Evaluation of Tolerance and Selection of Heat-Tolerant Woody Plants against Heat Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
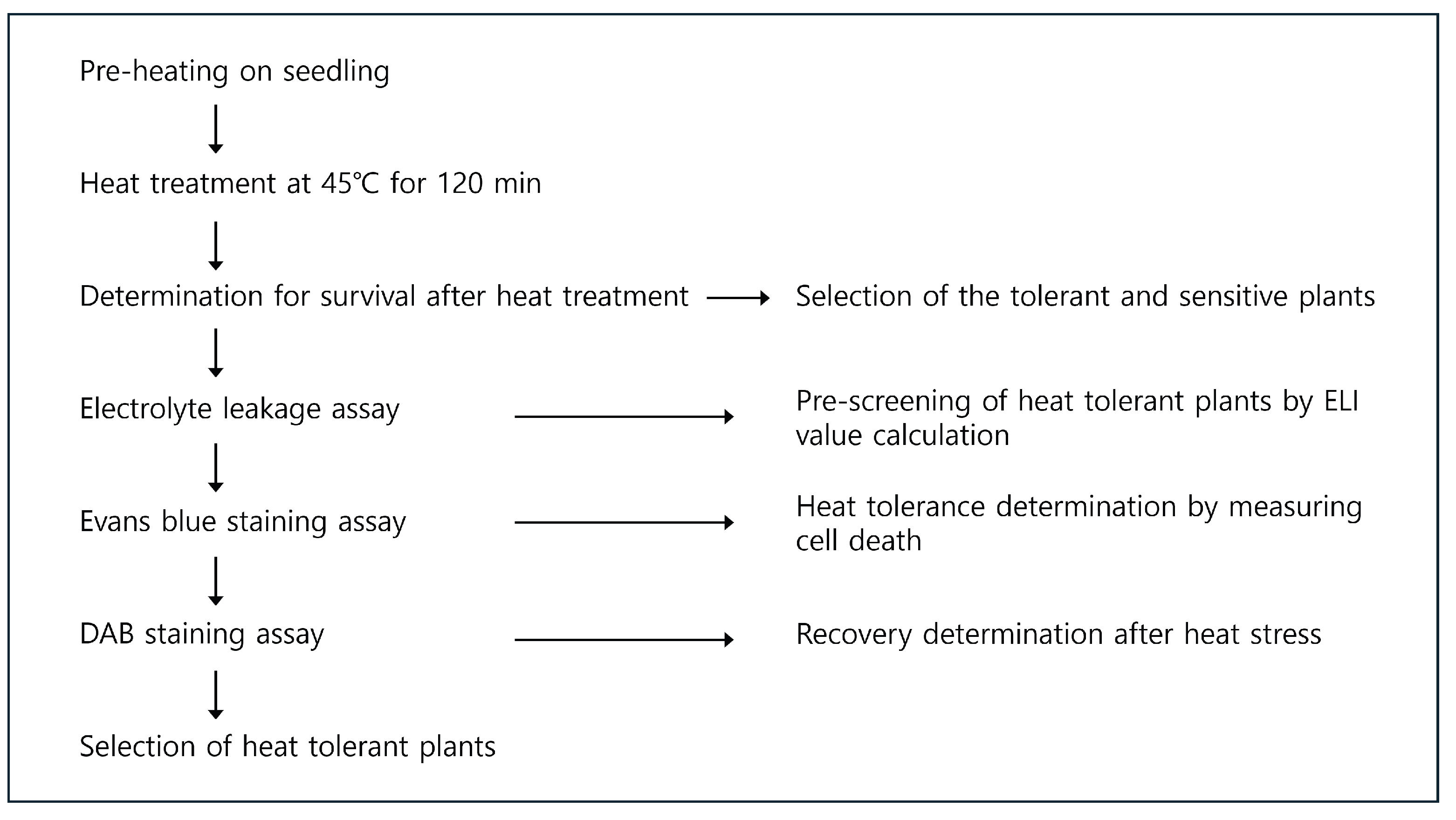
2.2. Heat Stress Treatment
2.3. Measuring Electrolyte Leakage Indexes
2.4. Investigation of Dead Tissue by Evans Blue Staining
2.5. Evaluation of Recovery of Heat-Stressed Plants by DAB Staining
2.6. Statistical Analysis
3. Results
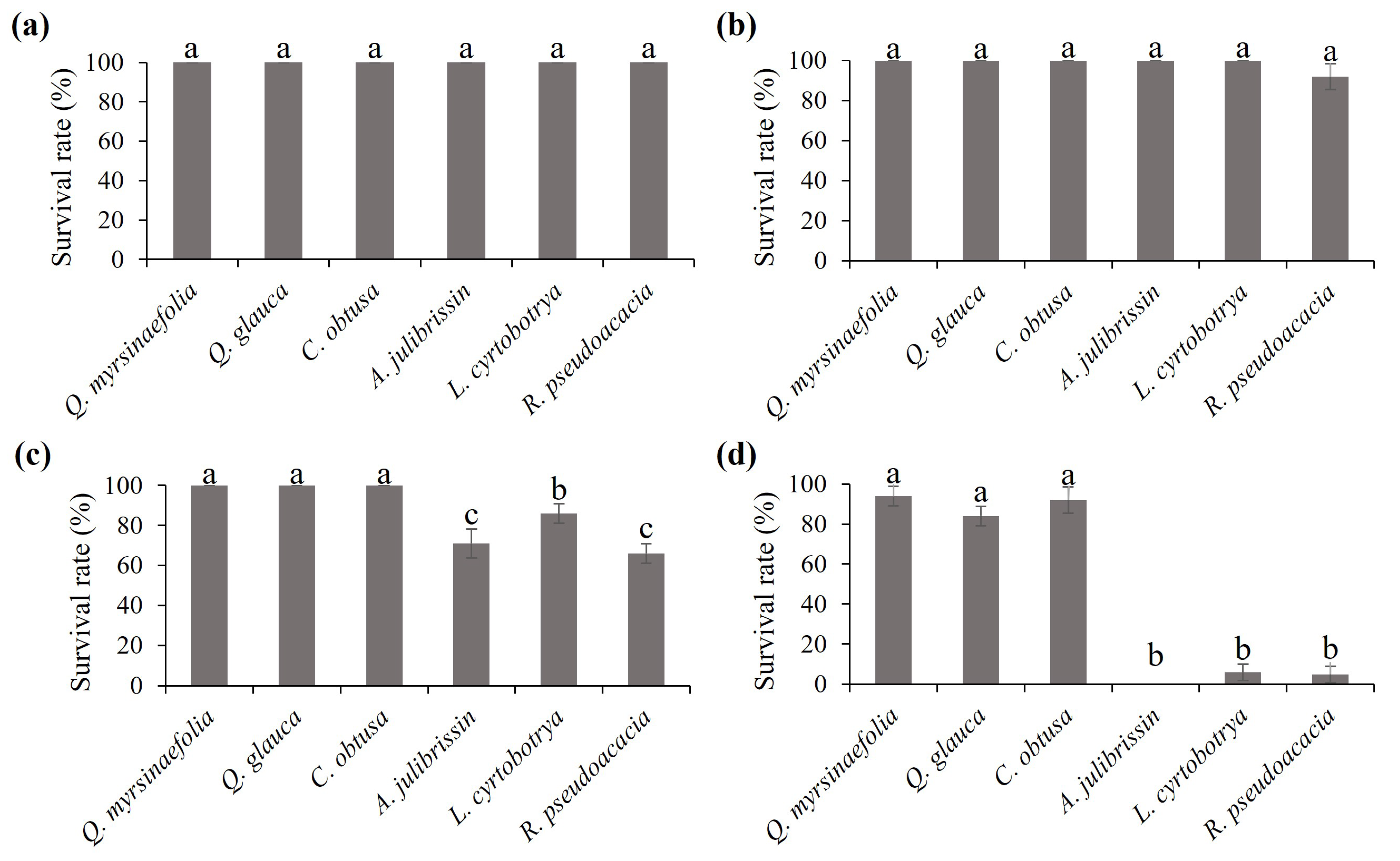
3.1. Measurement of the Response of Heat-Tolerant and Sensitive Woody Plants
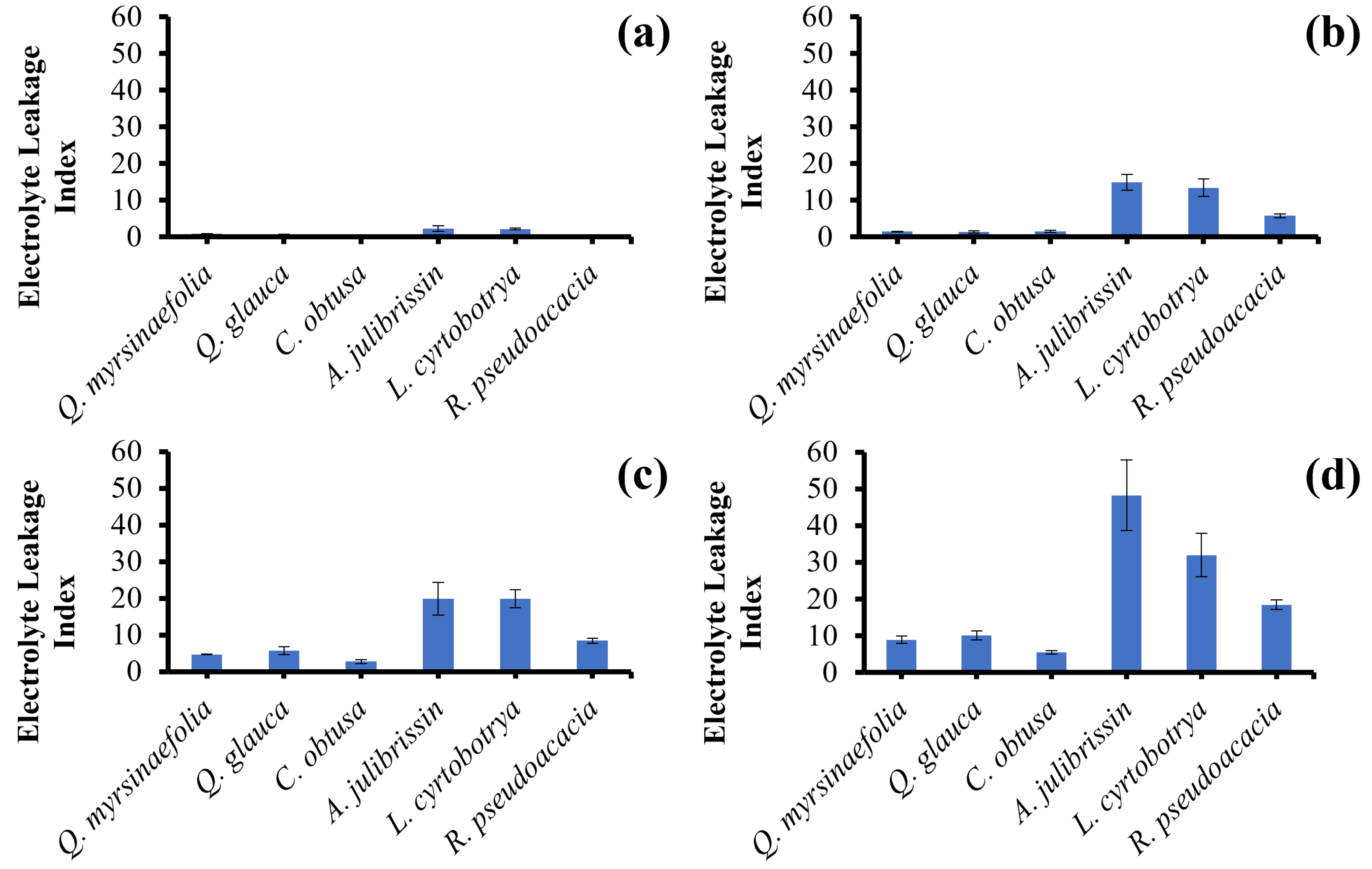
3.2. Pre-Screening of Heat-Tolerant Woody Plants Using Electrolyte Leakage Assay
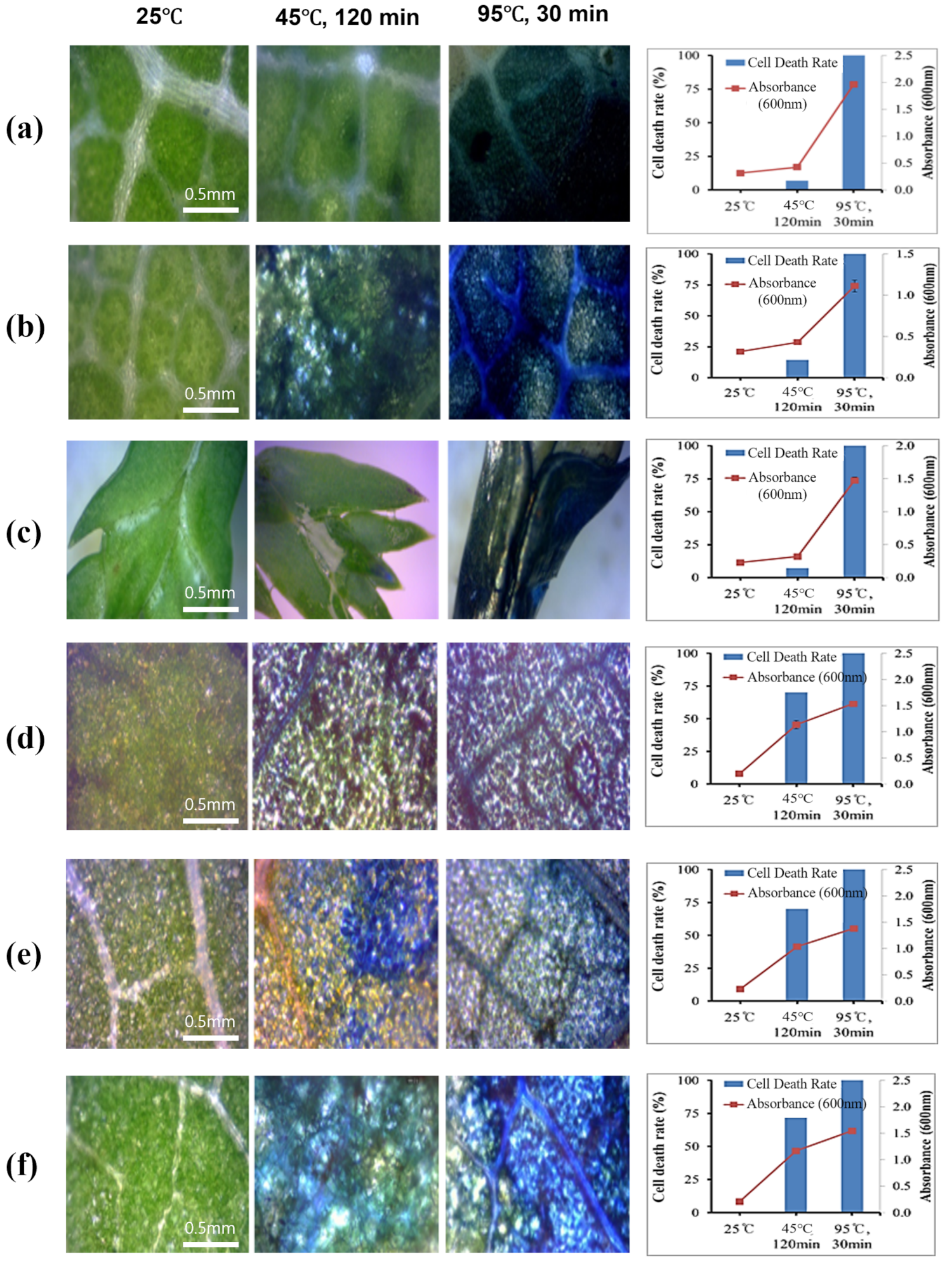
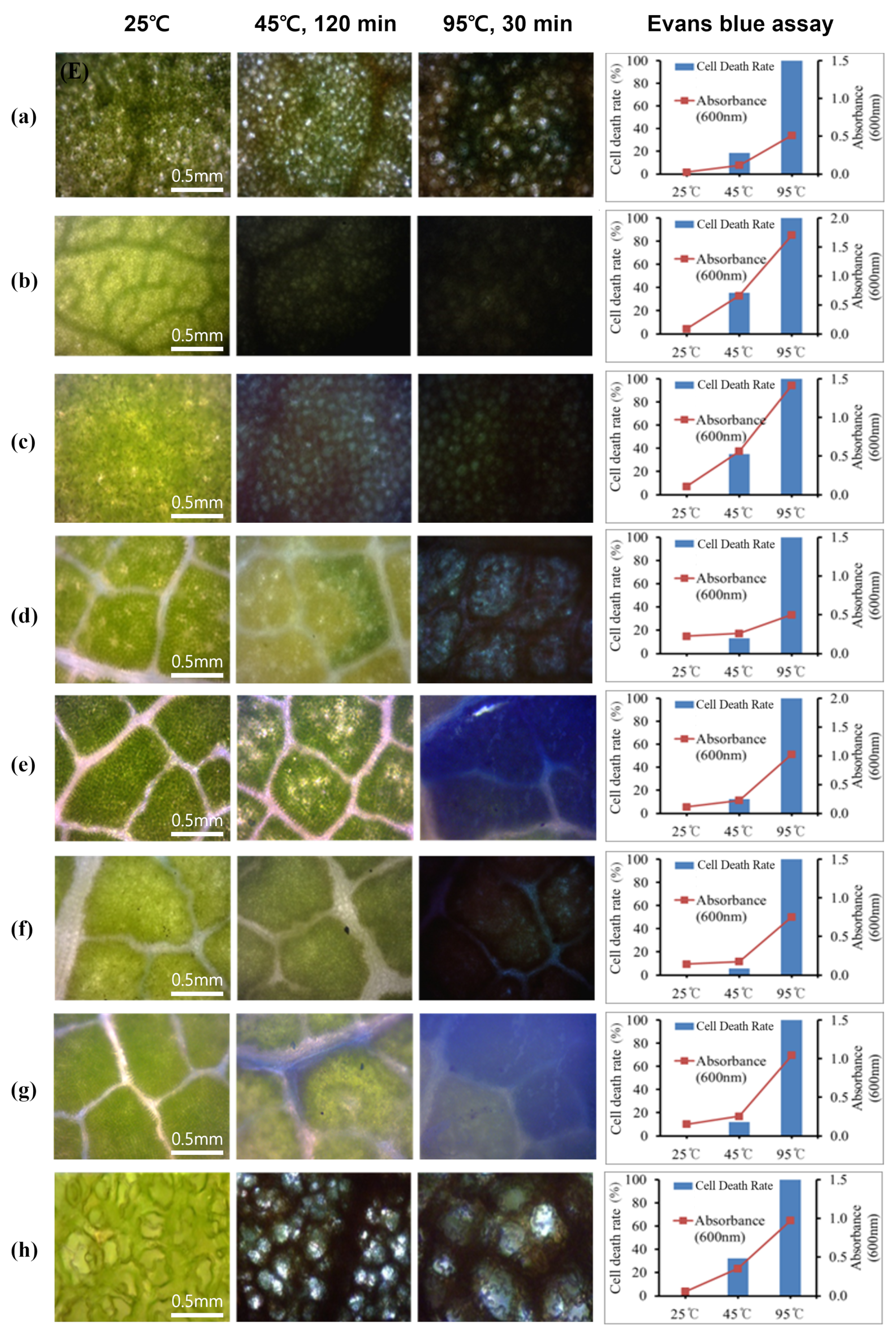
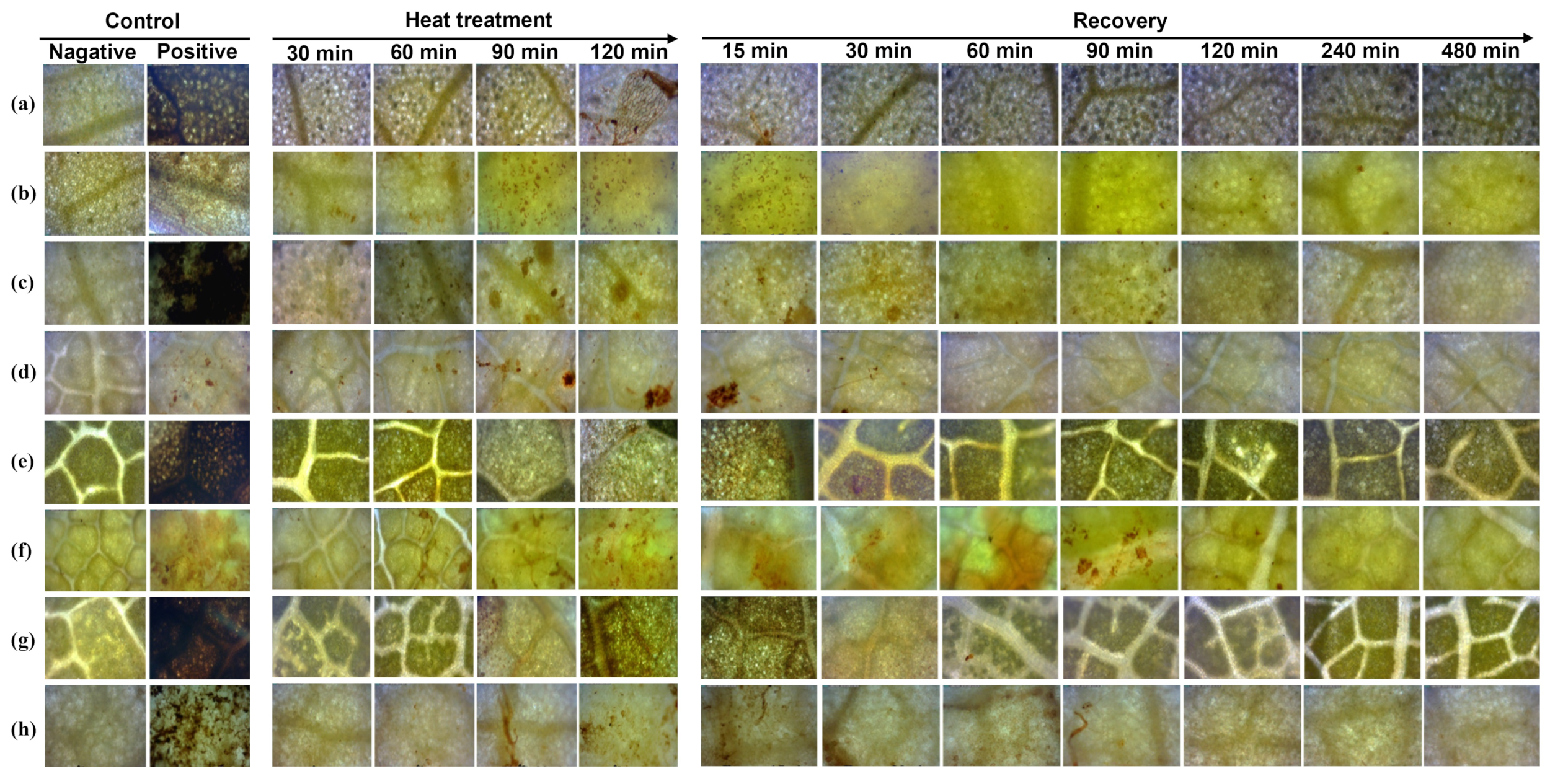
3.3. Verification of Heat Tolerance Using Evans Blue Staining and DAB Staining
3.4. Plant Recovery Ability of Selected Woody Plant Species under Heat Stress
3.5. Correlation Analysis of the Methods for Heat Tolerance Evaluation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- IPCC. Climate Change 2023: Synthesis Report. Contribution of Working Groups I, II and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change [Core Writing Team, H. Lee and J. Romero (eds.)]; IPCC: Geneva, Switzerland, 2023; pp. 42–74. [Google Scholar] [CrossRef]
- Climate Change Situation Map (Korea Meteorological Administration). Available online: http://www.climate.go.kr/atlas/dashboard (accessed on 12 June 2024).
- Zhao, J.; Lu, Z.; Wang, L.; Jin, B. Plant Responses to Heat Stress: Physiology, Transcription, Noncoding RNAs, and Epigenetics. Int. J. Mol. Sci. 2021, 22, 117. [Google Scholar] [CrossRef] [PubMed]
- Crafts-Brandner, S.J.; Salvucci, M.E. Sensitivity of Photosynthesis in a C4 Plant, Maize, to Heat Stress. Plant Physiol. 2002, 129, 1773–1780. [Google Scholar] [CrossRef] [PubMed]
