Impact of Different Land Use Types on Bacterial and Fungal Communities in a Typical Karst Depression in Southwestern China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Field Sampling
2.3. Physicochemical Analysis
2.4. PCR Analysis and Data Sequencing
2.5. Data Analysis
3. Results
3.1. Soil Physicochemical Properties
3.2. Soil Microbial Community Composition
3.3. Soil Microbial Diversity
3.4. Correlation between Soil Physicochemical Properties and Microorganisms
3.5. Soil Bacterial and Fungal Co-Occurrence Network
4. Discussion
4.1. Differences in Soil Microbial Communities across Land Use Types
4.2. Soil Microbial Networks among Different Land Use Types
4.3. Soil Property Factors and Microbial Communities
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, X.; Hui, C.; Bi, L.; Romantschuk, M.; Kontro, M.; Strommer, R.; Hui, N. Bacterial community structure in atrazine treated reforested farmland in Wuying China. Appl. Soil Ecol. 2016, 98, 39–46. [Google Scholar] [CrossRef]
- Tong, X.; Brandt, M.; Yue, Y.; Ciais, P.; Rudbeck Jepsen, M.; Penuelas, J.; Wigneron, J.-P.; Xiao, X.-P.; Song, X.-P.; Horion, S.; et al. Forest management in southern China generates short term extensive carbon sequestration. Nat. Commun. 2020, 11, 129. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.; Brandt, M.; Yue, Y.; Zhang, X.; Fensholt, R.; Ciais, P.; Wang, K.; Liu, S.; Zhang, W.; Mao, C.; et al. Reforestation policies around 2000 in southern China led to forest densification and expansion in the 2010s. Commun. Earth Environ. 2023, 4, 260. [Google Scholar] [CrossRef]
- Peng, X.; Dai, Q.; Ding, G.; Shi, D.; Li, C. Impact of vegetation restoration on soil properties in near-surface fissures located in karst rocky desertification regions. Soil Tillage Res. 2020, 200, 104620. [Google Scholar] [CrossRef]
- Szoboszlay, M.; Dohrmann, A.B.; Poeplau, C.; Don, A.; Tebbe, C.C. Impact of land-use change and soil organic carbon quality on microbial diversity in soils across Europe. Fems Microbiol. Ecol. 2017, 93, fix146. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Li, W.; Pang, X.; Liu, Q.; Yin, C. Soil properties and root traits are important factors driving rhizosphere soil bacterial and fungal community variations in alpine Rhododendron nitidulum shrub ecosystems along an altitudinal gradient. Sci. Total Environ. 2023, 864, 161048. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Gu, C.; Rodrigues, J.L.M.; Li, C.; Bohannan, B.; Nuesslein, K.; Margenot, A.J. Soil phosphorus cycling across a 100-year deforestation chronosequence in the Amazon rainforest. Glob. Change Biol. 2024, 30, e17077. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Liu, H.; Liu, Y.; Zhang, Q.; Luo, Y.; Liu, X.; Liu, Y.; Xu, J.; Di, H.; Li, Y. Understanding the relationships between grazing intensity and the distribution of nitrifying communities in grassland soils. Sci. Total Environ. 2018, 634, 1157–1164. [Google Scholar] [CrossRef]
