Non-Linear Relationships between Fine Root Functional Traits and Biomass in Different Semi-Arid Ecosystems on the Loess Plateau of China
Abstract
:1. Introduction
2. Materials and Methods
2.1. The Study Area
2.2. Research Methods and Sample Collection
2.3. Fine Root Functional Traits
2.4. Statistical Analysis
3. Result
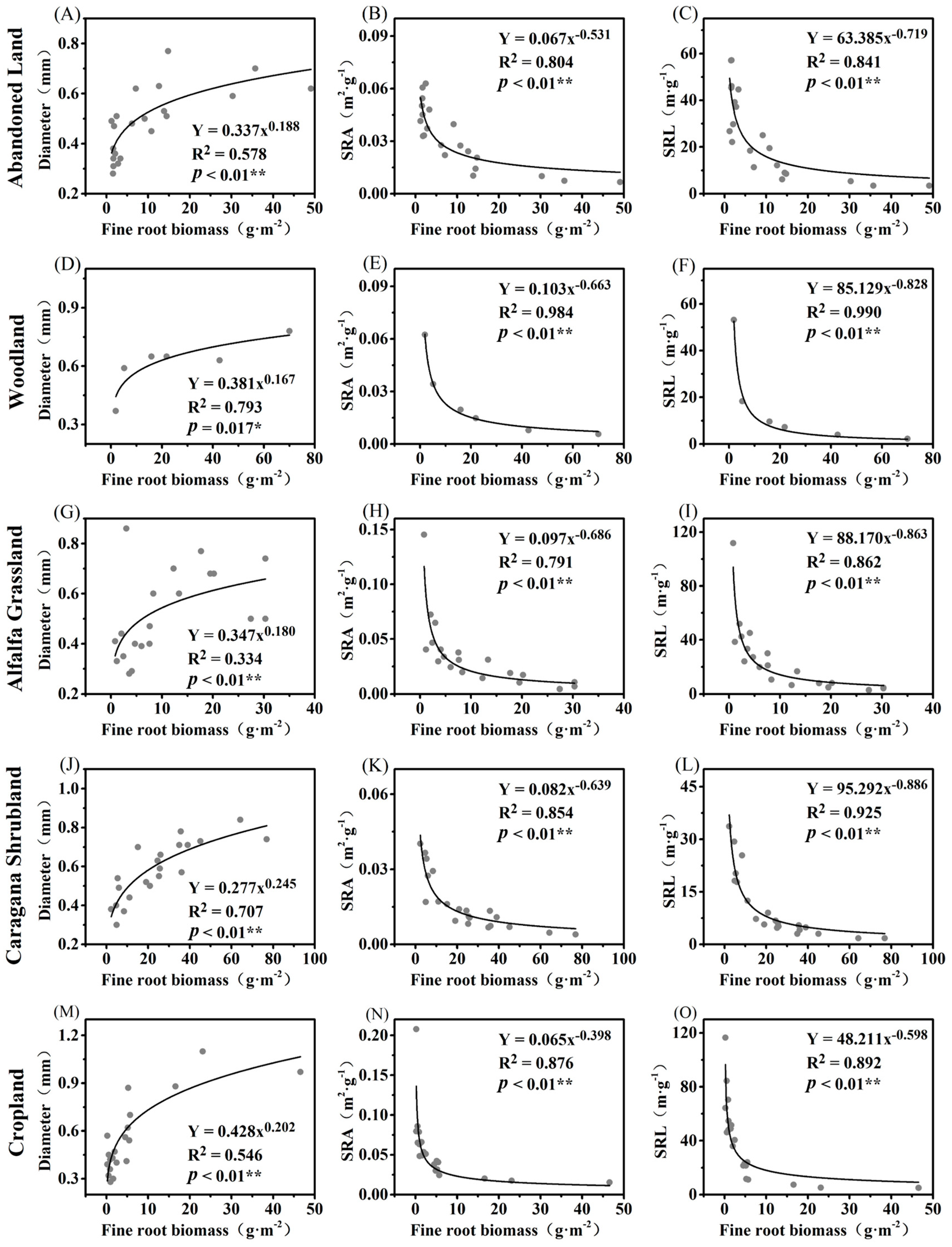
3.1. Root Functional Trait Model Based on Fine Root Biomass in Different Ecosystems
3.2. Test of the Regression Models
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Violle, C.; Navas, M.-L.; Vile, D.; Kazakou, E.; Fortunel, C.; Hummel, I.; Garnier, E. Let the concept of trait be functional! Oikos 2007, 116, 882–892. [Google Scholar] [CrossRef]
- Li, Y.; Sun, L.; Zhu, B. Trade-offs among fine-root phosphorus-acquisition strategies of 15 tropical woody species. For. Ecosyst. 2022, 9, 100055. [Google Scholar] [CrossRef]
- Pérez-Harguindeguy, N.; Díaz, S.; Garnier, E.; Lavorel, S.; Poorter, H.; Jaureguiberry, P.; Bret-Harte, M.S.; Cornwell, W.K.; Craine, J.M.; Gurvich, D.E.; et al. New handbook for standardised measurement of plant functional traits worldwide. Aust. J. Bot. 2013, 61, 167–234. [Google Scholar] [CrossRef]
- Stokes, A.; Atger, C.; Bengough, A.G.; Fourcaud, T.; Sidle, R.C. Desirable plant root traits for protecting natural and engineered slopes against landslides. Plant Soil 2009, 324, 1–30. [Google Scholar] [CrossRef]
- Roumet, C.; Lafont, F.; Sari, M.; Warembourg, F.; Garnier, E. Root traits and taxonomic affiliation of nine herbaceous species grown in glasshouse conditions. Plant Soil 2008, 312, 69–83. [Google Scholar] [CrossRef]
- Shen, Y.; Natalia Umaña, M.; Li, W.; Fang, M.; Chen, Y.; Lu, H.; Yu, S. Linking soil nutrients and traits to seedling growth: A test of the plant economics spectrum. For. Ecol. Manag. 2022, 505, 119941. [Google Scholar] [CrossRef]
- Han, M.; Sun, L.; Gan, D.; Fu, L.; Zhu, B. Root functional traits are key determinants of the rhizosphere effect on soil organic matter decomposition across 14 temperate hardwood species. Soil Biol. Biochem. 2020, 151, 108019. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, M.; Li, L.; Hou, J.; Zhang, X.; Li, H.; Yang, C.; Yang, L. Absorptive root-multidimension strategy links air temperature and species distribution in a montane forest. For. Ecosyst. 2023, 10, 100113. [Google Scholar] [CrossRef]
- Zhang, Y.; Cao, J.; Lu, M.; Kardol, P.; Wang, J.; Fan, G.; Kong, D. The origin of bi-dimensionality in plant root traits. Trends Ecol. Evol. 2024, 39, 78–88. [Google Scholar] [CrossRef]
- Roumet, C.; Birouste, M.; Picon-Cochard, C.; Ghestem, M.; Osman, N.; Vrignon-Brenas, S.; Cao, K.F.; Stokes, A. Root structure-function relationships in 74 species: Evidence of a root economics spectrum related to carbon economy. New Phytol. 2016, 210, 815–826. [Google Scholar] [CrossRef]
- Ye, Z.; Ryser, P. Root porosity contributes to root trait space of wetland monocotyledons independently of economics traits. Plant Soil 2021, 471, 301–314. [Google Scholar] [CrossRef]
