Abstract
The floodplain forests of the Danube Delta are among the important European biotopes and are protected in Slovakia under Natura 2000. In order to preserve these biotopes, their restoration is underway, which also restores the original fauna. These biotopes are sensitive to environmental and ecological changes, which is also reflected in the spatial distribution of epigeic arthropods. Between the years 2020 and 2023, we investigated the impact of floodplain restoration on the population structure of epigeic arthropods in eight study areas (two control study areas and six study areas with ongoing biotope restoration). We placed five pitfall traps in a transect for each biotope. In total, we recorded 66,771 individuals belonging to 15 arthropod taxa. We found differences in the taxonomic structures between forest stands with management and forest stands without management (larger number of taxa) using spatial modelling. We also confirmed interannual changes in the taxa composition of epigeic arthropods and their abundance. Over the years of restoration, the number of individual epigeic arthropods decreased. In the years following revitalization, when succession took place, it subsequently increased. Overall, the restoration management of floodplain forests had a positive effect on epigeic arthropods, as well as on their number of individuals, which is important for the preservation of these important habitats in Europe.
1. Introduction
The Danube River is the second-longest river in Europe and began to form 12,000 years ago. During this period, it was still a bay, and after it filled with sediment, lobes were formed, and the formation of its current state evolved. Its spring is located in Germany and ends in Romania [1].
The alluvial landscape of the Danube Delta has a mosaic-like, structured ecosystem comprising water courses, dead branches, wetlands, floodplain meadows, and floodplain forests. The dynamics of floodplain habitat communities are influenced by the water regime, and the taxa structure is diversified by the effects of surface flooding [2]. Currently, there are few floodplain forests in Europe; most of them have been destroyed by anthropogenic processes and the fragments they consist of are in poor condition. The alluvial forests of the Danube Delta belong to the most threatened ecosystems in Europe; therefore, for their preservation, it is necessary to record the current state of biodiversity and, subsequently, ensure their restoration [3]. The management and protection of these habitats requires an understanding of the relationships between taxa and environmental variables [4].
The floodplain forests of the Danube Delta belong to the important European biotopes that fall under Natura 2000. This is a network of protected areas in the member states of the European Union and aims to preserve Europe’s natural heritage. Under the Natura 2000 network of protected areas, the rarest and most endangered taxa of plants, animals, and natural habitats in the European Union should be protected. EU biodiversity should be preserved by safeguarding taxa and habitats of European significance [5].
The spatial structure and diversity of epigeic arthropods in alluvial forests is influenced by various environmental variables such as temperature, humidity, soil type, and vegetation density. Certain epigeic arthropods (Opilionida, Araneida, Coleoptera, Hymenoptera) are used as environmental bioindicators for monitoring and studying ecological changes and habitat disturbances [6,7,8]. Studies dealing with Opiliones confirmed that their presence is influenced by the presence of nitrogen in the litter, pH in the soil, the area of floodplain forests, and the structure of the habitat, which affects the microclimate [9,10,11]. The influence of humidity on spider communities in floodplain forests of the Danube Delta was confirmed by Majzlan [12]. The study confirmed an increase in the abundance of spiders with changes in humidity conditions. Beetles belonging to the family Carabidae and Staphylinidae are an important part of epigeic arthropod communities in wetlands. These taxa are highly specialised and stenotopically adapted to this dynamic environment, making them effective bioindicators with a high susceptibility to anthropogenic influences [13,14]. Several studies have examined the activity of ground beetles in floodplain forests and found that their spatial distribution is influenced by nitrogen in the litter, soil pH, soil and microclimatic conditions, and the species composition of plants in layers E3 and E1 [15,16]. The family Staphylinadae was studied under the different conditions of the floodplain forests, and it was found that their occurrence affected the pH of the soil and the species composition of plants in the E2 layer [17,18,19]. Knowledge of the interactions between epigeic groups and environmental variables of the habitat is important for designing or developing appropriate habitat management programs.
The aim of this work was to evaluate the impact of restoration interventions (restoration of the arms of the Danube Delta, simulated floods, and mowing the grass between the trees) of the important European alluvial forests of the Danube Delta on the spatial distribution of epigeic arthropods. The research hypothesis was that intervention on floodplain forest stands would increase taxonomic diversity.
2. Materials and Methods
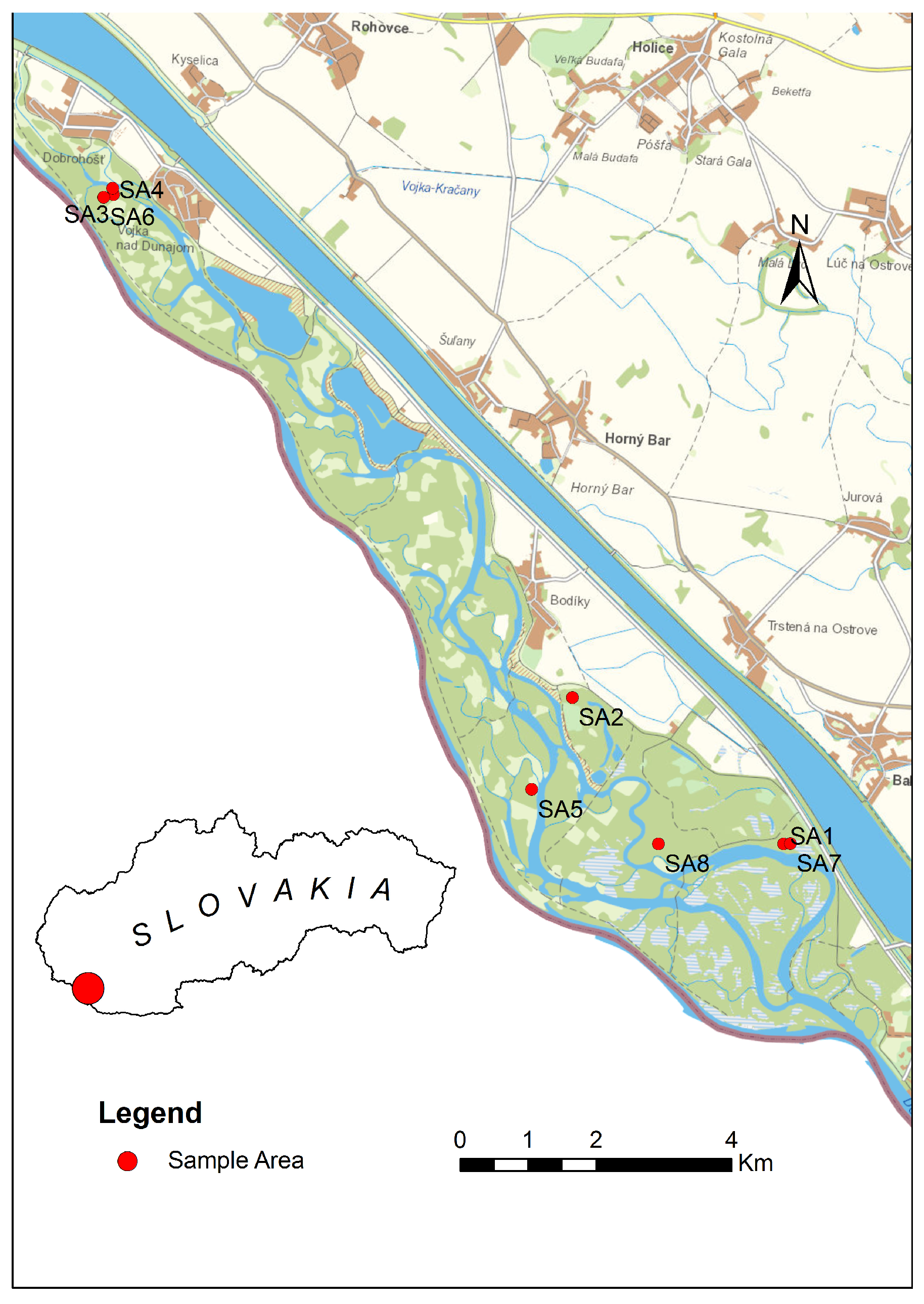
The study took place between 2020 and 2023 in eight study areas in the Dunajské luhy protected landscape area (PLA) in Western Slovakia. The area of the Dunajské luhy territory (PLA) is 122.8 km2, and it belongs to the Alpine-Himalayan system and the Danube Plain. The climate is warm with mild winters; the average winter temperature is −3 °C, and the average summer temperature is 25 °C. The soil type is clayey loam formed by the alluvium of the Danube River. In the selected study areas, habitat restoration aimed at restoring the original biotopes. We used two study areas as controls, which represented natural forest cover. The division of study areas was as follows (Figure 1):
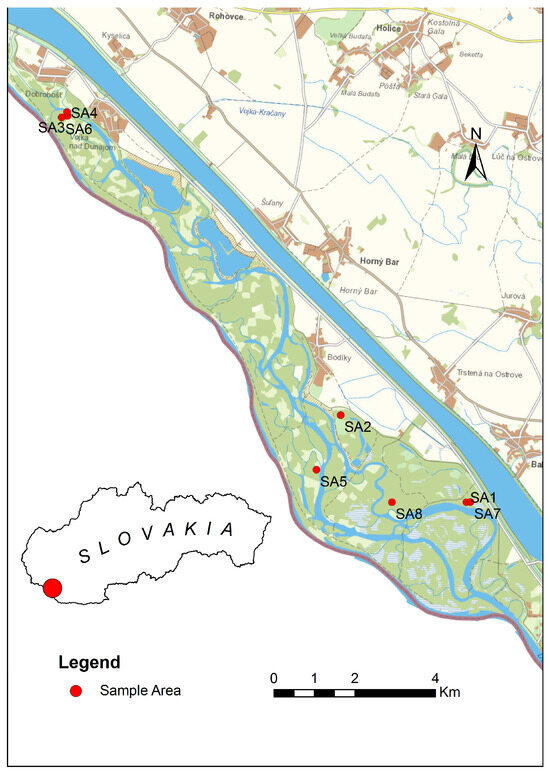
Figure 1.
