Responses of Soil Organic Carbon Fractions and Stability to Forest Conversion in the Nanling Nature Reserve, China
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area Description and Sample Collection
2.2. Analysis of Soil Organic Carbon and Fractions
2.3. Analysis of Soil Properties
2.4. Analysis of Enzyme Activity
2.5. Determination of Soil C Stocks and CPMI
2.6. Data Analysis
3. Results
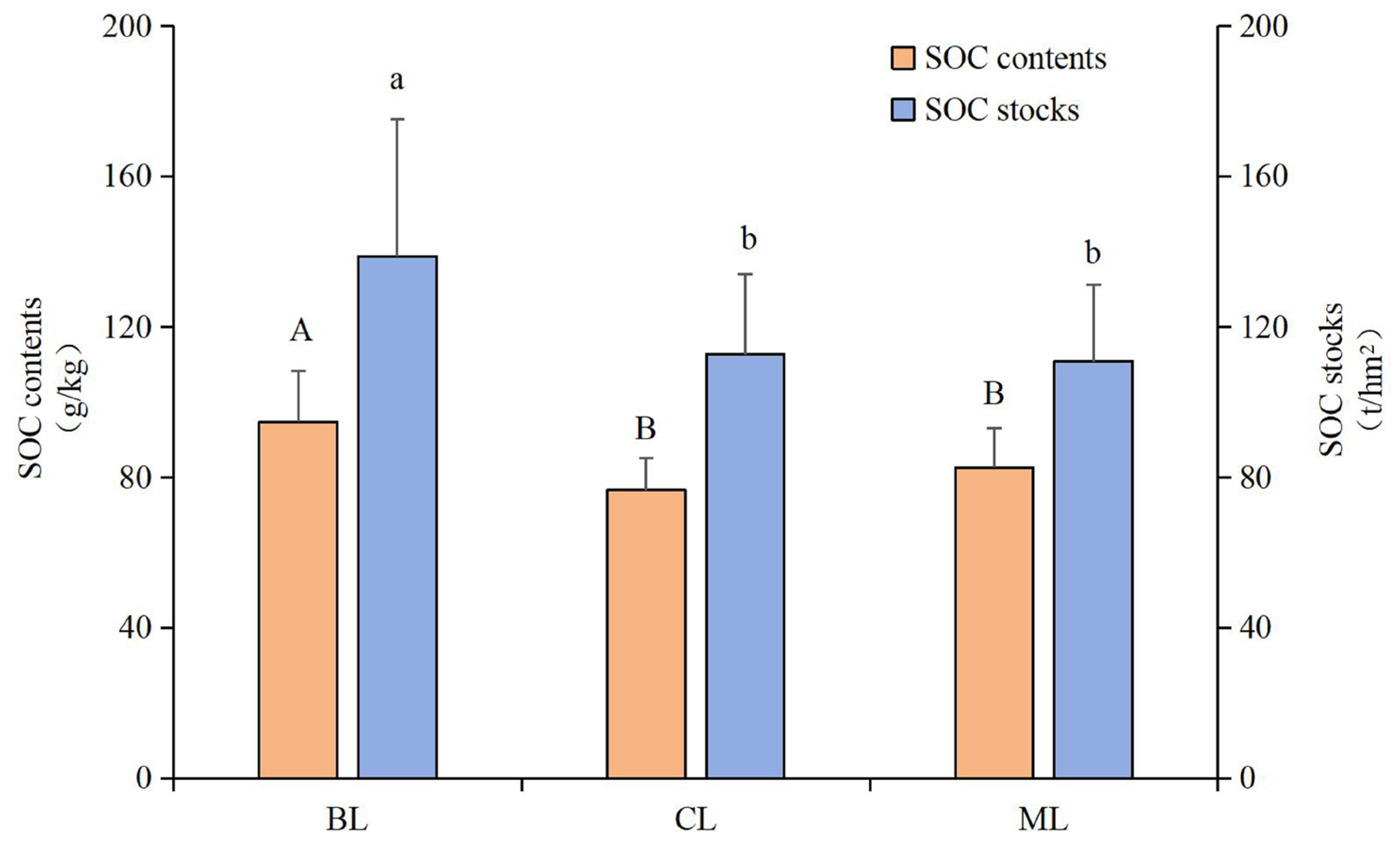
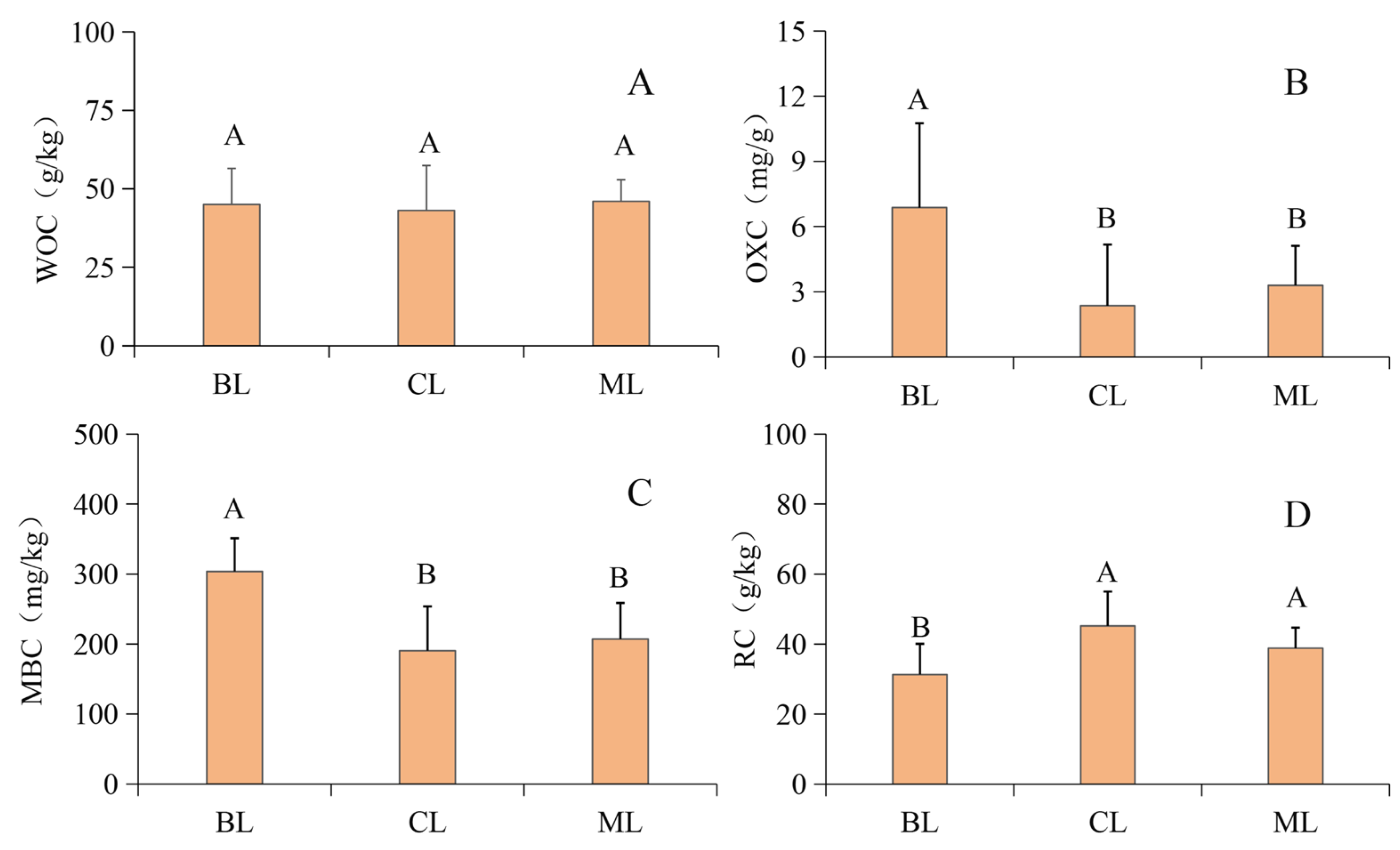
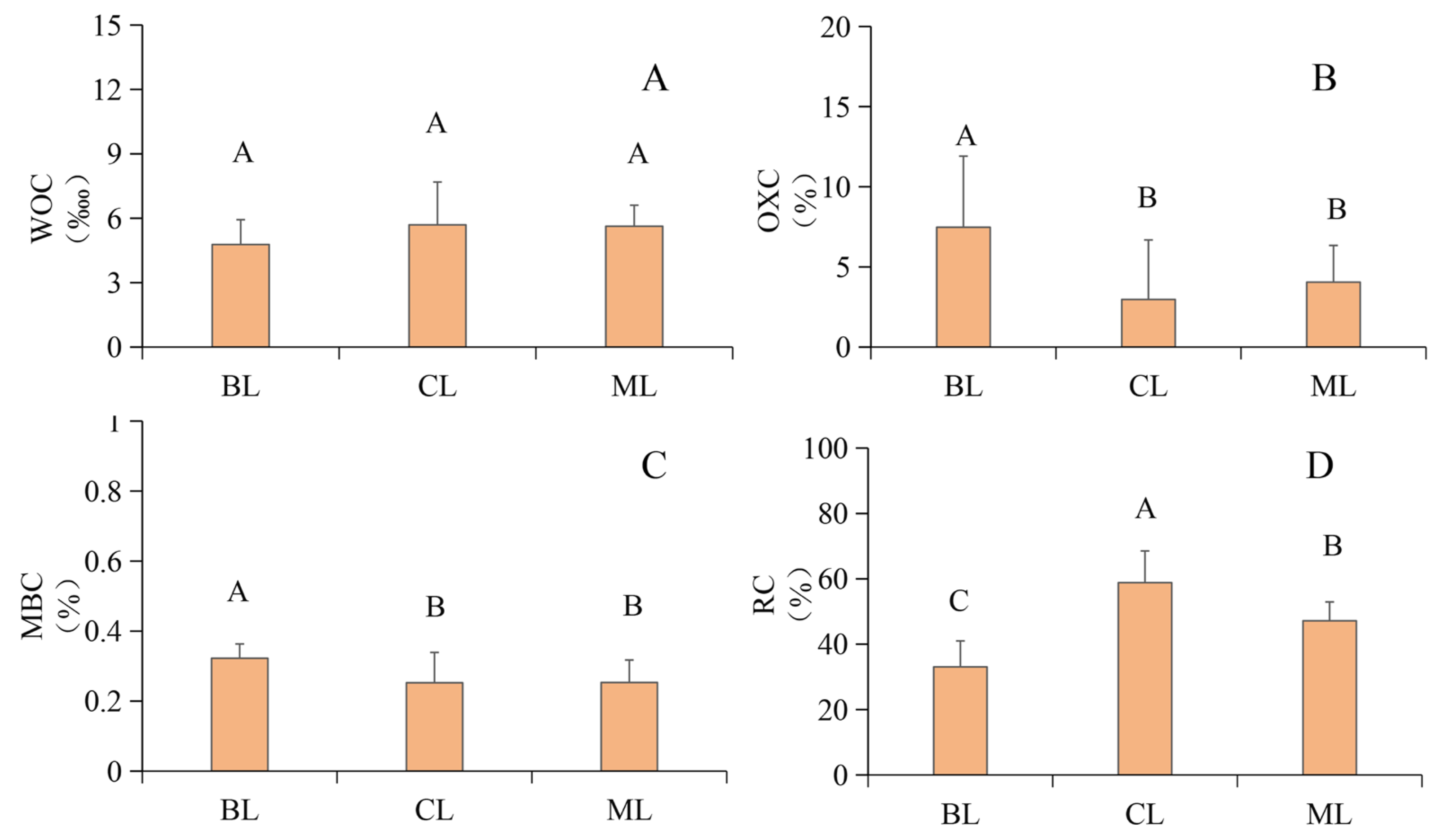
3.1. Soil SOC and Fractions
3.2. Carbon Pool Management Indices
3.3. Soil Properties
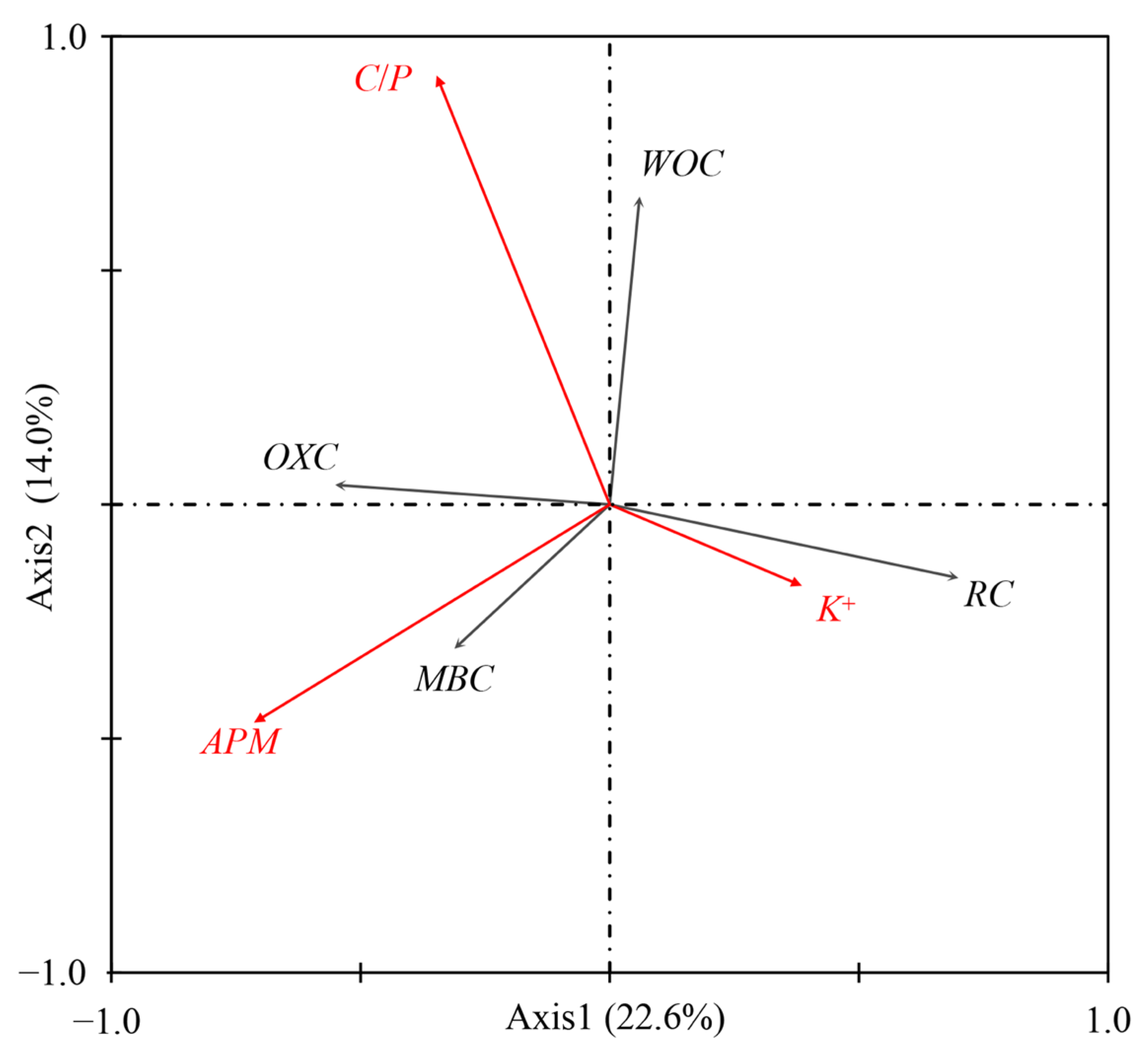
3.4. Correlation Analysis
4. Discussion
4.1. Variations in SOC Concentrations and Stocks
4.2. Variations in Soil LAC and NLAC Concentrations and Proportions
4.3. Variations in Soil CPMI
4.4. Relationships between Soil C Fractions and Soil Properties
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Peng, S.; Wang, B. Forest succession of Dinghushan, Guangdong, China. Chin. J. Bot. 1995, 7, 75–80. [Google Scholar]
- Fan, H.; Wu, J.; Liu, W.; Yuan, Y.; Hu, L.; Cai, Q. Linkages of plant and soil C: N: P stoichiometry and their relationships to forest growth in subtropical plantations. Plant Soil 2015, 392, 127–138. [Google Scholar] [CrossRef]
- Gong, C.; Wang, S.; Zeng, Z.; Ding, S.; Chen, J.; Long, K. Carbon storage and its distribution pattern of evergreen broad-leaved forests at different succession stages in mid-subtropical China. Chin. J. Ecol. 2011, 30, 1935–1941. [Google Scholar]
- Yarwood, S.A.; Högberg, M.N. Soil bacteria and archaea change rapidly in the first century of Fennoscandian boreal forest development. Soil Biol. Biochem. 2017, 114, 160–167. [Google Scholar] [CrossRef]
- Hu, Y.F.; Shu, X.Y.; He, J.; Zhang, Y.L.; Xiao, H.H.; Tang, X.Y.; Gu, Y.F.; Lan, T.; Xia, J.G.; Ling, J.; et al. Storage of C, N, and P affected by afforestation with Salix cupularis in an alpine semiarid desert ecosystem. Land Degrad. Dev. 2018, 29, 188–198. [Google Scholar] [CrossRef]
- Chu, X.; Zhan, J.; Li, Z.; Zhang, F.; Qi, W. Assessment on forest carbon sequestration in the Three-North Shelterbelt Program region, China. J. Clean. Prod. 2019, 215, 382–389. [Google Scholar] [CrossRef]
- Liao, C.; Tian, Q.; Wang, D.; Qu, L.; Wu, Y.; Liu, F. Effects of labile carbon addition on organic carbon mineralization and microbial growth strategies in subtropical forest soils. Chin. J. Appl. Ecol. 2016, 27, 2848–2854. [Google Scholar]
- Plaza-Bonilla, D.; Álvaro-Fuentes, J.; Cantero-Martínez, C. Identifying soil organic carbon fractions sensitive to agricultural management practices. Soil Tillage Res. 2014, 139, 19–22. [Google Scholar] [CrossRef]
- Duru, M.; Therond, O.; Martin, G.; Martin-Clouaire, R.; Magne, M.A.; Justes, E.; Journet, E.; Aubertot, J.; Savary, S.; Bergez, J.; et al. How to implement biodiversity-based agriculture to enhance ecosystem services: A review. Agron. Sustain. Dev. 2015, 35, 1259–1281. [Google Scholar] [CrossRef]
- Dong, X.; Zhao, K.; Wang, J.; Gui, H.; Xiao, Y.; Chen, Z.; Miao, Y.; Han, S. Effects of forest types on soil carbon content in aggregate faction under climate transition zone. Front. Environ. Sci. 2023, 10, 1052175. [Google Scholar] [CrossRef]
- Gao, F.; Cui, X.; Sang, Y.; Song, J. Changes in soil organic carbon and total nitrogen as affected by primary forest conversion. For. Ecol. Manag. 2020, 463, 118013. [Google Scholar] [CrossRef]
