Root Signaling Substances Regulate Carbon Allocation Mechanism in the Plant and Soil of Peatlands under Permafrost Degradation
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. 13C Pulse Labeling
2.3. Sample Collection and Testing
2.4. Statistical Analysis
3. Results
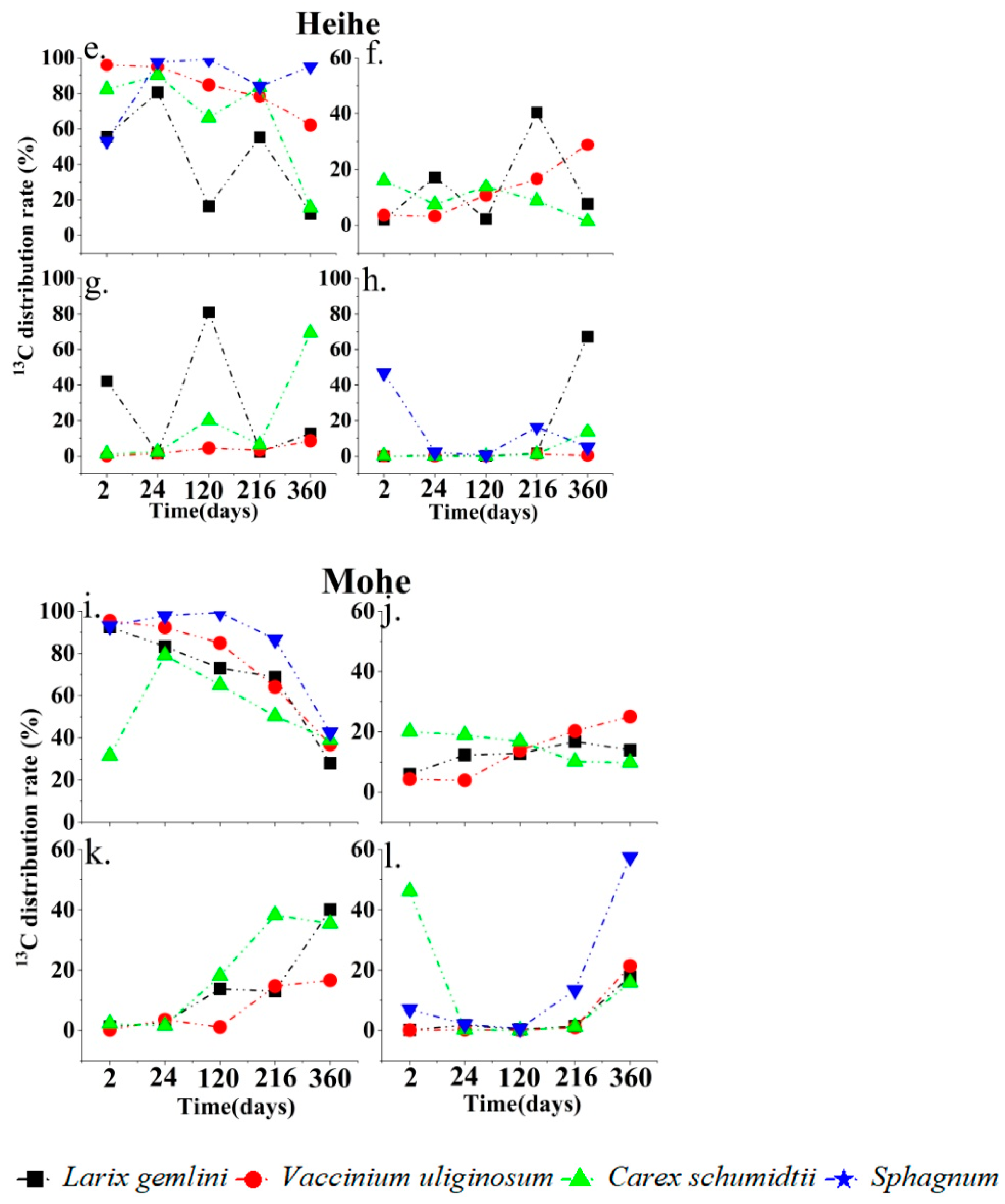
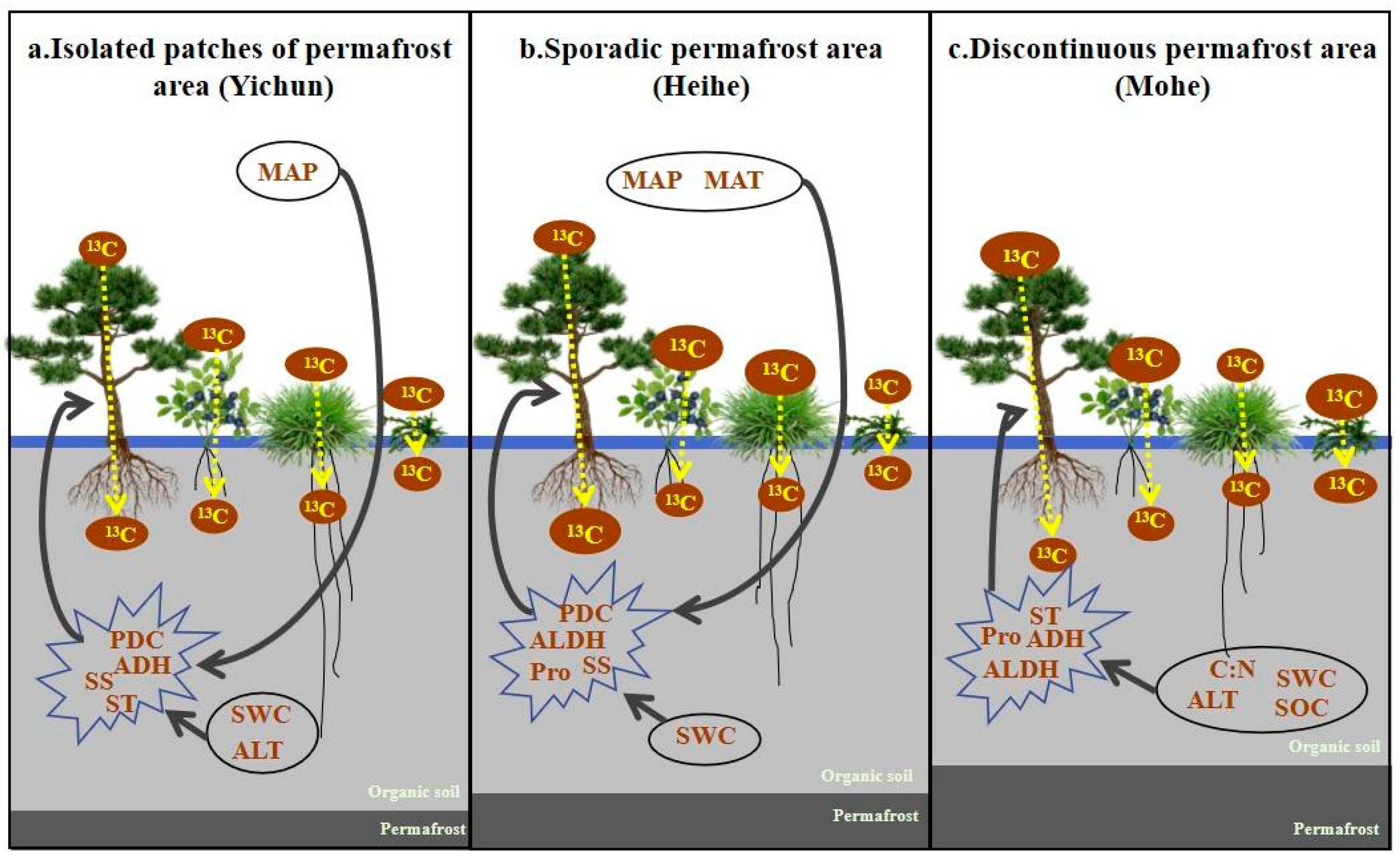
3.1. Carbon Allocation in the Plant–Soil System of Peatlands in Different Permafrost Regions
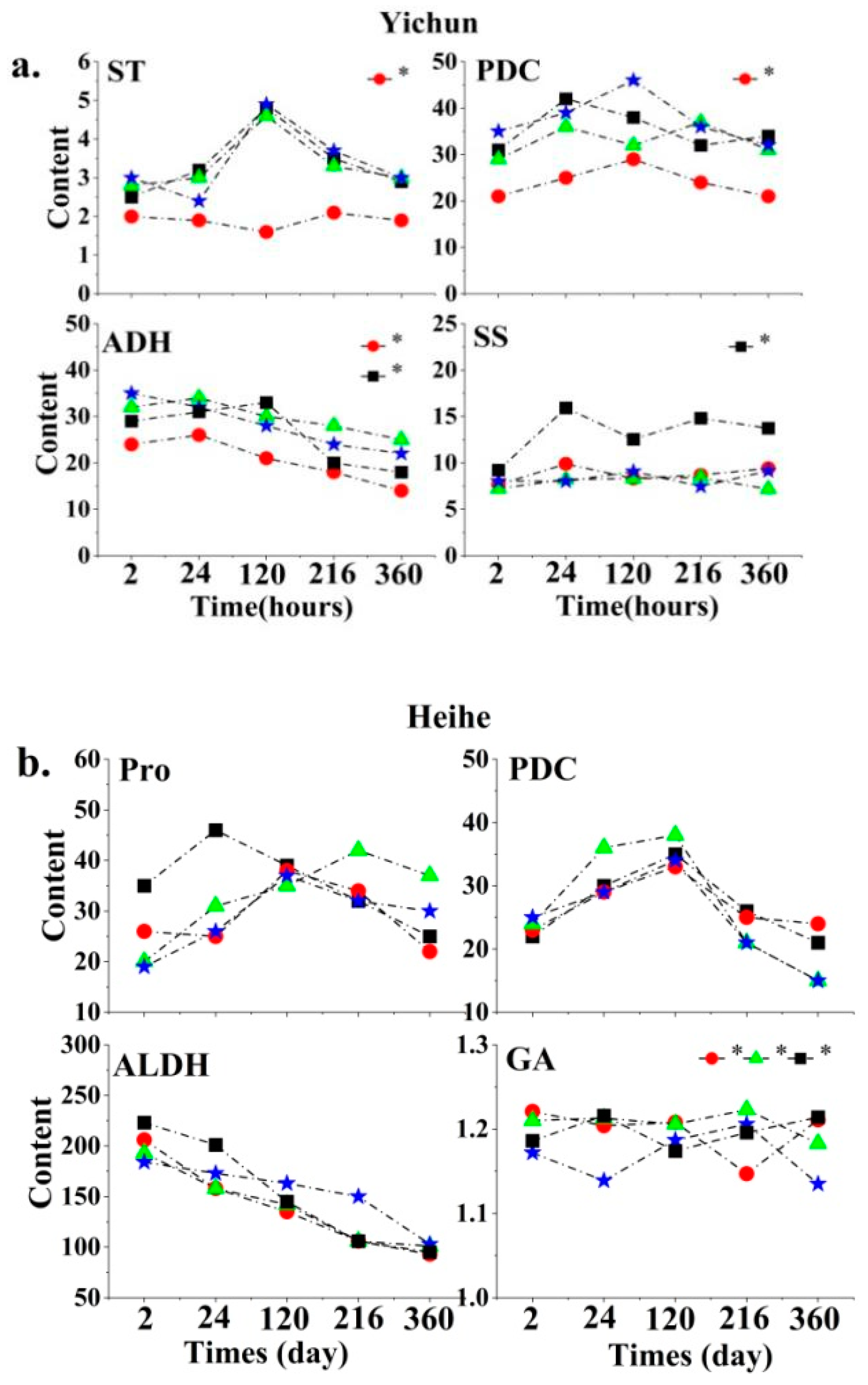
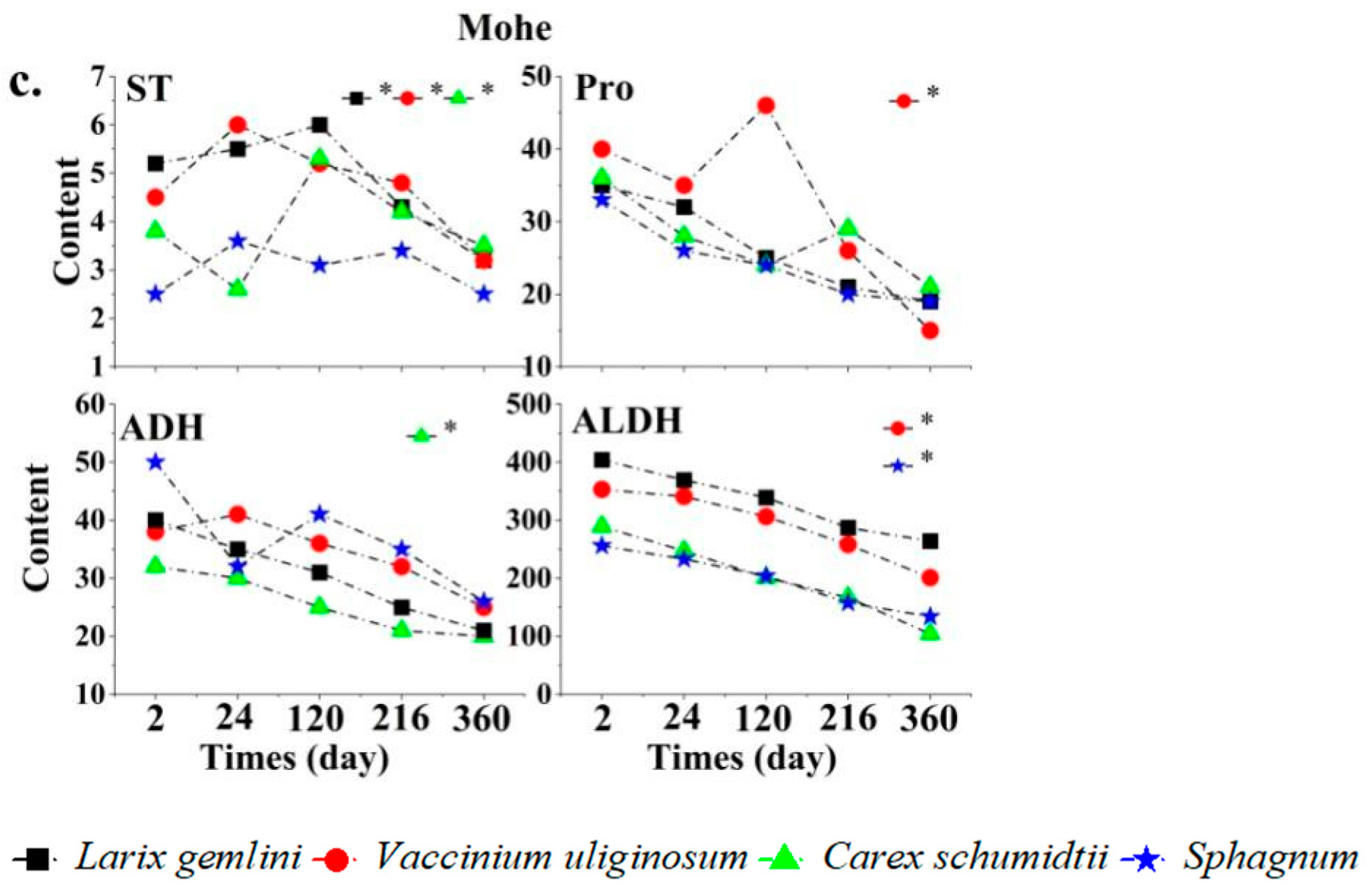
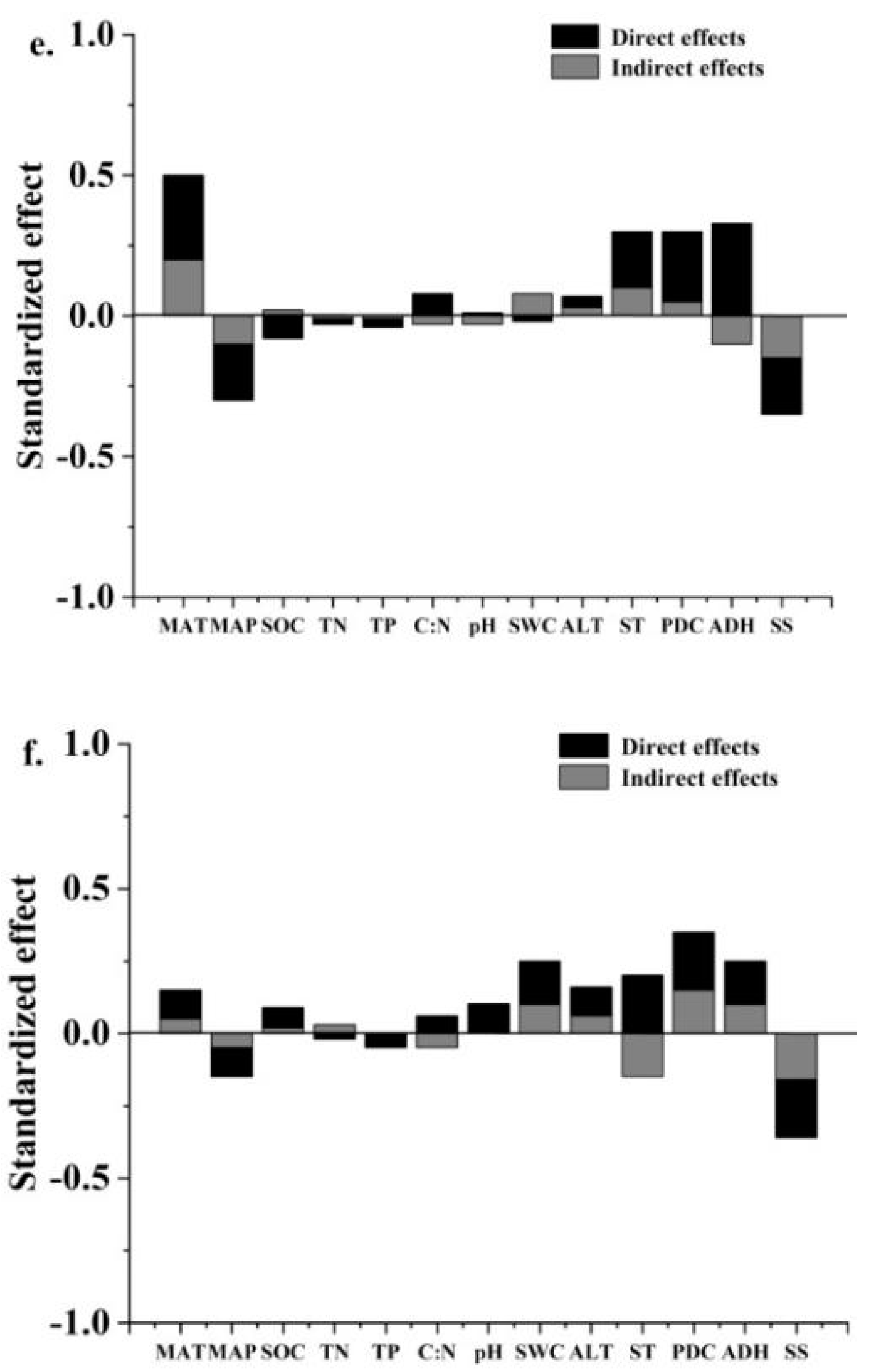
3.2. Main Root Signaling Substances Affecting Plant Growth in Peatlands in Different Permafrost Regions
3.3. Changes in Plant Root Signaling Substances with Labeling Time in Different Permafrost Regions
3.4. Root Signaling Substances Regulate Carbon Allocation in Plant–Soil Systems under Permafrost Degradation
4. Discussion
4.1. 13C Allocation for Plant–Soil Systems in Peatlands in Permafrost Regions
4.2. Effects of Permafrost Degradation on Plant Root Signaling Substances
4.3. Carbon Allocation in Plant–Soil System Affects Carbon Cycle under Permafrost Degradation
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wu, T.; Wang, D.; Mu, C.; Zhang, W.; Zhu, X.; Zhao, L.; Li, R.; Hu, G.; Zou, D.; Chen, J.; et al. Storage, patterns, and environmental controls of soil organic carbon stocks in the permafrost regions of the Northern Hemisphere. Sci. Total Environ. 2022, 828, 154464. [Google Scholar] [CrossRef] [PubMed]
- Keuper, F.; van Bodegom, P.M.; Dorrepaal, E.; Weedon, J.T.; van Hal, J.; van Logtestijn, R.S.P.; Aerts, R. A frozen feast: Thawing permafrost increases plant-available nitrogen in subarctic peatlands. Glob. Chang. Biol. 2012, 18, 1998–2007. [Google Scholar] [CrossRef]
- Mao, C.; Kou, D.; Chen, L.; Qin, S.; Zhang, D.; Peng, Y.; Yang, Y. Permafrost nitrogen status and its determinants on the Tibetan Plateau. Glob. Chang. Biol. 2020, 26, 5290–5302. [Google Scholar] [CrossRef]