- Camejo, D.; Rodríguez, P.; Morales, M.A.; Dell’Amico, J.M.; Torrecillas, A.; Alarcón, J.J. High temperature effects on photosynthetic activity of two tomato cultivars with different heat susceptibility. J. Plant Physiol. 2005, 165, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Havaux, M. Characterization of thermal damage to the photosynthetic electron transport system in potato leaves. Plant Sci. 1993, 94, 19–33. [Google Scholar] [CrossRef]
- Yin, H.; Chen, Q.; Yi, M. Effects of short-term heat stress on oxidative damage and responses of antioxidant system in Lilium longiflorum. Plant Growth Regul. 2008, 54, 45–54. [Google Scholar] [CrossRef]
- Xu, S.; Li, J.; Zhang, X.; Wei, H.; Cui, L. Effects of heat acclimation pretreatment on changes of membrane lipid peroxidation, antioxidant metabolites, and ultrastructure of chloroplasts in two cool-season turfgrass species under heat stress. Environ. Exp. Bot. 2006, 56, 274–285. [Google Scholar] [CrossRef]
- Sarkar, N.K.; Kundnani, P.; Grover, A. Functional analysis of Hsp70 superfamily proteins of rice (Oryza sativa). Cell Stress Chaperones 2013, 18, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Vickers, C.E.; Gershenzon, J.; Lerdau, M.T.; Loreto, F. A unified mechanism of action for volatile isoprenoids in plant abiotic stress. Nat. Chem. Biol. 2009, 5, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zou, Z.; Li, Q.; Sun, K.; Chen, X.; Xinghui, L. The CsHSP17.2 molecular chaperone is essential for thermotolerance in Camellia sinensis. Sci. Rep. 2017, 7, 1237. [Google Scholar] [CrossRef] [PubMed]
- Asthir, B. Protective mechanisms of heat tolerance in crop plants. J. Plant Interact. 2015, 10, 202–210. [Google Scholar] [CrossRef]
- Moreno, A.A.; Orellana, A. The physiological role of the unfolded protein response in plants. Biol. Res. 2011, 44, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Yeh, C.H.; Kaplinsky, N.J.; Hu, C.; Charng, Y. Some like it hot, some like it warm: Phenotyping to explore thermotolerance diversity. Plant Sci. 2012, 195, 10–23. [Google Scholar] [CrossRef] [PubMed]
