Leaf Trait Variations and Ecological Adaptation Mechanisms of Populus euphratica at Different Developmental Stages and Canopy Heights
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area, Sampling, and Measurements
2.2. Measurement and Data Collection
2.2.1. Structural Traits in Leaf Anatomy and Morphology
2.2.2. Leaf Nutrients, Carbon Isotopes, and Chemical Traits of Endogenous Hormones
2.2.3. Leaf Photosynthetic Indices and Physiological Traits of Proline and Malondialdehyde Concentrations
2.3. LTN Establishment
2.4. Statistical Analysis
3. Results
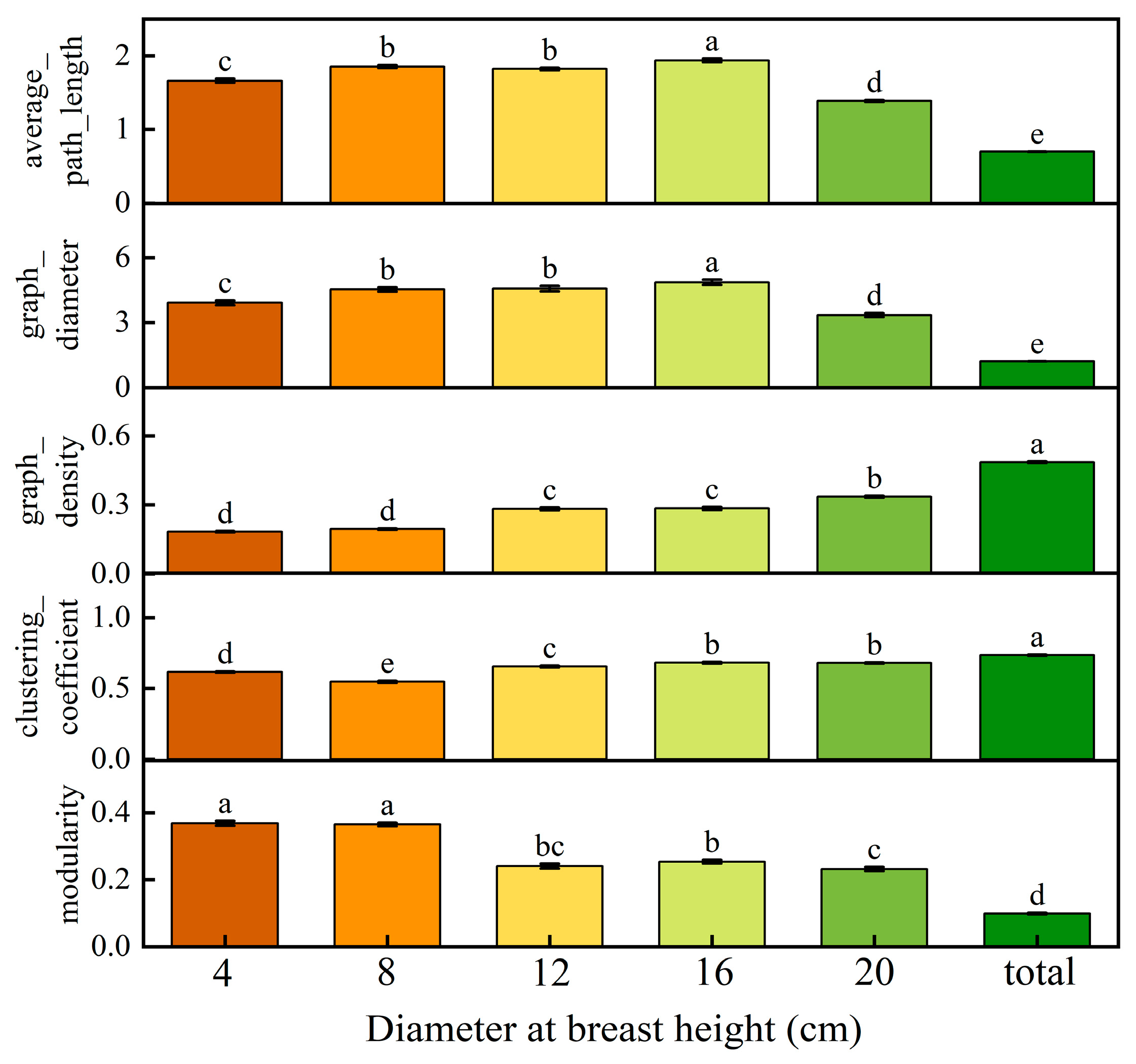
3.1. Developmental Stage P. euphratica LTN Characteristics
3.2. Central and Linkage Traits in Developmental Stage of P. euphratica (LTN)
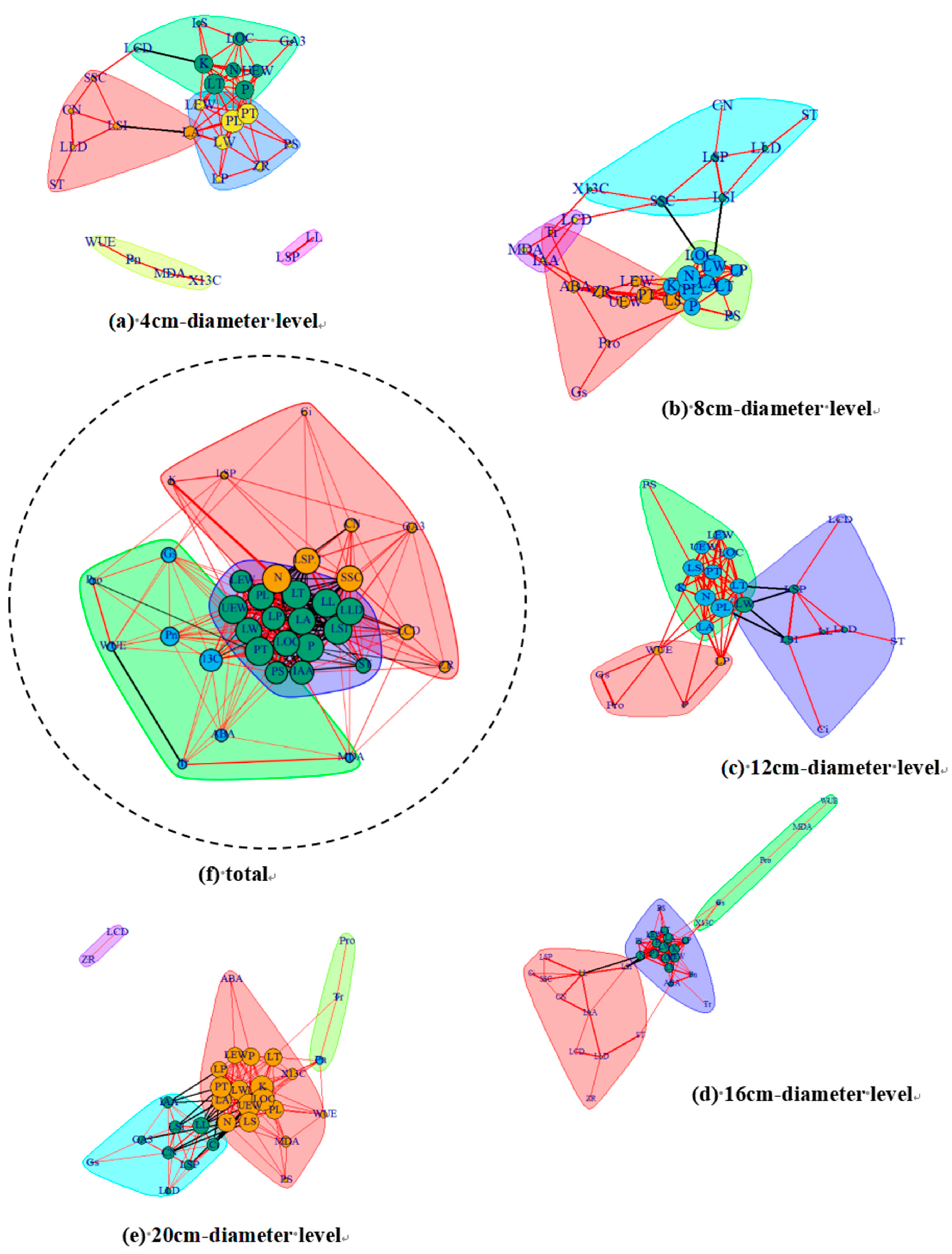
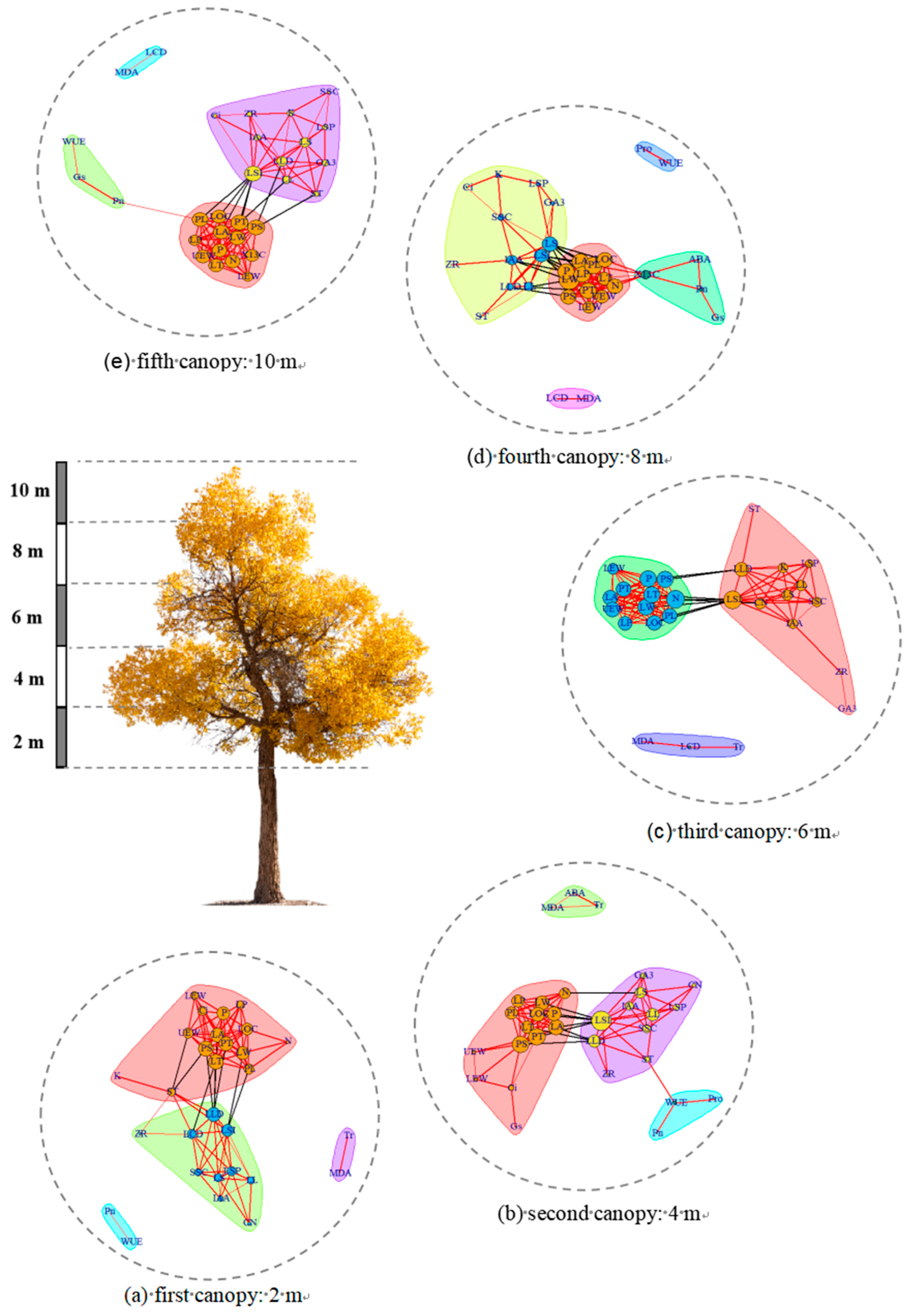
3.3. LTN Characterization in Different Mature P. euphratica Canopies
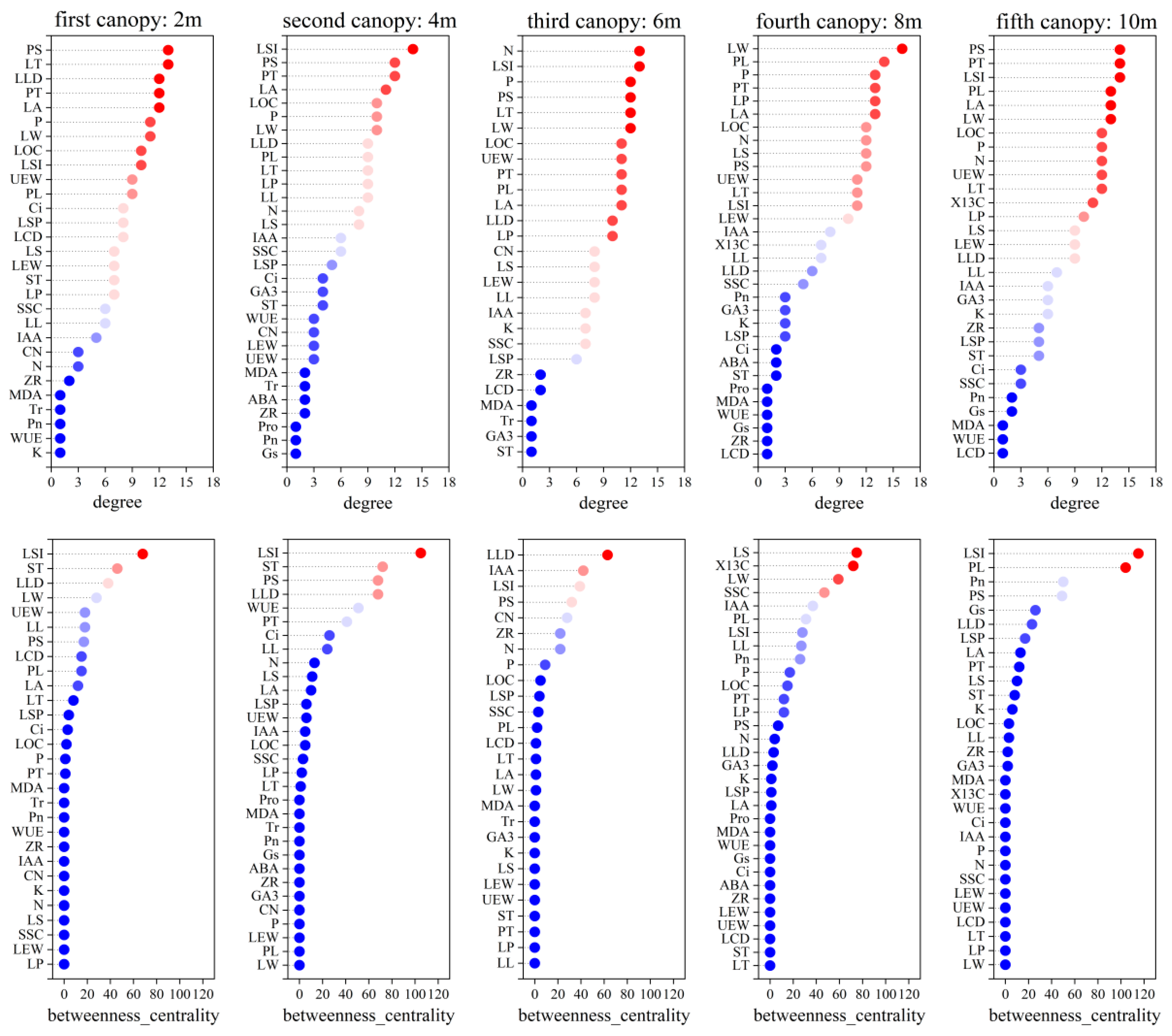
3.4. Identification of Central and Linkage Traits in Different P. euphratica Canopies
3.5. Connectivity between Structural, Chemical, and Physiological Traits in Developmental Stage P. euphratica Leaves
4. Discussion
4.1. Developmental Stage Poplar Leaf Trait Network Differences and Linkages
4.2. Highly Correlated Traits in Developmental-Stage Poplar Leaf Trait Network
4.3. Highly Correlated Traits in Developmental-Stage Poplar Leaf Trait Network
4.4. Highly Correlated Traits in a Network of Poplar Leaf Traits at Different Canopy Heights
4.5. Effects of Different Developmental Stages and Canopy Heights on the Composition of Trait Relationships
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bryant, K.; Fredericksen, B.; Hudiburg, T.; Rosenthal, D. Physiological Strategies for Handling Summer Water Stress Differ among Co-Existing Species and between Juvenile and Mature Trees. Front. For. Glob. Chang. 2023, 5, 1018789. [Google Scholar] [CrossRef]
