Effect of Planting Ground Treatments Using Artificial Rainfall Slope Simulating Degraded Forestland on Drought Stress Susceptibility of Pinus densiflora
Abstract
:1. Introduction
2. Materials and Methods
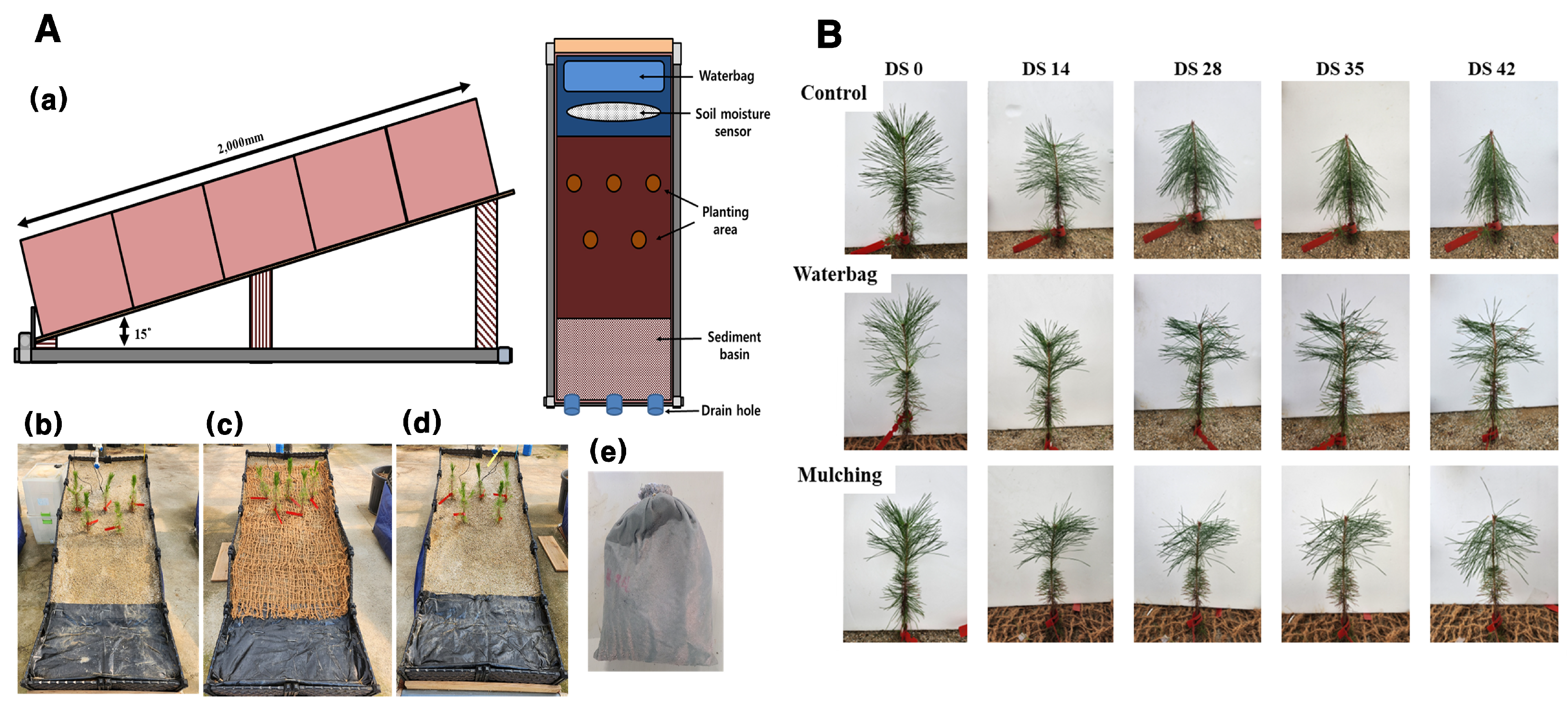
2.1. Artificial Rainfall Testing Plot for Simulating Forest Degradation
2.2. Growth Characteristics and Leaf Water Saturation Deficit
2.3. Photosynthetic and Stomatal Response
2.4. Chlorophyll a Fluorescence Response
2.5. Leaf Pigment Content
2.6. Data Analysis
3. Results
3.1. Soil Moisture Content
3.2. Diameter at Root-Collar and Leaf Water Saturation Deficit
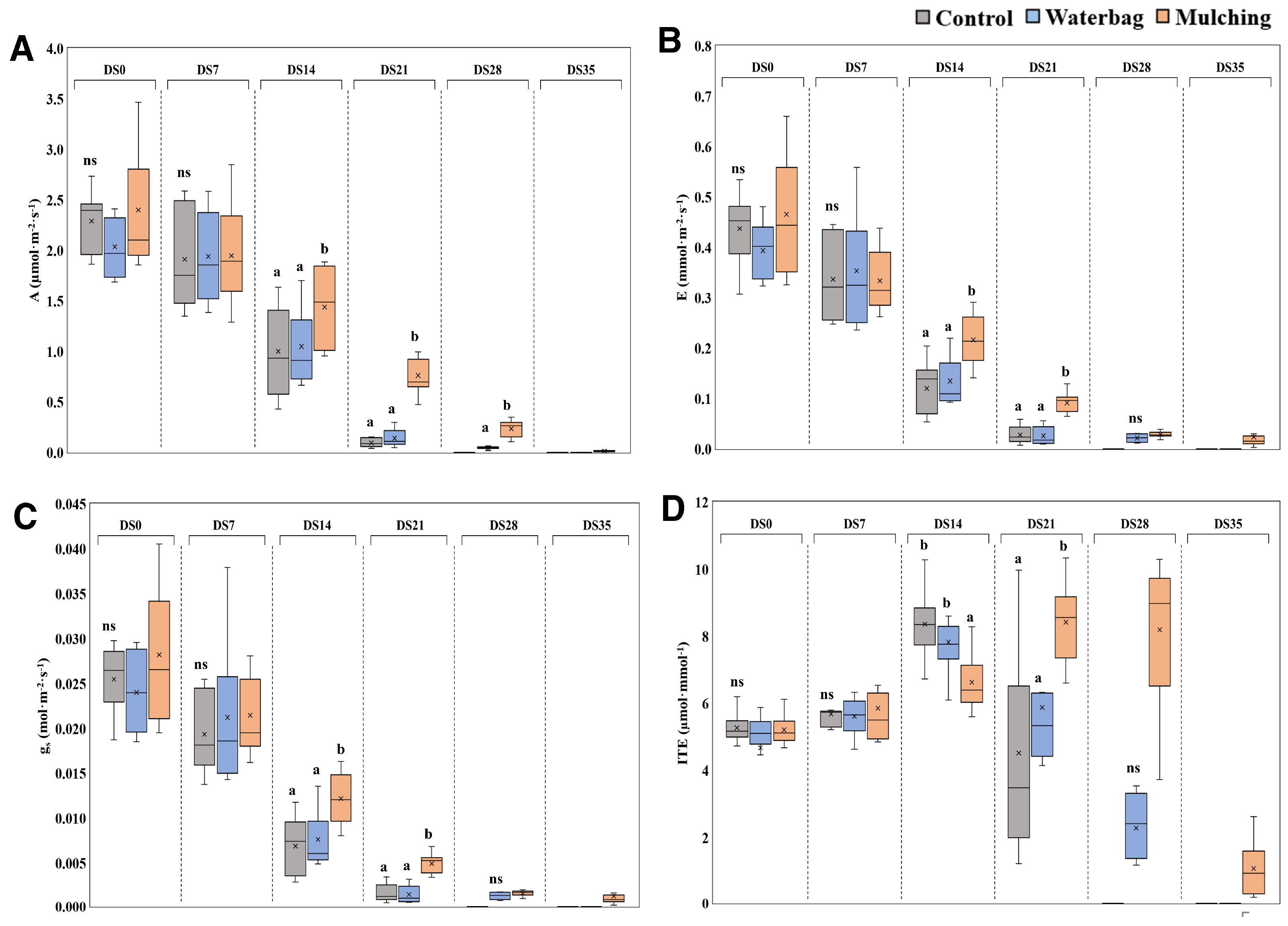
3.3. Photosynthesis and Stomatal Responses
3.4. Leaf Pigment Content
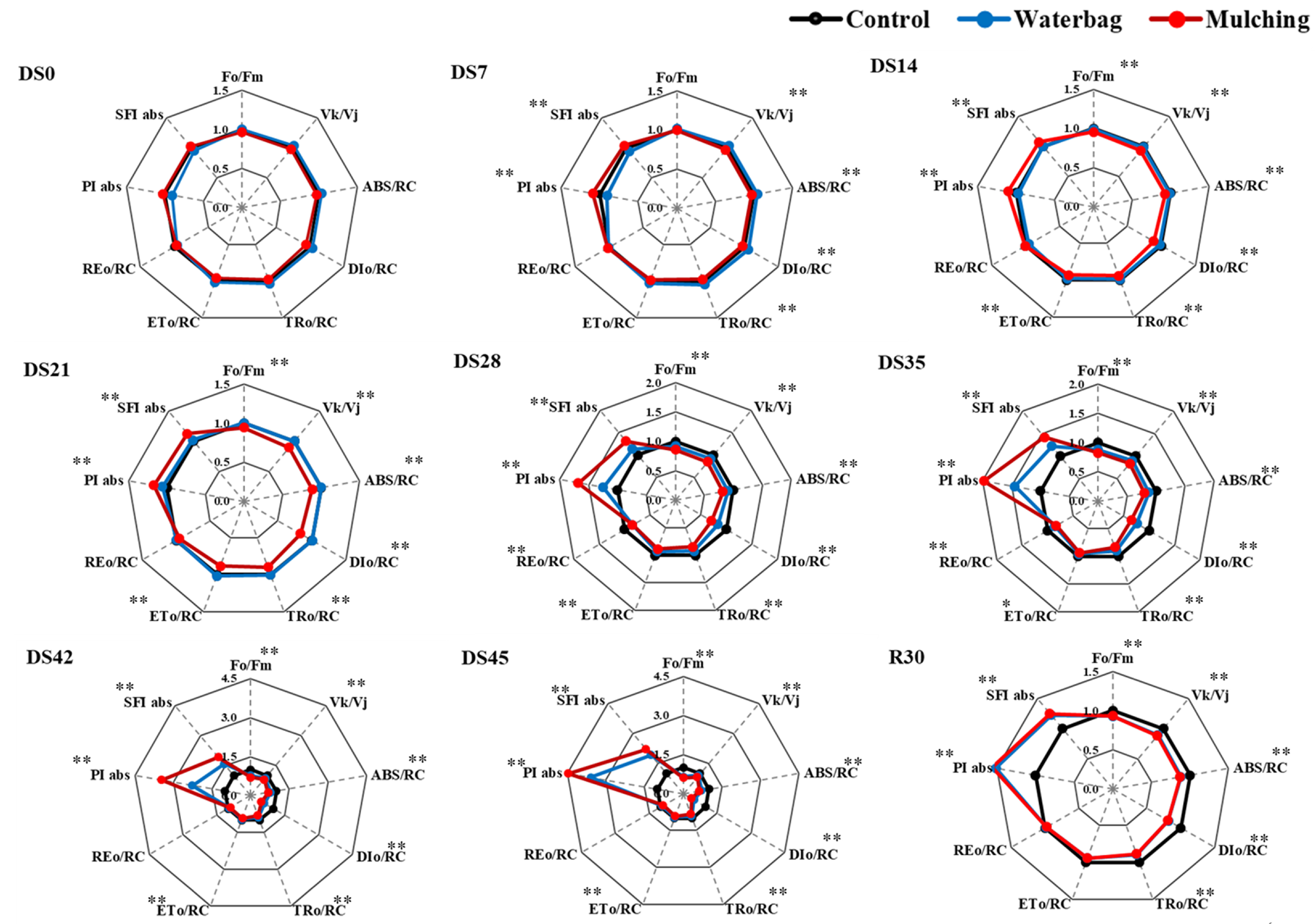
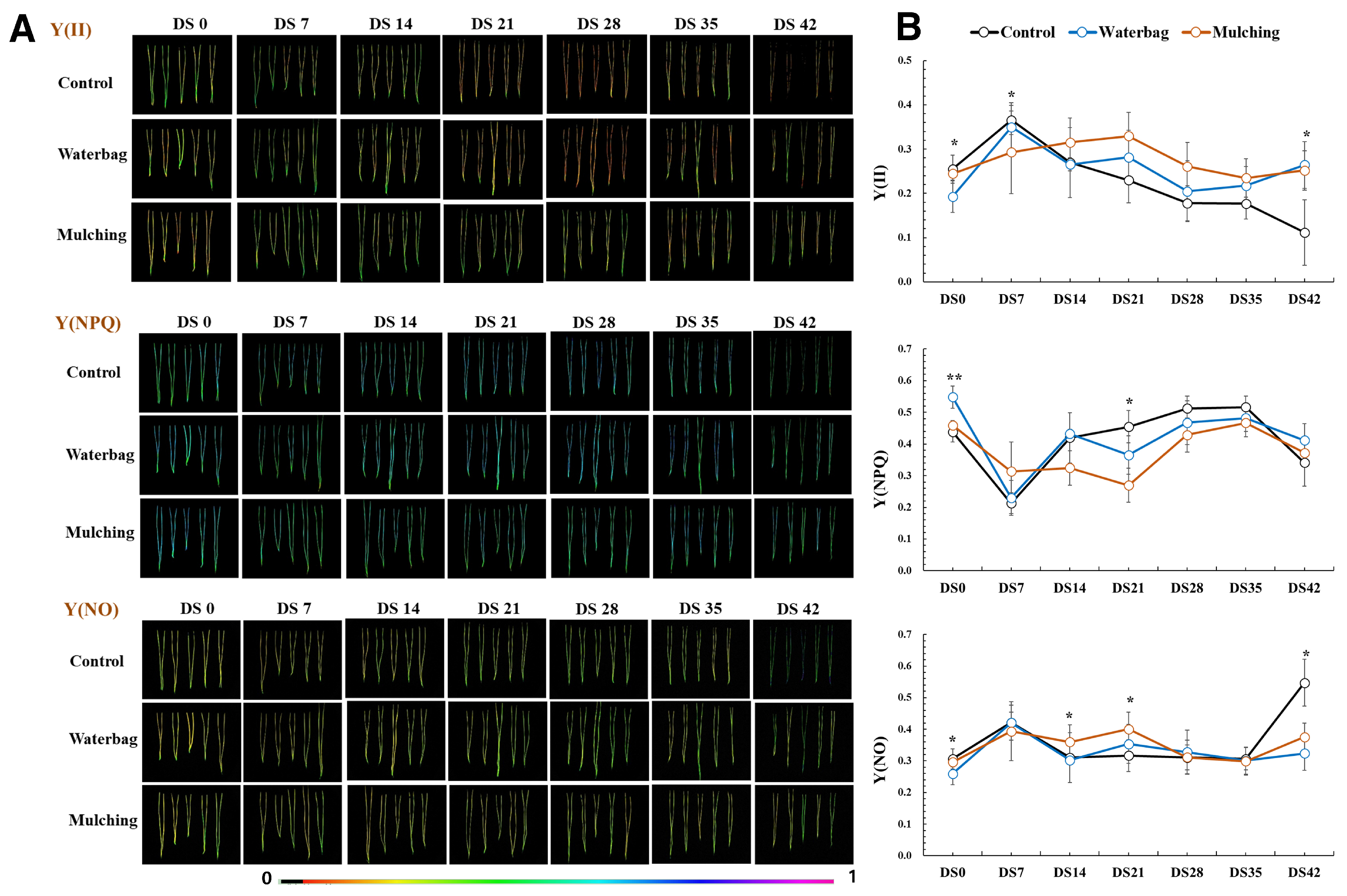
3.5. Chlorophyll Fluorescence and Imaging Fluorescence Responses
4. Discussion
4.1. Soil Moisture Content
4.2. Water Saturation Deficit and Root Collar Growth
4.3. Photosynthesis and Stomatal Response
4.4. Leaf Pigment Content
4.5. Chlorophyll Fluorescence and Imaging Fluorescence Response
4.6. Practical Application in Real Forest Sites
4.7. Future Research Directions
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, D.; Han, H.; Chung, J. Optimal Forest Management for Improving Economic and Public Functions in MT. Gari Leading Forest Management Zone. J. Korean Soc. For. Sci. 2021, 110, 665–677. [Google Scholar] [CrossRef]
- Yu, J.; Choi, W.; Lee, S.; Choi, J. Forest Degradation and Spatial Distribution of Forest Land Development. J. Korean Soc. Environ. Restor. Technol. 2016, 19, 101–110. [Google Scholar] [CrossRef]
- Kang, H.M.; Choi, S.H.; Kim, D.H.; Song, J.T. A Study on the Restoration Effects of Vegetation Restoration Types. Korean J. Environ. Ecol. 2017, 31, 174–187. [Google Scholar] [CrossRef]
- Jung, T.J.; Kim, Y.G.; Kim, Y.J.; Jung, M.H.; Park, K.H.; Shin, C.K.; Park, S.; Kim, Y.S. A Change of Vegetation at the Ecological Restoration Area of Simwon Valley in Jirisan National Park. Korean J. Environ. Ecol. 2021, 35, 294–304. [Google Scholar] [CrossRef]