- Hameed, M.; Keitel, C.; Ahmad, N.; Mahmood, T.; Trethowan, R. Screening of tomatoes germplasm for heat stress tolerance under controlled conditions. Procedia Environ. Sci. 2015, 29, 173–174. [Google Scholar] [CrossRef]
- Bibi, A.C.; Oosterhuis, D.M.; Gonias, E.G. Photosynthesis, quantum yield of photosystem II, and membrane leakage as affected by high temperature in cotton genotypes. Cotton Sci. 2008, 12, 150–159. [Google Scholar]
- Dexter, S.T.; Tottingham, W.E.; Graber, L.F. Preliminary results in measuring the hardiness of plants. Plant Physiol. 1930, 5, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Ilík, P.; Špundováet, M.; Šicner, M.; Melkovičová, H.; Kučerová, Z.; Krchňák, P.; Fürst, T.; Večeřová, K.; Panzarová, K.; Benediktyová, Z.; et al. Estimating heat tolerance of plants by ion leakage: A new method based on gradual heating. New Phytol. 2018, 218, 1278–1287. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Harguindeguy, N.; Díaz, S.; Garnier, E.; Lavorel, S.; Poorter, H.; Jaureguiberry, P.; Bret-Harte, M.S.; Cornwell, W.K.; Craine, J.M.; Gurvich, D.E.; et al. New handbook for standardised measurement of plant functional traits worldwide. Aust. J. Bot. 2013, 61, 167–234. [Google Scholar] [CrossRef]
- Mansour, M.M.F. Plasma membrane permeability as an indicator of salt tolerance in plants. Biol. Plant. 2013, 57, 1–10. [Google Scholar] [CrossRef]
- Murkowski, A. Heat stress and spermidine: Effect on chlorophyll fluorescence in tomato plants. Biol. Plant. 2001, 44, 53–57. [Google Scholar] [CrossRef]