- Voigt, C.; Marushchak, M.E.; Mastepanov, M.; Lamprecht, R.E.; Christensen, T.R.; Dorodnikov, M.; Jackowicz-Korczynski, M.; Lindgren, A.; Lohila, A.; Nykanen, H.; et al. Ecosystem carbon response of an Arctic peatland to simulated permafrost thaw. Glob. Chang. Biol. 2019, 25, 1746–1764. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Peng, Y.; Abbott, B.W.; Biasi, C.; Wei, B.; Zhang, D.; Wang, J.; Yu, J.; Li, F.; Wang, G.; et al. Phosphorus rather than nitrogen regulates ecosystem carbon dynamics after permafrost thaw. Glob. Chang. Biol. 2021, 27, 5818–5830. [Google Scholar] [CrossRef] [PubMed]
- Mekonnen, Z.A.; Riley, W.J.; Berner, L.T.; BouskiMAll, N.J.; Torn, M.S.; Iwahana, G.; Breen, A.L.; Myers-Smith, I.H.; Criado, M.G.; Liu, Y.; et al. Arctic tundra shrubification: A review of mechanisms and impacts on ecosystem carbon balance. Environ. Res. Lett. 2021, 16, 053001. [Google Scholar] [CrossRef]
- Dieleman, C.M.; Day, N.J.; Holloway, J.E.; Baltzer, J.; Douglas, T.A.; Turetsky, M.R. Carbon and nitrogen cycling dynamics following permafrost thaw in the Northwest Territories, Canada. Sci. Total Environ. 2022, 845, 157288. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xu, Y.; Wei, D.; Shi, L.; Jia, Z.; Yang, Y. Different chemical composition and storage mechanism of soil organic matter between active and permafrost layers on the Qinghai–Tibetan Plateau. J. Soils Sediments 2019, 20, 653–664. [Google Scholar] [CrossRef]
- Li, X.; Jin, H.; Sun, L.; Wang, H.; He, R.; Huang, Y.; Chang, X. Climate warming over 1961–2019 and impacts on permafrost zonation in Northeast China. J. For. Res. 2021, 33, 767–788. [Google Scholar] [CrossRef]
- Che, L.; Cheng, M.; Xing, L.; Cui, Y.; Wan, L. Effects of permafrost degradation on soil organic matter turnover and plant growth. Catena 2022, 208, 105721. [Google Scholar] [CrossRef]