- Li, J.; Le, X.; Chen, X.; Reich, P.B.; Niklas, K.J.; Li, X.; Wu, P.; Zhou, Y.; Zhong, Q.; Hu, D.; et al. Divergent intra- and interspecific root order variability identifies a two-dimensional root economics spectrum. Plant Soil 2024, 499, 473–490. [Google Scholar] [CrossRef]
- Weemstra, M.; Mommer, L.; Visser, E.J.; van Ruijven, J.; Kuyper, T.W.; Mohren, G.M.; Sterck, F.J. Towards a multidimensional root trait framework: A tree root review. New Phytol. 2016, 211, 1159–1169. [Google Scholar] [CrossRef]
- Fort, F. Grounding trait-based root functional ecology. Funct. Ecol. 2023, 37, 2159–2169. [Google Scholar] [CrossRef]
- Bardgett, R.D.; Mommer, L.; De Vries, F.T. Going underground: Root traits as drivers of ecosystem processes. Trends Ecol. Evol. 2014, 29, 692–699. [Google Scholar] [CrossRef]
- Jones, D.L.; Nguyen, C.; Finlay, R.D. Carbon flow in the rhizosphere: Carbon trading at the soil–root interface. Plant Soil 2009, 321, 5–33. [Google Scholar] [CrossRef]
- Balliu, A.; Sallaku, G. The environment temperature affects post-germination growth and root system architecture of pea (Pisum sativum L.) plants. Sci. Hortic. 2021, 278, 109858. [Google Scholar] [CrossRef]
- Walne, C.H.; Reddy, K.R. Temperature Effects on the Shoot and Root Growth, Development, and Biomass Accumulation of Corn (Zea mays L.). Agriculture 2022, 12, 443. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, Z.; Guan, Q.; Zhang, E.; Sun, Y.; Yan, Y.; Du, Q. Coupling mechanism between vegetation and multi-depth soil moisture in arid–semiarid area: Shift of dominant role from vegetation to soil moisture. For. Ecol. Manag. 2023, 546, 121323. [Google Scholar] [CrossRef]
- Zhou, G.; Zhou, X.; Nie, Y.; Bai, S.H.; Zhou, L.; Shao, J.; Cheng, W.; Wang, J.; Hu, F.; Fu, Y. Drought-induced changes in root biomass largely result from altered root morphological traits: Evidence from a synthesis of global field trials. Plant Cell Environ. 2018, 41, 2589–2599. [Google Scholar] [CrossRef]
- Beyer, F.; Hertel, D.; Leuschner, C. Fine root morphological and functional traits in Fagus sylvatica and Fraxinus excelsior saplings as dependent on species, root order and competition. Plant Soil 2013, 373, 143–156. [Google Scholar] [CrossRef]
- Huang, Y.; Ji, Z.; Tao, Y.; Wei, S.; Jiao, W.; Fang, Y.; Jian, P.; Shen, C.; Qin, Y.; Zhang, S.; et al. Improving rice nitrogen-use efficiency by modulating a novel monouniquitination machinery for optimal root plasticity response to nitrogen. Nat. Plants 2023, 9, 1902–1914. [Google Scholar] [CrossRef]
- Guo, Z.; Li, P.; Ma, L.; Yang, X.; Yang, J.; Wu, Y.; Liu, G.; Ritsema, C.J.; Geissen, V. Cascading effects from soil to maize functional traits explain maize response to microplastics disturbance in multi-nutrient soil environment. Geoderma 2024, 441, 116759. [Google Scholar] [CrossRef]