- Lee, K.; Song, Y.; Koo, H.; Kim, H.; Kweon, H.; Koo, N. Evaluation of Soil Loss Tolerance and Tree Growth Features Based on Planting Ground Methods in the Alpine Center, Degraded Forestland in the Republic of Korea. Forests 2023, 14, 200. [Google Scholar] [CrossRef]
- Nam, S.J.; Yeo, H.J.; Choi, J.Y.; Kim, N. Development of Revegetation Method Using Forest Topsoils for Ecological Restoration of the Slopes (I). J. Korean Soc. Environ. Restor. Technol. 2004, 7, 110–119. [Google Scholar]
- Cha, D.S.; Oh, J.H.; Ji, B.Y.; Cho, K.H.; Lee, H.J. Development of the Soil Bioengineering Techniques for Restoring of Degraded Forest Area (V)–Pull–out Resistance Characteristics of Shrubs’ roots. J. Forest Environ. Sci. 2008, 24, 111–118. [Google Scholar]
- Kim, M.; Choi, Y.; Lee, S.; Kim, H.; Kim, S.; Kim, Y. Polyacrylamide, Its Beneficial Application of Soil Erosion Control from Sloped Agricultural Fields. J. Korean Soc. Agric. Eng. 2015, 57, 123–128. [Google Scholar] [CrossRef]
- Jeong, S.J.; Oh, K.K.; Oh, J.G. A study on Restoration Measures of Vegetation for Devastated Ridge Line Area in National Park, Korea. Korean J. Environ. Ecol. 2001, 15, 69–78. [Google Scholar]
- Jang, S.J.; Lee, Y.T.; Lee, K.Y.; Kim, K.N.; Lee, J.H.; Chun, K.W. A Study of Disaster Prevention and Characteristics of Landslides Triggered by the 2019 Typhoon Mitag in Samcheok. J. Korean Soc. Hazard Mitig. 2020, 20, 221–227. [Google Scholar] [CrossRef]
- Chazdon, R.L. Beyond Deforestation: Restoring Forests and Ecosystem Services on Degraded Lands. Science 2008, 320, 1458–1460. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.D.; Lee, K.J.; Choi, J.W. Management Plan to Consider Ecological Characteristic of Pinus densiflora Community in Seoul. Korean J. Environ. Ecol. 2009, 23, 258–271. [Google Scholar]
- Lee, D.H.; Rhee, D.S.; Kim, M. Determination of Permissible Shear Stresses on Vegetation Mats by Soil Loss Evaluation. J. Korea Acad. Ind. Coop. Soc. 2013, 14, 5956–5963. [Google Scholar] [CrossRef]