- Standen, K.M.; Baltzer, J.L. Permafrost condition determines plant community composition and community-level foliar functional traits in a boreal peatland. Ecol. Evol. 2021, 11, 10133–10146. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Hu, Y.; Xiong, Z.; Yan, X.; Ren, B.; Bu, R. Spatiotemporal Variations of Growing-season NDVI Associated with Climate Change in Northeastern China’s Permafrost Zone. Pol. J. Environ. Stud. 2017, 26, 1521–1529. [Google Scholar] [CrossRef] [PubMed]
- Moore, T.R.; Alfonso, A.; Clarkson, B.R. Plant uptake of organic nitrogen in two peatlands. Plant Soil 2018, 433, 391–400. [Google Scholar] [CrossRef]
- Street, L.E.; Garnett, M.H.; Subke, J.A.; Baxter, R.; Dean, J.F.; Wookey, P.A. Plant carbon allocation drives turnover of old soil organic matter in permafrost tundra soils. Glob. Chang. Biol. 2020, 26, 4559–4571. [Google Scholar] [CrossRef]
- Wang, J.; Liu, D. Vegetation green-up date is more sensitive to permafrost degradation than climate change in spring across the northern permafrost region. Glob. Chang. Biol. 2021, 28, 1569–1582. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, N.; Westermann, S.; Lamba, S.; Shurpali, N.; Sannel, A.B.K.; Schurgers, G.; Miller, P.A.; Smith, B. Modelling past and future peatland carbon dynamics across the pan-Arctic. Glob. Chang. Biol. 2020, 26, 4119–4133. [Google Scholar] [CrossRef]
- Che, L.; Xuan, L.; Wan, L. Allocation and transfer of photosynthetic 13C in the vegetation-soil system and its response to permafrost degradation. Land Degrad. Dev. 2022, 34, 453–465. [Google Scholar] [CrossRef]
- Wan, X.; Yu, Z.; Wang, M.; Zhang, Y.; Huang, Z. Litter and root traits control soil microbial composition and enzyme activities in 28 common subtropical tree species. J. Ecol. 2022, 110, 3012–3022. [Google Scholar] [CrossRef]