- Xu, F.; Liao, H.; Yang, J.; Zhang, Y.; Yu, P.; Cao, Y.; Fang, J.; Chen, S.; Li, L.; Sun, L.; et al. Auxin-producing bacteria promote barley rhizosheath formation. Nat. Commun. 2023, 14, 5800. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Cong, M.; Hu, Y.; Qiu, C.; Yang, Z.; Tang, G.; Xu, W.; Zhu, X.; Sun, X.; Jia, H. Biochar-mediated changes in the microbial communities of rhizosphere soil alter the architecture of maize roots. Front. Microbiol. 2022, 13, 1023444. [Google Scholar] [CrossRef]
- Wang, B.; Li, P.; Huang, C.; Liu, G.; Yang, Y. Effects of root morphological traits on soil detachment for ten herbaceous species in the Loess Plateau. Sci. Total Environ. 2021, 754, 142304. [Google Scholar] [CrossRef]
- Singh, K.; Gupta, S.; Singh, A.P. Review: Nutrient-nutrient interactions governing underground plant adaptation strategies in a heterogeneous environment. Plant Sci. 2024, 342, 112024. [Google Scholar] [CrossRef] [PubMed]
- Tracy, S.R.; Black, C.R.; Roberts, J.A.; Sturrock, C.; Mairhofer, S.; Craigon, J.; Mooney, S.J. Quantifying the impact of soil compaction on root system architecture in tomato (Solanum lycopersicum) by X-ray micro-computed tomography. Ann. Bot. 2012, 110, 511–519. [Google Scholar] [CrossRef]
- Kang, Y.; Yang, H.; Zeng, S.; Jiang, S.; Xie, C.; Wang, Z.; Dong, C.; Xu, Y.; Shen, Q. Mitigation of soil acidification in orchards: A case study to alleviate early defoliation in pear (Pyrus pyrifolia) trees. Rhizosphere 2021, 20, 100445. [Google Scholar] [CrossRef]
- Chen, G.; Hobbie, S.E.; Reich, P.B.; Yang, Y.; Robinson, D. Allometry of fine roots in forest ecosystems. Ecol. Lett. 2019, 22, 322–331. [Google Scholar] [CrossRef]
- Zanvo, S.M.G.; Mensah, S.; Salako, K.V.; Glèlè Kakaï, R. Tree height-diameter, aboveground and belowground biomass allometries for two West African mangrove species. Biomass Bioenerg. 2023, 176, 106917. [Google Scholar] [CrossRef]
- Huang, G.; Fang, Q.; Peng, S.; Li, Y. Genotypic variation of plant biomass under nitrogen deficiency is positively correlated with conservative economic traits in wheat. J. Exp. Bot. 2022, 73, 2175–2189. [Google Scholar] [CrossRef] [PubMed]
- Romillac, N.; Piutti, S.; Amiaud, B.; Slezack-Deschaumes, S. Effects of organic inputs derived from pea and wheat root functional traits on soil protease activities. Pedobiologia 2019, 77, 150576. [Google Scholar] [CrossRef]
- Li, S.; Nie, Z.; Sun, J.; Li, X.; Yang, G. The physiological role of abscisic acid in regulating root system architecture of alfalfa in its adaptation to water deficit. Agronomy 2022, 12, 1882. [Google Scholar] [CrossRef]
- Chopart, J.L.; Azevedo, M.C.B.; Le Mézo, L.; Marion, D. Functional Relationship Between Sugarcane Root Biomass and Length for Cropping System Applications. Sugar Tech 2011, 12, 317–321. [Google Scholar] [CrossRef]
- Jiang, X.; Jia, X.; Gao, S.; Jiang, Y.; Wei, N.; Han, C.; Zha, T.; Liu, P.; Tian, Y.; Qin, S. Plant nutrient contents rather than physical traits are coordinated between leaves and roots in a desert shrubland. Front. Plant Sci. 2021, 12, 734775. [Google Scholar] [CrossRef] [PubMed]