- Kumar, D.; Scheiter, S.; Langan, L.; Koirala, S.; Pfeiffer, M.; Martens, C.; Weber, U.; Carvalhais, N. Investigating the Spatial and Temporal Variation of Plants Traits across Flux Sites Using a Trait-Based Dynamic Vegetation Model. In EGU General Assembly Conference Abstracts; EGU-6385; Astrophysics Data: Washington, DC, USA, 2023. [Google Scholar]
- Legner, N.; Fleck, S.; Leuschner, C. Within-Canopy Variation in Photosynthetic Capacity, SLA and Foliar N in Temperate Broad-Leaved Trees with Contrasting Shade Tolerance. Trees 2013, 28, 263–280. [Google Scholar] [CrossRef]
- Shen, T.; Corlett, R.T.; Collart, F.; Kasprzyk, T.; Guo, X.; Patiño, J.; Su, Y.; Hardy, O.J.; Ma, W.; Wang, J.; et al. Microclimatic Variation in Tropical Canopies: A Glimpse into the Processes of Community Assembly in Epiphytic Bryophyte Communities. J. Ecol. 2022, 110, 3023–3038. [Google Scholar] [CrossRef]
- Ryan, M.G.; Phillips, N.; Bond, B.J. The Hydraulic Limitation Hypothesis Revisited. Plant Cell Environ. 2006, 29, 367–381. [Google Scholar] [CrossRef]
- Dong, N.; Prentice, I.C.; Wright, I.J.; Evans, B.J.; Togashi, H.F.; Caddy-Retalic, S.; McInerney, F.A.; Sparrow, B.; Leitch, E.; Lowe, A.J. Components of Leaf-Trait Variation along Environmental Gradients. New Phytol. 2020, 228, 82–94. [Google Scholar] [CrossRef]
- Adler, P.B.; Salguero-Gomez, R.; Compagnoni, A.; Hsu, J.S.; Ray-Mukherjee, J.; Mbeau-Ache, C.; Franco, M. Functional Traits Explain Variation in Plant Life History Strategies. Proc. Natl. Acad. Sci. USA 2013, 111, 740–745. [Google Scholar] [CrossRef]
- Jones, T.A.; Thomas, S.C. Leaf-Level Acclimation to Gap Creation in Mature Acer Saccharum Trees. Tree Physiol. 2007, 27, 281–290. [Google Scholar] [CrossRef]