- Rytter, R.M. Afforestation of former agricultural land with Salicaceae species–initial effects on soil organic carbon, mineral nutrients, C: N and pH. For. Ecol. Manag. 2016, 363, 21–30. [Google Scholar] [CrossRef]
- Chen, Y.; Yu, S.; Liu, S.; Wang, X.; Zhang, Y.; Liu, T.; Zhou, L.; Zhang, W.; Fu, S. Reforestation makes a minor contribution to soil carbon accumulation in the short term: Evidence from four subtropical plantations. For. Ecol. Manag. 2017, 384, 400–405. [Google Scholar] [CrossRef]
- Haynes, R.J. Labile organic matter as an indicator of organic matter quality in arable and pastoral soils in New Zealand. Soil Biol. Biochem. 2000, 32, 211–219. [Google Scholar] [CrossRef]
- Manu, V.; Whitbread, A.; Blair, N.; Blair, G. Carbon status and structural stability of soils from differing land use systems in the Kingdom of Tonga. Soil Use Manag. 2014, 30, 517–523. [Google Scholar] [CrossRef]
- Yang, X.; Meng, J.; Lan, Y.; Chen, W.; Yang, T.; Yuan, J.; Liu, S.; Han, J. Effects of maize stover and its biochar on soil CO2 emissions and labile organic carbon fractions in Northeast China. Agric. Ecosyst. Environ. 2017, 240, 24–31. [Google Scholar] [CrossRef]
- Lepcha, N.T.; Devi, N.B. Effect of land use, season, and soil depth on soil microbial biomass carbon of Eastern Himalayas. Ecol. Process. 2020, 9, 1–14. [Google Scholar] [CrossRef]
- Karavanova, E.I.; Zolovkina, D.F. The effect of litter composition on the characteristics of their water-soluble organic matter. Mosc. Univ. Soil Sci. Bull. 2020, 75, 67–73. [Google Scholar] [CrossRef]
- Li, Y.; Chen, J.; Cui, J.; Zhao, X.; Zhang, T. Nutrient resorption in Caragana microphylla along a chronosequence of plantations: Implications for desertified land restoration in North China. Ecol. Eng. 2013, 53, 299–305. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, W.; Wang, K.; Pan, F.; Yang, S.; Shu, S. Factors controlling accumulation of soil organic carbon along vegetation succession in a typical karst region in Southwest China. Sci. Total Environ. 2015, 521, 52–58. [Google Scholar] [CrossRef]
- Yuan, Z.; Jin, X.; Xiao, W.; Wang, L.; Sun, Y.; Guan, Q.; Meshack, A.O. Comparing soil organic carbon stock and fractions under natural secondary forest and Pinus massoniana plantation in subtropical China. Catena 2022, 212, 106092. [Google Scholar] [CrossRef]
- Liang, Q.; Wang, C.; Zhang, K.; Shi, S.; Guo, J.; Gao, F.; Liu, J.; Wang, J.; Liu, Y. The influence of tree species on soil organic carbon stability under three temperate forests in the Baihua Mountain Reserve, China. Glob. Ecol. Conserv. 2021, 26, e01454. [Google Scholar] [CrossRef]
- Wang, Q.K.; Wang, S.L.; Den, S.J. Comparative study on active soil organic matter in Chinese fir plantation and native broad-leaved forest in subtropical China. J. For. Res. 2005, 16, 23–26. [Google Scholar]
- Datta, A.; Basak, N.; Chaudhari, S.K.; Sharma, D.K. Soil properties and organic carbon distribution under different land uses in reclaimed sodic soils of North-West India. Geoderma Reg. 2015, 4, 134–146. [Google Scholar] [CrossRef]
- Chen, X.M.; Yan, J.H.; Lin, M.Z.; Zhu, G.W.; Wu, J.P.; Zhang, D.Q. Variations in the fractions and stabilization of soil organic carbon with forest succession in Southern China. Adv. Earth Sci. 2016, 31, 86–93. [Google Scholar]
- Zhang, X.Y.; Chen, X.M.; Wei, H.; Lin, M.Z. Effect of urbanization on soil organic carbon fractions and carbon pool management index in remnant evergreen broad-leaved forests of the PearRiver Delta. J. Soil Water Conserv. 2017, 31, 184–190. [Google Scholar]