- Pierick, K.; Leuschner, C.; Homeier, J. Topography as a factor driving small-scale variation in tree fine root traits and root functional diversity in a species-rich tropical montane forest. New Phytol. 2020, 230, 129–138. [Google Scholar] [CrossRef]
- Tang, L.; Zhan, M.; Shang, C.; Yuan, J.; Wan, Y.; Qin, M. Dynamics of root exuded carbon and its relationships with root traits of rapeseed and wheat. Plant Soil Environ. 2021, 67, 317–323. [Google Scholar] [CrossRef]
- Zhang, L.; Xiao, J.; Zheng, Y.; Li, S.; Zhou, Y. Increased carbon uptake and water use efficiency in global semi-arid ecosystems. Environ. Res. Lett. 2020, 15, 034022. [Google Scholar] [CrossRef]
- Gong, X.; Li, Y.; Wang, X.; Zhang, Z.; Lian, J.; Ma, L.; Chen, Y.; Li, M.; Si, H.; Cao, W. Quantitative assessment of the contributions of climate change and human activities on vegetation degradation and restoration in typical ecologically fragile areas of China. Ecol. Indic. 2022, 144, 109536. [Google Scholar] [CrossRef]
- Liu, R.; Wang, D. Soil C, N, P and K stoichiometry affected by vegetation restoration patterns in the alpine region of the Loess Plateau, Northwest China. PLoS ONE 2020, 15, e0241859. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Jia, X.; An, J.; Huang, L.; Wang, L.; Shao, M. Afforestation vegetation uses water from very deep soil layers in the semi-arid Loess Plateau. Hydrol. Process. 2023, 37, e14933. [Google Scholar] [CrossRef]
- Corneo, P.E.; Keitel, C.; Kertesz, M.A.; Dijkstra, F.A. Variation in specific root length among 23 wheat genotypes affects leaf δ13C and yield. Agric. Ecosyst. Environ. 2017, 246, 21–29. [Google Scholar] [CrossRef]
- Zobel, R.W.; Kinraide, T.B.; Baligar, V.C. Fine root diameters can change in response to changes in nutrient concentrations. Plant Soil 2007, 297, 243–254. [Google Scholar] [CrossRef]
- Song, X.; Fang, C.; Yuan, Z.; Li, F. Long-Term Growth of Alfalfa Increased Soil Organic Matter Accumulation and Nutrient Mineralization in a Semi-Arid Environment. Front. Environ. Sci. 2021, 9, 649346. [Google Scholar] [CrossRef]
- Feng, X.; Wang, R.; Li, T.; Cai, J.; Liu, H.; Li, H.; Jiang, Y. Plant functional traits modulate the effects of soil acidification on above- and belowground biomass. Biogeosciences 2024, 21, 2641–2653. [Google Scholar] [CrossRef]
- Yuan, Z.; Feng, X.; Tian, J.; Song, X.; Li, G.; Fang, C. Controlling factors of soil organic carbon and nitrogen in lucerne grasslands in a semiarid environment. Catena 2022, 211, 105983. [Google Scholar] [CrossRef]
- Yuan, Z.; Fang, C.; Zhang, R.; Li, F.; Javaid, M.M.; Janssens, I.A. Topographic influences on soil properties and aboveground biomass in lucerne-rich vegetation in a semi-arid environment. Geoderma 2019, 344, 137–143. [Google Scholar] [CrossRef]