- Qiu, J.; Cao, J.; Lan, G.; Liang, Y.; Wang, H.; Li, Q. The Influence of Land Use Patterns on Soil Bacterial Community Structure in the Karst Graben Basin of Yunnan Province, China. Forests 2020, 11, 51. [Google Scholar] [CrossRef]
- Darriaut, R.; Martins, G.; Dewasme, C.; Mary, S.; Darrieutort, G.; Ballestra, P.; Marguerit, E.; Vivin, P.; Ollat, N.; Masneuf-Pomarede, I.; et al. Grapevine decline is associated with difference in soil microbial composition and activity. Oeno One 2021, 55, 67–84. [Google Scholar] [CrossRef]
- Bosso, L.; Lacatena, F.; Varlese, R.; Nocerino, S.; Cristinzio, G.; Russo, D. Plant pathogens but not antagonists change in soil fungal communities across a land abandonment gradient in a Mediterranean landscape. Acta Oecologica 2017, 78, 1–6. [Google Scholar] [CrossRef]
- Moscatelli, M.C.; Bonifacio, E.; Chiti, T.; Cudlin, P.; Dinca, L.; Gomoryova, E.; Grego, S.; La Porta, N.; Karlinski, L.; Pellis, G.; et al. Soil properties as indicators of treeline dynamics in relation to anthropogenic pressure and climate change. Clim. Res. 2017, 73, 73–84. [Google Scholar] [CrossRef]
- Chaparro, J.M.; Sheflin, A.M.; Manter, D.K.; Vivanco, J.M. Manipulating the soil microbiome to increase soil health and plant fertility. Biol. Fertil. Soils 2012, 48, 489–499. [Google Scholar] [CrossRef]
- Seaton, F.M.; Griffiths, R.I.; Goodall, T.; Lebron, I.; Norton, L.R. Soil bacterial and fungal communities show within field heterogeneity that varies by land management and distance metric. Soil Biol. Biochem. 2023, 177, 108920. [Google Scholar] [CrossRef]
- Kostin, J.E.; Cesarz, S.; Lochner, A.; Schaedler, M.; Macdonald, C.A.; Eisenhauer, N. Land-use drives the temporal stability and magnitude of soil microbial functions and modulates climate effects. Ecol. Appl. 2021, 31, e02325. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Zhai, D.; Ding, X.; Guo, Z.; Zhao, Y. Shifts in soil microbial diversity and functions during continuous cropping of strawberry. Land Degrad. Dev. 2023, 34, 4810–4820. [Google Scholar] [CrossRef]
- Sun, Y.; Luo, C.; Jiang, L.; Song, M.; Zhang, D.; Li, J.; Li, Y.; Ostle, N.J.; Zhang, G. Land-use changes alter soil bacterial composition and diversity in tropical forest soil in China. Sci. Total Environ. 2020, 712, 136526. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yang, L.; Fan, M.; Shangguan, Z. Plantation vegetation restoration enhances the relationship between rhizosphere microbial diversity and soil multifunctionality. Land Degrad. Dev. 2022, 33, 3630–3640. [Google Scholar] [CrossRef]
- Wang, M.; Lin, M.; Liu, Q.; Li, C.; Pang, X. Fungal, but not bacterial, diversity and network complexity promote network stability during roadside slope restoration. Sci. Total Environ. 2024, 922, 171007. [Google Scholar] [CrossRef]
- Seaton, F.M.; George, P.B.L.; Lebron, I.; Jones, D.L.; Creer, S.; Robinson, D.A. Soil textural heterogeneity impacts bacterial but not fungal diversity. Soil Biol. Biochem. 2020, 144, 107766. [Google Scholar] [CrossRef]