- Eclesia, R.P.; Jobbagy, E.G.; Jackson, R.B.; Biganzoli, F.; Piñeiro, G. Shifts in soil organic carbon for plantation and pasture establishment in native forests and grasslands of South America. Glob. Chang. Biol. 2012, 18, 3237–3251. [Google Scholar] [CrossRef]
- Wang, F.; Zhu, W.; Chen, H. Changes of soil C stocks and stability after 70-year afforestation in the Northeast USA. Plant Soil 2016, 401, 319–329. [Google Scholar] [CrossRef]
- Lugato, E.; Lavallee, J.M.; Haddix, M.L.; Panagos, P.; Cotrufo, M.F. Different climate sensitivity of particulate and mineral-associated soil organic matter. Nat. Geosci. 2021, 14, 295–300. [Google Scholar] [CrossRef]
- Feyissa, A.; Raza, S.T.; Cheng, X. Soil carbon stabilization and potential stabilizing mechanisms along elevational gradients in alpine forest and grassland ecosystems of Southwest China. Catena 2023, 229, 107210. [Google Scholar] [CrossRef]
- Luo, X.; Zhang, R.; Zhang, L.; Frew, A.; Yu, H.; Hou, E.; Wen, D. Mechanisms of soil organic carbon stabilization and its response to conversion of primary natural broadleaf forests to secondary forests and plantation forests. Catena 2024, 240, 108021. [Google Scholar] [CrossRef]
- Sainepo, B.M.; Gachene, C.K.; Karuma, A. Assessment of soil organic carbon fractions and carbon management index under different land use types in Olesharo Catchment, Narok County, Kenya. Carbon Balance Manag. 2018, 13, 4. [Google Scholar] [CrossRef] [PubMed]
- Diekow, J.; Mielniczuk, J.; Knicker, H.; Bayer, C.; Dick, D.P.; Kögel-Knabner, I. Soil C and N stocks as affected by cropping systems and nitrogen fertilisation in a southern Brazil Acrisol managed under no-tillage for 17 years. Soil Tillage Res. 2005, 81, 87–95. [Google Scholar] [CrossRef]
- Chaudhary, S.; Dheri, G.S.; Brar, B.S. Long-term effects of NPK fertilizers and organic manures on carbon stabilization and management index under rice-wheat cropping system. Soil Tillage Res. 2017, 166, 59–66. [Google Scholar] [CrossRef]
- Huang, B.; Zhang, L.; Cao, Y.; Yang, Y.; Wang, P.; Li, Z.; Lin, Y. Effects of land-use type on soil organic carbon and carbon pool management index through arbuscular mycorrhizal fungi pathways. Glob. Ecol. Conserv. 2023, 43, e02432. [Google Scholar] [CrossRef]
- Liao, J.; Zhao, D. Forestry landscape patterns changes and dynamic simulation of Nanling National Nature Reserve, Guangdong. Sci. Geogr. Sin. 2014, 34, 1099–1107. [Google Scholar]
- Bao, S. Soil and Agricultural Chemistry Analysis, 3rd ed.; China Agriculture Press: Beijing, China, 2000. [Google Scholar]
- Blair, G.J.; Lefroy, R.D.; Lisle, L. Soil carbon fractions based on their degree of oxidation, and the development of a carbon management index for agricultural systems. Aust. J. Agric. Res. 1995, 46, 1459–1466. [Google Scholar] [CrossRef]
- Sun, X.; Niu, J.; Zhao, G. Effects of previous crop stubbles and nitrogen applied amount on summer maize yield and soil organic carbon pool at a semiarid loess site of China. Agric. Res. Arid Areas 2018, 36, 19–27. [Google Scholar]
- McGill, W.B.; Cannon, K.R.; Robertson, J.A.; Cook, F.D. Dynamics of soil microbial biomass and water-soluble organic C in Breton L after 50 years of cropping to two rotations. Can. J. Soil Sci. 1986, 66, 1–19. [Google Scholar] [CrossRef]
- Xiang, H.; Wen, D.; Zhang, L.; Li, J. Altitudinal changes in active and recalcitrant soil carbon pools of forests in the Dinghu Mountains. Acta Ecol. Sin. 2015, 35, 6089–6099. [Google Scholar]
- Lu, R. Analysis of Soil Nutrients, 3rd ed.; China Agriculture Press: Beijing, China, 2019. [Google Scholar]