- Violle, C.; Enquist, B.J.; McGill, B.J.; Jiang, L.; Albert, C.H.; Hulshof, C.; Jung, V.; Messier, J. The Return of the Variance: Intraspecific Variability in Community Ecology. Trends Ecol. Evol. 2012, 27, 244–252. [Google Scholar] [CrossRef]
- Xu, R.; Cheng, S.; Zhou, J.; Tigabu, M.; Ma, X.; Li, M. Intraspecific Variations in Leaf Functional Traits of Cunninghamia lanceolata Provenances. BMC Plant Biol. 2023, 23, 92. [Google Scholar] [CrossRef]
- Ritter, L.J.; Medina, M.; Goya, J.F.; Campanello, P.I.; Pinazo, M.A.; Arturi, M.F. Functional Traits and Growth Rate Response to Stand Variables: Differences between Saplings and Seedlings of Native Trees Established in Loblolly Pine Plantations in the Atlantic Forest. New For. 2022, 54, 311–324. [Google Scholar] [CrossRef]
- Wright, I.J.; Reich, P.B.; Westoby, M.; Ackerly, D.D.; Baruch, Z.; Bongers, F.; Cavender-Bares, J.; Chapin, T.; Cornelissen, J.H.C.; Diemer, M.; et al. The Worldwide Leaf Economics Spectrum. Nature 2004, 428, 821–827. [Google Scholar] [CrossRef]
- Cao, J.; Chen, J.; Yang, Q.; Xiong, Y.; Ren, W.; Kong, D. Leaf Hydraulics Coordinated with Leaf Economics and Leaf Size in Mangrove Species along a Salinity Gradient. Plant Divers. 2023, 45, 309–314. [Google Scholar] [CrossRef]
- Liu, Z.; Zhao, M.; Zhang, H.; Ren, T.; Liu, C.; He, N. Divergent Response and Adaptation of Specific Leaf Area to Environmental Change at Different Spatio-Temporal Scales Jointly Improve Plant Survival. Glob. Chang. Biol. 2022, 29, 1144–1159. [Google Scholar] [CrossRef]
- Krieg, C.P.; Seeger, K.; Campany, C.; Watkins, J.E.; Mcclearn, D.; Mcculloh, K.A.; Sessa, E.B. Functional Traits and Trait Coordination Change over the Life of a Leaf in a Tropical Fern Species. Am. J. Bot. 2023, 110, e16151. [Google Scholar] [CrossRef]