- Baik, J.; Chiang, M.H. Effects of Different Soil on the Growth of Salicornia herbacea. J. Bio-Environ. Control 2011, 20, 216–220. [Google Scholar]
- Mannan, M.A.; Tithi, M.A.; Islam, M.R.; Al Mamun, M.A.; Mia, S.; Rahman, M.Z.; Awad, M.F.; ElSayed, A.I.; Mansour, E.; Hossain, M.S. Soil and Foliar Applications of Zinc Sulfate and Iron Sulfate Alleviate the Destructive Impacts of Drought Stress in Wheat. Cereal Res. Commun. 2022, 50, 1279–1289. [Google Scholar] [CrossRef]
- Lee, K.C.; Kweon, H.; Sung, J.W.; Kim, Y.S.; Song, Y.G.; Cha, S.; Koo, N. Physiological Response Analysis for the Diagnosis of Drought and Waterlogging Damage in Prunus Yedoensis. For. Sci. Technol. 2022, 18, 14–25. [Google Scholar] [CrossRef]
- Zheng, W.P.; Wang, P.; Zhang, H.X.; Zhou, D. Photosynthetic Characteristics of the Cotyledon and First True Leaf of Castor (Ricinus communis L.). Aust. J. Crop Sci. 2011, 5, 702–708. [Google Scholar]
- Xia, J.B.; Zhang, G.C.; Wang, R.R.; Zhang, S.Y. Effect of Soil Water Availability on Photosynthesis in Ziziphus Jujuba var. spinosus in a Sand Habitat Formed from Seashells: Comparison of Four Models. Photosynthetica 2014, 52, 253–261. [Google Scholar] [CrossRef]
- Sharkey, T.D.; Bernacchi, C.J.; Farquhar, G.D.; Singsaas, E.L. Fitting Photosynthetic Carbon Dioxide Response Curves for C3 Leaves. Plant Cell Environ. 2007, 30, 1035–1040. [Google Scholar] [CrossRef]
- Strasser, R.J.; Srivastava, A.; Tsimilli–Michael, M. The fluorescence transient as a tool to characterize and screen photosynthetic samples. In Probing Photosynthesis: Mechanism, Regulation, and Adaptation; Yunus, M., Pathre, U., Mohanty, P., Eds.; Taylor and Francis: London, UK; New York, NY, USA, 2000; pp. 445–483. [Google Scholar]
- de Sousa, C.A.F.; de Paiva, D.S.; Casari, R.A.D.C.N.; de Oliveira, N.G.; Molinari, H.B.C.; Kobayashi, A.K.; Magalhães, P.C.; Gomide, R.L.; Souza, M.T. A Procedure for Maize Genotypes Discrimination to Drought by Chlorophyll Fluorescence Imaging Rapid Light Curves. Plant Methods 2017, 13, 61. [Google Scholar] [CrossRef]
