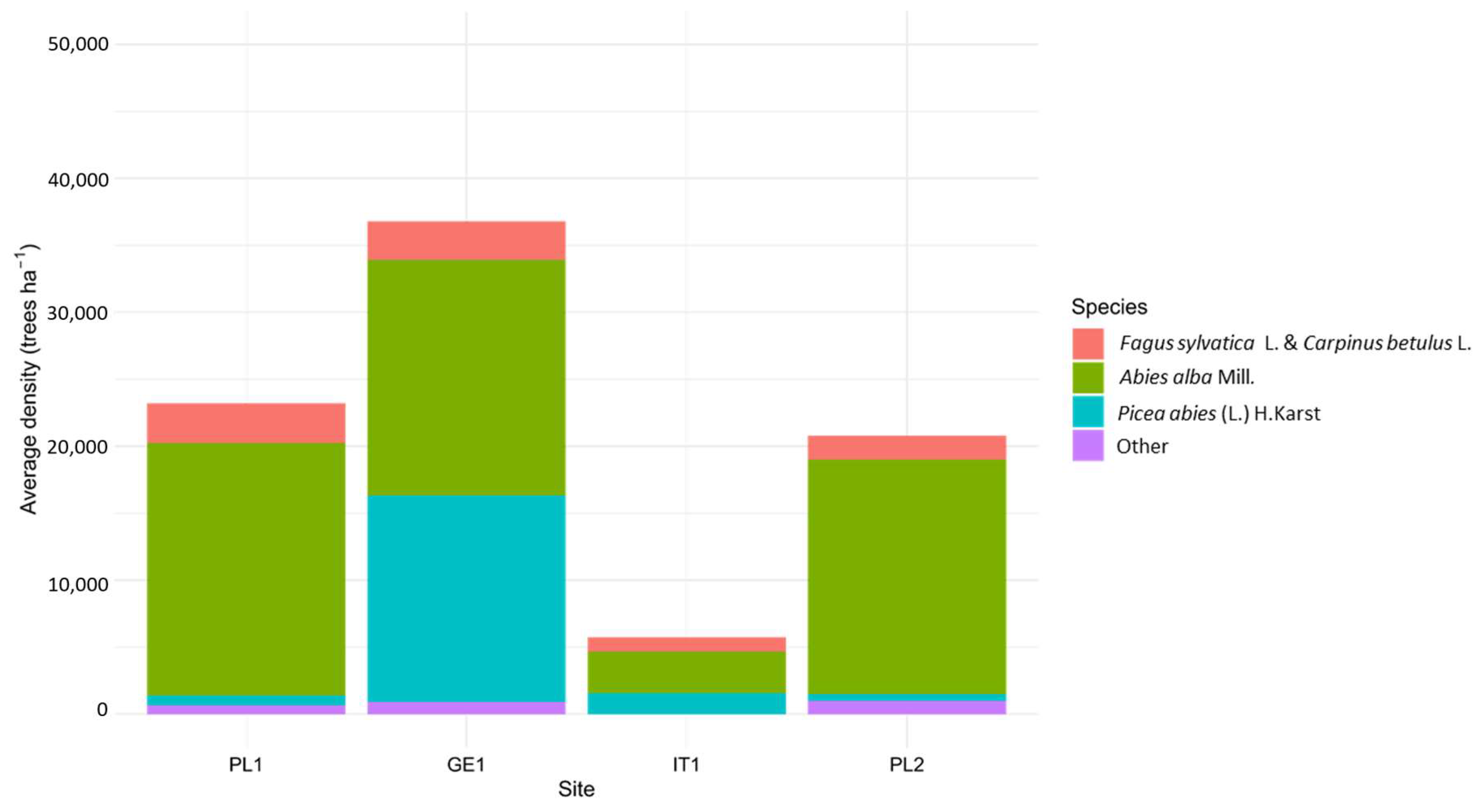
| Site | Elevation | Average Annual Temp. (C) * | Sum of Annual Precipitation (mm) * | Total Basal Area (m2 ha−1) | Admixture of Broadleaf Species (%) |
|---|---|---|---|---|---|
| Tissens-Laurein, Italy (IT1) | 1050–1750 (1320) | 3.1–8.8 (6.8) | 642–1336 (894) | 29.8–90.5 (53.4) | 0–34.3 (13.9) |
| Inzel, Germany (GE1) | 820–1140 (920) | 6.2–8.5 (7.6) | 1471–2081 (1805) | 40.7–70.1 (54.4) | 0–35.7 (11.5) |
| Zagnansk, Poland (PL1) | 320–400 (350) | 7.3–9.8 (8.7) | 531–816 (685) | 21.1–46.9 (34.1) | 0–36.5 (13.0) |
| Nawojowa, Poland (PL2) | 580–820 (650) | 8.1–10.5 (9.4) | 702–1087 (905) | 31.0–62.9 (45.4) | 0–58.8 (15.3) |
| Component | Variable | Description | Part of the Model/Model |
|---|---|---|---|
| Model 1 *—all species | |||
| Dependent Variable | Density_all_ha | The density of natural regeneration of all species per hectare | |
| Fixed Effect | Martonne | Martonne aridity index | Main Model and ZI Part |
| Fixed Effect | Admixture_broad | Admixture of broadleaf tree species | Main Model and ZI Part |
| Fixed Effect | TBA | Normalized by maximum total BA of living trees per hectare | Main Model |
| Interaction Term | Site × Admixture_broad | Interaction between site and admixture of broadleaf tree species | Main Model |
| Random Effect | Site | The factor for grouping plots by site | Main Model |
| Model 2—silver fir | |||
| Dependent Variable | Density_fir_ha | Density of natural regeneration of silver fir per hectare | |
| Fixed Effect | Martonne | Martonne aridity index | Main Model and ZI Part |
| Fixed Effect | Admixture_broad | Admixture of broadleaf tree species | Main Model |
| Interaction Term | Site × Admixture_broad | Interaction between site and admixture of broadleaf tree species | Main Model |
| Random Effect | Site | The factor for grouping plots by site | Main Model |
| Tested variables that were not included | |||
| Tree_size_div | Tree size diversity expressed as the normalized Shannon diversity index | Models 1 and 2 | |
| Ungulates_density | Density of the ungulates by site | Models 1 and 2 | |
| Admixture | Admixture of tree species other than fir by species | Models 1 and 2 | |
| TBA | Normalized by maximum total BA of living trees per hectare | Model 2 | |
| Component | Variable | Description |
|---|---|---|
| Dependent Variable | DS | Dominant developmental stage (DS) of silver fir regeneration |
| Fixed Effect | Martonne | Martonne aridity index |