- Qiu, Q.; Wu, J.; Liang, G.; Liu, J.; Chu, G.; Zhou, G.; Zhang, D. Effects of simulated acid rain on soil and soil solution chemistry in a monsoon evergreen broad-leaved forest in southern China. Environ. Monit. Assess. 2015, 187, 272. [Google Scholar] [CrossRef] [PubMed]
- Nannipieri, P.; Trasar-Cepeda, C.; Dick, R.P. Soil enzyme activity: A brief history and biochemistry as a basis for appropriate interpretations and meta-analysis. Biol. Fertil. Soils 2018, 54, 11–19. [Google Scholar] [CrossRef]
- Tabatabai, M.A.; Bremner, J.M. Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Biol. Biochem. 1969, 1, 301–307. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, P.B.; Fan, M.M.; Zheng, S.Y.; Wu, J.T.; Yang, X.H.; Zhang, M.; Yin, A.J.; Gao, C. Dynamics and driving factors of the organic carbon fractions in agricultural land reclaimed from coastal wetlands in eastern China. Ecol. Indic. 2018, 89, 639–647. [Google Scholar] [CrossRef]
- Yang, X.; Ren, W.; Sun, B.; Zhang, S. Effects of contrasting soil management regimes on total and labile soil organic carbon fractions in a loess soil in China. Geoderma 2012, 177, 49–56. [Google Scholar] [CrossRef]
- Qaswar, M.; Huang, J.; Ahmed, W.; Li, D.; Liu, S.; Zhang, L.; Cai, A.; Liu, L.; Xu, Y.; Gao, J.; et al. Yield sustainability, soil organic carbon sequestration and nutrients balance under long-term combined application of manure and inorganic fertilizers in acidic paddy soil. Soil Tillage Res. 2020, 198, 104569. [Google Scholar] [CrossRef]
- Benbi, D.K.; Brar, K.; Toor, A.S.; Singh, P. Total and labile pools of soil organic carbon in cultivated and undisturbed soils in northern India. Geoderma 2015, 237, 149–158. [Google Scholar] [CrossRef]
- Prescott, C.E.; Vesterdal, L. Decomposition and transformations along the continuum from litter to soil organic matter in forest soils. For. Ecol. Manag. 2021, 498, 119522. [Google Scholar] [CrossRef]
- Sun, T.; Wang, Y.; Hui, D.; Jing, X.; Feng, W. Soil properties rather than climate and ecosystem type control the vertical variations of soil organic carbon, microbial carbon, and microbial quotient. Soil Biol. Biochem. 2020, 148, 107905. [Google Scholar] [CrossRef]
- Huang, Y.Z.; Wang, S.Q.; Ye, S.M. Effects of Cunninghamia lanceolata stand types on the changes of aggregate-related organic carbon and nutrients in surface soil. J. Appl. Ecol. 2020, 31, 2857–2865. [Google Scholar]
- Pang, D.; Cui, M.; Liu, Y.; Wang, G.; Cao, J.; Wang, X.; Dan, X.; Zhou, J. Responses of soil labile organic carbon fractions and stocks to different vegetation restoration strategies in degraded karst ecosystems of southwest China. Ecol. Eng. 2019, 138, 391–402. [Google Scholar] [CrossRef]
- Zhang, Y.; Zou, J.; Meng, D.; Dang, S.; Zhou, J.; Osborne, B.; Ren, Y.; Liang, T.; Yu, K. Effect of soil microorganisms and labile C availability on soil respiration in response to litter inputs in forest ecosystems: A meta-analysis. Ecol. Evol. 2020, 10, 13602–13612. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Niu, B.; Hu, Y.; Luo, T.; Zhang, G. Warming and increased precipitation indirectly affect the composition and turnover of labile-fraction soil organic matter by directly affecting vegetation and microorganisms. Sci. Total Environ. 2020, 714, 136787. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.Y.; Du, C.; Jian, J.S.; Hou, L.; Wang, Z.K.; Wang, Q.; Geng, Z.C. The interplay of labile organic carbon, enzyme activities and microbial communities of two forest soils across seasons. Sci. Rep. 2021, 11, 5002. [Google Scholar] [CrossRef] [PubMed]