- Jiang, H.; Jiang, J.; Jia, Y.; Li, F.; Xu, J. Soil carbon pool and effects of soil fertility in seeded alfalfa fields on the semi-arid Loess Plateau in China. Soil Biol. Biochem. 2006, 38, 2350–2358. [Google Scholar] [CrossRef]
- McCormack, M.L.; Dickie, I.A.; Eissenstat, D.M.; Fahey, T.J.; Fernandez, C.W.; Guo, D.; Helmisaari, H.S.; Hobbie, E.A.; Iversen, C.M.; Jackson, R.B.; et al. Redefining fine roots improves understanding of below-ground contributions to terrestrial biosphere processes. New Phytol. 2015, 207, 505–518. [Google Scholar] [CrossRef]
- Ping, Q.; Fang, C.; Yuan, X.; Agathokleous, E.; He, H.; Zheng, H.; Feng, Z. Nitrogen addition changed the relationships of fine root respiration and biomass with key physiological traits in ozone-stressed poplars. Sci. Total Environ. 2023, 875, 162721. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, H.; Guo, K.; Li, W.; Feng, X.; Liu, X. Changes in soil properties induced by pioneer vegetation patches in coastal ecosystem. Catena 2021, 204, 105393. [Google Scholar] [CrossRef]
- Zhang, G.; Meng, W.; Pan, W.; Han, J.; Liao, Y. Effect of soil water content changes caused by ridge-furrow plastic film mulching on the root distribution and water use pattern of spring maize in the Loess Plateau. Agric. Water Manag. 2022, 261, 107338. [Google Scholar] [CrossRef]
- Fang, C.; Verbrigghe, N.; Sigurdsson, B.D.; Ostonen, I.; Leblans, N.I.W.; Marañón-Jiménez, S.; Fuchslueger, L.; Sigurðsson, P.; Meeran, K.; Portillo-Estrada, M.; et al. Decadal soil warming decreased vascular plant above and belowground production in a subarctic grassland by inducing nitrogen limitation. New Phytol. 2023, 240, 565–576. [Google Scholar] [CrossRef] [PubMed]
- Raklami, A.; Quintas-Nunes, F.; Nascimento, F.X.; Jemo, M.; Oufdou, K.; Syed, A.; Bahkali, A.H.; Verma, M.; Nafis, A. Assessing the growth-promoting traits of actinobacteria spp. isolated from Cleome africana: Implications on growth and root enhancement of Medicago sativa. J. King Saud Univ. Sci. 2023, 35, 102722. [Google Scholar] [CrossRef]
- Montagnoli, A.; Baronti, S.; Alberto, D.; Chiatante, D.; Scippa, G.S.; Terzaghi, M. Pioneer and fibrous root seasonal dynamics of Vitis vinifera L. are affected by biochar application to a low fertility soil: A rhizobox approach. Sci. Total Environ. 2021, 751, 141455. [Google Scholar] [CrossRef]
- Tan, W.H.; Ibrahim, H.; Chan, D.J.C. Estimation of mass, chlorophylls, and anthocyanins of Spirodela polyrhiza with smartphone acquired images. Comput. Electron. Agric. 2021, 190, 106449. [Google Scholar] [CrossRef]
- Hobson, D.J.; Harty, M.A.; Langton, D.; McDonnell, K.; Tracy, S.R. The establishment of winter wheat root system architecture in field soils: The effect of soil type on root development in a temperate climate. Soil Use Manag. 2023, 39, 198–208. [Google Scholar] [CrossRef] [PubMed]
- Clark, L.J.; Price, A.H.; Steele, K.A.; Whalley, W.R. Evidence from near-isogenic lines that root penetration increases with root diameter and bending stiffness in rice. Funct. Plant Biol. 2008, 35, 1163–1171. [Google Scholar] [CrossRef]