- Moran, M.E.; Aparecido, L.M.T.; Koepke, D.F.; Cooper, H.F.; Doughty, C.E.; Gehring, C.A.; Throop, H.L.; Whitham, T.G.; Allan, G.J.; Hultine, K.R. Limits of Thermal and Hydrological Tolerance in a Foundation Tree Species (Populus fremontii) in the Desert Southwestern United States. New Phytol. 2023, 240, 2298–2311. [Google Scholar] [CrossRef]
- Cao, D.; Li, J.; Huang, Z.; Baskin, C.C.; Baskin, J.M.; Hao, P.; Zhou, W.; Li, J. Reproductive Characteristics of a Populus euphratica Population and Prospects for Its Restoration in China. PLoS ONE 2012, 7, e39121. [Google Scholar] [CrossRef]
- Huang, W.J.; Li, Z.J.; Yang, Z.P.; Bai, G.Z. The structural traits of Populus euphratica heteromorphic leaves and their correlations. Acta Ecol. Sin. 2010, 30, 4636–4642. [Google Scholar]
- Zhai, J.; Zhang, X.; Li, Z.; Han, X.; Zhang, S. Differences in the Functional Traits of Populus Pruinosa Leaves in Different Developmental Stages. Plants 2023, 12, 2262. [Google Scholar] [CrossRef]
- Li, Y.; Liu, C.; Xu, L.; Li, M.; Zhang, J.; He, N. Leaf Trait Networks Based on Global Data: Representing Variation and Adaptation in Plants. Front. Plant Sci. 2021, 12, 710530. [Google Scholar] [CrossRef]
- Sun, J.; Xu, J.; Qiu, C.; Zhai, J.; Zhang, S.; Zhang, X.; Wu, Z.; Li, Z. The Chromosome-Scale Genome and Population Genomics Reveal the Adaptative Evolution of Populus pruinosa to Desertification Environment. Hortic. Res. 2024, 11, uhae034. [Google Scholar] [CrossRef]
- Zheng, Y.; Fei, M.; Li, Z. Investigation of Bud Burst, Shoot Growth and Leaf Expansion in Populus euphraticaof Different Ages. Shengtai Xuebao 2015, 35, 1198–1207. [Google Scholar] [CrossRef][Green Version]