- Yin, L.; Dijkstra, F.A.; Wang, P.; Zhu, B.; Cheng, W. Rhizosphere priming effects on soil carbon and nitrogen dynamics among tree species with and without intraspecific competition. New Phytol. 2018, 218, 1036–1048. [Google Scholar] [CrossRef]
- AbuQamar, S.; Moustafa, K.; Tran, L.S. Mechanisms and strategies of plant defense against Botrytis cinerea. Crit. Rev. Biotechnol. 2017, 37, 262–274. [Google Scholar] [CrossRef]
- Mundim, F.M.; Alborn, H.T.; Vieira-Neto, E.H.; Bruna, E.M. A whole-plant perspective reveals unexpected impacts of above- and belowground herbivores on plant growth and defense. Ecology 2017, 98, 70–78. [Google Scholar] [CrossRef]
- Desalme, D.; Priault, P.; Gerant, D.; Dannoura, M.; Maillard, P.; Plain, C.; Epron, D. Seasonal variations drive short-term dynamics and partitioning of recently assimilated carbon in the foliage of adult beech and pine. New Phytol. 2017, 213, 140–153. [Google Scholar] [CrossRef]
- Wang, X.; Li, M.; Jannasch, A.H.; Jiang, Y. Submergence stress alters fructan and hormone metabolism and gene expression in perennial ryegrass with contrasting growth habits. Environ. Exp. Bot. 2020, 179, 104202. [Google Scholar] [CrossRef]
- Gao, J.Q.; Gao, J.J.; Zhang, X.W.; Xu, X.L.; Deng, Z.H.; Yu, F.H. Effects of waterlogging on carbon assimilate partitioning in the Zoige alpine wetlands revealed by 13CO2 pulse labeling. Sci. Rep. 2015, 5, 9411. [Google Scholar] [CrossRef] [PubMed]
- Obersteiner, S.; Klein, T. Closing in on the last frontier: C allocation in the rhizosphere. Glob. Chang. Biol. 2022, 28, 6835–6837. [Google Scholar] [CrossRef]
- Li, L.; Shao, T.; Yang, H.; Chen, M.; Gao, X.; Long, X.; Shao, H.; Liu, Z.; Rengel, Z. The endogenous plant hormones and ratios regulate sugar and dry matter accumulation in Jerusalem artichoke in salt-soil. Sci. Total Environ. 2017, 578, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Ding, C.; Baerson, S.R.; Lian, F.; Lin, X.; Zhang, L.; Wu, C.; Hwang, S.Y.; Zeng, R.; Song, Y. The roles of jasmonate signalling in nitrogen uptake and allocation in rice (Oryza sativa L.). Plant Cell Environ. 2019, 42, 659–672. [Google Scholar] [CrossRef]
