Tidal Freshwater Forested Wetlands in the Mobile-Tensaw River Delta along the Northern Gulf of Mexico
Abstract
:1. Introduction
- (1)
- Characterize the TFFW communities using multivariate clustering techniques to create fine-scale forest structure community types, which can help delineate the upstream extent of tidal forests in the MT River Delta.
- (2)
- Assess how contributing environmental factors (river distance and relative elevation) relate to TFFW community composition and forest structure.
2. Materials and Methods
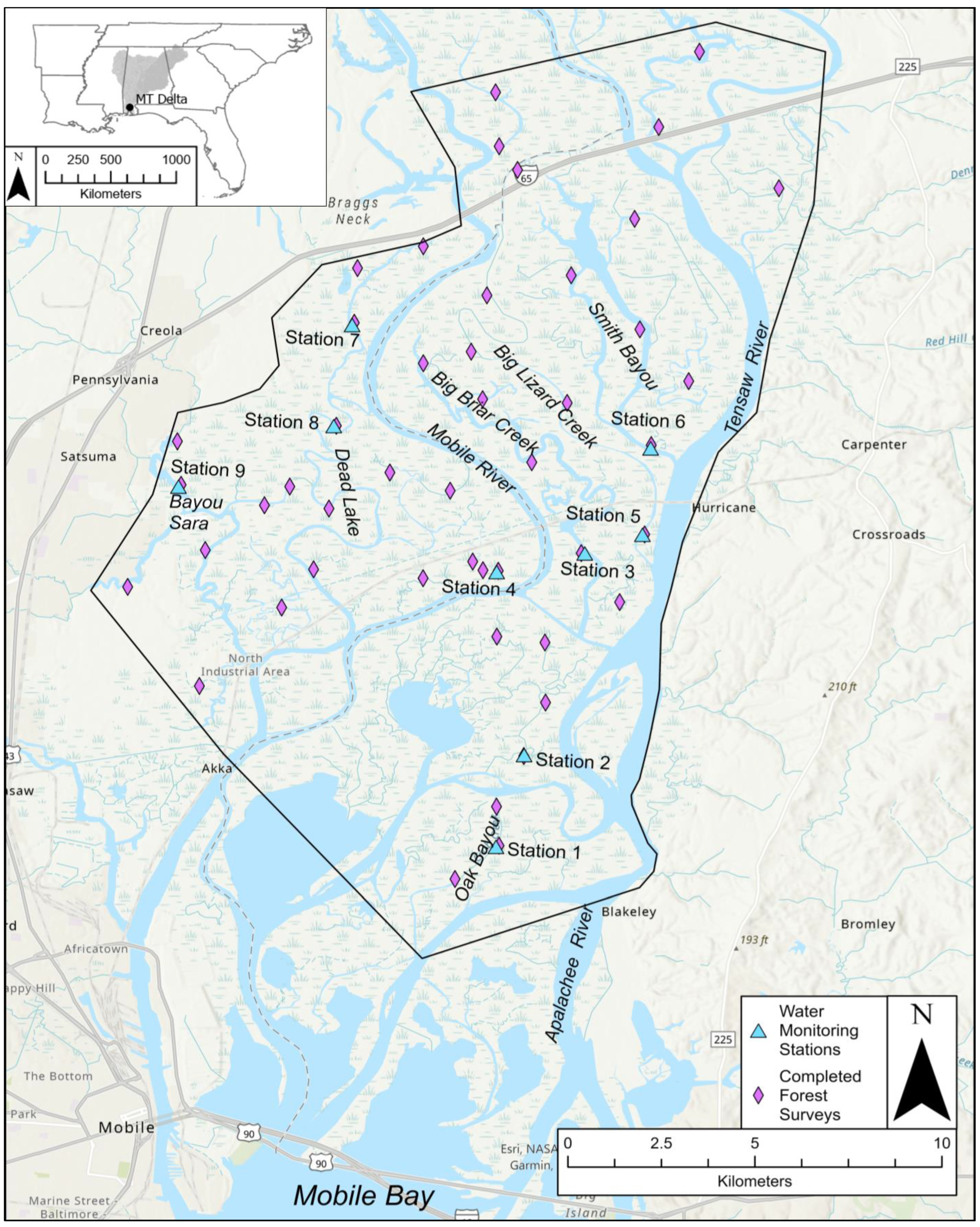
2.1. Study Site
2.2. Water Monitoring Stations
2.3. Forest Surveys
2.4. Assessment of Canopy Communities
2.5. Multivariate Clustering Approach
2.6. Environmental Variables and Their Effects on Community Composition
2.7. Descriptive Statistics
3. Results
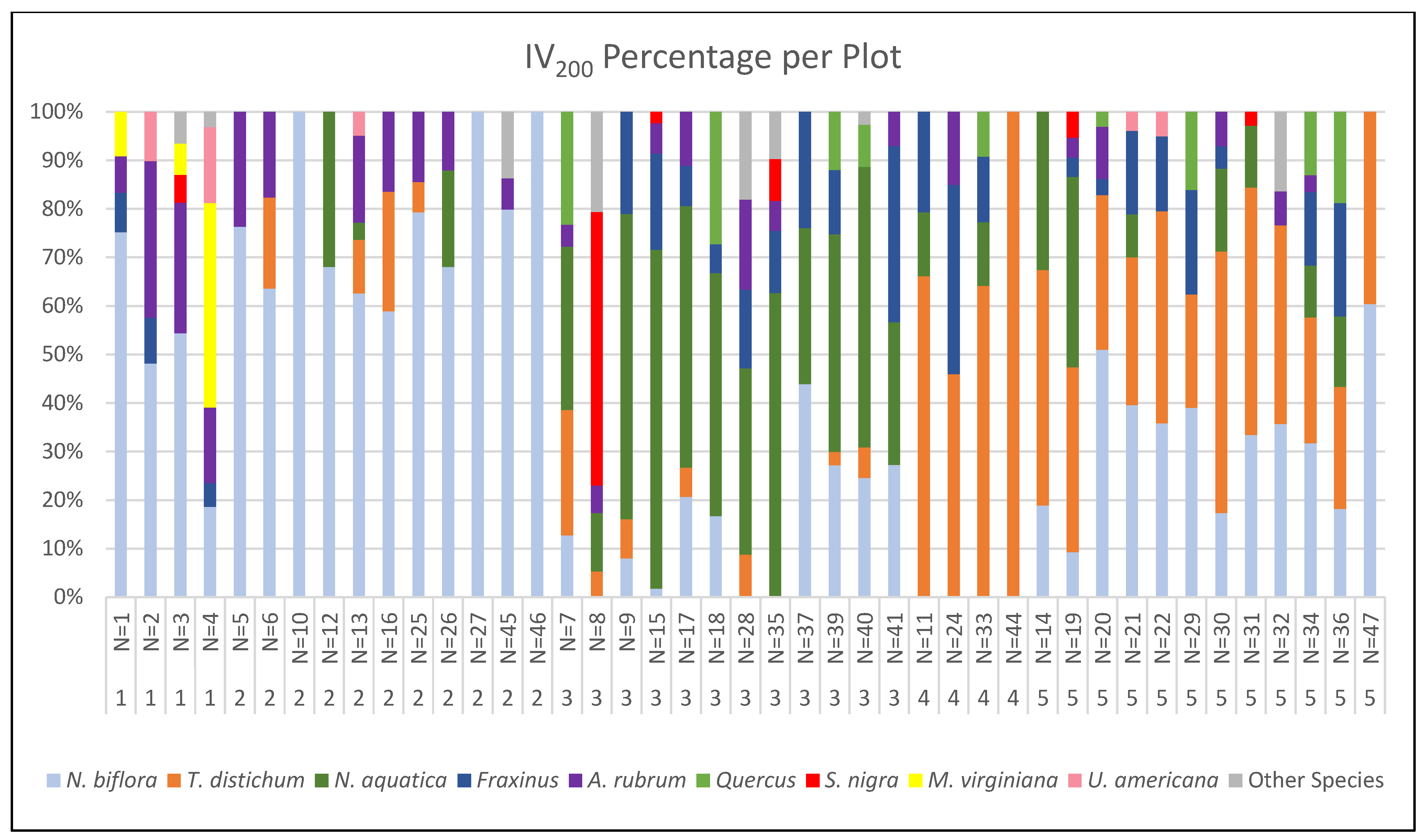
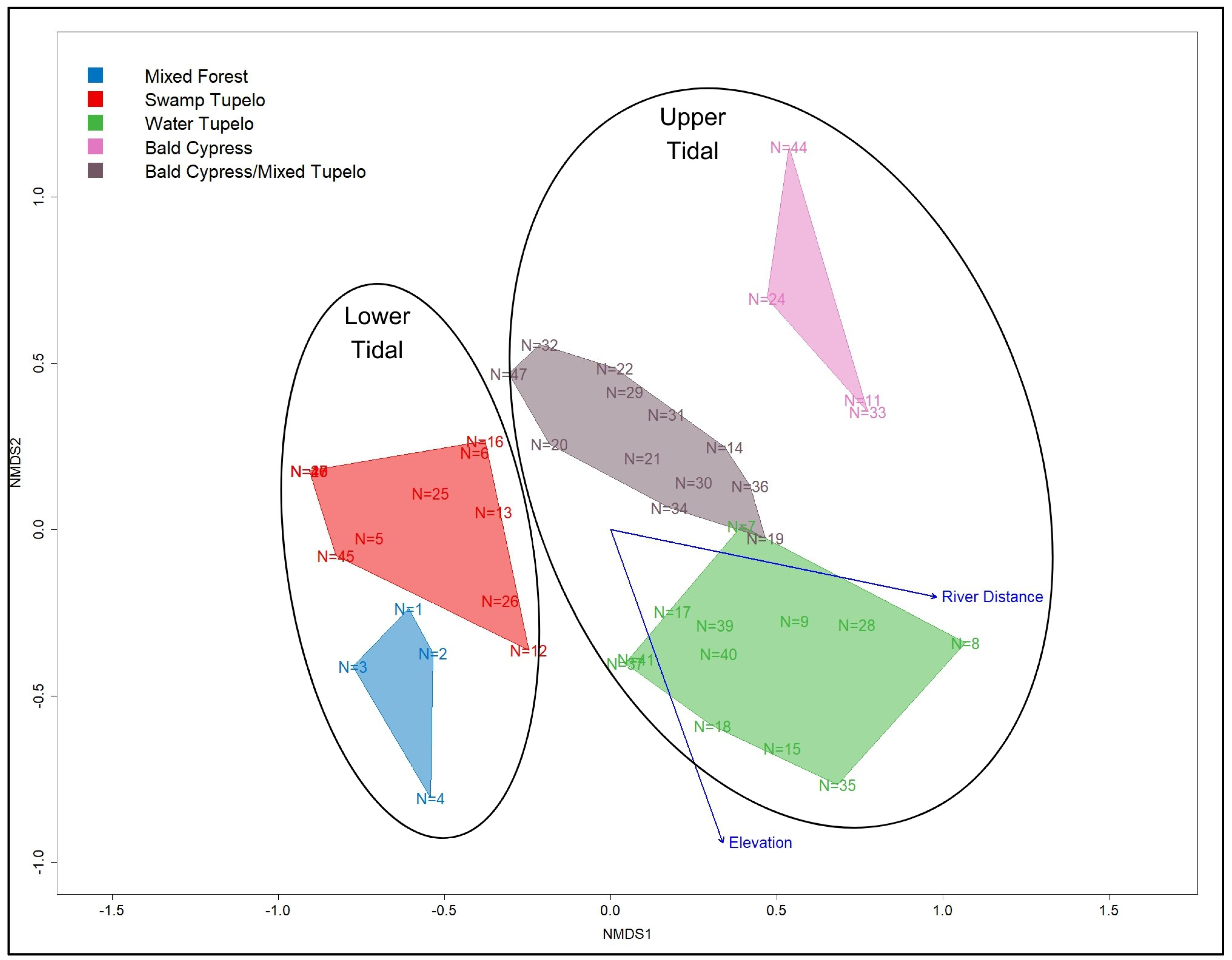
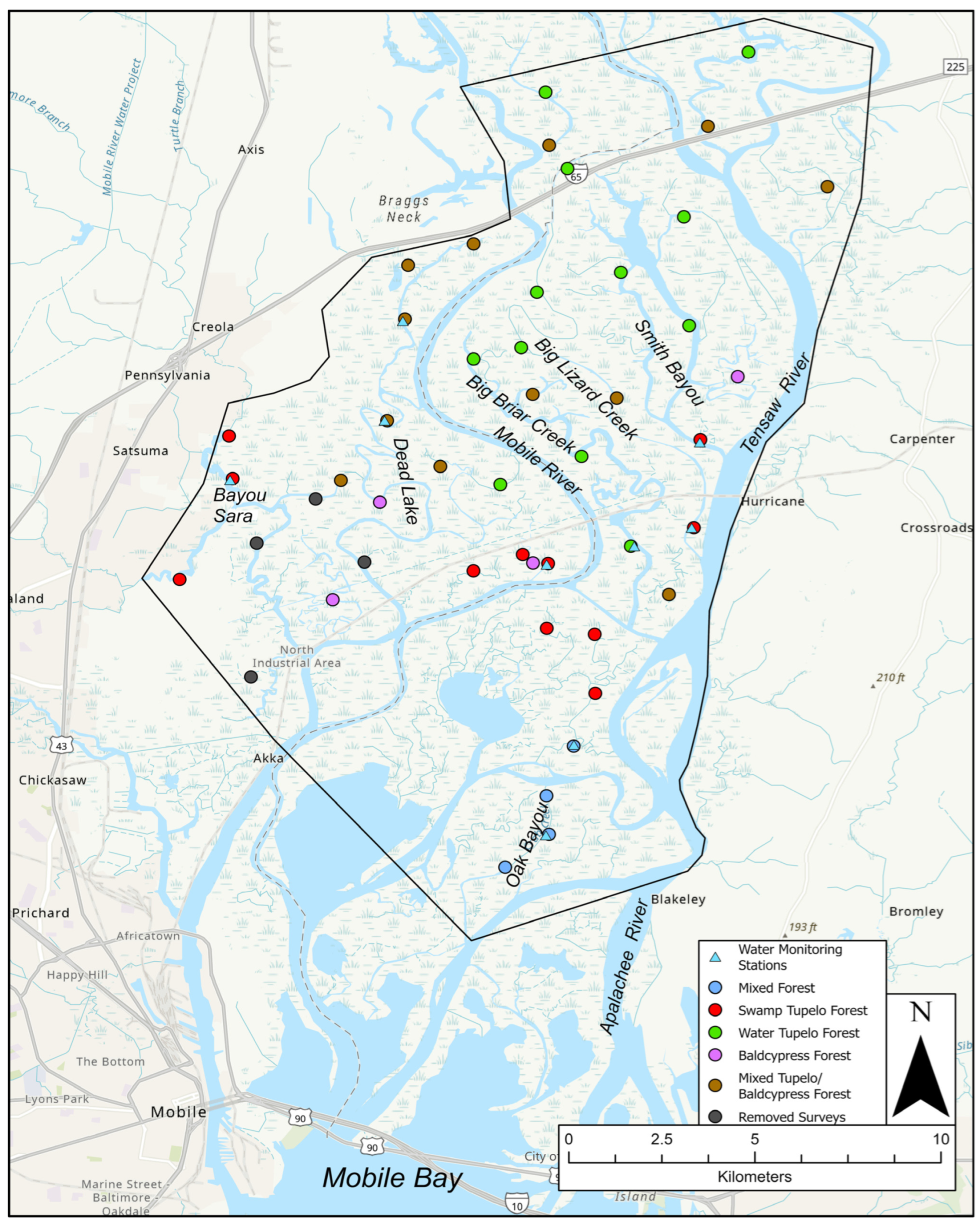
3.1. Forest Community Classification
- (1)
- Mixed Forest—Significant indicator value index species: Magnolia virginiana (sweetbay magnolia), Nyssa biflora (swamp tupelo), Fraxinus spp., and Ulmus americana (American elm).
- (2)
- Swamp Tupelo—Significant indicator value index species: Nyssa biflora.
- (3)
- Water Tupelo—Significant indicator value index species: Nyssa biflora, Nyssa aquatica, Fraxinus spp.
- (4)
- Bald Cypress—Significant indicator value index species: Taxodium distichum, Nyssa aquatica, and Fraxinus spp.