Map of study areas (SA1–SA8).
- Study area 1 (SA1)—biotope = willow–poplar floodplain forest (main species: Salix fragilis (Linne, 1753), S. alba (Linne, 1753); age of growth, 35 years); altitude, 114 m.a.s.l.; geographic coordinates (47°53′31.1″ N 17°30′25.4″ E). The area served as a reference location where no restoration was taking place.
- Study area 2 (SA2)—biotope = willow–poplar floodplain forest (main species: Salix alba (Linne, 1753), S. fragilis (Linne, 1753), Populus x canadensis (Moench, 1750), Populus x canescens (Aiton, 1750); age of growth, 30 years); altitude, 119 m.a.s.l.; geographic coordinates (47°54′36.0″ N 17°27′51.3″ E). The area served as a reference location where no restoration was taking place.
- Study area 3 (SA3)—biotope = ash–alder floodplain forests (main species: Acer negundo (Linne, 1753), Fraxinus excelsior (Linne, 1753); age of growth, 15 years); altitude, 121 m.a.s.l.; geographic coordinates (47°58′24.5″ N 17°22′09.7″ E). During the year 2021, the arms of the Danube Delta were undergoing restoration, and simulated floods were carried out.
- Study area 4 (SA4)—biotope = Pannonian poplar forest (poplar) (main species: Acer negundo (Linne, 1753), Fraxinus excelsior (Linne, 1753); age of growth, 20 years); altitude, 121 m.a.s.l.; geographic coordinates (47°58′27.4″ N 17°22′09.1″ E). During the year 2021, the arms of the Danube Delta were undergoing restoration, and simulated floods were carried out.
- Study area 5 (SA5)—biotope = poplar nursery (main species: Populus alba (Linne, 1753); age of growth, 2 years); altitude, 118 m.a.s.l.; geographic coordinates (47°53′51.5″ N 17°27′25.5″ E). During the years 2020–2021, the area was undergoing restoration, e.g., the grass was mowed among the trees.
- Study area 6 (SA6)—biotope = poplar nursery (main species: Populus alba (Linne, 1753); age of growth, 2 years); altitude, 127 m.a.s.l.; geographic coordinates (47°58′22.9″ N 17°22′02.9″ E). During the years 2020–2021, the area was undergoing restoration, e.g., the grass was mowed among the trees.
- Study area 7 (SA7)—biotope = willow–poplar floodplain forest (main species: Populus x canadensis (Moench, 1750); age of growth, 15 years); altitude, 114 m.a.s.l.; geographic coordinates (47°53′31.5″ N 17°30′30.2″ E). During the year 2021, the arms of the Danube Delta were undergoing restoration, and simulated floods were carried out.
- Study area 8 (SA8)—biotope = willow–poplar floodplain forest forest (main species: Populus x canadensis (Moench, 1750), Salix fragilis (Linne, 1753); age of growth, 30 years); altitude, 115 m.a.s.l., geographic coordinates (47°53′28.5″ N 17°28′56.9″ E). During the year 2021, the arms of the Danube Delta were undergoing restoration, and simulated floods were carried out.
The newly created arms of the Danube Delta went through forest habitats. Simulated floods took place twice a year (during spring and summer). All the above-mentioned management activities took place in forest habitats (SA3, SA4, SA7, SA8). Mowing the grass between the trees in the poplar nursery was carried out 2 times a year (during spring and summer). The entire area of the poplar nursery was mowed. These management activities took place in study areas 5 and 6.
We collected arthropods using pitfall traps at regular monthly intervals. In each study area, 5 pitfall traps were placed in a transect. We collected the catches from April to October. The distance between the traps was 10 m, so the total line was 50 m long. In total, 40 pitfall traps were placed throughout the year over the period of all 4 years. Formalin (4%) was used as a preservative, and epigeic arthropods were identified (to the order) according to [20]. We used epigeic arthropods because they can be rapidly identified and due to their quick reaction to changes in the environment.
Statistical Analysis
Spatial distribution of epigeic arthropods due to biotopes was analyzed using redundancy analysis (RDA). We tested the statistical significance of biotopes using the Monte Carlo test (iteration 499) in the Canoco5 programme [21].
In the statistical programme R 4.1.3. [22], an analysis was aimed at determining the normality of the data distribution using the Shapiro–Wilk W-test. Based on the violated normality of the data distribution (p = 0.00001), we determined the difference in the number of individuals between the years 2020 to 2023 using the nonparametric Kruskal–Wallis test. Using the Friedman test, we determined the difference in number of individuals of Coleoptera between the years 2022 and 2023 in forest stands and poplar nursery. We determined the taxa diversity and balance of the investigated study areas (SA1–SA8) with the help of the Shannon–Wiener index (H) and equitability of order.
3. Results
Between the years 2020 and 2023, we recorded a total of 66,771 individuals belonging to 15 arthropod taxa in the eight study areas. We confirmed the eudominant representation of Collembola (29.16%), Coleoptera (16.54%), and Isopoda (12%). We found the highest number of individuals in study areas 3 (11,569 = 17.33%) and 4 (10,684 = 16%) and the lowest number of individuals in study areas 5 (5109 = 7.65%) and 6 (5622 = 8.42%) (Table 1).

Table 1.
The taxa identified in the study areas (SA1–SA8).
We calculated the taxa diversity of taxa using the Shannon–Wiener index (H) and the balance of equitability of taxa. The highest value in the Shannon–Wiener index and in equitability was in SA7 (H = 2.336, E = 0.8625). The decrease in values in individual study areas was as follows: SA2 (H = 2.308, E = 0.8525), SA3 (H = 2.301, E = 0.8497), SA4 (H = 2.279, E = 0.8416), SA5 (H = 2.207, E= 0.8151), SA6 (H = 2.242, E = 0.7539), and SA8 (H = 1.817, E = 0.671). Differences in the values of taxa diversity and equitability of taxa can also be caused by the different vegetation in SA (1–8) (Table 2).