- Six, J.; Feller, C.; Denef, K.; Ogle, S.M.; Moraes, S.C.J.; Albrecht, A. Soil organic matter, biota and aggregation in temperate and tropical soils—Effects of no-tillage. Agronomie 2002, 22, 755–775. [Google Scholar] [CrossRef]
- Ahirwal, J.; Gogoi, A.; Sahoo, U.K. Stability of soil organic carbon pools affected by land use and land cover changes in forests of eastern Himalayan region, India. Catena 2022, 215, 106308. [Google Scholar] [CrossRef]
- Yang, F.; Zhong, Y.; Han, G.; Li, X.; Luo, L.; Cai, X.; Long, X.; Li, T.; Huang, L. Effect of different vegetation restoration on soil organic carbon dynamics and fractions in the Rainy Zone of Western China. J. Environ. Manag. 2023, 331, 117296. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Zhong, Y.; Liu, J.; Zhu, G.; Shangguan, Z.; Yan, W. Effects of nitrogen enrichment on soil microbial characteristics: From biomass to enzyme activities. Geoderma 2020, 366, 114256. [Google Scholar] [CrossRef]
- Powlson, D.S.; Prookes, P.C.; Christensen, B.T. Measurement of soil microbial biomass provides an early indication of changes in total soil organic matter due to straw incorporation. Soil Biol. Biochem. 1987, 19, 159–164. [Google Scholar] [CrossRef]
- Kaiser, K.; Kaupenjohann, M.; Zech, W. Sorption of dissolved organic carbon in soils: Effects of soil sample storage, soil-to-solution ratio, and temperature. Geoderma 2001, 99, 317–328. [Google Scholar] [CrossRef]
- Tian, Y.; Haibara, K.; Toda, H.; Ding, F.; Liu, Y.; Choi, D. Microbial biomass and activity along a natural pH gradient in forest soils in a karst region of the upper Yangtze River, China. J. For. Res. 2008, 13, 205–214. [Google Scholar] [CrossRef]
- Silva-Sánchez, A.; Soares, M.; Rousk, J. Testing the dependence of microbial growth and carbon use efficiency on nitrogen availability, pH, and organic matter quality. Soil Biol. Biochem. 2019, 134, 25–35. [Google Scholar] [CrossRef]
- Hessen, D.O.; Ågren, G.I.; Anderson, T.R.; Elser, J.J.; De Ruiter, P.C. Carbon sequestration in ecosystems: The role of stoichiometry. Ecology 2004, 85, 1179–1192. [Google Scholar] [CrossRef]
- Knorr, M.; Frey, S.D.; Curtis, P.S. Nitrogen additions and litter decomposition: A meta-analysis. Ecology 2005, 86, 3252–3257. [Google Scholar] [CrossRef]
- Jenkinson, D.S.; Ladd, J.N. Microbial biomass in soil: Measurement and turnover. Soil Biochem. 1981, 5, 415–471. [Google Scholar]
- Sparling, G.P. Ratio of microbial biomass carbon to soil organic carbon as a sensitive indicator of changes in soil organic matter. Soil Res. 1992, 30, 195–207. [Google Scholar] [CrossRef]
- Li, D.J.; Wen, L.; Zhang, W.; Yang, L.Q.; Xiao, K.C.; Chen, H.; Wang, K.L. Afforestation effects on soil organic carbon and nitrogen pools modulated by lithology. For. Ecol. Manag. 2017, 400, 85–92. [Google Scholar] [CrossRef]
- Li, Z.Q.; Zhao, B.Z.; Hao, X.Y.; Zhang, J.B. Effects of residue incorporation and plant growth on soil labile organic carbon and microbial function and community composition under two soil moisture levels. Environ. Sci. Pollut. Res. 2017, 24, 18849–18859. [Google Scholar] [CrossRef]
- Li, M.; Zhang, A.; Wu, H.; Liu, H.; Lv, J. Predicting potential release of dissolved organic matter from biochars derived from agricultural residues using fluorescence and ultraviolet absorbance. J. Hazard. Mater. 2017, 334, 86–92. [Google Scholar] [CrossRef]
- Sruthi, S.N.; Ramasamy, E.V. Enrichment of soil organic carbon by native earthworms in a patch of tropical soil, Kerala, India: First report. Sci. Rep. 2018, 8, 5784. [Google Scholar] [CrossRef]