- Nasiri, M.R.; Amiri, E.; Behzadi, J.; Shahinrokhsar, P.; Mohammadian Roshan, N. A linear model for predicting olive yield using root characteristics. Rhizosphere 2024, 29, 100859. [Google Scholar] [CrossRef]
- Campbell, M.; Ortuño, J.; Koidis, A.; Theodoridou, K. The use of near-infrared and mid-infrared spectroscopy to rapidly measure the nutrient composition and the in vitro rumen dry matter digestibility of brown seaweeds. Anim. Feed Sci. Technol. 2022, 285, 115239. [Google Scholar] [CrossRef]
- Zhang, Y.; Freedman, Z.B.; Hartemink, A.E.; Whitman, T.; Huang, J. Characterizing soil microbial properties using MIR spectra across 12 ecoclimatic zones (NEON sites). Geoderma 2022, 409, 115647. [Google Scholar] [CrossRef]
- Yusup, A.; Halik, Ü.; Keyimu, M.; Aishan, T.; Abliz, A.; Dilixiati, B.; Wei, J. Trunk volume estimation of irregular shaped Populus euphratica riparian forest using TLS point cloud data and multivariate prediction models. For. Ecosyst. 2023, 10, 100082. [Google Scholar] [CrossRef]
- Sherratt, E.; McCullough, E.L.; Painting, C.J. Commentary: The ecological and evolutionary implications of allometry. Evol. Ecol. 2022, 36, 431–437. [Google Scholar] [CrossRef]
- Zhao, M.; Tian, S.; Zhu, Y.; Li, Z.; Zeng, S.; Liu, S. Allometric relationships, functional differentiations, and scaling of growth rates across 151 tree species in China. Ecosphere 2021, 12, e03522. [Google Scholar] [CrossRef]
- Peichl, M.; Arain, M.A. Allometry and partitioning of above- and belowground tree biomass in an age-sequence of white pine forests. For. Ecol. Manag. 2007, 253, 68–80. [Google Scholar] [CrossRef]
- Ishihara, M.I.; Utsugi, H.; Tanouchi, H.; Aiba, M.; Kurokawa, H.; Onoda, Y.; Nagano, M.; Umehara, T.; Ando, M.; Miyata, R.; et al. Efficacy of generic allometric equations for estimating biomass: A test in Japanese natural forests. Ecol. Appl. 2015, 25, 1433–1446. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Wang, X. Biomass partitioning and allometric relations of the Reaumuria soongorica shrub in Alxa steppe desert in NW China. For. Ecol. Manag. 2020, 468, 118178. [Google Scholar] [CrossRef]
- Zhang, J.; Fiddler, G.O.; Young, D.H.; Shestak, C.; Carlson, R. Allometry of tree biomass and carbon partitioning in ponderosa pine plantations grown under diverse conditions. For. Ecol. Manag. 2021, 497, 119526. [Google Scholar] [CrossRef]
- Annighöfer, P.; Mund, M.; Seidel, D.; Ammer, C.; Ameztegui, A.; Balandier, P.; Bebre, I.; Coll, L.; Collet, C.C.; Hamm, T.; et al. Examination of aboveground attributes to predict belowground biomass of young trees. For. Ecol. Manag. 2022, 505, 119942. [Google Scholar] [CrossRef]
- Hiernaux, P.; Issoufou, H.B.-A.; Igel, C.; Kariryaa, A.; Kourouma, M.; Chave, J.; Mougin, E.; Savadogo, P. Allometric equations to estimate the dry mass of Sahel woody plants mapped with very-high resolution satellite imagery. For. Ecol. Manag. 2023, 529, 120653. [Google Scholar] [CrossRef]