- Nakayama, M.; Imamura, S.; Taniguchi, T.; Tateno, R. Does conversion from natural forest to plantation affect fungal and bacterial biodiversity, community structure, and co-occurrence networks in the organic horizon and mineral soil? For. Ecol. Manag. 2019, 446, 238–250. [Google Scholar] [CrossRef]
- Wang, C.; Zheng, M.M.; Chen, J.; Shen, R.F. Land-use change has a greater effect on soil diazotrophic community structure than the plant rhizosphere in acidic ferralsols in southern China. Plant Soil 2021, 452, 445–458. [Google Scholar] [CrossRef]
- Xu, A.; Liu, J.; Guo, Z.; Wang, C.; Pan, X. Soil microbial community composition but not diversity is affected by land-use types in the agro-pastoral ecotone undergoing frequent conversions between cropland and grassland. Geoderma 2021, 401, 115165. [Google Scholar] [CrossRef]
- Hu, P.; Xiao, J.; Zhang, W.; Xiao, L.; Yang, R.; Xiao, D.; Zhao, J.; Wang, K. Response of soil microbial communities to natural and managed vegetation restoration in a subtropical karst region. Catena 2020, 195, 104849. [Google Scholar] [CrossRef]
- Xiao, K.; He, T.; Chen, H.; Peng, W.; Song, T.; Wang, K.; Li, D. Impacts of vegetation restoration strategies on soil organic carbon and nitrogen dynamics in a karst area, southwest China. Ecol. Eng. 2017, 101, 247–254. [Google Scholar] [CrossRef]
- Wang, J.; Feng, L.; Palmer, P.I.; Liu, Y.; Fang, S.; Bösch, H.; O’Dell, C.W.; Tang, X.; Yang, D.; Liu, L.; et al. Large Chinese land carbon sink estimated from atmospheric carbon dioxide data. Nature 2020, 586, 720–723. [Google Scholar] [CrossRef] [PubMed]
- Xiao, D.; He, X.; Zhang, W.; Hu, P.; Sun, M.; Wang, K. Comparison of bacterial and fungal diversity and network connectivity in karst and non-karst forests in southwest China. Sci. Total Environ. 2022, 822, 153179. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Long, J.; Liao, H.; Zheng, C.; Li, J.; Liu, L.; Zhang, M. Dynamics of soil microbial communities following vegetation succession in a karst mountain ecosystem, Southwest China. Sci. Rep. 2019, 9, 2160. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Zhao, M.; Cong, J.; Qi, Q.; Zhang, Y. Soil pH exerts stronger impacts than vegetation type and plant diversity on soil bacterial community composition in subtropical broad-leaved forests. Plant Soil 2020, 450, 273–286. [Google Scholar] [CrossRef]
- Zhang, W.; Zhao, J.; Pan, F.J.; Li, D.J.; Chen, H.S.; Wang, K.L. Changes in nitrogen and phosphorus limitation during secondary succession in a karst region in southwest China. Plant Soil 2015, 391, 77–91. [Google Scholar] [CrossRef]
- Huang, X.; Xian, T.; Long, T.; He, L.; Donald, M.L.; Deng, D.; Dong, T. Impacts of vegetation restoration strategies on soil nutrients and stoichiometry of the earthquake-induced landslides in Jiuzhaigou, eastern Qinghai-Tibet Plateau. Front. Environ. Sci. 2024, 11, 1296187. [Google Scholar] [CrossRef]
- Mora, J.L.; Armas-Herrera, C.; Gomez, D.; Badia-Villas, D. Mosaic coexistence of two subalpine grassland types as a consequence of soil nutrient heterogeneity. Catena 2024, 243, 108192. [Google Scholar] [CrossRef]