- Wang, W.; Wang, C.; Pan, D.; Zhang, Y.; Luo, B.; Ji, J. Effects of Drought Stress on Photosynthesis and Chlorophyll Fluorescence Images of Soybean (Glycine max) seedlings. Int. J. Agric. Biol. Eng. 2018, 11, 196–201. [Google Scholar] [CrossRef]
- Hiscox, J.D.; Israelstam, G.F.A. Method for the Extraction of Chlorophyll from Leaf Tissue Without Maceration. Can. J. Bot. 1979, 57, 1332–1334. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Jajoo, A.; Oukarroum, A.; Brestic, M.; Zivcak, M.; Samborska, I.A.; Cetner, M.D.; Łukasik, I.; Goltsev, V.; Ladle, R.J. Chlorophyll A Fluorescence as a Tool to Monitor Physiological Status of Plants Under Abiotic Stress Conditions. Acta Physiol. Plant. 2016, 38, 102. [Google Scholar] [CrossRef]
- Klughammer, C.; Schreiber, U. Complementary PS II Quantum Yields Calculated from Simple Fluorescence Parameters Measured by PAM Fluorometry and the Saturation Pulse Method. PAM Appl. Notes 2008, 1, 201–247. [Google Scholar]
- Irvine, J.; Perks, M.P.; Magnani, F.; Grace, J. The response of Pinus sylvestris to drought: Stomatal control of transpiration and hydraulic conductance. Tree Physiol. 1998, 18, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Lee, K. Photosynthetic responses and growth performances affected by organic compost level in seedling of Synurus deltoides(aiton) nakai. J. Agric. Life Sci. 2017, 51, 65–78. [Google Scholar] [CrossRef]
- Tardieu, F.; Simonneau, T. Variability among species of stomatal control under fluctuating soil water status and evaporative demand: Modelling isohydric and anisohydric behaviours. J. Exp. Bot. 1998, 49, 419–432. [Google Scholar] [CrossRef]
- Meinzer, F.C.; Woodruff, D.R.; Marias, D.E.; McCulloh, K.A.; Sevanto, S. Dynamics of Leaf Water Relations Components in Co-occurring Iso-and Anisohydric Conifer Species. Plant Cell Environ. 2014, 37, 2577–2586. [Google Scholar] [CrossRef]