- (5)
- Bald Cypress and Mixed Tupelo—Significant indicator value index species: Nyssa biflora, Taxodium distichum, Nyssa aquatica, and Fraxinus spp.
3.2. Upper and Lower Tidal Groupings
3.3. Canopy Composition across Plots
3.4. Relation between Environmental Variables and Forest Communities
3.5. Midstory Composition
3.6. Community Descriptions
- (1)
- Mixed Forest
- (2)
- Swamp Tupelo Forest
- (3)
- Water Tupelo Forest
- (4)
- Bald Cypress Forest
- (5)
- Bald Cypress/Mixed Tupelo Forest
4. Discussion
4.1. Community Change across a Tidal Gradient
4.2. Comparing TFFWs along the Gulf of Mexico
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Doyle, T.W.; O’Neil, C.P.; Melder, M.P.V.; From, A.S.; Palta, M.M. Tidal Freshwater Swamps of the Southeastern United States: Effects of Land Use, Hurricanes, Sea-Level Rise, and Climate Change. In Ecology of Tidal Freshwater Forested Wetlands of the Southeastern United States; Conner, W.H., Doyle, T.W., Krauss, K.W., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 1–28. [Google Scholar] [CrossRef]
- Day, R.H.; Williams, T.M.; Swarzenski, C.M. Ecology of Tidal Freshwater Forested Wetlands of the Southeastern United States; Conner, W.H., Doyle, T.W., Krauss, K.W., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 29–64. [Google Scholar] [CrossRef]
- Anderson, C.J.; Lockaby, B.G. Forested wetland communities as indicators of tidal influence along the Apalachicola River, Florida, USA. Wetlands 2011, 31, 895–906. [Google Scholar] [CrossRef]
- Duberstein, J.A.; Conner, W.H.; Krauss, K.W. Woody vegetation communities of tidal freshwater swamps in South Carolina, Georgia and Florida (US) with comparisons to similar systems in the US and South America. J. Veg. Sci. 2014, 25, 848–862. [Google Scholar] [CrossRef]
- Hackney, C.T.; Avery, G.B.; Leonard, L.A.; Posey, M.; Alphin, T. Biological, chemical, and physical characteristics of tidal freshwater swamp forests of the Lower Cape Fear River/Estuary, North Carolina. In Ecology of Tidal Freshwater Forested Wetlands of the Southeastern United States; Conner, W.H., Doyle, T.W., Krauss, K.W., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 183–222. [Google Scholar] [CrossRef]
- Carter, V. An overview of the hydrologic concerns related to wetlands in the United States. Can. J. Bot. 1986, 64, 364–374. [Google Scholar] [CrossRef]
- Mitsch, W.J.; Gosselink, J.G. The value of wetlands: Importance of scale and landscape setting. Ecol. Econ. 2000, 35, 25–33. [Google Scholar] [CrossRef]
- Shen, X.; Jiang, M.; Lu, X.; Liu, X.; Liu, B.; Zhang, J.; Wang, X.; Tong, S.; Lei, G.; Wang, S.; et al. Aboveground biomass and its spatial distribution pattern of herbaceous marsh vegetation in China. Sci. China Earth Sci. 2021, 64, 1115–1125. [Google Scholar] [CrossRef]
- Letshele, K.P.; Atekwana, E.A.; Molwalefhe, L.; Ramatlapeng, G.J.; Masamba, W.R.L. Stable hydrogen and oxygen isotopes reveal aperiodic non-river evaporative solute enrichment in the solute cycling of rivers in arid watersheds. Sci. Total Environ. 2023, 859, 159113. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.J.; Lockaby, B.G. Seasonal patterns of river connectivity and saltwater intrusion in tidal freshwater forested wetlands. River Res. Appl. 2012, 28, 814–826. [Google Scholar] [CrossRef]
- Ensign, S.H.; Noe, G.; Hupp, C.R.; Skalak, K.J. Head-of-tide bottleneck of particulate material transport from watersheds to estuaries. Geophys. Res. Lett. 2015, 42, 10671–10679. [Google Scholar] [CrossRef]
- Brittain, R.A.; Craft, C.B. Effects of Sea-Level Rise and Anthropogenic Development on Priority Bird Species Habitats in Coastal Georgia, USA. Environ. Manag. 2012, 49, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Korol, A.R.; Noe, G.B. Patterns of Denitrification Potential in Tidal Freshwater Forested Wetlands. Estuaries Coasts 2020, 43, 329–346. [Google Scholar] [CrossRef]
- Mcleod, E.; Chmura, G.L.; Bouillon, S.; Salm, R.; Björk, M.; Duarte, C.M.; Lovelock, C.E.; Schlesinger, W.H.; Silliman, B.R. A blueprint for blue carbon: Toward an improved understanding of the role of vegetated coastal habitats in sequestering CO2. Front. Ecol. Environ. 2011, 9, 552–560. [Google Scholar] [CrossRef]
- Adame, M.F.; Kelleway, J.; Krauss, K.W.; Lovelock, C.E.; Adams, J.B.; Trevathan-Tackett, S.M.; Noe, G.; Jeffrey, L.; Ronan, M.; Zann, M.; et al. All tidal wetlands are blue carbon ecosystems. BioScience 2024, 74, 253–268. [Google Scholar] [CrossRef] [PubMed]