- Chou, Q.; Cao, T.; Ni, L.; Xie, P.; Jeppesen, E. Leaf Soluble Carbohydrates, Free Amino Acids, Starch, Total Phenolics, Carbon and Nitrogen Stoichiometry of 24 Aquatic Macrophyte Species along Climate Gradients in China. Front. Plant Sci. 2019, 10, 442. [Google Scholar] [CrossRef] [PubMed]
- Rehman, A.U.; Bashir, F.; Ayaydin, F.; Kóta, Z.; Páli, T.; Vass, I. Proline Is a Quencher of Singlet Oxygen and Superoxide Both in In Vitro Systems and Isolated Thylakoids. Physiol. Plant. 2020, 172, 7–18. [Google Scholar] [CrossRef]
- Ghosh, U.K.; Islam, M.N.; Siddiqui, M.N.; Cao, X.; Khan, M.A.R. Proline, a Multifaceted Signalling Molecule in Plant Responses to Abiotic Stress: Understanding the Physiological Mechanisms. Plant Biol. 2022, 24, 227–239. [Google Scholar] [CrossRef]
- Kleyer, M.; Trinogga, J.; Cebrián-Piqueras, M.A.; Trenkamp, A.; Fløjgaard, C.; Ejrnaes, R.; Bouma, T.J.; Minden, V.; Maier, M.; Mantilla-Contreras, J.; et al. Trait Correlation Network Analysis Identifies Biomass Allocation Traits and Stem Specific Length as Hub Traits in Herbaceous Perennial Plants. J. Ecol. 2018, 107, 829–842. [Google Scholar] [CrossRef]
- Armbruster, W.S.; Pélabon, C.; Bolstad, G.H.; Hansen, T.F. Integrated Phenotypes: Understanding Trait Covariation in Plants and Animals. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130245. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, C.D.; Scoffoni, C.; John, G.P.; Bartlett, M.K.; Inman-Narahari, F.; Ostertag, R.; Cordell, S.; Giardina, C.; Sack, L. An Extensive Suite of Functional Traits Distinguishes Hawaiian Wet and Dry Forests and Enables Prediction of Species Vital Rates. Funct. Ecol. 2018, 33, 712–734. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, H.; Harrison, S.P.; Prentice, I.C.; Wright, I.J.; Peng, C.; Lin, G. Quantifying Leaf-Trait Covariation and Its Controls across Climates and Biomes. New Phytol. 2018, 221, 155–168. [Google Scholar] [CrossRef]