- de Santana, T.A.; da Silva, L.D.; de Oliveira, P.S.; Benjamin, C.S.; Ramos, E.P.; de Souza, J.O.; Gomes, F.P. Leaf Gas Exchange and Biomass Partitioning in Jatropha curcas L. Young Plants Subjected to Flooding and Drought Stresses. Aust. J. Crop Sci. 2017, 11, 792–798. [Google Scholar] [CrossRef]
- Matyssek, R.; Kozovits, A.R.; Wieser, G.; Augustaitiene, I.; Augustaitis, A. Biological Reactions of Forests to Climate Change and Air Pollution. Eur. J. For. Res. 2014, 133, 671–673. [Google Scholar] [CrossRef]
- Balekoglu, S.; Caliskan, S.; Dirik, H.; Rosner, S. Response to Drought Stress Differs Among Pinus pinea Provenances. For. Ecol. Manag. 2023, 531, 120779. [Google Scholar] [CrossRef]
- Collatz, G.J.; Ball, J.T.; Grivet, C.; Berry, J.A. Physiological and Environmental Regulation of Stomatal Conductance, Photosynthesis and Transpiration: A Model that Includes a Laminar Boundary Layer. Agric. For. Meteorol. 1991, 54, 107–136. [Google Scholar] [CrossRef]
- Jo, H.; Chang, H.; An, J.; Cho, M.S.; Son, Y. Species Specific Physiological Responses of Pinus densiflora and Larix kaempferi Seedlings to Open–field Experimental Warming and Precipitation Manipulation. For. Sci. Technol. 2019, 15, 44–50. [Google Scholar] [CrossRef]
- Espadafor, M.; Orgaz, F.; Testi, L.; Lorite, I.J.; González–Dugo, V.; Fereres, E. Responses of Transpiration and Transpiration Efficiency of Almond Trees to Moderate Water Deficits. Sci. Hortic. 2017, 225, 6–14. [Google Scholar] [CrossRef]
- Hopkins, W.G.; Hüner, N.P.A. Introduction to Plant Physiology, 4th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2008; pp. 223–230. [Google Scholar]
- Song, Y.; Kim, C.W.; Kweon, H.; Yoon, K.K.; Shim, Y.J.; Lee, U.; Lee, K.C. Effects of Drought Stress on the Growth and Physiological Characteristics of Grafted Ziziphus jujuba var. inermis under RCP 6.0 Climate Change Scenarios. Hortic. Sci. Technol. 2023, 41, 11–26. [Google Scholar] [CrossRef]