- Costanza, R.; d’Arrée, R.; De Groot, R.; Farber, S.; Grasso, M.; Hannon, B.; Limburg, K.; Naeem, S.; O’Neill, R.V.; Paruelo, J.; et al. The value of the world’s ecosystem services and natural capital. Nature 1997, 387, 253–260. [Google Scholar] [CrossRef]
- Williams, T.; Amatya, D.; Connor, W.H.; Panda, S.; Xu, G.; Dong, J.; Trettin, C.; Dong, C.; Gao, X.; Shi, H.; et al. Tidal forested wetlands: Mechanisms, threats, and management tools. In Wetlands: Ecosystem Services, Restoration and Wise Use; An, S., Verhoeven, J.T.A., Eds.; Springer: Berlin/Heidelberg, Germany, 2019; pp. 129–158. [Google Scholar] [CrossRef]
- Grieger, R.; Capon, S.J.; Hadwen, W.L.; Mackey, B. Between a bog and a hard place: A global review of climate change effects on coastal freshwater wetlands. Clim. Chang. 2020, 163, 161–179. [Google Scholar] [CrossRef]
- Kirwan, M.L.; Gedan, K.B. Sea-Level Driven Land Conversion and the Formation of Ghost Forests. Nat. Clim. Chang. 2019, 9, 450–457. [Google Scholar] [CrossRef]
- Sweet, W.V.; Hamlington, B.D.; Kopp, R.E.; Weaver, C.P.; Barnard, P.L.; Bekaert, D.; Brooks, W.; Craghan, M.; Dusek, G.; Frederikse, T.; et al. Global and Regional Sea Level Rise Scenarios for the United States: Updated Mean Projections and Extreme Water Level Probabilities Along U.S. Coastlines; Technical Report NOS 01; National Oceanic and Atmospheric Administration, National Ocean Service (NOAA): Silver Spring, MD, USA, 2022. [Google Scholar]
- Field, D.W.; Reyer, A.J.; Genovese, P.V.; Shearer, B.D. Coastal Wetlands of the United States, an Accounting of a Valuable National Resource; Technical Report; National Oceanic and Atmospheric Administration: Rockville, MD, USA, 1991; p. 58. [Google Scholar]
- De Oliveira, G.; Lehrter, J.; Powers, S.; Mataveli, G.; Santos, C. Safely Manage Alabama’s Mobile-Tensaw Delta. Science 2024, 384, 966. [Google Scholar] [CrossRef] [PubMed]
- Wilson, E.O. Naturalist; Island Press: Washington, DC, USA, 2006; 416p. [Google Scholar]
- Bartram, W. The Travels of William Bartram; University of Georgia Press: Athens, GA, USA, 1998; 824p. [Google Scholar]
- Mohr, C. Plant life of Alabama. An Account of the Distribution, Modes of Association, and Adaptations of the Flora of Alabama, Together with a Systematic Catalogue of the Plant Growing in the State; Brown Printing Co.: Montgomery, AL, USA, 1901; 137p. [Google Scholar]
- Dufrêne, M.; Legendre, P. Species assemblage and indicator species: The need for a flexible asymmetrical approach. Ecol. Monogr. 1997, 67, 345–366. [Google Scholar] [CrossRef]
- Brown, J.H. On the relationship between abundance and distribution of species. Am. Nat. 1984, 124, 255–279. [Google Scholar] [CrossRef]
- Malik, R.N.; Husain, S.Z. Linking remote sensing and ecological vegetation communities: A multivariate approach. Pak. J. Bot. 2008, 40, 337–349. [Google Scholar]
- Szabo, M.W.; Osborne, E.W.; Copeland, C.W., Jr.; Neathery, T.L. Geologic Map of Alabama; Special Map 220; Geological Society of Alabama: Tuscaloosa, AL, USA, 1988. [Google Scholar]
- Dykstra, S.L.; Dzwonkowski, B. The propagation of fluvial flood waves through a backwater-estuarine environment. Water Resour. Res. 2020, 56, e2019WR025743. [Google Scholar] [CrossRef]
- U.S. Geological Survey. National Water Information System; USGS Water Data for the Nation. Location ID 02428400. 2016. Available online: https://waterdata.usgs.gov/monitoring-location/02428400/ (accessed on 1 June 2024).
- U.S. Geological Survey. National Water Information System; USGS Water Data for the Nation. Location ID 02469761. 2016. Available online: https://waterdata.usgs.gov/monitoring-location/02469761/ (accessed on 1 June 2024).
- Waselkov, G.A.; Andrus, C.F.; Plumb, G.E. A State of Knowledge of the Natural, Cultural, and Economic Resources of the Greater Mobile-Tensaw River Area; Natural Resource Report NPS/NRSS/BRD/NRR—2016/1243; Biological Resources Division, National Park Service: Fort Collins, CO, USA, 2016.
- U.S. Department of Agriculture, Natural Resources Conservation Service. Soil survey of Mobile County, Alabama. Available online: https://www.nrcs.usda.gov/wps/portal/nrcs/main/soils/survey/ (accessed on 29 April 2024).
- National Centers for Environmental Information. U.S. Climate Normals, Monthly Normals, Station USW00013894, Alabama. Available online: https://www.ncei.noaa.gov/access/us-climate-normals/#dataset=normals-monthly&timeframe=15&location=AL&station=USW00013894 (accessed on 17 July 2024).