- Ramírez, P.B.; Fuentes-Alburquenque, S.; Díez, B.; Vargas, I.; Bonilla, C.A. Soil microbial community responses to labile organic carbon fractions in relation to soil type and land use along a climate gradient. Soil Biol. Biochem. 2020, 141, 107692. [Google Scholar] [CrossRef]
- Tian, Q.; Jiang, Y.; Tang, Y.; Wu, Y.; Tang, Z.; Liu, F. Soil pH and organic carbon properties drive soil bacterial communities in surface and deep layers along an elevational gradient. Front. Microbiol. 2021, 12, 646124. [Google Scholar] [CrossRef] [PubMed]
- Badagliacca, G.; Romeo, M.; Lo Presti, E.; Gelsomino, A.; Monti, M. Factors governing total and permanganate oxidizable C pools in agricultural soils from southern Italy. Agriculture 2020, 10, 99. [Google Scholar] [CrossRef]
- Mueller, K.E.; Eissenstat, D.M.; Hobbie, S.E.; Oleksyn, J.; Jagodzinski, A.M.; Reich, P.B.; Chadwick, O.A.; Chorover, J. Tree species effects on coupled cycles of carbon, nitrogen, and acidity in mineral soils at a common garden experiment. Biogeochemistry 2012, 111, 601–614. [Google Scholar] [CrossRef]
- Li, Z.W.; Nie, X.D.; Chen, X.L.; Lu, Y.M.; Jiang, W.G.; Zeng, G.M. The effects of land use and landscape position on labile organic carbon and carbon management index in red soil hilly region, southern China. J. Mt. Sci. 2015, 12, 626–636. [Google Scholar] [CrossRef]
- Rudrappa, L.; Purakayastha, T.J.; Singh, D.; Bhadraray, S. Long-term manuring and fertilization effects on soil organic carbon pools in a Typic Haplustept of semi-arid sub-tropical India. Soil Tillage Res. 2006, 88, 180–192. [Google Scholar] [CrossRef]
- Huang, Z.; Xu, X.; Zhang, H.; Cai, B.; Li, L. Advances in effects of root input on forest soil carbon pool and carbon cycle. J. Nanjing For. Univ. (Nat. Sci. Ed.) 2022, 46, 25–32. [Google Scholar]
- Bowering, K.L.; Edwards, K.A.; Wiersma, Y.F.; Billings, S.A.; Warren, J.; Skinner, A.; Ziegler, S.E. Dissolved organic carbon mobilization across a climate transect of mesic boreal forests is explained by air temperature and snowpack duration. Ecosystems 2023, 26, 55–71. [Google Scholar] [CrossRef]
- Bhattacharyya, R.; Das, T.K.; Sudhishri, S.; Dudwal, B.; Sharma, A.R.; Bhatia, A.; Singh, G. Conservation agriculture effects on soil organic carbon accumulation and crop productivity under a rice–wheat cropping system in the western Indo-Gangetic Plains. Eur. J. Agron. 2015, 70, 11–21. [Google Scholar] [CrossRef]
- Wang, Z.; Ren, J.; Xu, C.; Geng, Z.; Du, X.; Li, Y. Characteristics of water extractable organic carbon fractions in the soil profiles of Picea asperata and Betula albosinensis forests. J. Soils Sediments 2021, 21, 3580–3589. [Google Scholar] [CrossRef]
- Dai, W.; Gao, H.; Sha, Z.; Penttinen, P.; Fang, K.; Wang, J.; Cao, L. Changes in soil organic carbon fractions in response to wheat straw incorporation in a subtropical paddy field in China. J. Plant Nutr. Soil Sci. 2021, 184, 198–207. [Google Scholar] [CrossRef]
- Zhang, G.; Zhao, J.; Song, X.; Liu, H.; Zhang, R.; Ji, Y.; Yang, D. Effects of fertilization on soil organic carbon and carbon pool management index. J. Plant Nutr. Fertil. 2012, 18, 359–365. [Google Scholar]
- Li, Y.; Wang, Y.; Zhang, W. Impact of simulated acid rain on the composition of soil microbial communities and soil respiration in typical subtropical forests in Southwest China. Ecotoxicol. Environ. Saf. 2021, 215, 112152. [Google Scholar] [CrossRef] [PubMed]