- Kong, D.; Wang, J.; Wu, H.; Valverde-Barrantes, O.J.; Wang, R.; Zeng, H.; Kardol, P.; Zhang, H.; Feng, Y. Nonlinearity of root trait relationships and the root economics spectrum. Nat. Commun. 2019, 10, 2203. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Li, K.M.; Liu, M.; Wang, W.; Fan, C.; Xu, X.; Cui, X. Variations in plant root traits shaped by intraspecific interactions are species-specific. Rhizosphere 2024, 30, 100889. [Google Scholar] [CrossRef]
- Yang, L.; Wu, P.-P.; Liao, M.-F.; Peng, J.; Tang, Z.-Z.; Long, H.-B.; Yu, X.-Y.; Zhang, H.-H. Three- dimensional modeling and visualization of rice root system based on the improved dual-scale automaton and L-system. Comput. Electron. Agric. 2022, 195, 106823. [Google Scholar] [CrossRef]
- Xu, H.; Wang, X.; Liu, C.; Chen, J.; Zhang, C. A 3D root system morphological and mechanical model based on L-Systems and its application to estimate the shear strength of root-soil composites. Soil Till. Res. 2021, 212, 105074. [Google Scholar] [CrossRef]
- Lu, H.; Yuan, W.; Chen, X. A processes-based dynamic root growth model integrated into the ecosystem model. J. Adv. Model. Earth Syst. 2019, 11, 4614–4628. [Google Scholar] [CrossRef]
- Pagès, L.; Vercambre, G.; Drouet, J.L.; Lecompte, F.; Collet, C.; Bot, J.L. Root Typ: A generic model to depict and analyse the root system architecture. Plant Soil 2004, 258, 103–119. [Google Scholar] [CrossRef]
- Zhou, Y.; Ma, H.; Lu, Q.; Ma, J.; Shen, Y.; Wang, G. Different responses of leaf and root economics spectrum to grazing time at the community level in desert steppe, China. Sci. Total Environ. 2024, 909, 168547. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Wang, X.; Hou, F. A general pattern of plant traits and their relationships with environmental factors and microbial life-history strategies. Sci. Total Environ. 2024, 931, 172670. [Google Scholar] [CrossRef]
- Zhang, L.-L.; Khamphilavong, K.; Zhu, H.-C.; Li, H.; He, X.-J.; Shen, X.-F.; Wang, L.-R.; Kang, Y.-X. Allometric scaling relationships of Larix potaninii subsp. chinensis traits across topographical gradients. Ecol. Indic. 2021, 125, 107492. [Google Scholar] [CrossRef]
- Guo, Z.; Lin, H.; Chen, S.; Yang, Q. Altitudinal Patterns of Leaf Traits and Leaf Allometry in Bamboo Pleioblastus amarus. Front. Plant Sci. 2018, 9, 1110. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Hu, Y.; Zhao, P. Interspecific variations in tree allometry and functional traits in subtropical plantations in southern China. Funct. Plant Biol. 2020, 47, 558–564. [Google Scholar] [CrossRef] [PubMed]
- McIntire, C.D.; Cunliffe, A.M.; Boschetti, F.; Litvak, M.E. Allometric Relationships for Predicting Aboveground Biomass, Sapwood, and Leaf Area of Two-Needle Piñon Pine (Pinus edulis) Amid Open-Grown Conditions in Central New Mexico. For. Sci. 2022, 68, 152–161. [Google Scholar] [CrossRef]