- Ensor, H.B.; Wilson, E.M.; Hill, M.C. Historic Resources Assessment, Tennessee-Tombigbee Waterway Wildlife Mitigation Project, Mobile and Tensaw River Deltas, Alabama; COESAM/PDER-93-002; Panamerican Consultants Inc.: Tuscaloosa, AL, USA, 1993; 80p. [Google Scholar]
- Weaver, D.C. Cultural Resources Reconnaissance Study of the Black Warrior-Tombigbee System Corridor, Alabama; DACWOI-8 1-C-OOO1; University of South Alabama: Mobile, AL, USA, 1983; Volume III. [Google Scholar]
- Mader, S.F. Recovery of Functions and Plant Community Structure by a Tupelo-Baldcypress Wetland Ecosystem following Timber Harvesting. Ph.D. Thesis, College of Forest Resources, North Carolina State University, Raleigh, NC, USA, 1990. [Google Scholar]
- Aust, W.M.; Fristoe, T.; Gellerstedt, P.; Giese, L.; Miwa, M. Long-term effects of helicopter and ground-based skidding on site properties and stand growth in a tupelo-cypress wetland. Ecol. Manag. 2006, 226, 72–79. [Google Scholar] [CrossRef]
- Matheny, N.; Clark, J. A photographic Guide to the Evaluation of Hazard Trees in Urban Areas; International Society of Arboriculture: Savoy, IL, USA, 1994; 84p. [Google Scholar]
- Mcintosh, R.P. The York Woods: A Case History of Forest Succession in Southern Wisconsin. Ecology 1957, 38, 29. [Google Scholar] [CrossRef]
- Light, H.M.; Darst, M.R.; Lewis, L.J.; Howell, D.A. Hydrology, Vegetation, and Soils of Riverine and Tidal Floodplain Forests of the Lower Suwannee River, Florida, and Potential Impacts of Flow Reductions; 1656A; US Geological Survey: Reston, VA, USA, 2002.
- Burk, C.J. The Hybrid Nature of Quercus laurifolia. J. Elisha Mitchell Sci. Soc. 1963, 79, 159–163. [Google Scholar]
- Keeland, B.D.; Gorham, L.E. Delayed tree mortality in the Atchafalaya Basin of southern Louisiana following Hurricane Andrew. Wetlands 2009, 29, 101–111. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 27 March 2024).
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package. Version 2.6. 2024. Available online: https://CRAN.R-project.org/package=vegan (accessed on 27 March 2024).
- Legendre, P.; De Cáceres, M. Beta diversity as the variance of community data: Dissimilarity coefficients and partitioning. Ecol. Lett. 2013, 16, 951–963. [Google Scholar] [CrossRef] [PubMed]
- McCune, B.; Grace, J.B. Analysis of Ecological Communities; MjM Software Design: Gleneden Beach, OR, USA, 2002; Volume 28, 300p. [Google Scholar]
- Costomiris, G.; Hladik, C.M.; Craft, C. Multivariate Analysis of the Community Composition of Tidal Freshwater Forests on the Altamaha River, Georgia. Forests 2024, 15, 200. [Google Scholar] [CrossRef]
- De Câceres, M.; Legendre, P. Associations between species and groups of sites: Indices and statistical inference. Ecology 2009, 90, 3566–3574. [Google Scholar] [CrossRef] [PubMed]
- Mielke, P.W. 34 Meteorological applications of permutation techniques based on distance functions. In Handbook of Statistics, 1st ed.; Krishnaiah, P.R., Sen, P.K., Eds.; Elsevier: Amsterdam, The Netherlands, 1984; Volume 7, pp. 813–830. [Google Scholar]
- Dunn, O.J. Multiple comparisons using rank sums. Technometrics 1964, 6, 241–252. [Google Scholar] [CrossRef]
- Mantel, N.A. The detection of disease clustering and a generalized regression approach. Cancer Res. 1967, 27, 209–220. [Google Scholar] [PubMed]
- Shannon, C.E. A mathematical theory of communication. Bell Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef]
- Pielou, E.C. The measurement of diversity in different types of biological collections. J. Theor. Biol. 1966, 13, 131–144. [Google Scholar] [CrossRef]
- Connor, W.H.; Krauss, K.W.; Doyle, T.W. Ecology of Tidal Freshwater Forests in Coastal Deltaic Louisiana and Northern South Carolina. In Ecology of Tidal Freshwater Forested Wetlands of the Southeastern United States; Conner, W.H., Doyle, T.W., Krauss, K.W., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 223–253. [Google Scholar] [CrossRef]
- Penfound, W.T.; Hathaway, E.S. Plant communities in the marshlands of southeastern Louisiana. Ecol. Monogr. 1958, 8, 3–5. [Google Scholar] [CrossRef]
- Light, H.M.; Vincent, K.R.; Darst, M.R.; Price, F.D. Water-Level Decline in the Apalachicola River, Florida from 1954 to 2004, and Effects on Floodplain Habitats; USGS Scientific Investigations Report 2006–5173; U.S. Geological Survey: Reston, VA, USA, 2006.