- Medriano, C.A.; Amabel Chan; Ryan De Sotto; Bae, S. Different types of land use influence soil physiochemical properties, the abundance of nitrifying bacteria, and microbial interactions in tropical urban soil. Sci. Total Environ. 2023, 869, 161722. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Yun, Y.; Wang, H.; Ma, L.; Tian, W.; Man, B.; Liu, C. Contrasting bacterial communities and their assembly processes in karst soils under different land use. Sci. Total Environ. 2021, 751, 142263. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho, T.S.; Jesus, E.d.C.; Barlow, J.; Gardner, T.A.; Soares, I.C.; Tiedje, J.M.; de Souza Moreira, F.M. Land use intensification in the humid tropics increased both alpha and beta diversity of soil bacteria. Ecology 2016, 97, 2760–2771. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, J.L.H.; Pellizari, V.H.; Mueller, R.; Baek, K.; Jesus, E.D.C.; Paula, F.S.; Mirza, B.; Hamaoui, G.S.; Tsai, S.M.; Feigl, B.; et al. Conversion of the Amazon rainforest to agriculture results in biotic homogenization of soil bacterial communities. Proc. Natl. Acad. Sci. USA 2013, 110, 988–993. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Liu, L.; Yang, X.; Deng, M.; Wang, Z.; Wang, P.; Yang, S.; Li, P.; Peng, Z.; Yang, L.; et al. Long-term nitrogen input alters plant and soil bacterial, but not fungal beta diversity in a semiarid grassland. Glob. Chang. Biol. 2021, 27, 3939–3950. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Li, S.; Avera, B.N.; Strahm, B.D.; Badgley, B.D. Soil bacterial and fungal communities show distinct recovery patterns during forest ecosystem restoration. Appl. Environ. Microbiol. 2017, 83, e00966-17. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Li, Q.; Yan, J.; Liu, C.; Zhong, J. Vegetation restoration facilitates belowground microbial network complexity and recalcitrant soil organic carbon storage in southwest China karst region. Sci. Total Environ. 2022, 820, 153137. [Google Scholar] [CrossRef]
- Bonfante, P.; Anca, I.A. Plants, mycorrhizal fungi, and bacteria: A network of interactions. Annu. Rev. Microbiol. 2009, 63, 363–383. [Google Scholar] [CrossRef]
- Yun, Y.; Wang, H.; Man, B.; Xiang, X.; Zhou, J.; Qiu, X.; Duan, Y.; Engel, A.S. The Relationship between pH and Bacterial Communities in a Single Karst Ecosystem and Its Implication for Soil Acidification. Front. Microbiol. 2016, 7, 1955. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Liu, Y.; Cui, M.; Zhou, Z.; Zhang, Q.; Li, Y.; Ha, W.; Pang, D.; Luo, J.; Zhou, J. Effects of secondary succession on soil fungal and bacterial compositions and diversities in a karst area. Plant Soil 2021, 475, 91–102. [Google Scholar] [CrossRef]
- Pankratov, T.A.; Ivanova, A.O.; Dedysh, S.N.; Liesack, W. Bacterial populations and environmental factors controlling cellulose degradation in an acidic Sphagnum peat. Environ. Microbiol. 2011, 13, 1800–1814. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gao, M.; Yu, C.; Zhang, H.; Yan, N.; Wu, Q.; Song, Y.; Li, X. Soil nutrients, enzyme activities, and microbial communities differ among biocrust types and soil layers in a degraded karst ecosystem. Catena 2022, 212, 106057. [Google Scholar] [CrossRef]