- Takahashi, F.; Suzuki, T.; Osakabe, Y.; Betsuyaku, S.; Kondo, Y.; Dohmae, N.; Fukuda, H.; Yamaguchi-Shinozaki, K.; Shinozaki, K. A small peptide modulates stomatal control via abscisic acid in long-distance signalling. Nature 2018, 556, 235–238. [Google Scholar] [CrossRef]
- Hikino, K.; Danzberger, J.; Riedel, V.P.; Hesse, B.D.; Hafner, B.D.; Gebhardt, T.; Rehschuh, R.; Ruehr, N.K.; Brunn, M.; Bauerle, T.L.; et al. Dynamics of initial carbon allocation after drought release in mature Norway spruce-Increased belowground allocation of current photoassimilates covers only half of the carbon used for fine-root growth. Glob. Chang. Biol. 2022, 28, 6889–6905. [Google Scholar] [CrossRef]
- Liu, Y.; Ge, T.; Ye, J.; Liu, S.; Shibistova, O.; Wang, P.; Wang, J.; Li, Y.; Guggenberger, G.; Kuzyakov, Y.; et al. Initial utilization of rhizodeposits with rice growth in paddy soils: Rhizosphere and N fertilization effects. Geoderma 2019, 338, 30–39. [Google Scholar] [CrossRef]
- Heinrich, S.; Dippold, M.A.; Werner, C.; Wiesenberg, G.L.; Kuzyakov, Y.; Glaser, B. Allocation of freshly assimilated carbon into primary and secondary metabolites after in situ 13C pulse labelling of Norway spruce (Picea abies). Tree Physiol. 2015, 35, 1176–1191. [Google Scholar] [PubMed]
- Che, L.; Zhang, H.; Wan, L. Spatial distribution of permafrost degradation and its impact on NDVI and vegetation phenology from 2000 to 2020. Sci. Total Environ. 2023, 887, 162889. [Google Scholar] [CrossRef] [PubMed]
- Obu, J.; Westermann, S.; Bartsch, A.; Berdnikov, N.; Christiansen, H.H.; Dashtseren, A.; Delaloye, R.; Elberling, B.; Etzelmüller, B.; Kholodov, A.; et al. Northern Hemisphere permafrost map based on TTOP modelling for 2000–2016 at 1 km2 scale. Earth-Sci. Rev. 2019, 193, 299–316. [Google Scholar] [CrossRef]