- Rheinhardt, R. A Multivariate Analysis of Vegetation Patterns in Tidal Freshwater Swamps of Lower Chesapeake Bay, USA. Bull. Torrey Bot. Club 1992, 119, 192–207. [Google Scholar] [CrossRef]
- Duberstein, J.; Kitchens, W. Community composition of select areas of tidal freshwater forest along the Savannah River. In Ecology of Tidal Freshwater Forested Wetlands of the Southeastern United States; Springer: Berlin/Heidelberg, Germany, 2007; pp. 321–348. [Google Scholar] [CrossRef]
- Krauss, K.W.; Duberstein, J.A.; Doyle, T.W.; Conner, W.H.; Day, R.H.; Inabinette, L.W.; Whitbeck, J.L. Site condition, structure, and growth of bald cypress along tidal/non-tidal salinity gradients. Wetlands 2009, 29, 505–519. [Google Scholar] [CrossRef]
- Liu, X.; Conner, W.H.; Song, B.; Jayakaran, A.D. Forest composition and growth in a freshwater forested wetland community across a salinity gradient in South Carolina, USA. Forest Ecol. Manag. 2017, 389, 211–219. [Google Scholar] [CrossRef]
- Celik, S.; Anderson, C.J.; Kalin, L.; Rezaeianzadeh, M. Long-term salinity, hydrology, and forested wetlands along a tidal freshwater gradient. Estuaries Coasts 2021, 44, 1816–1830. [Google Scholar] [CrossRef]
- Anderson, C.J.; Lockaby, B.G.; Click, N. Changes in Wetland Forest Structure, Basal Growth, and Composition across a Tidal Gradient. Am. Midl. Nat. 2013, 170, 1–13. [Google Scholar] [CrossRef]
- Conner, W.; Whitmire, S.; Duberstein, J.; Stalter, R.; Baden, J. Changes within a South Carolina Coastal Wetland Forest in the Face of Rising Sea Level. Forests 2022, 13, 414. [Google Scholar] [CrossRef]
- Noe, G.B.; Bourg, N.A.; Krauss, K.W.; Duberstein, J.A.; Hupp, C.R. Watershed and estuarine controls both influence plant Community and tree growth changes in tidal freshwater forested wetlands along two US Mid-Atlantic Rivers. Forests 2021, 12, 1182. [Google Scholar] [CrossRef]
- Janousek, C.N.; Folger, C.L. Variation in Tidal Wetland Plant Diversity and Composition Within and among Coastal Estuaries: Assessing the Relative Importance of Environmental Gradients. J. Veg. Sci. 2014, 25, 534–545. [Google Scholar] [CrossRef]
- Johnson, L.K.; Simenstad, C.A. Variation in the Flora and Fauna of Tidal Freshwater Forest Ecosystems Along the Columbia River Estuary Gradient: Controlling Factors in the Context of River Flow Regulation. Estuaries Coasts 2015, 38, 679–698. [Google Scholar] [CrossRef]
- Grieger, R.; Capon, S.J.; Hadwen, W.L.; Mackey, B. Spatial Variation and Drivers of Vegetation Structure and Composition in Coastal Freshwater Wetlands of Subtropical Australia. Mar. Freshw. Res. 2021, 72, 1746–1759. [Google Scholar] [CrossRef]
- Sande, E.; Young, D.R. Effect of sodium chloride on growth and nitrogenase activity in seedlings of Myrica cerifera L. New Phytol. 1992, 120, 345–350. [Google Scholar] [CrossRef]
- Crain, C.M.; Silliman, B.R.; Bertness, S.L.; Bertness, M.D. Physical and Biotic Drivers of Plant Distribution across Estuarine Salinity Gradients. Ecology 2004, 85, 2539–2549. [Google Scholar] [CrossRef]



| Monitoring Station | Mean Salinity (PSU) | Max Salinity (PSU) |
|---|---|---|
| Station 1 | 1.53 | 14.06 |
| Station 2 | 1.49 | 12.26 |
| Station 3 | 1.03 | 10.33 |
| Station 4 | 1.05 | 14.66 |
| Station 5 | 0.24 | 2.88 |
| Station 6 | 0.23 | 4.56 |
| Station 7 | 0.26 | 3.61 |
| Station 8 | 0.46 | 5.79 |
| Station 9 | 1.74 | 9.71 |
| Forest Type | Hydrologic Group | ||||||
|---|---|---|---|---|---|---|---|
| Species | Mixed Forest (n = 4) | Swamp Tupelo (n = 11) | Water Tupelo (n = 12) | Bald Cypress (n = 4) | Bald Cypress/Mixed Tupelo (n = 12) | Lower Tidal (n = 15) | Upper Tidal (n = 28) |
| Acer rubrum | 41.1 (6.0) | 19.8 (5.4) | 10.3 (3.4) | 7.5 (5.8) | 5.4 (2.2) | 25.5 (5.3) | 7.8 (2.0) |
| Fraxinus spp. | 11.2 (3.3) | - | 26.4 (6.4) | 36.6 (12.6) | 17.4 (5.2) | 3.0 (1.7) | 24.0 (4.2) |
| Nyssa aquatica | - | 10.1 (6.5) | 92.2 (9.5) | 13.1 (5.9) | 22.6 (7.6) | 7.4 (4.8) | 51 (8.6) |
| Nyssa biflora | 98.2 (18.1) | 155.7 (9.5) | 30.4 (8.1) | - | 65.0 (8.5) | 140 (11.2) | 40.9 (6.6) |
| Persea palustris | 1.6 (1.2) | 2.5 (2.5) | - | - | - | 2.2 (1.8) | - |