- Harsh, A.; Sharma, Y.K.; Joshi, U.; Rampuria, S.; Singh, G.; Kumar, S.; Sharma, R. Effect of short-term heat stress on total sugars, proline and some antioxidant enzymes in moth bean (Vigna aconitifolia). Ann. Agric. Sci. 2016, 61, 57–64. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Z.; Li, D.; Wei, J.; Qiao, W. Meng, X.; Sun, S.; Li, H.; Zhao, M.; Chen, X.; Zhao, F. Evaluation of a new method for quantification of heat tolerance in different wheat cultivars. J. Integr. Agric. 2018, 17, 786–795. [Google Scholar] [CrossRef]
- Hossain, A.; Sarker, M.A.Z.; Saifuzzaman, M.; Teixeira da Silva, J.A.; Lozovskaya, M.V.; Akhter, M.M. Evaluation of growth, yield, relative performance and heat susceptibility of eight wheat (Triticum aestivum L.) genotypes grown under heat stress. Int. J. Plant Prod. 2013, 7, 615–636. [Google Scholar] [CrossRef]
- Wassie, M.; Zhang, W.; Zhang, Q.; Ji, K.; Chen, L. Effect of Heat Stress on Growth and Physiological Traits of Alfalfa (Medicago sativa L.) and a Comprehensive Evaluation for Heat Tolerance. Agronomy 2019, 9, 597. [Google Scholar] [CrossRef]
- Dorado, F.J.; Solla, A.; Alcaide, F.; Martín, M.Á. Assessing heat stress tolerance in Castanea sativa. Forestry 2022, 95, 667–677. [Google Scholar] [CrossRef]
- Marias, D.E.; Meinzer, F.C.; Woodruff, D.R.; McCulloh, K.A. Thermotolerance and heat stress responses of Douglas-fir and ponderosa pine seedling populations from contrasting climates. Tree Physiol. 2017, 37, 301–315. [Google Scholar] [CrossRef] [PubMed]
- Daudi, A.; O’Brien, J.A. Detection of hydrogen peroxide by DAB staining in Arabidopsis leaves. Bio-Protocol 2012, 2, e263. [Google Scholar] [CrossRef]
- Lin, C.Y.; Roberts, J.K.; Key, J.L. Acquisition of thermotolerance in soybean seedlings: Synthesis and accumulation of heat shock proteins and their cellular localization. Plant Physiol. 1984, 74, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Jin, E.J.; Cho, M.G.; Bae, E.J.; Park, J.H.; Lee, K.S.; Choi, M.S. Physiological responses to drought stress of seven evergreen hardwood species. J. Korean For. Soc. 2017, 106, 397–407. [Google Scholar] [CrossRef]