- Lefcheck, J.S.; Freckleton, R. piecewiseSEM: Piecewise structural equation modelling in r for ecology, evolution, and systematics. Methods Ecol. Evol. 2015, 7, 573–579. [Google Scholar] [CrossRef]
- Li, X.; Wen, B.; Yang, F.; Hartley, A.; Li, X. Effects of alternate flooding-drought conditions on degenerated Phragmites australis salt marsh in Northeast China. Restor. Ecol. 2022, 25, 810–819. [Google Scholar] [CrossRef]
- Zhao, T.; Sun, S.; Liu, Y. Regulating the drought-responsive element (DRE) mediated signaling pathway by synergic functions of transactive and trans-inactive DRE binding factors in Brassica napus. J. Biol. Chem. 2006, 281, 10752–10759. [Google Scholar] [CrossRef]
- Masyagina, O.; Prokushkin, A.; Kirdyanov, A.; Artyukhov, A.; Udalova, T.; Senchenkov, S.; Rublev, A. Intraseasonal carbon sequestration and allocation in larch trees growing on permafrost in Siberia after 13C labeling (two seasons of 2013–2014 observation). Photosynth. Res. 2016, 130, 267–274. [Google Scholar] [CrossRef]
- Hafner, S.; Unteregelsbacher, S.; Seeber, E.; Lena, B.; Xu, X.; Li, X.; Guggenberger, G.; Miehe, G.; Kuzyakov, Y. Effect of grazing on carbon stocks and assimilate partitioning in a Tibetan montane pasture revealed by 13CO2 pulse labeling. Glob. Chang. Biol. 2012, 18, 528–538. [Google Scholar] [CrossRef]
- Strauss, J.; Schirrmeister, L.; Grosse, G.; Fortier, D.; Hugelius, G.; Knoblauch, C.; Romanovsky, V.; Schädel, C.; Schneider von Deimling, T.; Schuur, E.A.G.; et al. Deep Yedoma permafrost: A synthesis of depositional characteristics and carbon vulnerability. Earth-Sci. Rev. 2017, 172, 75–86. [Google Scholar] [CrossRef]
- Schuur, E.A.G.; McGuire, A.D.; Schadel, C.; Grosse, G.; Harden, J.W.; Hayes, D.J.; Hugelius, G.; Koven, C.D.; Kuhry, P.; Lawrence, D.M.; et al. Climate change and the permafrost carbon feedback. Nature 2015, 520, 171–179. [Google Scholar] [CrossRef]