- Kielak, A.M.; Barreto, C.C.; Kowalchuk, G.A.; van Veen, J.A.; Kuramae, E.E. The ecology of acidobacteria: Moving beyond genes and genomes. Front. Microbiol. 2016, 7, 744. [Google Scholar] [CrossRef] [PubMed]
- Sui, X.; Frey, B.; Yang, L.; Liu, Y.; Zhang, R.; Ni, H.; Li, M.-H. Soil Acidobacterial community composition changes sensitively with wetland degradation in northeastern of China. Front. Microbiol. 2022, 13, 1052161. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, B.S.; Philippot, L. Insights into the resistance and resilience of the soil microbial community. FEMS Microbiol. Rev. 2013, 37, 112–129. [Google Scholar] [CrossRef] [PubMed]
- Qu, Z.; Liu, B.; Ma, Y.; Xu, J.; Sun, H. The response of the soil bacterial community and function to forest succession caused by forest disease. Funct. Ecol. 2020, 34, 2548–2559. [Google Scholar] [CrossRef]
- McNamara, P.J.; Krzmarzick, M.J. Triclosan enriches for Dehalococcoides-like Chloroflexi in anaerobic soil at environmentally relevant concentrations. Fems Microbiol. Lett. 2013, 344, 48–52. [Google Scholar] [CrossRef]
- Wan, P.; He, R. Soil microbial community characteristics under different vegetation types at the national nature reserve of Xiaolongshan Mountains, Northwest China. Ecol. Inform. 2020, 55, 101020. [Google Scholar] [CrossRef]
- Li, Q.; Zhu, C.; Yu, J.; Wu, X.; Huang, S.; Yang, F.; Tigabu, M.; Hou, X. Response of Soil Bacteria of Dicranopteris dichotoma Populations to Vegetation Restoration in Red Soil Region of China. J. Soil Sci. Plant Nutr. 2023, 23, 456–468. [Google Scholar] [CrossRef]
- Tedersoo, L.; Bahram, M.; Põlme, S.; Kõljalg, U.; Yorou, N.S.; Wijesundera, R.; Ruiz, L.V.; Vasco-Palacios, A.M.; Thu, P.Q.; Suija, A.; et al. Global diversity and geography of soil fungi. Science 2014, 346, 1256688. [Google Scholar] [CrossRef] [PubMed]
- Dong, P.; Zhang, Z.; Zhang, M. Combination of Phytoextraction and Biochar Improves Available Potassium and Alters Microbial Community Structure in Soils. Water 2024, 16, 118. [Google Scholar] [CrossRef]
- Yuan, M.M.; Guo, X.; Wu, L.; Zhang, Y.; Xiao, N.; Ning, D.; Shi, Z.; Zhou, X.; Wu, L.; Yang, Y.; et al. Climate warming enhances microbial network complexity and stability. Nat. Clim. Chang. 2021, 11, 343–348. [Google Scholar] [CrossRef]
- Li, Q.; Song, A.; Yang, H.; G.Müller, W.E. Impact of Rocky Desertification Control on Soil Bacterial Community in Karst Graben Basin, Southwestern China. Front. Microbiol. 2021, 12, 636405. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Zhang, P.; Qin, Y.; Tu, Q.; Yang, Y.; He, Z.; Schadt, C.W.; Zhou, J. Network succession reveals the importance of competition in response to emulsified vegetable oil amendment for uranium bioremediation. Environ. Microbiol. 2016, 18, 205–218. [Google Scholar] [CrossRef]
- Xiao, H.; Sheng, H.; Zhang, L.; Zhang, L.; Pan, B.; Zhou, P. How does land-use change alter soil microbial diversity, composition, and network in subtropical China? Catena 2023, 231, 107335. [Google Scholar] [CrossRef]