- Bhattarai, S.; Harvey, J.T.; Djidonou, D.; Leskovar, D.I. Exploring morpho-physiological variation for heat stress tolerance in tomato. Plants 2021, 10, 347. [Google Scholar] [CrossRef] [PubMed]
- McNabb, K.; Takahashi, E. Freeze Damage to Loblolly Pine Seedlings as Indicated by Conductivity Measurements and Outplanting Survival; Research Report 00-4; Auburn University Southern Forest Nursery Management Cooperative: Auburn, AL, USA, 2000. [Google Scholar]
- Orlova, I.V.; Serebriiskaya, T.S.; Popov, V.; Merkulova, N.; Nosov, A.M.; Trunova, T.I.; Tsydendambaev, V.D.; Los, D.A. Transformation of Tobacco with a gene for the thermophilic Acyl-lipid desaturase enhances the chilling tolerance of plants. Plant Cell Physiol. 2003, 44, 447–450. [Google Scholar] [CrossRef] [PubMed]
- Heidarvand, L.; Maali Amiri, R.; Naghavi, M.R.; Farayedi, Y.; Sadeghzadeh, B.; Alizadeh, K. Physiological and morphological characteristics of chickpea accessions under low temperature stress. Russ. J. Plant Physiol. 2011, 58, 157–163. [Google Scholar] [CrossRef]
- Wahid, A.; Gelani, S.; Ashraf, M.; Foolad, M.R. Heat tolerance in plants: An overview. Environ. Exp. Bot. 2007, 61, 199–223. [Google Scholar] [CrossRef]
- Petrov, V.; Hille, J.; Mueller-Roeber, B.; Gechev, T.S. ROS-mediated abiotic stress-induced programmed cell death in plants. Front. Plant Sci. 2015, 6, 69. [Google Scholar] [CrossRef] [PubMed]
- Hemantaranjan, A.; Bhanu, A.N.; Singh, M.N.; Yadav, D.K.; Patel, P.K.; Singh, R.; Katiyar, D. Heat stress responses and thermotolerance. Adv. Plants Agric. Res. 2014, 1, 62–70. [Google Scholar] [CrossRef]
- Zhu, J.K. Abiotic stress signaling and responses in plants. Cell 2008, 167, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Breusegem, F.V.; Dat, J.F. Reactive oxygen species in plant cell death. Plant Physiol. 