Long-Term Planting of Taxodium Hybrid ‘Zhongshanshan’ Can Effectively Enhance the Soil Aggregate Stability in Saline–Alkali Coastal Areas
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Situation of the Study Area and Plot Setting
2.2. Soil Sample Collection
2.3. Determination of Physical and Chemical Properties of Soil
2.4. Soil Aggregate Classification and Stability Evaluation
2.5. Determination of Soil Enzyme Activity
2.6. Data Analysis
3. Results
3.1. Physical and Chemical Properties of Soil
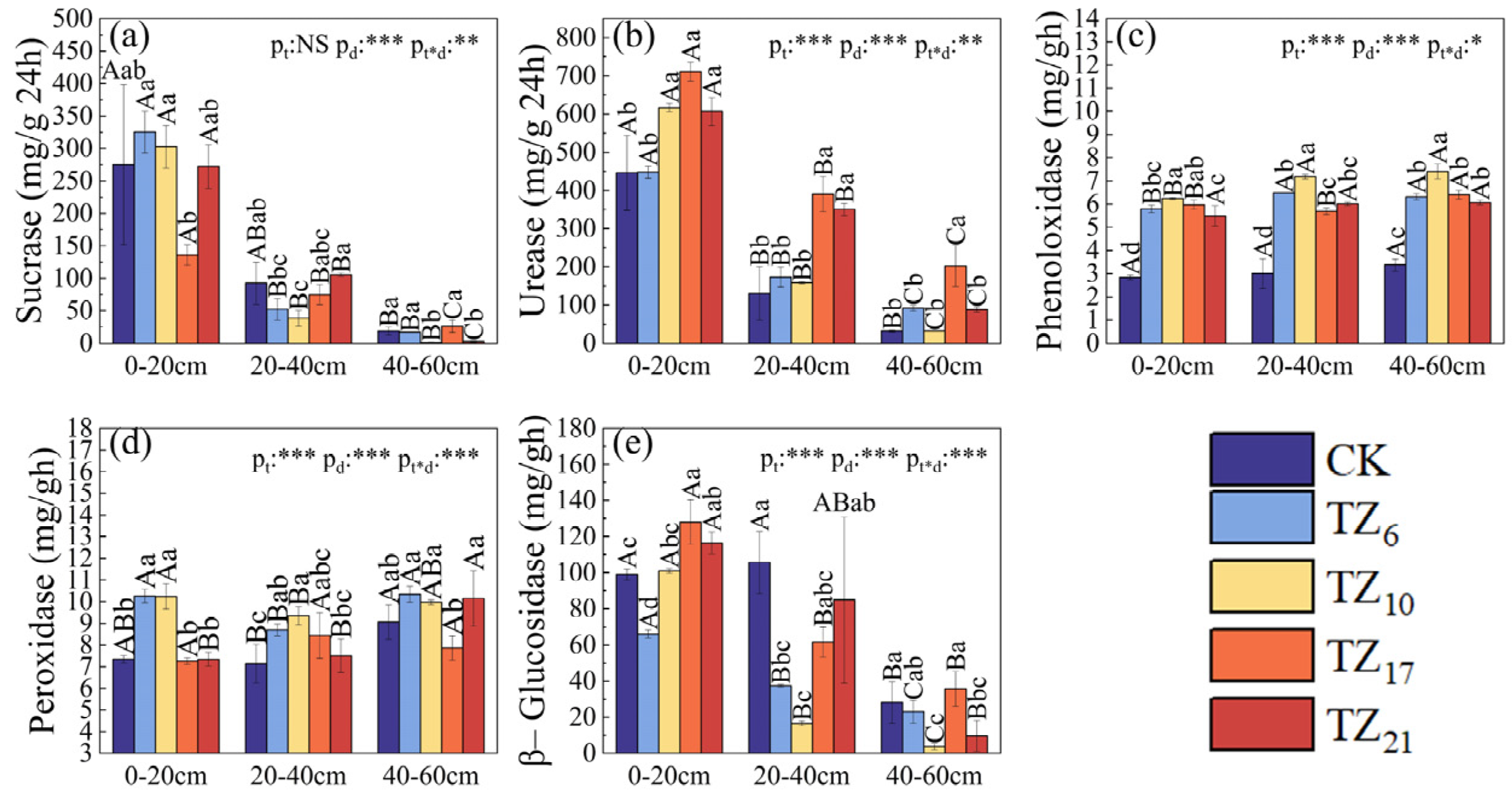
3.2. Analysis of Soil Enzyme Activity
3.3. Soil Water Stability Aggregate Size Content Characteristics
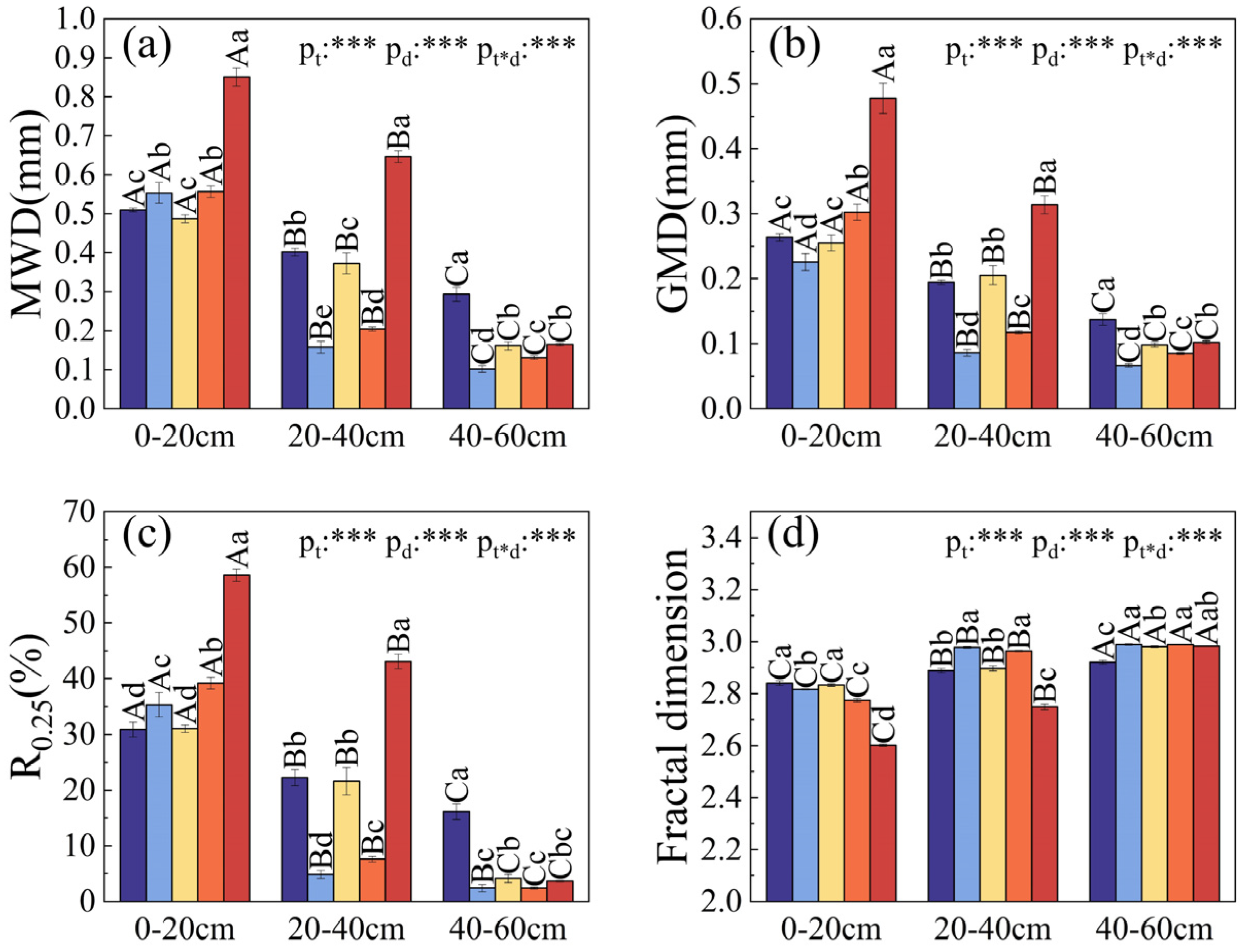
3.4. Stability Analysis of Soil Aggregates
3.5. Impact of the Physical and Chemical Characteristics of Soil on the Activity of Enzymes
3.6. Enzyme Activity and Soil Physicochemical Characteristics’ Effects on Aggregate Stability
3.7. Mechanism of Aggregate Stability Impacts of Soil Physical and Chemical Characteristics, Enzyme Activity, and Planting Time
4. Discussion
4.1. Differences in Soil Physical and Chemical Properties with Planting Time and Soil Depth in Coastal Saline–Alkali Land
4.2. Analysis and Influencing Factors of Soil Enzyme Activity in Different Planting Times in Saline–Alkali Coastal Land
4.3. Analysis and Influencing Mechanism of Soil Aggregate Stability at Different Planting Times in Saline–Alkali Coastal Land