- Thomson, B.C.; Tisserant, E.; Plassart, P.; Uroz, S.; Griffiths, R.I.; Hannula, S.E.; Buée, M.; Mougel, C.; Ranjard, L.; Van Veen, J.A.; et al. Soil conditions and land use intensification effects on soil microbial communities across a range of European field sites. Soil Biol. Biochem. 2015, 88, 403–413. [Google Scholar] [CrossRef]
- Chu, H.; Fierer, N.; Lauber, C.L.; Caporaso, J.G.; Knight, R.; Grogan, P. Soil bacterial diversity in the Arctic is not fundamentally different from that found in other biomes. Environ. Microbiol. 2010, 12, 2998–3006. [Google Scholar] [CrossRef]
- Ivanova, A.A.; Kulichevskaya, I.S.; Merkel, A.Y.; Toshchakov, S.V.; Dedysh, S.N. High Diversity of Planctomycetes in Soils of Two Lichen-Dominated Sub-Arctic Ecosystems of Northwestern Siberia. Front. Microbiol. 2016, 7, 2065. [Google Scholar] [CrossRef]
- Chen, W.H.; Liang, J.D.; Ren, X.X.; Zhao, J.H.; Han, Y.F.; Liang, Z.Q. Species Diversity of Cordyceps-like Fungi in the Tiankeng Karst Region of China. Microbiol. Spectr. 2022, 10, e0197522. [Google Scholar] [CrossRef]
- Manici, L.M.; Caputo, F.; De Sabata, D.; Fornasier, F. The enzyme patterns of Ascomycota and Basidiomycota fungi reveal their different functions in soil. Appl. Soil Ecol. 2024, 196, 105323. [Google Scholar] [CrossRef]
- TlÁskal, V.; Brabcová, V.; Větrovský, T.; Jomura, M.; López-Mondéjar, R.; Monteiro, L.M.O.; Saraiva, J.P.; Human, Z.R.; Cajthaml, T.; da Rocha, U.N.; et al. Complementary Roles of Wood-Inhabiting Fungi and Bacteria Facilitate Deadwood Decomposition. Msystems 2021, 6, e01078-20. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Walder, F.; Buechi, L.; Meyer, M.; Held, A.Y.; Gattinger, A.; Keller, T.; Charles, R.; van der Heijden, M.G.A. Agricultural intensification reduces microbial network complexity and the abundance of keystone taxa in roots. Isme J. 2019, 13, 1722–1736. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Li, Q.; Tong, L.; Wu, R.; Xu, J. Rhizospheric soil organic carbon accumulated but its molecular groups redistributed via rhizospheric soil microorganisms along multi-root Cerasus humilis plantation chronosequence at the karst rocky desertification control area. Environ. Sci. Pollut. Res. 2023, 30, 72993–73007. [Google Scholar] [CrossRef] [PubMed]
- Kerfahi, D.; Guo, Y.; Dong, K.; Wang, Q.; Adams, J.M. pH is the major predictor of soil microbial network complexity in Chinese forests along a latitudinal gradient. Catena 2024, 234, 107595. [Google Scholar] [CrossRef]
- Shang, R.; Li, S.; Huang, X.; Liu, W.; Lang, X.; Xu, C.; Su, J. Soil bacterial community and ecosystem multifunctionality regulated by keystone plant species during secondary succession. Land Degrad. Dev. 2023, 34, 5997–6008. [Google Scholar] [CrossRef]
- Dai, Z.; Su, W.; Chen, H.; Barberán, A.; Zhao, H.; Yu, M.; Yu, L.; Brookes, P.C.; Schadt, C.W.; Chang, S.X.; et al. Long-term nitrogen fertilization decreases bacterial diversity and favors the growth of Actinobacteria and Proteobacteria in agro-ecosystems across the globe. Glob. Change Biol. 2018, 24, 3452–3461. [Google Scholar] [CrossRef] [PubMed]