- Gray, E.F.; Wright, I.J.; Falster, D.S.; Eller, A.S.D.; Lehmann, C.E.R.; Bradford, M.G.; Cernusak, L.A. Leaf:wood allometry and functional traits together explain substantial growth rate variation in rainforest trees. AoB Plants 2019, 11, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Guo, D.; Xu, X.; Lu, M.; Bardgett, R.D.; Eissenstat, D.M.; McCormack, M.L.; Hedin, L.O. Evolutionary history resolves global organization of root functional traits. Nature 2018, 555, 94–97. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, Y.; Li, W.; Liu, W.; Gu, X.; Guan, Z. Effects of tree functional diversity and environmental gradients on belowground biomass in a natural old-growth forest ecosystem. Can. J. For. Res. 2019, 49, 1623–1632. [Google Scholar] [CrossRef]
- Comas, L.H.; Anderson, L.J.; Dunst, R.M.; Lakso, A.N.; Eissenstat, D.M. Canopy and environmental control of root dynamics in a long-term study of Concord grape. New Phytol. 2005, 167, 829–840. [Google Scholar] [CrossRef] [PubMed]
- Comas, L.H.; Bauerle, T.L.; Eissenstat, D.M. Biological and environmental factors controlling root dynamics and function: Effects of root ageing and soil moisture. Aust. J. Grape Wine Res. 2010, 16, 131–137. [Google Scholar] [CrossRef]
- Roybal, C.M.; Butterfield, B.J. Species-specific trait-environment relationships among populations of widespread grass species. Oecologia 2019, 189, 1017–1026. [Google Scholar] [CrossRef]
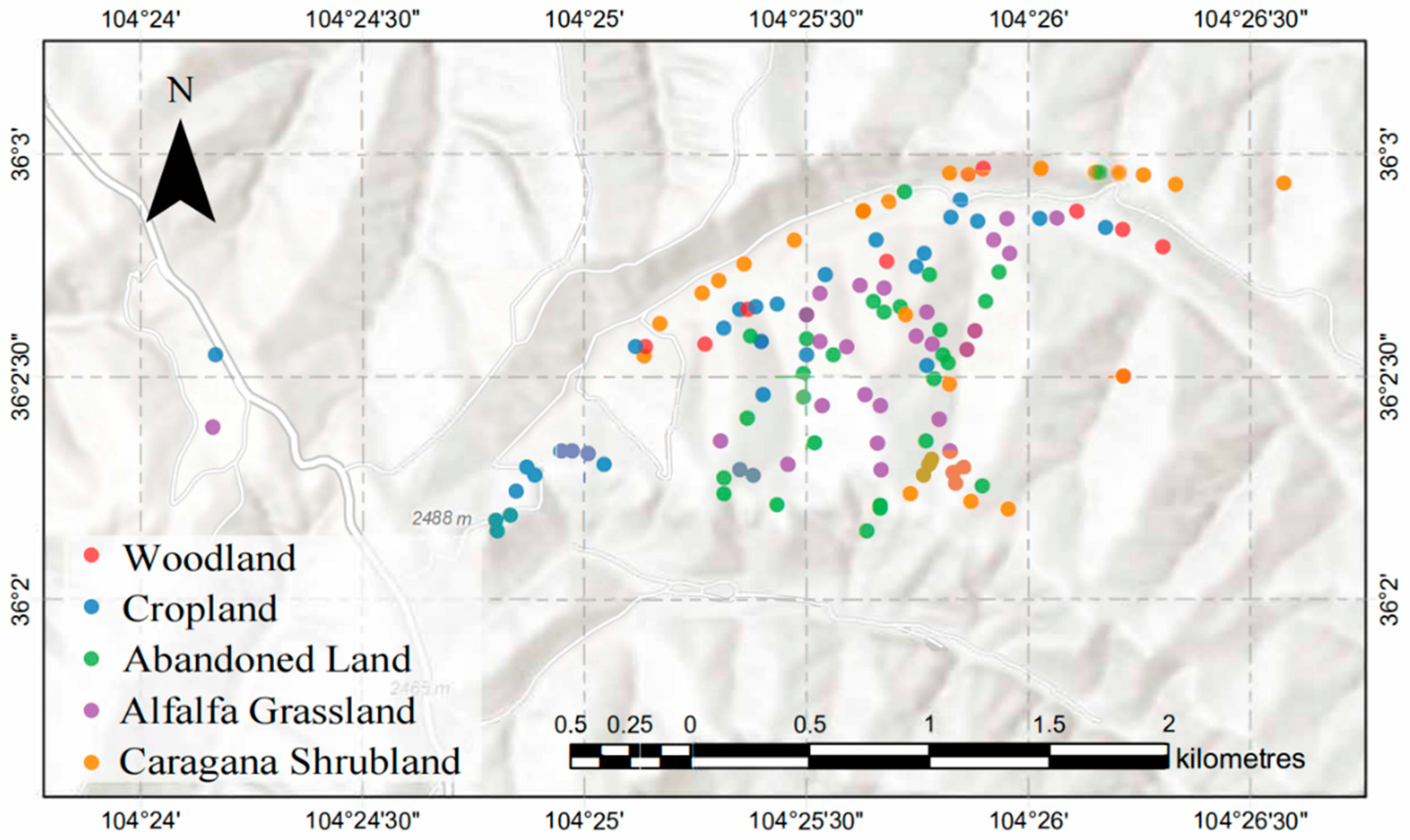



| Ecosystem Type | pH | BD | SOC | TN | TP |
|---|---|---|---|---|---|
| (g cm−3) | (g kg−1) | (g kg−1) | (g kg−1) | ||
| Abandoned lands | 8.44 ± 0.16 | 1.19 ± 0.14 | 9.29 ± 3.22 | 1.19 ± 0.38 | 0.61 ± 0.06 |
| Woodlands | 8.33 ± 0.19 | 1.14 ± 0.15 | 9.78 ± 4.52 | 1.27 ± 0.58 | 0.66 ± 0.05 |
| Alfalfa grasslands | 8.39 ± 0.20 | 1.24 ± 0.12 | 10.01 ± 3.80 | 1.38 ± 0.55 | 0.64 ± 0.05 |
| Caragana shrublands | 8.36 ± 0.23 | 1.16 ± 0.10 | 9.75 ± 3.39 | 1.56 ± 0.57 | 0.57 ± 0.04 |
| Croplands | 8.38 ± 0.20 | 1.26 ± 0.08 | 9.17 ± 3.93 | 1.24 ± 0.48 | 0.71 ± 0.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, Z.; Wang, R.; Sun, Z.; Peng, Y.; Jiang, M.; Wu, S.; Yuan, Z.; Song, X.; Fang, C.; Sardans, J. Non-Linear Relationships between Fine Root Functional Traits and Biomass in Different Semi-Arid Ecosystems on the Loess Plateau of China. Forests 2024, 15, 1226. https://doi.org/10.3390/f15071226
Tian Z, Wang R, Sun Z, Peng Y, Jiang M, Wu S, Yuan Z, Song X, Fang C, Sardans J. Non-Linear Relationships between Fine Root Functional Traits and Biomass in Different Semi-Arid Ecosystems on the Loess Plateau of China. Forests. 2024; 15(7):1226. https://doi.org/10.3390/f15071226
Chicago/Turabian StyleTian, Zhun, Rui Wang, Zihan Sun, Yang Peng, Mingfeng Jiang, Shiqi Wu, Ziqiang Yuan, Xin Song, Chao Fang, and Jordi Sardans. 2024. "Non-Linear Relationships between Fine Root Functional Traits and Biomass in Different Semi-Arid Ecosystems on the Loess Plateau of China" Forests 15, no. 7: 1226. https://doi.org/10.3390/f15071226