- Amarh, F.; Voegborlo, R.B.; Essuman, E.K.; Agorku, E.S.; Tettey, C.O.; Kortei, N.K. Effects of soil depth and characteristics on phosphorus adsorption isotherms of different land utilization types: Phosphorus adsorption isotherms of soil. Soil Tillage Res. 2021, 213, 105139. [Google Scholar] [CrossRef]
- Li, L. Distribution Pattern and Influencing Factors of Total Phosphorus in Natural Forests and Plantation Forests in China. Ph.D. Thesis, Nanjing University of Information Science & Technology, Nanjing, China, 2023. [Google Scholar]
- Zhang, H.; Shi, L.; Lu, H.; Shao, Y.; Liu, S.; Fu, S. Drought promotes soil phosphorus transformation and reduces phosphorus bioavailability in a temperate forest. Sci. Total Environ. 2020, 732, 139295. [Google Scholar] [CrossRef]
- Read, J.J.; Reddy, K.R.; Jenkins, J.N. Yield and fiber quality of upland cotton as influenced by nitrogen and potassium nutrition. Eur. J. Agron. 2006, 24, 282–290. [Google Scholar] [CrossRef]
- Uroz, S.; Calvaruso, C.; Turpault, M.P.; Frey-Klett, P. Mineral weathering by bacteria: Ecology, actors and mechanisms. Trends Microbiol. 2009, 17, 378–387. [Google Scholar] [CrossRef]


| Site Factor | Forest Stand Characteristics | Major Tree Species | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Altitude/m | Slope /(°) | Aspect | Forest Types | Origin | Coordinates | Canopy Density | Stand Density /Trees·hm−2 | Mean Diameter at Breast Height /cm | Mean Tree Height /m | |
| 1265 | 32 | Southwest | broad-leaved forests | Natural | 113°1′57.73″ E, 24°53′24.53″ N | 0.88 | 6149 | 5.63 | 5.88 | Rhododendron ovatum (12.8%), Pentaphylax euryoides (12.1%), Rhododendron moulmainense (5.9%), Castanopsis eyrei (5.4%), Schima superba (2.5%), etc. |
| 1058 | 31 | Northwest | coniferous broad-leaved mixed forests | Secondary | 113°1′6.01″ E, 24°52′59.88″ N | 0.87 | 7408 | 4.62 | 5.10 | Litsea elongata (8.5%), Adinandra bockiana var. Acutifolia (6.1%), Rhododendron moulmainense (8.1%), Cunninghamia lanceolata (15.5%), etc. |
| 869 | 30 | Southwest | Chinese fir plantations | Plantation | 113°2′56.99″ E, 24°52′59.88″ N | 0.82 | 6739 | 6.14 | 5.08 | Cunninghamia lanceolata (72.6%), Eurya acuminatissima (11.7%) |
| Variable | Source | SS | df | MS | F-Ratio | p |
|---|---|---|---|---|---|---|
| SOC content | FS | 2044.08 | 2 | 1022.04 | 7.77 | <0.01 |
| Error | 4338.54 | 33 | 131.47 | |||
| SOC storage | FS | 5873.55 | 2 | 2936.77 | 3.68 | 0.04 |
| Error | 26,336.15 | 33 | 798.07 | |||
| WOC content | FS | 53.12 | 2 | 26.56 | 0.19 | 0.83 |
| Error | 4628.24 | 33 | 140.25 | |||
| OXC content | FS | 136.46 | 2 | 68.23 | 7.17 | <0.01 |
| Error | 314.13 | 33 | 9.52 | |||
| MBC content | FS | 89,690.54 | 2 | 44,845.27 | 13.81 | <0.001 |
| Error | 107,153.37 | 33 | 3247.07 | |||
| RC content | FS | 1160.10 | 2 | 580.05 | 7.67 | <0.01 |
| Error | 2495.66 | 33 | 75.63 | |||
| WOC proportion | FS | 6.25 | 2 | 3.13 | 1.37 | 0.27 |
| Error | 75.31 | 33 | 2.28 | |||
| OXC proportion | FS | 129.16 | 2 | 64.58 | 4.59 | 0.02 |
| Error | 464.26 | 33 | 14.07 | |||
| MBC proportion | FS | 3999.08 | 2 | 1999.54 | 28.86 | <0.001 |
| Error | 2286.17 | 33 | 69.28 | |||
| IOC proportion | FS | 0.04 | 2 | 0.02 | 4.00 | 0.03 |
| Error | 0.16 | 33 | 0.01 |
| Variables | Broad-Leaved Evergreen Forest (BL) | Artificial Chinese Fir Forest (CL) | Mixed Coniferous and Broad-Leaved Forest (ML) |
|---|---|---|---|
| LBOC | 0.25 ±0.18 a | 0.05 ±0.07 b | 0.08 ±0.05 b |
| LI | 7.91 ±5.70 a | 1.70 ±2.07 b | 2.65 ±1.60 b |
| CPI | 1.48 ±0.21 a | 1.20 ±0.13 b | 1.29 ±0.16 ab |