- Rousk, J.; Baath, E. Growth of saprotrophic fungi and bacteria in soil. Fems Microbiol. Ecol. 2011, 78, 17–30. [Google Scholar] [CrossRef]
- Su, Y.; Yu, M.; Xi, H.; Lv, J.; Ma, Z.; Kou, C.; Shen, A. Soil microbial community shifts with long-term of different straw return in wheat-corn rotation system. Sci. Rep. 2020, 10, 6360. [Google Scholar] [CrossRef]
- Aguilera-Huertas, J.; Cuartero, J.; Ros, M.; Pascual, J.A.; Parras-Alcantara, L.; Gonzalez-Rosado, M.; Ozbolat, O.; Zornoza, R.; Egea-Cortines, M.; Hurtado-Navarro, M.; et al. How binomial (traditional rainfed olive grove-Crocus sativus) crops impact the soil bacterial community and enhance microbial capacities. J. Environ. Manag. 2023, 345, 118572. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; de Vries, W.T.; Wu, C.; Zheng, H. Improvement of subsoil physicochemical and microbial properties by short-term fallow practices. Peerj 2019, 7, e7501. [Google Scholar] [CrossRef] [PubMed]
- Lidbury, I.D.E.A.; Borsetto, C.; Murphy, A.R.J.; Bottrill, A.; Jones, A.M.E.; Bending, G.D.; Hammond, J.P.; Chen, Y.; Wellington, E.M.H.; Scanlan, D.J. Niche-adaptation in plant-associated Bacteroidetes favours specialisation in organic phosphorus mineralisation. Isme J. 2021, 15, 1040–1055. [Google Scholar] [CrossRef] [PubMed]
- Pascazio, S.; Crecchio, C.; Scagliola, M.; Mininni, A.N.; Dichio, B.; Xiloyannis, C.; Sofo, A. Microbial-based soil quality indicators in irrigated and rainfed soil portions of Mediterranean olive and peach orchards under sustainable management. Agric. Water Manag. 2018, 195, 172–179. [Google Scholar] [CrossRef]
- Sofo, A.; Ricciuti, P.; Fausto, C.; Mininni, A.N.; Crecchio, C.; Scagliola, M.; Malerba, A.D.; Xiloyannis, C. The metabolic and genetic diversity of soil bacterial communities depends on the soilmanagement system and C/N dynamics: The case of sustainable and conventional olive groves. Appl. Soil Ecol. 2019, 137, 21–28. [Google Scholar] [CrossRef]
- Liu, W.; Qiu, K.; Xie, Y.; Wang, R.; Li, H.; Meng, W.; Yang, Y.; Huang, Y.; Li, Y.; He, Y. Years of sand fixation with Caragana korshinskii drive the enrichment of its rhizosphere functional microbes by accumulating soil N. Peerj 2022, 10, e14271. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.; Wang, T.; Xu, Y.; Deng, J.; Zhao, F.; Yang, G.; Han, X.; Feng, Y.; Ren, G. Differential soil microbial community responses to the linkage of soil organic carbon fractions with respiration across land-use changes. For. Ecol. Manag. 2018, 409, 170–178. [Google Scholar] [CrossRef]
- Bosso, L.; Scelza, R.; Testa, A.; Cristinzio, G.; Rao, M.A. Depletion of Pentachlorophenol Contamination in an Agricultural Soil Treated with Byssochlamys nivea, Scopulariopsis brumptii and Urban Waste Compost: A Laboratory Microcosm Study. Water Air Soil Pollut. 2015, 226, 183. [Google Scholar] [CrossRef]