Table 2.
Indices of diversity in the study areas (SA1–SA8).
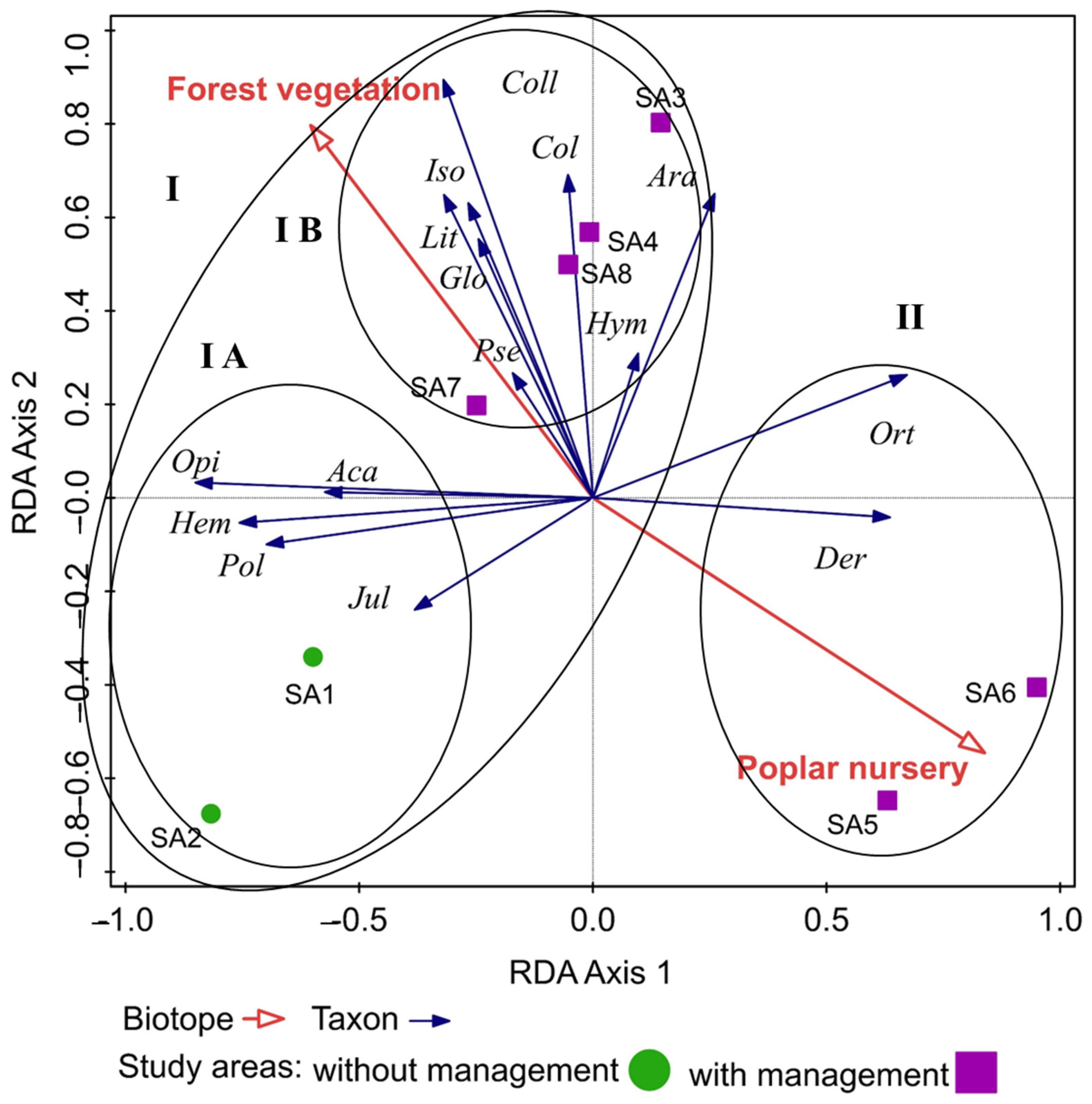
The spatial distribution of epigeic arthropods linked to biotopes (forest stand, poplar nursery) was tested using redundancy analysis (RDA, SD = 0.8 on the first ordination axis). The variability of taxa was 28.79% on the first ordination axis and 39.11% on the second cumulative axis. Due to the influence of environmental variables, the variability increased to 73.61% (first ordination axis), and on the second cumulative axis, it increased to 95%. We found a statistically significant influence on the spatial structure of epigeic arthropods in both types of biotopes: forest vegetation (p = 0.012, df = 2, F = 2.8) and poplar nursery (p = 0.03, df = 2, F = 2.21).
From the results of the analysis, we observed that epigeic arthropods are organised into two main clusters on the graph. The first (I) group consisted of epigeic arthropods preferring the conditions of forest stands. This cluster was divided into two smaller subclusters (subsets: IA, IB). Cluster IA is represented by epigeic arthropods that prefer original forest stands without intervention in the environment. Cluster IB is formed by epigeic arthropods preferring the conditions of forest stands in which restoration of floodplain forests took place (flowing of watercourse arms). The second cluster (II) was represented by epigeic arthropods preferring poplar nursery conditions. The second cluster preferred a smaller number of epigeic arthropods, which is also influenced by the management of the areas, which leads to the disturbance of the habitat. We recorded a larger number of epigeic arthropods in forest stands, where they had their optimum food. Subset I B included study areas where the restoration of the watercourses of the Danube Delta took place and simulated flooding, which also affected the occurrence of taxa preferring a wetter environment and the habitat of watercourses. The differences can also be caused by the different vegetation in SAs 1–8 (Figure 2).
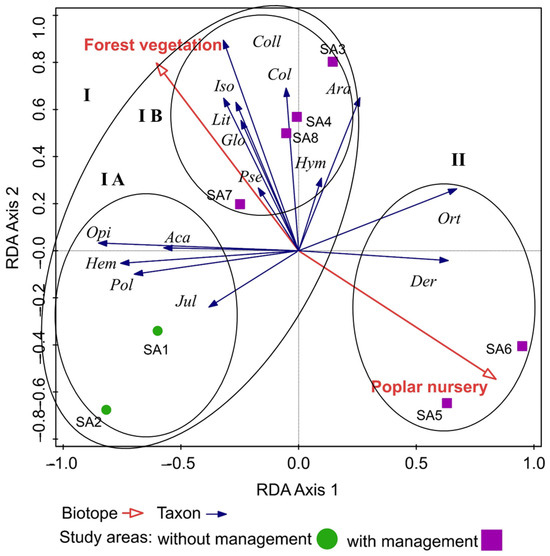
Figure 2.
Spatial distribution of epigeic arthropods in floodplain forests of the Danube Delta. Explanations: Sco = Scorpionida, Opi = Opilionida, Ara = Araneida, Aca = Acarina, Iso = Isopoda, Lit = Lithobiomorpha, Jul = Julida, Pol = Polydesmida, Glo = Glomerida, Col = Collembola, Der = Dermaptera, Ort = Orthoptera, Hem = Hemiptera, Coll = Coleoptera, Hym = Hymenoptera.