- Deng, Y.; Jiang, Y.-H.; Yang, Y.; He, Z.; Luo, F.; Zhou, J. Molecular Ecological Network Analyses. BMC Bioinform. 2012, 13, 113. [Google Scholar] [CrossRef]
- Kurokawa, H.; Oguro, M.; Takayanagi, S.; Aiba, M.; Shibata, R.; Mimura, M.; Yoshimaru, H.; Nakashizuka, T. Plant Characteristics Drive Ontogenetic Changes in Herbivory Damage in a Temperate Forest. J. Ecol. 2022, 110, 2772–2784. [Google Scholar] [CrossRef]
- Rao, Q.; Chen, J.; Chou, Q.; Ren, W.; Cao, T.; Zhang, M.; Xiao, H.; Liu, Z.; Chen, J.; Su, H.; et al. Linking Trait Network Parameters with Plant Growth across Light Gradients and Seasons. Funct. Ecol. 2023, 37, 1732–1746. [Google Scholar] [CrossRef]
- Flores-Moreno, H.; Fazayeli, F.; Banerjee, A.; Datta, A.; Kattge, J.; Butler, E.E.; Atkin, O.K.; Wythers, K.; Chen, M.; Anand, M.; et al. Robustness of Trait Connections across Environmental Gradients and Growth Forms. Glob. Ecol. Biogeogr. 2019, 28, 1806–1826. [Google Scholar] [CrossRef]
- Liu, C.; Li, Y.; Yan, P.; He, N. How to Improve the Predictions of Plant Functional Traits on Ecosystem Functioning? Front. Plant Sci. 2021, 12, 622260. [Google Scholar] [CrossRef]
- Wang, X.; Ji, M.; Zhang, Y.; Zhang, L.; Akram, M.A.; Dong, L.; Hu, W.; Xiong, J.; Sun, Y.; Li, H.; et al. Plant Trait Networks Reveal Adaptation Strategies in the Drylands of China. BMC Plant Biol. 2023, 23, 266. [Google Scholar] [CrossRef]
- Koschützki, D.; Schreiber, F. Centrality Analysis Methods for Biological Networks and Their Application to Gene Regulatory Networks. Gene Regul. Syst. Biol. 2008, 2, 193–201. [Google Scholar] [CrossRef]