| CPMI | 1161.80 ±932.91 a | 205.76 ±247.39 b | 337.74 ±202.35 b |
| Variables | Broad-Leaved Evergreen Forest (BL) | Artificial Chinese Fir Forest (CL) | Mixed Coniferous and Broad-Leaved Forest (ML) |
|---|---|---|---|
| pH | 4.17 ± 0.24 b | 4.47 ± 0.14 a | 4.27 ± 0.14 b |
| microbial biomass nitrogen (MBN) (mg/kg) | 42.55 ± 17.86 a | 59.41 ± 18.15 a | 49.91 ± 13.75 a |
| acid phospho-monoesterase (APM) (μmol·g−1 dry·soil h−1) | 17.90 ± 8.18 a | 8.67 ± 1.79 b | 12.11 ± 2.33 b |
| β-1,4-glucosidase (BG) (μmol·g−1 dry·soil h−1) | 0.49 ± 0.10 a | 0.38 ± 0.09 b | 0.42 ± 0.08 ab |
| β-1,4-N-acetaminophen glucosidase (NAG) (μmol·g−1 dry·soil h−1) | 0.24 ± 0.04 a | 0.16 ± 0.05 b | 0.21 ± 0.02 a |
| available P (AP) (mg/kg) | 0.80 ± 0.20 a | 0.71 ± 0.19 a | 0.76 ± 0.13 a |
| total N (TN) (g/kg) | 3.45 ± 1.33 a | 3.08 ± 0.59 a | 3.58 ± 0.59 a |
| total P (TP) (g/kg) | 0.11 ± 0.03 b | 0.13 ± 0.03 a | 0.11 ± 0.02 b |
| C/N | 31.69 ± 12.02 a | 25.86 ± 6.15 a | 24.13 ± 6.68 a |
| C/P | 978.24 ± 316.28 a | 603.56 ± 143.24 b | 790.98 ± 179.23 ab |
| K+ (mg/kg) | 61.02 ± 15.91 a | 70.99 ± 12.26 a | 74.01 ± 9.71 a |
| Ca2+ (mg/kg) | 18.40 ± 8.42 b | 47.22 ± 24.85 a | 33.72 ± 10.73 a |
| Mg2+ (mg/kg) | 16.09 ± 5.37 a | 15.31 ± 2.89 a | 13.74 ± 2.36 a |
| Variables | SOC | WOC | OXC | RC | MBC | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| r | p Value | r | p Value | r | p Value | r | p Value | r | p Value | |
| pH | −0.32 | 0.06 | −0.03 | 0.84 | −0.446 ** | <0.01 | 0.30 | 0.07 | −0.45 ** | <0.01 |
| MBN | 0.03 | 0.87 | −0.41 * | <0.05 | −0.15 | 0.38 | 0.34 * | <0.05 | −0.06 | 0.74 |
| APM | 0.27 | 0.11 | −0.15 | 0.40 | 0.488 ** | <0.01 | −0.29 | 0.09 | 0.46 ** | <0.01 |
| BG | 0.30 | 0.07 | −0.27 | 0.12 | 0.438 ** | <0.01 | −0.10 | 0.58 | 0.33 | 0.05 |
| NAG | 0.33 | 0.05 | −0.15 | 0.38 | 0.31 | 0.07 | −0.23 | 0.18 | 0.23 | 0.18 |
| AP | 0.00 | 0.99 | 0.19 | 0.26 | 0.28 | 0.10 | −0.37 * | <0.05 | 0.11 | 0.53 |
| TN | −0.01 | 0.96 | −0.35 * | <0.05 | 0.19 | 0.26 | 0.08 | 0.64 | 0.13 | 0.46 |
| TP | −0.16 | 0.35 | −0.51 ** | <0.001 | −0.27 | 0.11 | 0.30 | 0.08 | −0.22 | 0.19 |
| C/N | 0.51 ** | <0.01 | 0.36 * | 0.029 | 0.06 | 0.73 | −0.02 | 0.93 | 0.24 | 0.16 |
| C/P | 0.63 ** | <0.01 | 0.32 | 0.06 | 0.355 * | <0.05 | −0.13 | 0.47 | 0.46 ** | <0.01 |
| K+ | −0.05 | 0.76 | −0.22 | 0.21 | −0.11 | 0.51 | 0.29 | 0.09 | −0.20 | 0.23 |
| Ca2+ | −0.49 ** | <0.01 | −0.01 | 0.97 | −0.29 | 0.09 | 0.05 | 0.78 | −0.33 * | <0.05 |
| Mg2+ | 0.16 | 0.36 | −0.38 * | <0.05 | 0.17 | 0.34 | 0.01 | 0.97 | 0.19 | 0.26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Huang, F.; Huang, Y.; Li, W.; Liu, M. Responses of Soil Organic Carbon Fractions and Stability to Forest Conversion in the Nanling Nature Reserve, China. Forests 2024, 15, 1330. https://doi.org/10.3390/f15081330
Li Y, Huang F, Huang Y, Li W, Liu M. Responses of Soil Organic Carbon Fractions and Stability to Forest Conversion in the Nanling Nature Reserve, China. Forests. 2024; 15(8):1330. https://doi.org/10.3390/f15081330
Chicago/Turabian StyleLi, Yifan, Fangfang Huang, Yuhui Huang, Wenjuan Li, and Mengyun Liu. 2024. "Responses of Soil Organic Carbon Fractions and Stability to Forest Conversion in the Nanling Nature Reserve, China" Forests 15, no. 8: 1330. https://doi.org/10.3390/f15081330
APA StyleLi, Y., Huang, F., Huang, Y., Li, W., & Liu, M. (2024). Responses of Soil Organic Carbon Fractions and Stability to Forest Conversion in the Nanling Nature Reserve, China. Forests, 15(8), 1330. https://doi.org/10.3390/f15081330