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Muhammad, K.; Dan, W.; Xie, K.; Lu, Y.; Shi, C.; Sabagh, A.E.; Gu, W.; Xu, P. Pre-sowing seed treatment with kinetin and calcium mitigates salt induced inhibition of seed germination and seedling growth of choysum (Brassica rapa var. parachinensis). Ecotoxicol. Environ. Saf. 2021, 227, 112921. [Google Scholar]
- Xie, W.; Chen, Q.; Wu, L.; Yang, H.; Xu, J.; Zhang, Y. Coastal saline soil aggregate formation and salt distribution are affected by straw and nitrogen application: A 4-year field study. Soil Tillage Res. 2020, 198, 104535. [Google Scholar] [CrossRef]
- Panta, S.; Flowers, T.; Lane, P.; Doyle, R.; Haros, G.; Shabala, S. Halophyte agriculture: Success stories. Environ. Exp. Bot. 2014, 107, 71–83. [Google Scholar] [CrossRef]
- Nadal-Romero, E.; Cammeraat, E.; Pérez-Cardiel, E.; Lasanta, T. Effects of secondary succession and afforestation practices on soil properties after cropland abandonment in humid Mediterranean mountain areas. Agric. Ecosyst. Environ. 2016, 228, 91–100. [Google Scholar] [CrossRef]
- Shao, T.; Gu, X.; Zhu, T.; Pan, X.; Zhu, Y.; Long, X.; Shao, H.; Liu, M.; Rengel, Z. Industrial crop Jerusalem artichoke restored coastal saline soil quality by reducing salt and increasing diversity of bacterial community. Appl. Soil Ecol. 2019, 138, 195–206. [Google Scholar] [CrossRef]
- Toktar, M.; Papa, G.L.; Kozybayeva, F.E.; Dazzi, C. Ecological restoration in contaminated soils of Kokdzhon phosphate mining area (Zhambyl region, Kazakhstan). Ecol. Eng. 2016, 86, 1–4. [Google Scholar] [CrossRef]