- Poorter, H.; Niklas, K.J.; Reich, P.B.; Oleksyn, J.; Poot, P.; Mommer, L. Biomass Allocation to Leaves, Stems and Roots: Meta-Analyses of Interspecific Variation and Environmental Control. New Phytol. 2011, 193, 30–50. [Google Scholar] [CrossRef] [PubMed]
- Medina-Vega, J.A.; Bongers, F.; Poorter, L.; Schnitzer, S.A.; Sterck, F.J. Lianas Have More Acquisitive Traits than Trees in a Dry but Not in a Wet Forest. J. Ecol. 2021, 109, 2367–2384. [Google Scholar] [CrossRef]
- Barton, K.E.; Koricheva, J. The Ontogeny of Plant Defense and Herbivory: Characterizing General Patterns Using Meta-Analysis. Am. Nat. 2010, 175, 481–493. [Google Scholar] [CrossRef]
- Meiforth, J.J.; Buddenbaum, H.; Hill, J.; Shepherd, J. Monitoring of Canopy Stress Symptoms in New Zealand Kauri Trees Analysed with AISA Hyperspectral Data. Remote Sens. 2020, 12, 926. [Google Scholar] [CrossRef]
- Bramesh Reddy, B.R.; Kiran, B.O.; Patil, S.B.; Ash Vathama, V.H. Canopy Temperature in Sorghum under Drought Stress: Influence of Gas-Exchange Parameters. J. Cereal Res. 2022, 14, 2582–2675. [Google Scholar]
- Dong, X.; Zhang, X. Some Observations of the Adaptations of Sandy Shrubs to the Arid Environment in the Mu Us Sandland: Leaf Water Relations and Anatomic Features. J. Arid. Environ. 2001, 48, 41–48. [Google Scholar]
- Shi, P.; Yu, K.; Niinemets, Ü.; Gielis, J. Can Leaf Shape Be Represented by the Ratio of Leaf Width to Length? Evidence from Nine Species of Magnolia and Michelia (Magnoliaceae). Forests 2020, 12, 41. [Google Scholar] [CrossRef]
- Wei, X.; Yang, Y.; Yao, J.; Han, J.; Yan, M.; Zhang, J.; Shi, Y.; Wang, J.; Mu, C. Improved Utilization of Nitrate Nitrogen through Within-Leaf Nitrogen Allocation Trade-Offs in Leymus chinensis. Front. Plant Sci. 2022, 13, 870681. [Google Scholar] [CrossRef]