2006, 141, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Demidchik, V.; Straltsova, D.; Medvedev, S.S.; Pozhvanov, G.A.; Sokolik, A.; Yurin, V. Stress-induced electrolyte leakage: The role of K+-permeable channels and involvement in programmed cell death and metabolic adjustment. J. Exp. Bot. 2014, 65, 1259–1270. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.H.; Meskauskiene, R.; Apel, K.; Laloi, C. No single way to understand singlet oxygen signalling in plants. EMBO Rep. 2008, 9, 435–439. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.N.; Cornejo, M.J.; Bush, D.S.; Jones, R.L. Estimating viability of plant protoplasts using double and single staining. Protoplasma 1986, 135, 80–87. [Google Scholar] [CrossRef]
- Silva, F.D.; Menedez-Yuffa, A. Viability in protoplasts and cell suspensions of Coffea arabica cv. Catimor. Electron. J. Biotechn. 2006, 9, 593–597. [Google Scholar] [CrossRef]
- Castro-Concha, L.A.; Escobedo, R.M.; Miranda-Ham, M.L. Measurement of cell viability in in vitro cultures. In Plant Cell Culture Protocols. Methods in Molecular Biology™; Loyola-Vargas, V.M., Vázquez-Flota, F., Eds.; Humana Press: Totowa, NJ, USA, 2006; Volume 318, pp. 71–76. [Google Scholar] [CrossRef]
- Gonzalez-Mendoz, D.; Quiroz-Moreno, A.; Medrano, R.E.; Grimaldo-Juarez, O.; Zapata-Perez, O. Cell viability and leakage of electrolytes in Avicennia germinans exposed to heavy metals. Z. Naturforsch. 2008, 64, 391–394. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Huang, B. Physiological recovery of Kentucky bluegrass from simultaneous drought and heat stress. Crop Sci. 2004, 44, 1729–1736. [Google Scholar] [CrossRef]
- Yan, Y.; Guo, X. Overexpression of GhWRKY27a reduce tolerance to drought stress and resistance to Rhizoctonia solani infection in transgenic Nicotiana benthamiana. Front. Physiol. 2015, 6, 265. [Google Scholar] [CrossRef] [PubMed]