| Fixed Effect | Tree_size_div | Tree size diversity expressed as the normalized Shannon diversity index |
| Interaction Term | Martonne × Tree_size_div | Interaction between site and tree size diversity |
| Random Effect | Site | A random intercept for each site |
| Tested variables that were not included | ||
| Admixture_broad | Admixture of broadleaf tree species | |
| TBA | Normalized by maximum total BA of living trees per hectare | |
| Ungulates_density | Density of the ungulates by site | |
| Predictors | Regeneration Presence | |||||
|---|---|---|---|---|---|---|
| All Species | Silver Fir | |||||
| Odds Ratios | CI | p | Odds Ratios | CI | p | |
| (Intercept) | 3.75 | 1.14–16.95 | 0.047 | 0.00 | 0.00–0.69 | 0.037 |
| Structure [Uneven-aged] | 6.91 | 2.09–26.26 | 0.002 | 3.42 | 1.29–9.06 | 0.014 |
| Site [IT1] | 0.13 | 0.02–0.51 | 0.007 | |||
| Site [PL1] | 1.75 | 0.26–14.62 | 0.568 | |||
| Temp | 2.43 | 1.16–5.08 | 0.018 | |||
| Random Effects | ||||||
| σ2 | 3.29 | |||||
| τ00 | 0.42 Site | |||||
| ICC | 0.11 | |||||
| N | 4 Site | |||||
| Observations | 105 | 139 | ||||
| R2 Tjur | 0.252 | 0.255/0.340 | ||||
| Predictors | sqrt(DS) | ||
|---|---|---|---|
| Estimates | CI | p | |
| (Intercept) | 0.96 | 0.68–1.35 | 0.813 |
| Martonne | 1.00 | 1.00–1.01 | 0.185 |
| Tree_size_div | 2.19 | 1.31–3.68 | 0.003 |
| Martonne × Tree_size_div | 0.99 | 0.98–1.00 | 0.035 |
| Random Effects | |||
| σ2 | 0.07 | ||
| τ00 Site | 0.01 | ||
| ICC | 0.07 | ||
| N Site | 4 | ||
| Observations | 77 | ||
| Marginal R2/Conditional R2 | 0.119/0.179 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kolisnyk, B.; Wellstein, C.; Czacharowski, M.; Drozdowski, S.; Bielak, K. Contrasting Regeneration Patterns in Abies alba-Dominated Stands: Insights from Structurally Diverse Mountain Forests across Europe. Forests 2024, 15, 1182. https://doi.org/10.3390/f15071182
Kolisnyk B, Wellstein C, Czacharowski M, Drozdowski S, Bielak K. Contrasting Regeneration Patterns in Abies alba-Dominated Stands: Insights from Structurally Diverse Mountain Forests across Europe. Forests. 2024; 15(7):1182. https://doi.org/10.3390/f15071182
Chicago/Turabian StyleKolisnyk, Bohdan, Camilla Wellstein, Marcin Czacharowski, Stanisław Drozdowski, and Kamil Bielak. 2024. "Contrasting Regeneration Patterns in Abies alba-Dominated Stands: Insights from Structurally Diverse Mountain Forests across Europe" Forests 15, no. 7: 1182. https://doi.org/10.3390/f15071182