- Hikosaka, K.; Terashima, I. A Model of the Acclimation of Photosynthesis in the Leaves of C3 Plants to Sun and Shade with Respect to Nitrogen Use. Plant Cell Environ. 1995, 18, 605–618. [Google Scholar] [CrossRef]
- Saraswathi, S.G.; Paliwal, K. Drought Induced Changes in Growth, Leaf Gas Exchange and Biomass Production in Albizia lebbeck and Cassia siamea Seedlings. J. Environ. Biol. 2011, 32, 173–178. [Google Scholar] [PubMed]
- Lee, K.C.; Kim, S.H.; Park, W.G.; Kim, Y.S. Effects of Drought Stress on Photosynthetic Capacity and Photosystem II Activity in Oplopanax elatus. Korean J. Med. Crop Sci. 2014, 22, 38–45. [Google Scholar] [CrossRef]
- Kramer, D.M.; Johnson, G.; Kiirats, O.; Edwards, G.E. New Fluorescence Parameters for the Determination of QA Redox State and Excitation Energy Fluxes. Photosynth. Res. 2004, 79, 209–218. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, M.; Hu, J.; Pan, M.; Shen, L.; Ye, J.; Tan, J. Early Diagnosis of Pine Wilt Disease in Pinus thunbergii Based on Chlorophyll Fluorescence Parameters. Forests 2023, 14, 154. [Google Scholar] [CrossRef]
- Faseela, P.; Sinisha, A.K.; Brestič, M.; Puthur, J.T. Chlorophyll a Fluorescence Parameters as Indicators of a Particular Abiotic Stress in Rice. Photosynthetica 2020, 58, 293–300. [Google Scholar] [CrossRef]
- Antunović Dunić, J.; Mlinarić, S.; Pavlović, I.; Lepeduš, H.; Salopek-Sondi, B. Comparative Analysis of Primary Photosynthetic Reactions Assessed by OJIP Kinetics in Three Brassica Crops after Drought and Recovery. Appl. Sci. 2023, 13, 3078. [Google Scholar] [CrossRef]
- Faria–Silva, L.; Gallon, C.Z.; Filgueiras, P.R.; Silva, D.M. Irrigation Improves Plant Vitality in Specific Stages of Mango Tree Development According to Photosynthetic Efficiency. Photosynthetica 2019, 57, 820–829. [Google Scholar] [CrossRef]
- Khatri, K.; Rathore, M.S. Photosystem Photochemistry, Prompt and Delayed Fluorescence, Photosynthetic Responses and Electron Flow in Tobacco Under Drought and Salt Stress. Photosynthetica 2019, 57, 61–74. [Google Scholar] [CrossRef]