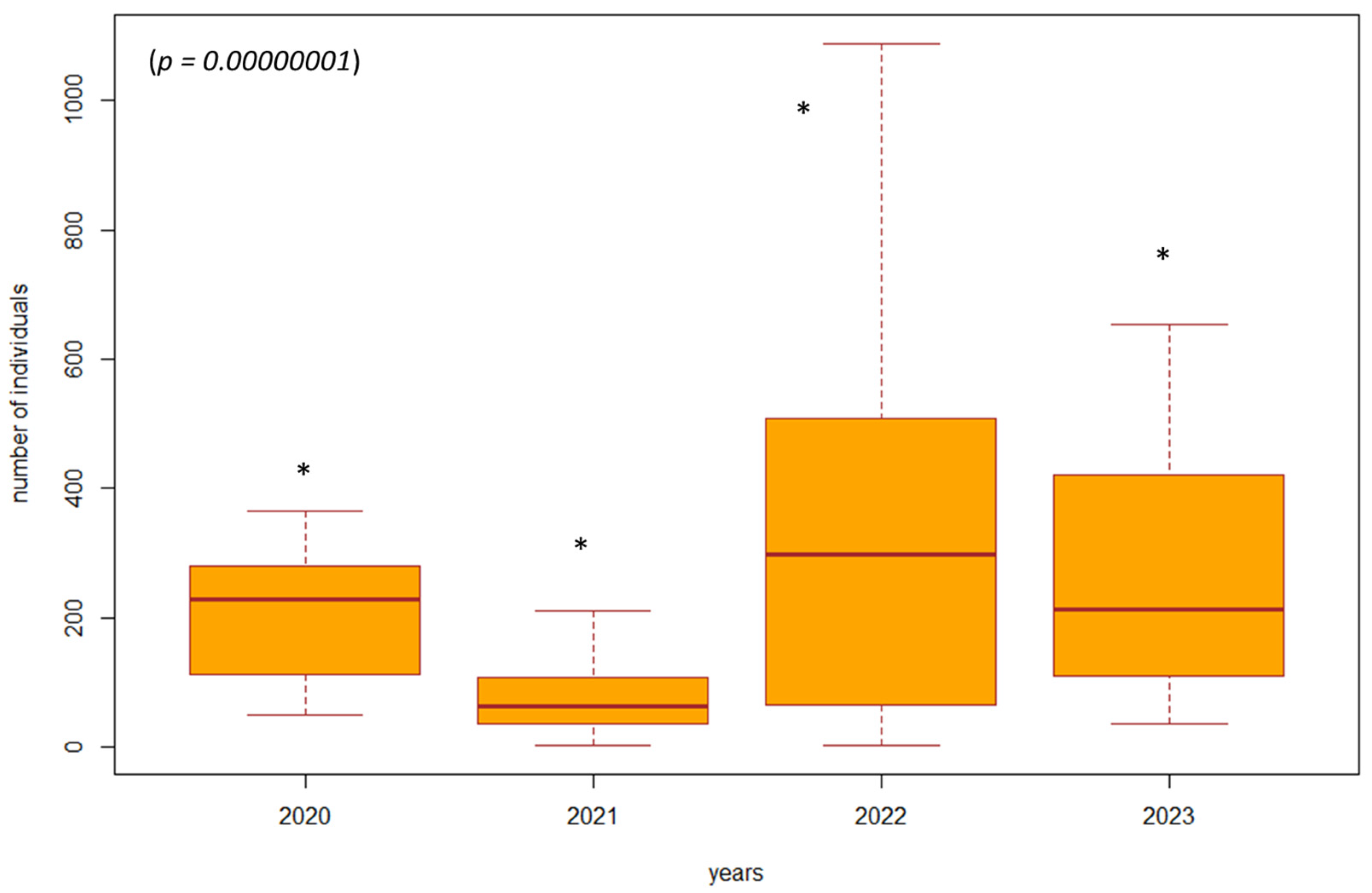
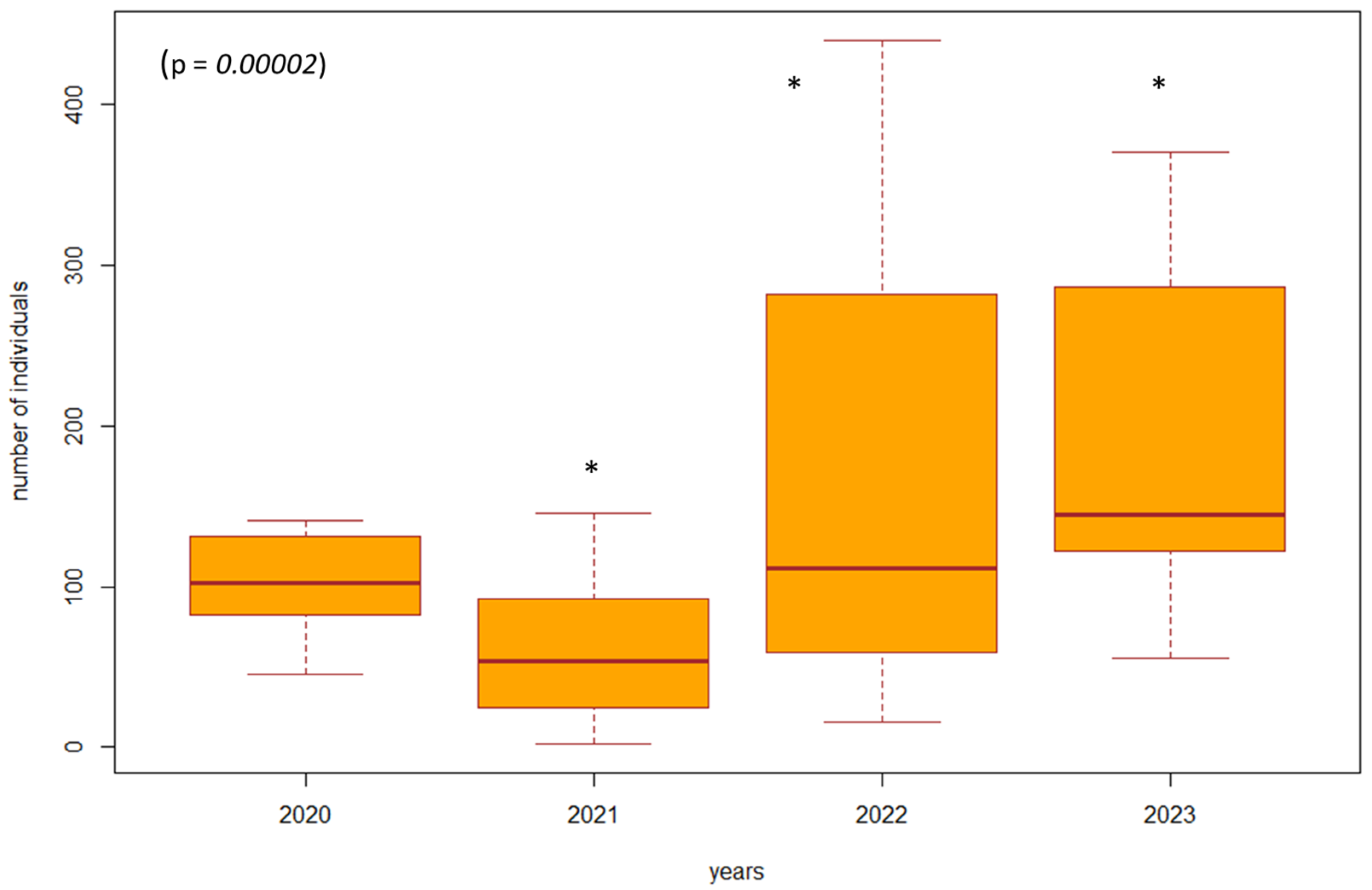
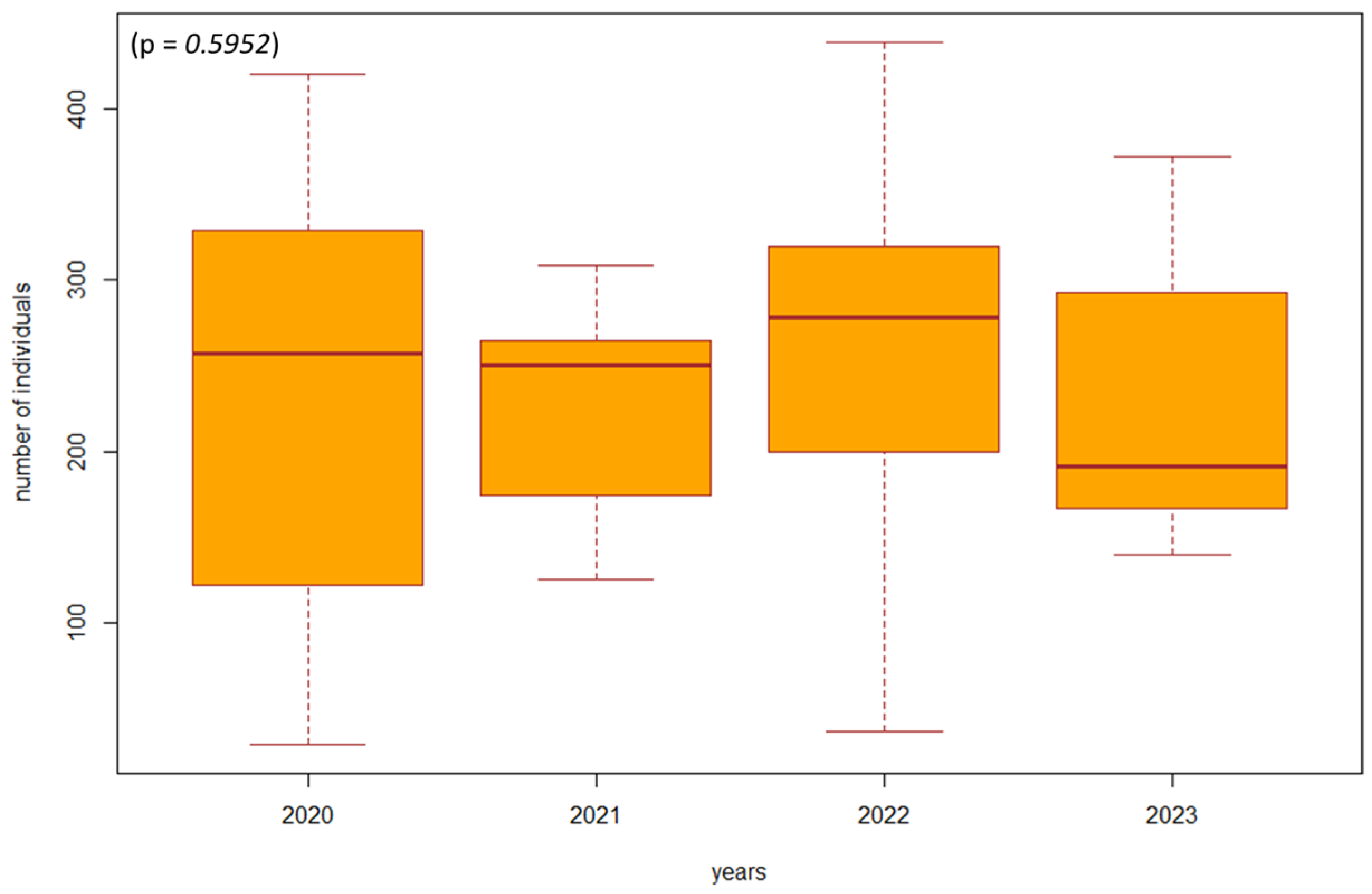
Based on the violated normality of the data distribution (p = 0.00001), we used the non-parametric Kruskal–Wallis test to determine the difference in the number of individuals between the years 2020 and 2023 in the investigated biotopes. In the biotopes under study where habitat restoration took place (restoration of the arms of the Danube Delta, simulated floods), we found a significant difference (p = 0.00000001, df = 3, F = 39.8413) in the number of individuals of epigeic arthropods over the years. The largest decrease in the number of individuals was in 2021, when management adjustments were also underway. In the following years, there was a gradual succession and an increase in the number of individuals; this increase was also higher compared to the beginning of 2020, when restoration operations were not yet underway (Figure 3). In poplar nurseries, we found a significant difference (p = 0.00002, df = 3, F = 24.454) in the number of individuals between the years 2020 and 2023. From the results, we can see that as the succession took place over the years 2022 and 2023, there was also an increase in the number of individuals. In the previous years, 2020 and 2021, restoration modifications were carried out (mowing the grass among the trees); when carried out with great intensity, this mowing reduced number of individuals (Figure 4). We did not find a