- Ping, C.L.; Jastrow, J.D.; Jorgenson, M.T.; Michaelson, G.J.; Shur, Y.L. Permafrost soils and carbon cycling. Soil 2015, 1, 147–171. [Google Scholar] [CrossRef]
- Li, F.; Peng, Y.; Chen, L.; Yang, G.; Abbott, B.W.; Zhang, D.; Fang, K.; Wang, G.; Wang, J.; Yu, J.; et al. Warming alters surface soil organic matter composition despite unchanged carbon stocks in a Tibetan permafrost ecosystem. Funct. Ecol. 2019, 34, 911–922. [Google Scholar] [CrossRef]
- Wei, H.; Man, X. Increased Litter Greatly Enhancing Soil Respiration in Betula platyphylla Forests of Permafrost Region, Northeast China. Forests 2021, 12, 89. [Google Scholar] [CrossRef]
- Liu, Y.; Ge, T.; Zhu, Z.; Liu, S.; Luo, Y.; Li, Y.; Wang, P.; Gavrichkova, O.; Xu, X.; Wang, J.; et al. Carbon input and allocation by rice into paddy soils: A review. Soil Biol. Biochem. 2019, 133, 97–107. [Google Scholar] [CrossRef]
- Schuur, E.A.G.; Crummer, K.G.; Vogel, J.G.; Mack, M.C. Plant Species Composition and Productivity following Permafrost Thaw and Thermokarst in Alaskan Tundra. Ecosystems 2007, 10, 280–292. [Google Scholar] [CrossRef]
- Jin, X.-Y.; Jin, H.-J.; Iwahana, G.; Marchenko, S.S.; Luo, D.-L.; Li, X.-Y.; Liang, S.-H. Impacts of climate-induced permafrost degradation on vegetation: A review. Adv. Clim. Chang. Res. 2021, 12, 29–47. [Google Scholar] [CrossRef]
- Fisher, J.P.; Estop-Aragones, C.; Thierry, A.; Charman, D.J.; Wolfe, S.A.; Hartley, I.P.; Murton, J.B.; Williams, M.; Phoenix, G.K. The influence of vegetation and soil characteristics on active-layer thickness of permafrost soils in boreal forest. Glob. Chang. Biol. 2016, 22, 3127–3140. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhuang, Q.; Zhao, D.; Zheng, D.; Kou, D.; Yang, Y. Permafrost Degradation Diminishes Terrestrial Ecosystem Carbon Sequestration Capacity on the Qinghai-Tibetan Plateau. Glob. Biogeochem. Cycles 2022, 36, e2021GB007068. [Google Scholar] [CrossRef]