- Murchie, E.H.; Lawson, T. Chlorophyll Fluorescence Analysis: A Guide to Good Practice and Understanding Some New Applications. J. Exp. Bot. 2013, 64, 3983–3998. [Google Scholar] [CrossRef]


| Parameters | Description |
|---|---|
| Fo/Fm | A parameter related to changes in heat dissipation in the PS II antenna |
| VK/VJ | Ratio of variable fluorescence in time 0.3 ms to variable fluorescence in time 2 ms as an indicator of the PS II donor side limitation |
| ABS/RC | Absorption flux per reaction center |
| TRo/RC | Trapping of electrons per reaction center |
| ETo/RC | Electron flux per reaction center beyond QA− |
| DIo/RC | Energy dissipation flux per reaction center |
| REo/RC | Electron transport flux until PS I acceptors per reaction center |
| PIabs | Performance index on absorption basis. |
| SFIabs | The structure function index on absorption basis. |
| Y(II) | PS II actual photochemical quantum yield |
| Y(NPQ) | Quantum yield of regulated energy dissipation in PS II |
| Y(NO) | Quantum yield of non-regulated energy dissipation in PS II |
| Treatment | Vcmax (µmol·m−2·s−1) | Jmax (µmol·m−2·s−1) | ||||||
|---|---|---|---|---|---|---|---|---|
| DS0 | DS7 | DS14 | DS21 | DS0 | DS7 | DS14 | DS21 | |
| Control | 8.61 ns | 6.62 ns | 7.25 ns | nd | 20.66 ns | 16.41 ns | 15.30 ns | nd |
| Waterbag | 8.43 | 6.81 | 8.00 | nd | 20.65 | 16.94 | 15.29 | nd |
| Mulching | 8.94 | 6.99 | 8.36 | 8.31 | 22.65 | 17.42 | 19.32 | 17.33 |
| DAT | Treatment | Chl (mg·g−1) | Car (mg·g−1) | T Chl/Car | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| a | b | a + b | |||||||||
| DS0 | Control | 9.68 ns | 2.83 b | 12.52 b | 1.98 b | 6.40 ns | |||||
| Waterbag | 8.51 | 1.87 a | 10.38 a | 1.68 a | 6.21 | ||||||
| Mulching | 9.50 | 2.53 b | 12.03 b | 1.95 b | 6.18 | ||||||
| DS21 | Control | 10.19 b | 2.75 b | 12.94 b | 2.12 b | 6.14 ns | |||||
| Waterbag | 8.30 a | 2.27 a | 10.57 a | 1.70 a | 6.25 | ||||||