significant difference in the number of individuals between 2020 and 2023 (p = 0.5952, df = 3, F = 1.8916) in the forest stand where no management adjustments were made. It was also observed that the number of individuals was more balanced over the entire research period. Changes in the number of individuals during the studied years are caused by management works and also by different vegetation in SAs 1–8 (Figure 5).
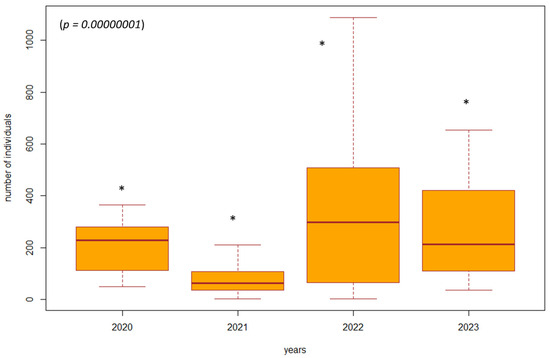
Figure 3.
The difference in the number of individuals between 2020 and 2023 in forest stands with ongoing habitat restoration (restoration of the arms of the Danube Delta, simulated floods) (SA3, SA4, SA7, SA8). Explanations: the boxplot shows the median (Q2), lower quartile (Q1), upper quartile (Q3), maximum, and minimum value. * = significant difference in the year compared to the others.
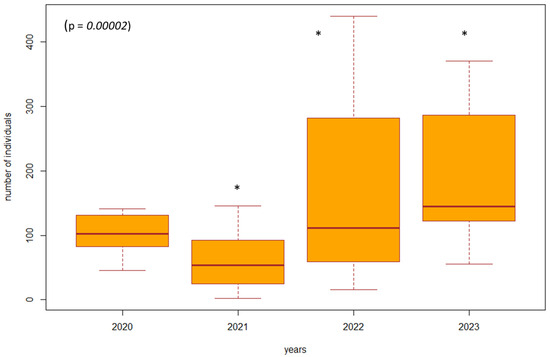
Figure 4.
The difference in the number of individuals between 2020 and 2023 in the poplar nursery (SA5, SA6). Explanations: the boxplot shows the median (Q2), lower quartile (Q1), upper quartile (Q3), maximum, and minimum value. * = significant difference in the year compared to the others.
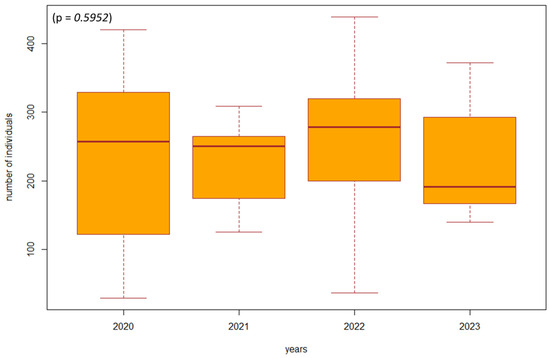
Figure 5.
The difference in the number of individuals between 2020 and 2023 in forest stands where management adjustments did not take place (SA1, SA2). Explanations: the boxplot shows the median (Q2), lower quartile (Q1), upper quartile (Q3), maximum, and minimum value.