- Bruhwiler, L.; Parmentier, F.-J.W.; Crill, P.; Leonard, M.; Palmer, P.I. The Arctic Carbon Cycle and Its Response to Changing Climate. Curr. Clim. Chang. Rep. 2021, 7, 14–34. [Google Scholar] [CrossRef]
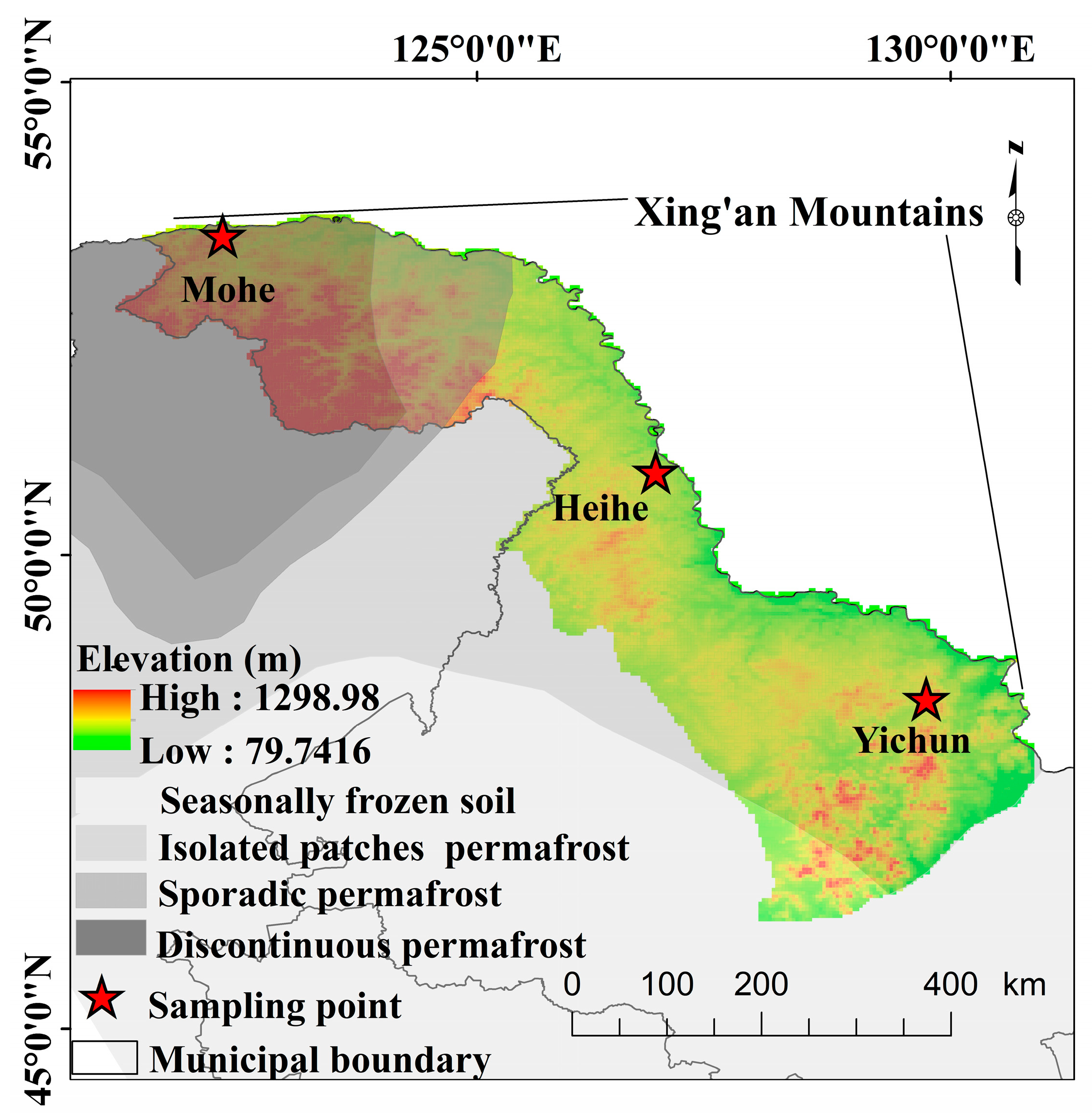




| Sites | Permafrost Zone | Forest Type | Active Layer Depth (m) |
|---|---|---|---|
| Yichun | Seasonally frozen soil | Shrub wetlands | 1.5 |
| Heihe | Sparsely patched permafrost | Shrub wetlands | 1.2 |
| Mohe | Predominantly continuous and island permafrost | Shrub wetlands | 1 |
| Scale of Inference | Scale at Which the Factor of Interest is Applied | Number of Replicates at the Appropriate Scale |
|---|---|---|
| Permafrost zones | Plant | 3 permafrost zones, 3 peatlands, 4 plant species, 3 samples were collected from each species at each time |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Che, L.; Qi, S.; Liu, S.; Wan, L. Root Signaling Substances Regulate Carbon Allocation Mechanism in the Plant and Soil of Peatlands under Permafrost Degradation. Forests 2024, 15, 1199. https://doi.org/10.3390/f15071199
Che L, Qi S, Liu S, Wan L. Root Signaling Substances Regulate Carbon Allocation Mechanism in the Plant and Soil of Peatlands under Permafrost Degradation. Forests. 2024; 15(7):1199. https://doi.org/10.3390/f15071199
Chicago/Turabian StyleChe, Lina, Shaoqun Qi, Shuo Liu, and Luhe Wan. 2024. "Root Signaling Substances Regulate Carbon Allocation Mechanism in the Plant and Soil of Peatlands under Permafrost Degradation" Forests 15, no. 7: 1199. https://doi.org/10.3390/f15071199
APA StyleChe, L., Qi, S., Liu, S., & Wan, L. (2024). Root Signaling Substances Regulate Carbon Allocation Mechanism in the Plant and Soil of Peatlands under Permafrost Degradation. Forests, 15(7), 1199. https://doi.org/10.3390/f15071199