| Mulching | 9.44 ab | 2.62 ab | 12.06 ab | 1.92 ab | 6.28 | ||||||
| DS45 | Control | 8.81 ns | 2.47 b | 11.28 ns | 2.08 ns | 5.43 a | |||||
| Waterbag | 7.28 | 2.00 a | 9.28 | 1.72 | 5.39 a | ||||||
| Mulching | 7.89 | 2.14 ab | 10.03 | 1.78 | 5.65 b | ||||||
| R30 | Control | 8.85 ns | 2.29 ns | 11.14 ns | 2.18 ns | 5.1 ns | |||||
| Waterbag | 8.33 | 1.94 | 10.27 | 2.07 | 4.94 | ||||||
| Mulching | 9.97 | 2.28 | 12.25 | 2.35 | 5.21 | ||||||
| Source | F-value | p-value | F-value | p-value | F-value | p-value | F-value | p-value | F-value | p-value | |
| DAT (D) | 5.40 | <0.001 | 4.03 | <0.008 | 4.79 | <0.003 | 8.20 | <0.001 | 44.96 | <0.001 | |
| Treatment (T) | 9.21 | <0.001 | 14.05 | <0.001 | 11.17 | <0.001 | 9.60 | <0.001 | 0.78 | 0.461 | |
| T × D | 1.10 | 0.36 | 1.02 | 0.42 | 0.88 | 0.51 | 1.20 | 0.31 | 0.59 | 0.74 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, K.; Song, Y.; Kim, M.; Choi, W.; Ju, H.; Koo, N. Effect of Planting Ground Treatments Using Artificial Rainfall Slope Simulating Degraded Forestland on Drought Stress Susceptibility of Pinus densiflora. Forests 2024, 15, 1323. https://doi.org/10.3390/f15081323
Lee K, Song Y, Kim M, Choi W, Ju H, Koo N. Effect of Planting Ground Treatments Using Artificial Rainfall Slope Simulating Degraded Forestland on Drought Stress Susceptibility of Pinus densiflora. Forests. 2024; 15(8):1323. https://doi.org/10.3390/f15081323
Chicago/Turabian StyleLee, Kyeongcheol, Yeonggeun Song, Minsu Kim, Wooyoung Choi, Hyoseong Ju, and Namin Koo. 2024. "Effect of Planting Ground Treatments Using Artificial Rainfall Slope Simulating Degraded Forestland on Drought Stress Susceptibility of Pinus densiflora" Forests 15, no. 8: 1323. https://doi.org/10.3390/f15081323
APA StyleLee, K., Song, Y., Kim, M., Choi, W., Ju, H., & Koo, N. (2024). Effect of Planting Ground Treatments Using Artificial Rainfall Slope Simulating Degraded Forestland on Drought Stress Susceptibility of Pinus densiflora. Forests, 15(8), 1323. https://doi.org/10.3390/f15081323