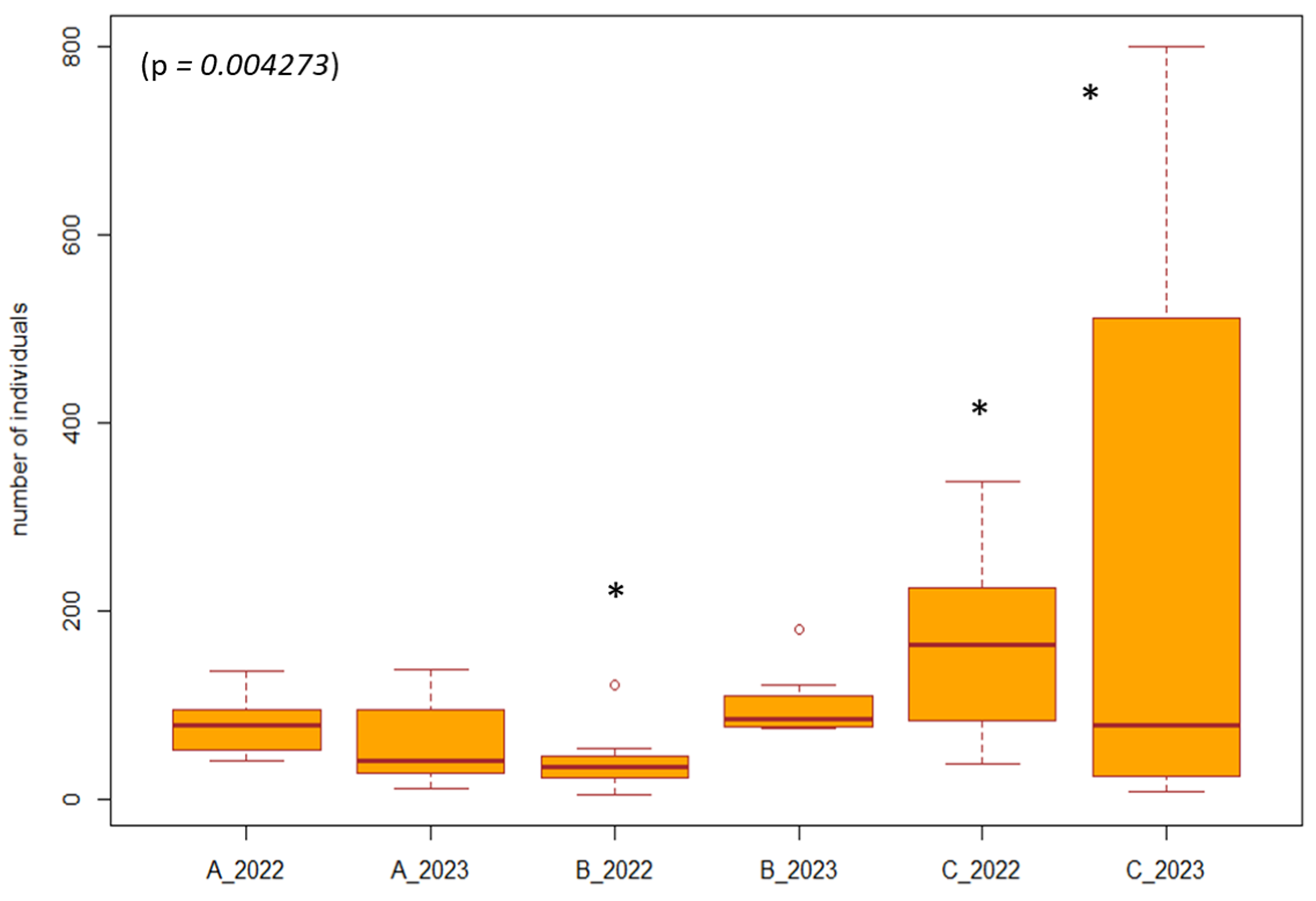
For the Coleoptera, which was among the eudominant taxa and was represented in all study areas (1–8) during all years and months, we performed a separate analysis. The analysis was focused on the period after the restoration of forest stands (2022, 2023) between forest stands with management, without management, and on the poplar nursery. Using the Friedman test, we found a significant difference (p = 0.004273, df = 5, F = 17.122) between years and types of management. From the results, we observed that after the end of the restoration of the forest stands, the number of individuals gradually increased in the managed forest stands (restoration of the arms of the Danube Delta, simulated floods) and also in the poplar nursery (mowing the grass among the trees). In forest stands without management, the number of individuals was equal in both years (2022, 2023). Differences in the number of individuals of the order Coleoptera can also be caused by the different vegetation in SAs 1–8 (Figure 6).
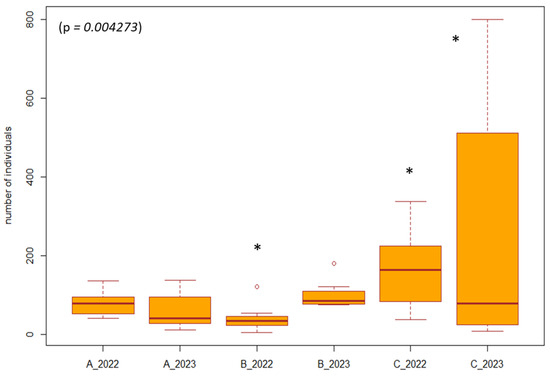
Figure 6.
Difference in the number of individuals between the years 2022, 2023 and management measures. Explanations: A_2022, A_2023 = forest stand without management; B_2022, B_2023 = poplar nursery (with management mowing the grass among the trees); C_2022, C_2023 = forest stand with management (restoration of the arms of the Danube Delta, simulated floods). * = significant difference in the year compared to the others.
4. Discussion
Over the last decades, emphasis has been placed on determining the state of ecosystems which change due to anthropogenic influence [23]. For that reason, the countries of the European Union created Natura 2000, which is a system of protected areas of European importance [5]. In our work, we also focused on the forests of the Danube Delta, which fall under the Natura 2000 biotopes of European importance.
Over the last 150 years, humanity has had an increasingly negative influence on the Danube River, on its basin, and on its water courses. The hydrological regime of the Danube was also influenced by the construction of the hydroelectric power plant—Gabčíkovo Hydroelectric Works. Its construction is expected to have a long-term impact on ecosystems as well as epigeic fauna [24,25,26,27]. In our research, simulated floods took place in the study areas (3, 4, 7, 8), which were regulated by the Gabčíkovo waterworks.
We recorded 66,771 individuals belonging to 15 arthropod taxa in the study areas. We recorded the greatest taxa diversity (Shannon–Wiener index (H)) and equitability at SA7, where the management works (restoration of the arms of the Danube Delta, simulated floods) were taking place, which points to their positive impact. We found the second highest Shannon–Wiener index (H) and equitability value in SA2, which served as our reference area. In the natural forest biotope (SA2), there was a high diversity of taxa, but a higher diversity of taxa was found in the forest biotope (SA7) where management works were carried out, which enriched the diversity of taxa with taxa preferring higher humidity. Thus, restoration had the effect of increasing the diversity of taxa. Another aspect that could influence the different taxa diversity may be the variety of vegetation throughout SAs 1–8. Taxa diversity and abundance is related to biotope factors (vegetation structure, microhabitats, soil conditions) and, thus, to the diversity of biotopes. Management works have encroached on the biotope and partially disturbed the vegetation. By creating water arms and increasing the water inflow, hygrophilous and epigeic arthropods, preferring wetter biotopes, appeared. These differences in taxa diversity and abundance can also be caused by the variety of vegetation throughout SAs 1–8.
Taxa preferring floodplain forest habitats find microhabitats with varying conditions, ranging from wet to dry environments, which are suitable for them to live. These microhabitats with different humidity levels provide suitable conditions for certain taxa such as xerophilic taxa [28]. In global forest policy, the main requirement is the protection of biodiversity. For sustainable forest management, natural renewal (restoration) of tree stands is a necessary way to improve biodiversity. Some epigeic arthropods (Araneida, Coleoptera, Hymenoptera) serve as environmental bioindicators showing the state of the habitat [29,30,31,32,33,34,35]. In our work, a eudominant representation in Coleoptera and dominant representation in Aranea and Hymenoptera was noted from the above-mentioned epigeic arthropods suitable as bioindicators.
Floodplains are centres of carbon sequestration, providing an important ecosystem service. Soil is the basis of carbon cycle processes such as organic matter degradation, mineralisation into inorganic compounds, as well as carbon sequestration. Epigeic arthropods, mainly Araneida, Isopoda, Collembola, Coleoptera, and Hymenoptera, act as regulators of these processes, where they influence the activities of soil microorganisms through their feeding activity. The high feeding activity of Epigeic arthropods is usually accompanied by increased activity of microorganisms and positive effects on the carbon cycle [36,37,38]. Using multivariate analysis (RDA), we confirmed the preference of these taxa for forest stands where restoration treatments were occurring (restoration of the arms of the Danube Delta, simulated floods). Simulated floods also occurred in these study areas, which affect carbon sequestration; the taxa Coleoptera, Araneae, Collembola, Isopoda, and Hymenoptera are their regulators. Epigeic arthropods also play a key role in maintaining the natural balance of elements in the ecosystem, including potassium, nitrogen, phosphorus, iron, and carbon. In addition, they contribute to the decomposition of plant parts and aeration of the soil [39].