- Yan, Y.; Sun, X.; Sun, F.; Zhao, Y.; Sun, W.; Guo, J.; Zhang, T. Sensitivity of soil fungal and bacterial community compositions to nitrogen and phosphorus additions in a temperate meadow. Plant Soil 2022, 471, 477–490. [Google Scholar] [CrossRef]
- Li, M.; Wei, Y.; Yin, Y.; Ding, H.; Zhu, W.; Zhou, Y. The effect of intercropping Mulberry (Morus alba L.) with Peanut (Arachis hypogaea L.), on the Soil Rhizosphere Microbial Community. Forests 2022, 13, 1757. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, L.; Huang, Z.; Wu, Z.; Tan, M.; Zhang, X.; Jiang, L.; Qin, X.; Huang, J.; Li, H. Effect of Anoxic Atmosphere on the Physicochemical and Pelletization Properties of Pinus massoniana Sawdust during Storage. Int. J. Environ. Res. Public Health 2023, 20, 791. [Google Scholar] [CrossRef] [PubMed]


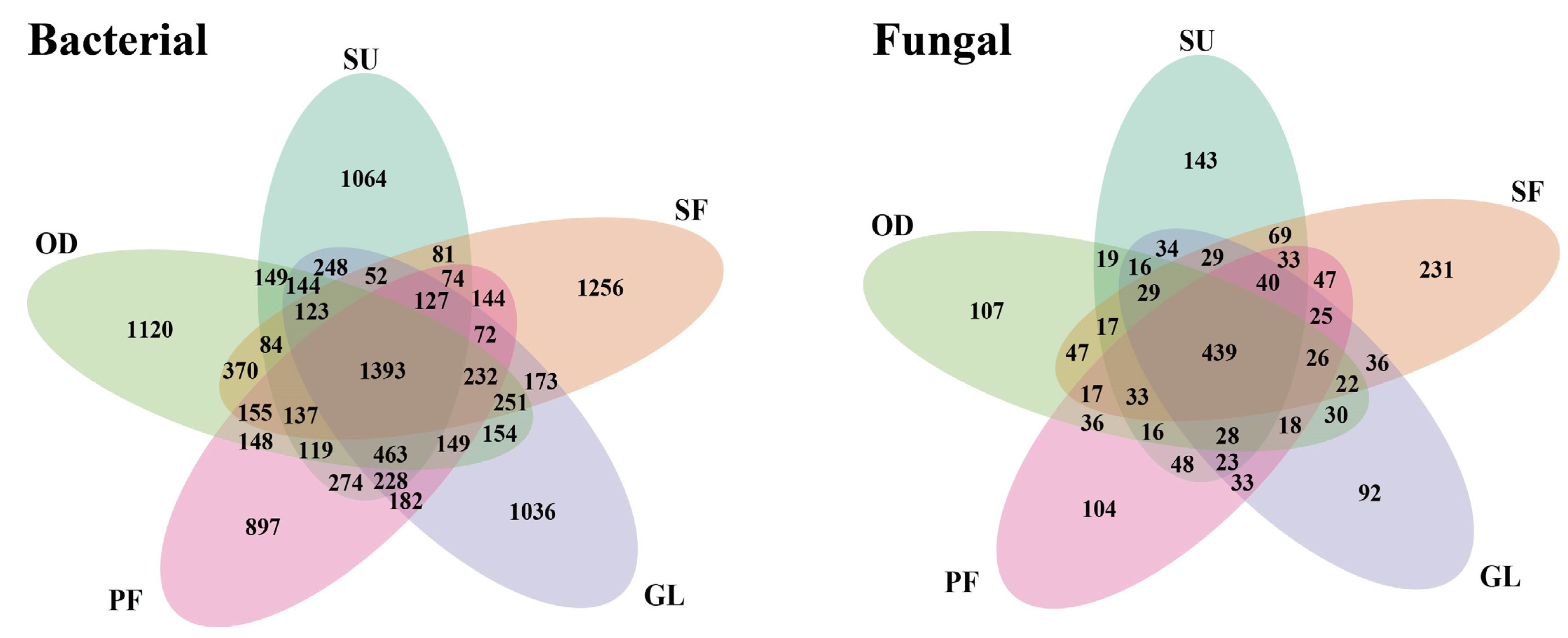
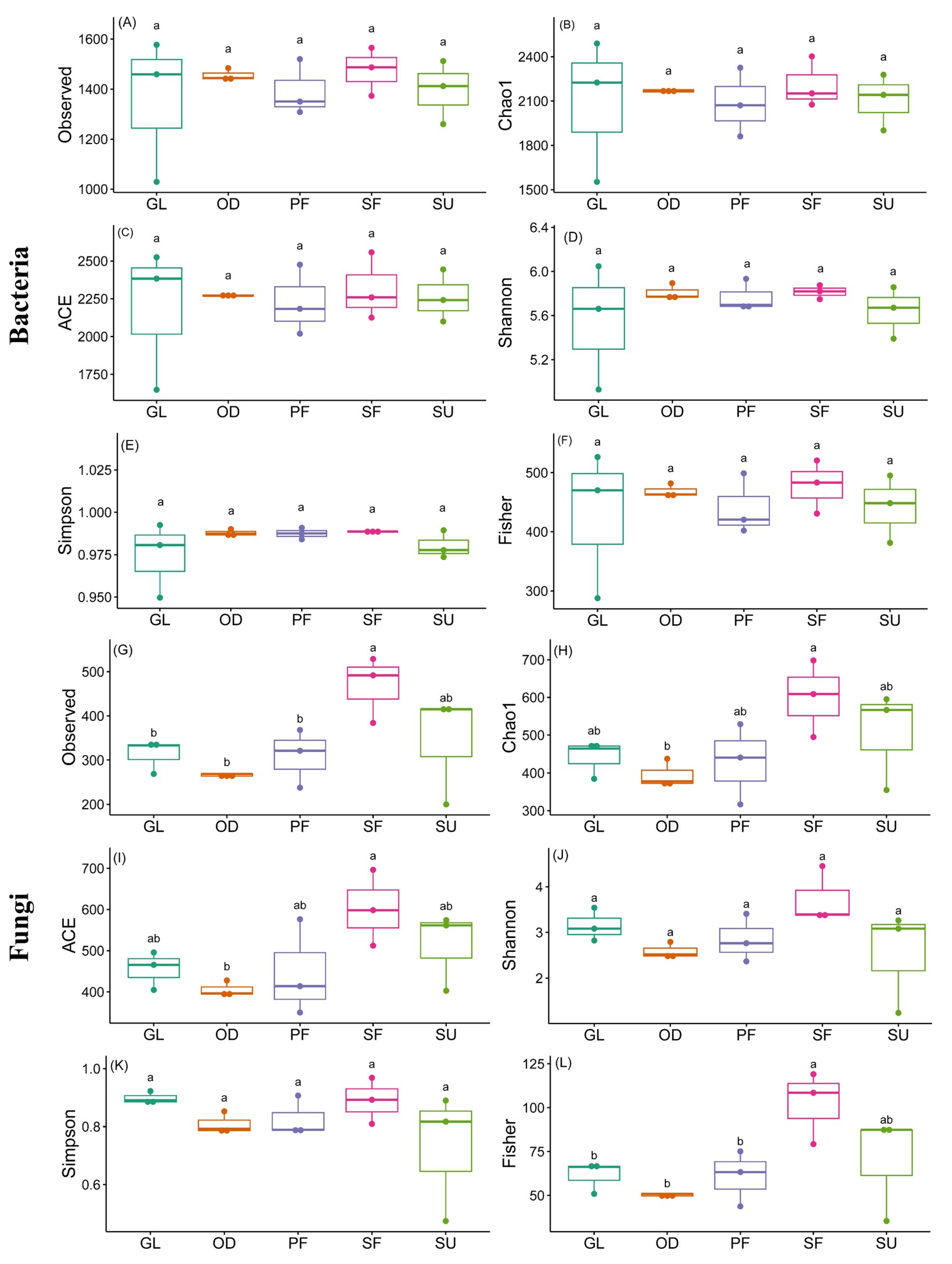


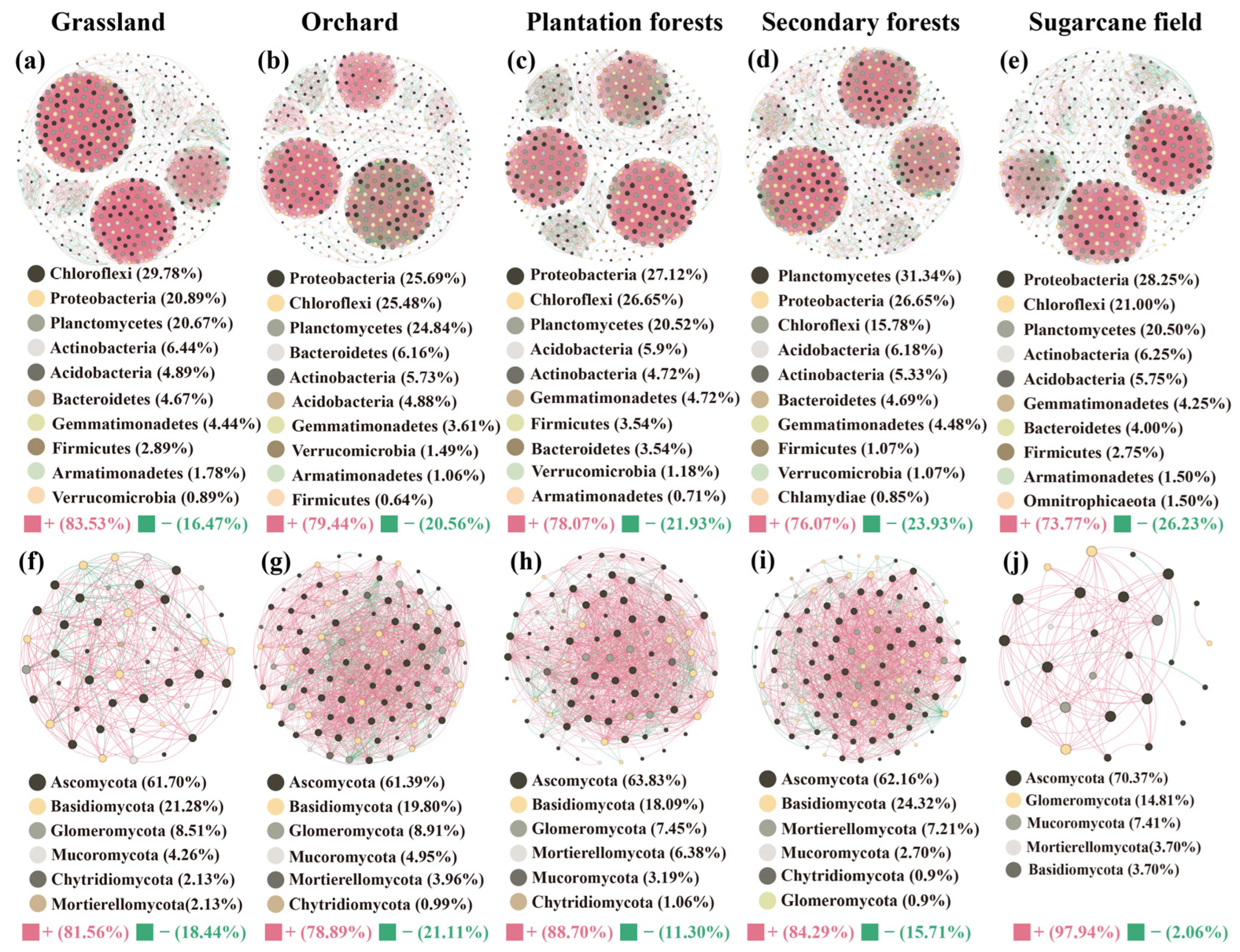
| Properties | SU | OD | GL | PF | SF |
|---|---|---|---|---|---|
| pH | 5.32 ± 0.05 b | 5.94 ± 0.26 ab | 5.60 ± 0.54 ab | 5.56 ± 0.11 ab | 6.39 ± 0.17 a |
| EC | 44.70 ± 6.66 a | 60.10 ± 13.03 a | 55.60 ± 8.31 a | 36.87 ± 1.29 a | 61.40 ± 4.30 a |
| SOC | 18.34 ± 1.17 a | 19.93 ± 3.33 a | 15.54 ± 1.38 a | 18.54 ± 2.69 a | 21.54 ± 2.08 a |
| TN | 1.83 ± 0.08 a | 2.32 ± 0.34 a | 1.81 ± 0.05 a | 1.81 ± 0.14 a | 2.01 ± 0.10 a |
| TP | 1.32 ± 0.14 ab | 0.91 ± 0.05 b | 0.90 ± 0.05 b | 1.29 ± 0.22 ab | 1.69 ± 0.27 a |
| C:N | 11.77 ± 1.3 a | 10.00 ± 0.50 a | 9.98 ± 0.62 a | 11.82 ± 1.05 a | 12.47 ± 0.63 a |
| C:P | 36.41 ± 3.06 a | 56.93 ± 10.41 a | 45.06 ± 5.09 a | 38.26 ± 6.81 a | 34.48 ± 5.89 a |
| N:P | 3.17 ± 0.47 b | 5.65 ± 0.88 a | 4.49 ± 0.29 ab | 3.27 ± 0.57 b | 2.76 ± 0.43 b |
| Microbe | Nodes | Edges | Degree | Density | Modularity | Clustering Coefficient | |
|---|---|---|---|---|---|---|---|
| Bacteria | OD | 471 | 10,037 | 21.31 | 0.05 | 0.65 | 0.45 |
| SU | 400 | 7826 | 19.57 | 0.05 | 0.67 | 0.45 | |
| GL | 450 | 12,082 | 26.85 | 0.06 | 0.59 | 0.45 | |
| SF | 469 | 10,420 | 22.22 | 0.05 | 0.69 | 0.47 | |
| PF | 424 | 8862 | 20.90 | 0.05 | 0.72 | 0.45 | |
| Fungi | OD | 101 | 1227 | 12.15 | 0.12 | 0.64 | 0.43 |
| SU | 27 | 97 | 3.54 | 0.14 | 0.33 | 0.35 | |
| GL | 47 | 244 | 5.19 | 0.11 | 0.55 | 0.42 | |
| SF | 111 | 1133 | 10.21 | 0.09 | 0.65 | 0.45 | |
| PF | 94 | 991 | 10.54 | 0.11 | 0.36 | 0.47 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, C.; Zhang, Z.; Zhong, C.; Hu, G.; Xu, C. Impact of Different Land Use Types on Bacterial and Fungal Communities in a Typical Karst Depression in Southwestern China. Forests 2024, 15, 1299. https://doi.org/10.3390/f15081299
Hu C, Zhang Z, Zhong C, Hu G, Xu C. Impact of Different Land Use Types on Bacterial and Fungal Communities in a Typical Karst Depression in Southwestern China. Forests. 2024; 15(8):1299. https://doi.org/10.3390/f15081299
Chicago/Turabian StyleHu, Cong, Zhonghua Zhang, Chaofang Zhong, Gang Hu, and Chaohao Xu. 2024. "Impact of Different Land Use Types on Bacterial and Fungal Communities in a Typical Karst Depression in Southwestern China" Forests 15, no. 8: 1299. https://doi.org/10.3390/f15081299