- Nakabayashi, R.; Yonekura-Sakakibara, K.; Urano, K.; Suzuki, M.; Yamada, Y.; Nishizawa, T.; Matsuda, F.; Kojima, M.; Sa-kakibara, H.; Shinozaki, K.; et al. Enhancement of oxidative and drought tolerance in Arabidopsis by overaccumulation of antioxidant flavonoids. Plant J. 2014, 77, 367–379. [Google Scholar] [CrossRef] [PubMed]
- Pellinen, R.I.; Korhonen, M.S.; Tauriainen, A.A.; Palva, E.T.; Kangasjärvi, J. Hydrogen peroxide activates cell death and defense gene expression in birch. Plant Physiol. 2002, 130, 549–560. [Google Scholar] [CrossRef] [PubMed]
- Ikei, H.; Song, C.; Miyazaki, Y. Physiological effect of olfactory stimulation by Hinoki cypress (Chamaecyparis obtusa) leaf oil. J. Physiol. Anthropol. 2008, 34, 44. [Google Scholar] [CrossRef] [PubMed]
- Park, D.J.; Yong, S.H.; Choi, M.S. Establishment of selection method for cold-tolerant individuals through evaluating tolerance of evergreen Quercus spp. against cold stress. J. For. Environ. Sci. 2019, 35, 232–239. [Google Scholar] [CrossRef]


| Scientific Name | Family | Common Name |
|---|---|---|
| Albizia julibrissin Durazz. | Fabaceae | Silk Tree |
| Amorpha fruticosa L. | Fabaceae | False indigo bush |
| Camellia sinensis L. | Theaceae | Tea camellia |
| Castanopsis sieboldii (Makino) Hatus. | Fabaceae | Siebold’s chinquapin |
| Chamaecyparis obtusa (Siebold and Zucc.) | Cupressaceae | Japanese false cypress |
| Cinnamomum yabunikkei H.Ohba | Lauraceae | Japanese camphor tree |
| Dendropanax morbiferus H. Lév | Araliaceae | Korean dendropanax |
| Eucommia ulmoides Oliv. | Eucommiaceae | Gutta-percha tree |
| Ilex cornuta Lindl. | Aquifoliaceae | Horned holly |
| Ilex crenata Thunb. | Aquifoliaceae | Box-leaf holly |
| Indigofera kirilowii Maxim. | Fabaceae | Kirilow’s indigo |
| Indigofera pseudotinctoria Matsum. | Fabaceae | Dwarf false-indigo |
| Lespedeza bicolor Turcz. | Fabaceae | Shrub lespedeza |
| Lespedeza cyrtobotrya Miq. | Fabaceae | Leafy lespedeza |
| Ligustrum japonicum Thunb. | Oleaceae | Wax-leaf privet |
| Morus bombycis Koidz. | Moraceae | Korean mulberry |