- Arora, R.L.; Tripathi, S.; Singh, R. Effect of Nitrogen on Leaf Mineral Nutrient Status, Growth and Fruiting in Peach. Indian J. Hortic. 1999, 56, 286–294. [Google Scholar]
- Zhi, X.; Hammer, G.; Borrell, A.; Tao, Y.; Wu, A.; Hunt, C.; van Oosterom, E.; Massey-Reed, S.R.; Cruickshank, A.; Potgieter, A.B.; et al. Genetic Basis of Sorghum Leaf Width and Its Potential as a Surrogate for Transpiration Efficiency. Theor. Appl. Genet. 2022, 135, 3057–3071. [Google Scholar] [CrossRef] [PubMed]
- Lambers, H.; Oliveira, R.S. Plant Physiological Ecology; Springer International Publishing: Cham, Switzerland, 2019; ISBN 9783030296384. [Google Scholar]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Zhai, J.; Zhang, J.; Han, X.; Ge, X.; Si, J.; Li, J.; Li, Z. Leaf Trait Variations and Ecological Adaptation Mechanisms of Populus euphratica at Different Developmental Stages and Canopy Heights. Forests 2024, 15, 1283. https://doi.org/10.3390/f15081283
Wang J, Zhai J, Zhang J, Han X, Ge X, Si J, Li J, Li Z. Leaf Trait Variations and Ecological Adaptation Mechanisms of Populus euphratica at Different Developmental Stages and Canopy Heights. Forests. 2024; 15(8):1283. https://doi.org/10.3390/f15081283
Chicago/Turabian StyleWang, Jie, Juntuan Zhai, Jinlong Zhang, Xiaoli Han, Xiaokang Ge, Jianhua Si, Jingwen Li, and Zhijun Li. 2024. "Leaf Trait Variations and Ecological Adaptation Mechanisms of Populus euphratica at Different Developmental Stages and Canopy Heights" Forests 15, no. 8: 1283. https://doi.org/10.3390/f15081283
APA StyleWang, J., Zhai, J., Zhang, J., Han, X., Ge, X., Si, J., Li, J., & Li, Z. (2024). Leaf Trait Variations and Ecological Adaptation Mechanisms of Populus euphratica at Different Developmental Stages and Canopy Heights. Forests, 15(8), 1283. https://doi.org/10.3390/f15081283