Natural floods are an important part of floodplain forest ecosystems, and artificial regime changes negatively affect the biodiversity of riparian and adjacent forest ecosystems. Water dams are barriers on watercourses, reducing seasonal and interannual hydrological fluctuations which, in turn, alters the dynamics of floodplain forests in the Danube Delta. Changing the water flow regime negatively affects the ecological balance of watercourses and their adjacent floodplain ecosystems. The dynamics of the water flow creates a specific structure of the river landscape, which is made up of a mosaic of contrasting surfaces with different levels of disturbance [40,41,42,43,44,45]. From our results, we also confirmed that a change in the water regime (restoration of the arms of the Danube Delta, simulated floods) in the forest stands (study areas 3, 4, 7, 8), disrupted the spatial dispersion of epigeic arthropods and reduced the number of individuals in 2021. In the following years (2022 and 2023), when these simulated floods no longer took place, the taxonomic structure and their abundance stabilised. The number of individuals was even higher in 2022 and 2023 compared to the first year before the flood simulation. In the forest stands (study areas 1, 2), where restoration was not carried out, both taxa and abundance were balanced over the entire research period (2020–2023).
Annual poplar seedlings are planted in poplar nurseries, with a distance of 2 m between them, which makes the nursery vulnerable to weed competition, especially in the initial stage of establishing the nursery. The main growth of poplar trees takes place from March to October, depending on the weather. However, these conditions also affect the growth of weeds. During this period, management adjustments must be implemented to remove weeds, which also affects epigeic arthropods [46,47]. Our results showed a decrease in taxa and the number of individuals in 2020 and 2021, coinciding with management adjustments. In the following years, natural succession occurred, leading to an increase in the number of individuals and balanced taxonomic dispersion.
5. Conclusions
The results of our research provided new information on the impact of restoring floodplain forests in the Danube Delta and on the spatial distribution of epigeic arthropods. Over the four years of research (2020–2023), we recorded 66,771 individuals belonging to 15 arthropod taxa. Using multivariate analyses, we found the connection between a smaller number of taxa in habitats where restoration adjustments took place (restoration of the arms of the Danube Delta, simulated floods, mowing the grass among the trees), compared to forest stands without restoration intervention where epigeic arthropods and their number of individuals were balanced. In forest stands and poplar nurseries where management adjustments were made, there was a decrease in taxa and the number of individuals in the years when restoration adjustments were made. In the following years, there was an increase in the number of individuals and a more balanced spatial structure of epigeic arthropods, which points to continued succession and stabilisation of biotopes. Furthermore, the number of individuals was higher compared to the first year of collection, before restoration treatments had been implemented. This suggests that the restoration management treatments had a positive effect on the biodiversity of the epigeic fauna. For the preservation of important European biotopes and their epigeic arthropods, it is important to have appropriate restoration measures.
Author Contributions
Conceptualization, V.L. and K.P.; methodology, V.L. and K.P.; software, V.L.; validation, V.L. and K.P.; formal analysis, V.L. and K.P.; investigation, V.L.; resources, K.P.; data curation, V.L.; writing—original draft preparation, V.L. and K.P.; writing—review and editing, V.L., S.D., and K.P.; visualization, V.L. and S.D.; supervision, V.L.; project administration, V.L.; funding acquisition, K.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the grant KEGA No. 037SPU-4/2024, Data integrity in biological and ecological databases.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Panin, N. The Black Sea Coastal Zone—An overview. Geo-Eco-Marina 2005, 11, 21–40. [Google Scholar]
- Penka, M.; Vyskot, M.; Klimo, E.; Vašíček, F. Floodplain Forest Ecosystem. 1. Before Water Management Measures; Academia-Elsevier: Praha, Czech Republic; Amsterdam, The Netherlands, 1985; 466p. [Google Scholar]
- Hughes, F.M.R.; del Tánago, M.G.; Mountford, J.O. A Goal-Oriented Approach to Forest Landscape Restoration. In World Forests; Springer: Dordrecht, The Netherlands, 2012; 422p. [Google Scholar]
- Boháč, J.; Jahnová, Z. Environmental Indicators; Springer: Dordrecht, The Netherlands, 2015; 419p. [Google Scholar]
- Directive, H. Natura 2000. Council Directive 92/43/EEC on the conservation of natural habitats and of wild fauna and flora. Off. J. Eur. Union 1992, 206, 50. [Google Scholar]
- Langraf, V.; Petrovičová, K. Seasonal Dynamics of Epigeic Arthropods under the Conditions of Ecological Management of the Triticum aestivum Crop. Agriculture 2024, 14, 482. [Google Scholar] [CrossRef]
- Langraf, V.; Petrovičová, K.; Schlarmannová, J. The Compositionand Seasonal Variation of EpigeicArthropods in Different Types ofAgricultural Crops and Their Ecotones. Agronomy 2021, 11, 2276. [Google Scholar] [CrossRef]
- Brygadyrenko, V.V. Community structure oflitter invertebrates of forest belt ecosystems in the Ukrainian steppe zone. Int. J. Environ. Res. 2015, 9, 1183–1192. [Google Scholar]
- Brygadyrenko, V.V. Influence of tree crowndensity and density of the herbaceous layer on the structure of litter macrofauna of deciduous forestsof Ukraine’s steppe zone. Visnyk Dnipropetr. Univ. Biol. Ecol. 2015, 23, 134–148. [Google Scholar]
- Lavelle, P.; Mathieu, J.; Spain, A.; Brown, G.; Fragoso, C.; Lapied, E.; De Aquino, A.; Barois, I.; Barrios, E.; Barros, M.E.; et al. Soil macroinvertebrate communities: A world-wide assessment. Glob. Ecol. Biogeogr. 2022, 31, 1261–1276. [Google Scholar] [CrossRef]
- Litavský, J.; Stašiov, S.; Svitok, M.; Michalková, E.; Majzlan, O.; Žarnovičan, H.; Fedor, P. Epigean communities of harvestmen (Opiliones) in Pannonian Basin floodplain forests: An interaction with environmental parameters. Biologia 2018, 73, 753–763. [Google Scholar] [CrossRef]
- Majzlan, O.; Hazuchová, A. Abundance and seasonal dynamics of grasshoppers (Opiliones) in the soil of floodplain forests of Podunajska. Folia Faun. Slov. 1997, 2, 47–51. [Google Scholar]
- Merino-Sáinz, I.; Anadón, A. Local distribution patterns of harvestmen (Arachnida: Opiliones) in a northern temperate biosphere reserve landscape: Influence of orientation and soil richness. Belg. J. Zool. 2015, 145, 3–16. [Google Scholar] [CrossRef]
- Krumpálova, Z. Epigeic spiders (Araneae) of the one Middle Danube floodplain forest. Bologia 2002, 57, 161–169. [Google Scholar]
- Litavský, J.; Majzlan, O.; Stašiov, S.; Svitok, M.; Fedor, P. The associations between ground beetle (Coleoptera: Carabidae) communities and environmental condition in floodplain forests in the Pannonian Basin. Eur. J. Entomol. 2021, 118, 14–23. [Google Scholar] [CrossRef]
- Igondová, E.; Majzlan, O. Assemblages of ground beetles (Carabidae, Coleoptera) in peatland habitat, surrounding dry pine forests and meadows. Folia Oecol. 2015, 42, 21–28. [Google Scholar]
- Stork, N.E. The Role of Ground Beetles in Ecological and Environmental Studies; Intercept: UK, 1990; 424p. [Google Scholar]
- Porhajašová, J.; Šustek, Z.; Noskovič, J.; Urminská, J.; Ondrišík, P. Spatial changes and succession of carabid communities (Coleoptera, Insecta) in seminatural wetland habitats of the Žitava river foodplain. Folia Oecol. 2010, 37, 75–85. [Google Scholar]
- Stašiov, S.; Litavský, J.; Majzlan, O.; Svitok, M.; Fedor, P. Influence of Selected Environmental Parameters on Rove Beetle (Coleoptera: Staphylinidae) Communities in Central European Floodplain Forests. Wetlands 2021, 41, 115. [Google Scholar] [CrossRef]
- Schierwater, B.; DeSalle, R. Invertebrate Zoology: A Tree of Life Approach; CRC Press: London, UK, 2021; 644p. [Google Scholar]
- Ter Braak, C.J.F.; Šmilauer, P. Canoco Reference Manual and User’s Guide: Software for Ordination, Version 5.0; Microcomputer Power: Ithaca, NY, USA, 2012. [Google Scholar]
- R, Version 4.1.3; The R Foundation for Statistical Computing: Vienna, Austria, 2020.