| Quercus lauriflora/nigra | - | - | 11.9 (5.7) | 4.6 (3.6) | 8.5 (4.2) | - | 8.7 (3.1) |
| Taxodium distichum | - | 11.0 (5.3) | 10.9 (4.3) | 138.1 (17.5) | 75.4 (6.0) | 8.1 (4.1) | 56.7 (6.9) |
| Ulmus americana | 12.9 (6.0) | 0.9 (0.9) | - | - | 1.5 (1.0) | 4.1 (2.4) | 0.6 (0.4) |
| Magnolia virginiana | 28.9 (14.6) | - | - | - | - | 7.7 (5.7) | - |
| Populus heterophylla | - | - | 5.9 (4.5) | - | - | - | 2.5 (2.0) |
| Salix nigra | 2.9 (2.2) | - | 9.3 (7.5) | - | 1.4 (1.0) | 0.8 (0.8) | 5.2 (3.3) |
| Triadica sebifera | 3.3 (2.5) | - | 2.2 (2.2) | - | 2.7 (2.7) | 0.9 (0.9) | 2.1 (1.5) |
| Forest/Environmental Variables | |||||||
| Mean basal area (m2/ha) | 17.2 (3.7) | 14.3 (3.8) | 31.8 (3.9) | 15.9 (4.4) | 32.9 (4.5) | 15.0 (3.0) | 30.0 (2.8) |
| Mean density (no./ha) | 363 (36) | 266 (44) | 475 (68) | 181 (47) | 379 (38) | 292 (36) | 392 (38) |
| Proportion of stressed trees (%) | 20 (10) | 21 (7) | 14 (0) | 16 (7) | 5 (2) | 20 (10) | 10 (0) |
| Mean river distance (rkm) | 11.6 (1.2) | 21.6 (1.0) | 32.4 (1.9) | 24.3 (1.9) | 31.5 (1.8) | 18.9 (1.4) | 30.9 (1.2) |
| Mean elevation (m) | 0.62 (0) | 0.53 (0.1) | 0.70 (0) | 0.51 (0.1) | 0.45 (0.1) | 0.5 (0.0) | 0.6 (0.0) |
| Forest Type | Hydrologic Group | ||||||
|---|---|---|---|---|---|---|---|
| Species | Mixed Forest (n = 4) | Swamp Tupelo (n = 11) | Water Tupelo (n = 12) | Bald Cypress (n = 4) | Bald Cypress/Mixed Tupelo (n = 12) | Lower Tidal (n = 15) | Upper Tidal (n = 28) |
| Sabal minor | 6144 (984) | 2330 (567) | 2052 (599) | 2544 (907) | 1369 (485) | 3347 (679) | 1829 (352) |
| Persea palustris | 113 (46) | 77 (40) | - | 44 (34) | 6 (4) | 87 (34) | 9 (6) |
| Acer rubrum | 188 (92) | 193 (40) | 167 (67) | 63 (26) | 127 (38) | 192 (38) | 135 (33) |
| Morella cerifera | 313 (97) | 489 (210) | 38 (31) | 44 (34) | 17 (12) | 442 (169) | 29 (15) |
| Cephalanthus occidentalis | 19 (6) | 80 (22) | 50 (28) | 44 (21) | 65 (31) | 63 (19) | 55 (18) |
| Cornus foemina | 6 (6) | 23 (13) | 8 (6) | - | - | 18 (11) | 4 (3) |
| Ilex cassine | 44 (16) | 23 (10) | - | - | 6 (6) | 28 (9) | 3 (3) |
| Ilex vomitoria | 38 (22) | 18 (9) | - | 6 (5) | 4 (4) | 23 (9) | 3 (2) |
| Ilex verticillata | - | 102 (57) | 150 (87) | 119 (80) | 288 91 | 75 (47) | 204 (56) |
| Quercus lauriflora/nigra | - | 21 (11) | 35 (21) | 19 (15) | 46 (23) | 15 (9) | 38 (13) |
| Ulmus americana | 6 (6) | 2 (2) | 21 (11) | - | 25 (14) | 3 (2) | 20 (8) |
| Triadica sebifera | 6 (6) | 5 (4) | 2 (2) | - | 35 (20) | 5 (4) | 16 (9) |
| Salix nigra | 13 (13) | 39 (15) | 6 (6) | 13 (10) | 4 (3) | 32 (13) | 6 (3) |
| Nyssa biflora | 31 (12) | 150 (55) | 50 (10) | 63 (37) | 44 (15) | 118 (46) | 49 (10) |
| Populus heterophylla | - | 16 (15) | 38 (31) | - | 29 (27) | 12 (12) | 29 (17) |
| Nyssa aquatica | - | 25 (18) | 54 (25) | 6 (5) | 60 (35) | 18 (15) | 50 (18) |
| Taxodium distichum | - | 20 (7) | 33 (8) | 81 (57) | 50 (31) | 15 (6) | 47 (16) |
| Itea virginica | - | 89 (59) | 15 (9) | 19 (9) | 77 (35) | 65 (48) | 42 (16) |
| Planera aquatica | - | - | 6 (6) | - | 4 (4) | - | 4 (3) |
| Baccharis halimifolia | - | 34 (23) | - | - | - | 25 (19) | - |
| Fraxinus spp. | 13 (7) | 102 (35) | 325 (92) | 512 (153) | 142 (54) | 78 (29) | 273 (57) |
| Species diversity (H) | 0.5 (0.2) | 1.2 (0.1) | 1.1 (0.1) | 0.9 (0.1) | 1.3 (0.1) | 1.0 (0.1) | 1.1 (0.1) |
| Species evenness (J) | 0.2 (0.1) | 0.6 (0.1) | 0.5 (0.1) | 0.5 (0.1) | 0.6 (0.1) | 0.5 (0.1) | 0.6 (0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balder, A.; Anderson, C.J.; Billor, N. Tidal Freshwater Forested Wetlands in the Mobile-Tensaw River Delta along the Northern Gulf of Mexico. Forests 2024, 15, 1359. https://doi.org/10.3390/f15081359
Balder A, Anderson CJ, Billor N. Tidal Freshwater Forested Wetlands in the Mobile-Tensaw River Delta along the Northern Gulf of Mexico. Forests. 2024; 15(8):1359. https://doi.org/10.3390/f15081359
Chicago/Turabian StyleBalder, Andrew, Christopher J. Anderson, and Nedret Billor. 2024. "Tidal Freshwater Forested Wetlands in the Mobile-Tensaw River Delta along the Northern Gulf of Mexico" Forests 15, no. 8: 1359. https://doi.org/10.3390/f15081359