| Neolitsea sericea (Blume) Koidz. | Lauraceae | Sericeous newlitsea |
| Pinus rigida Mill. | Pinaceae | Pitch pine |
| Quercus acuta Thunb. | Fagaceae | Red-wood evergreen oak |
| Quercus glauca Thunb. | Fagaceae | Ring-cup oak |
| Quercus myrsinaefolia Bl. | Fagaceae | Bamboo-leaf oak |
| Quercus phillyraeoides A. Gray | Fagaceae | Ubame oak |
| Quercus salicina Bl. | Fagaceae | Willow-leaf evergreen oak |
| Robinia pseudoacacia L. | Fabaceae | Black locust |
| Sorbaria sorbifolia var. stellipila Max. | Rosaceae | False spiraea |
| Spiraea prunifolia f. simpliciflora Nakai. | Rosaceae | Simple bridalwreath spiraea |
| Ternstroemia japonica Thunb. | Pentaphylacaceae | Naked-anther ternstroemia |
| - a | - | - | - | - | - | - | - | - | |
| 1 | 0.57 * | 0.35 | 0.23 | 0.07 | 0.11 | 0.06 | −0.34 | ||
| 1 | 0.88 ** | −0.25 | −0.53 * | −0.43 | −0.54 * | −0.87 ** | |||
| 1 | −0.59 ** | −0.81 ** | −0.76 ** | −0.76 ** | −0.99 ** | ||||
| 1 | 0.87 ** | 0.92 * | 0.88 ** | 0.65** | |||||
| 1 | 0.98 ** | 0.95 ** | 0.80** | ||||||
| 1 | 0.96 ** | 0.78 ** | |||||||
| 1 | 0.77 ** | ||||||||
| 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, D.J.; Yong, S.H.; Kim, D.H.; Park, K.B.; Cha, S.A.; Lee, J.-H.; Kim, S.A.; Choi, M.S. Evaluation of Tolerance and Selection of Heat-Tolerant Woody Plants against Heat Stress. Forests 2024, 15, 1366. https://doi.org/10.3390/f15081366
Park DJ, Yong SH, Kim DH, Park KB, Cha SA, Lee J-H, Kim SA, Choi MS. Evaluation of Tolerance and Selection of Heat-Tolerant Woody Plants against Heat Stress. Forests. 2024; 15(8):1366. https://doi.org/10.3390/f15081366
Chicago/Turabian StylePark, Dong Jin, Seong Hyeon Yong, Do Hyun Kim, Kwan Been Park, Seung A. Cha, Ji-Hyun Lee, Seon A. Kim, and Myung Suk Choi. 2024. "Evaluation of Tolerance and Selection of Heat-Tolerant Woody Plants against Heat Stress" Forests 15, no. 8: 1366. https://doi.org/10.3390/f15081366