- Hadley, D. Habits and Traits of Rove Beetles, Family Staphylinidae; ThoughtCo., 2020; p. 25. Available online: https://www.thoughtco.com/rove-beetles-family-staphylinidae-1968139 (accessed on 2 January 2020).
- Čejka, T.; Beracko, P.; Matečný, I. The impact of the Gabčíkovo hydroelectric power barrier on the Danube floodplain environment the results of long-term monitoring of land snail fauna. Environ. Monit. Assess. 2019, 192, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Adis, J.; Junk, W.J. Terrestrial invertebrates inhabiting lowland river floodplains of Central Amazonia and Central Europe: A review. Freshw. Biol. 2002, 47, 711–731. [Google Scholar] [CrossRef]
- Bunn, S.E.; Arthington, A.H. Basic principles and ecological consequences of altered flow regimes for aquatic biodiversity. Environ. Manag. 2002, 30, 492–507. [Google Scholar] [CrossRef]
- Čejka, T.; Hamerlík, L. Land snails as indicators of soil humidity in Danubian woodland (SW Slovakia). Pol. J. Ecol. 2009, 57, 741–747. [Google Scholar]
- Stašiov, S. Ecology of Soil Organisms (Soil Animals); Technická Univerzita vo Zvolene: Zvolen, Slovakia, 2015; 150p. [Google Scholar]
- Rainio, J.; Niemela, J. Ground Beetles (Coleoptera: Carabidae) as Bioindicators. Biodivers. Conserv. 2003, 12, 487–506. [Google Scholar] [CrossRef]
- Dobrovodská, M.; Kanka, R.; Gajdoš, P.; Krištín, A.; Kollár, J.; Stašiov, S.; Lieskovsky, J. Factors affecting the biodiversity of historical landscape elements: Detailed analyses from three case studies in Slovakia. Environ. Monit. Assess. 2023, 195, 674. [Google Scholar] [CrossRef]
- Dobrovodská, M.; Kanka, R.; David, S.; Kollár, J.; Špulerová, J.; Štefunková, D.; Mojses, M.; Petrovič, F.; Krištín, A.; Stašiov, S.; et al. Assessment of the biocultural value of traditional agricultural landscape on a plot-by-plot level: Case studies from Slovakia. Biodivers. Conserv. 2019, 28, 2615–2645. [Google Scholar] [CrossRef]
- Kalivoda, H.; Petrovič, F.; Kalivodová, E.; Kürthy, A. Influence of the landscape structure on the butterfly (Lepidoptera, Hesperioidea and Papilionoidea) and bird (Aves) taxocoenoses in Vel’ké Leváre (SW Slovakia). Ekol. Bratisl. 2010, 29, 337–359. [Google Scholar] [CrossRef]
- Machar, I.; Šimek, P.; Schlossárek, M.; Pechanec, V.; Petrovič, F.; Brus, J.; Špinlerová, Z.; Seják, J. Comparison of bird diversity between temperate floodplain forests and urban parks. Urban For. Urban Green. 2022, 67, 127427. [Google Scholar] [CrossRef]
- Fazekašová, D.; Petrovič, F.; Fazekaš, J.; Štofejová, L.; Baláž, I.; Tulis, F.; Tóth, T. Soil contamination in the problem areas of agrarian Slovakia. Land 2021, 10, 1248. [Google Scholar] [CrossRef]
- Fazekašová, D.; Bobul’ovská, L. Soil organisms as an Indicator of Quality and Environmental Stress in the Soil Ecosystem. Zivotn. Prostr. 2012, 46, 103–106. [Google Scholar]
- Shupe, H.A.; Jensen, K.; Oldeland, J.; Ludewig, K. Droughts decrease and floods increase carbon sequestration rates of Quercus robur in hardwood floodplain forests. Trees For. People 2022, 9, 100294. [Google Scholar] [CrossRef]
- Saccá, M.L.; Caracciolo, A.B.; Di Lenola, M.; Grenni, P. Ecosystem services provided by soil microorganisms. In Soil Biological Communities and Ecosystem Resilience; Springer: Cham, Switzerland, 2017; pp. 1–24. [Google Scholar]
- Adhikari, K.; Hartemink, A.E. Linking soils to ecosystem services—A global review. Geoderma 2016, 262, 101–111. [Google Scholar] [CrossRef]
- Sticht, C.; Schrader, S.; Giesemann, A.; Weigel, H.J. Atmospheric CO2 enrichment induces life strategy- and species-specificresponses of collembolans in the rhizosphere of sugar beet and winter wheat. Soil Biol. Biochem. 2008, 40, 1432–1445. [Google Scholar] [CrossRef]
- Poff, N.L.; Zimmermann, J.K.H. Ecological responses to altered flow regimes: A literature review to inform the science and management of environmental flows. Freshw. Biol. 2009, 55, 194–205. [Google Scholar] [CrossRef]
- Poff, N.L.; Olden, J.D.; Merritt, D.M.; Pepin, D.M. Homogenization of regional river dynamics by dams and global biodiversity implications. Proc. Natl. Acad. Sci. USA 2007, 104, 5732–5737. [Google Scholar] [CrossRef] [PubMed]
- Zuzulová, V.; Šiška, B. Identification of drought in western Slovakia by palmer drought severity index (PDSI). Acta Reg. Et Environ. 2017, 1, 7–14. [Google Scholar] [CrossRef]
- Vale, V.S.; Schiavini, I.; Araujo, G.M.; Gussons, A.E.; Lopes, S.F.; Oliveira, A.P.; Prado, J.A.; Arantes, C.S.; Dias-Neto, O.C. Effects of reduced water flow in a riparian forest community: A conservation approach. J. Trop. Sci. 2015, 27, 13–24. [Google Scholar]
- Pazourkova, E.; Krecek, J.; Bitušík, P.; Chvojka, P.; Kamasová, L.; Senoo, T.; Špaček, J.; Stuchlik, E. Impacts of an extreme flood on the ecosystem of a headwater stream. J. Limnol. 2021, 80, 1998. [Google Scholar]
- Ungermanová, L.; Dockalova, K.; Stuchlik, E.; Senoo, T.; Horecký, J.; Kopáček, J.; Chvojka, P.; Tatosova, J.; Bitušík, P.; Fjellheim, A. Littoral macroinvertebrates of acidified lakes in the Bohemian Forest. Biologia 2014, 69, 1190–1201. [Google Scholar] [CrossRef]
- Dhiman, R.C.; Gandhi, J.N. Testing of mechanical and chemical methods for weed control in poplar (Populus deltoides Bartr.) nurseries. J. Tree Sci. 2011, 30, 60–67. [Google Scholar]
- Sixto, H.; Grau, J.M.; GarcmHa BaudmHn, J.M. Assessment of the effect of broadspectrum preemergence herbicides in poplar nurseries. Crop. Prot. 2001, 20, 121–126. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).