- Blanco-Canqui, H.; Lal, R. Mechanisms of Carbon Sequestration in Soil Aggregates. Crit. Rev. Plant Sci. 2004, 23, 481–504. [Google Scholar] [CrossRef]
- Wu, T.; Schoenau, J.J.; Li, F.; Qian, P.; Malhi, S.S.; Shi, Y. Effect of tillage and rotation on organic carbon forms of chernozemic soils in Saskatchewan. J. Plant Nutr. Soil Sci. 2003, 166, 328–335. [Google Scholar] [CrossRef]
- Ma, L.; Wang, Q.; Shen, S.T.; Li, F.C.; Li, L. Heterogeneity of soil structure and fertility during desertification of alpine grassland in northwest Sichuan. Ecosphere 2020, 11, e03161. [Google Scholar] [CrossRef]
- Xiao, L.; Yao, K.; Li, P.; Liu, Y.; Chang, E.; Zhang, Y.; Zhu, T. Increased soil aggregate stability is strongly correlated with root and soil properties along a gradient of secondary succession on the Loess Plateau. Ecol. Eng. 2020, 143, 105671. [Google Scholar] [CrossRef]
- Paz-Ferreiro, J.; Fu, S. Biological Indices for Soil Quality Evaluation: Perspectives and Limitations. Land Degrad. Dev. 2016, 27, 14–25. [Google Scholar] [CrossRef]
- Feng, C.; Ma, Y.; Fu, S.; Chen, H.Y.H. Soil Carbon and Nutrient Dynamics Following Cessation of Anthropogenic Disturbances in Degraded Subtropical Forests. Land Degrad. Dev. 2017, 28, 2457–2467. [Google Scholar] [CrossRef]
- Yunchao, L.; Lixin, C.; Wenbiao, D.; Yongan, B.; Xiaolan, L. Effects of Litter Decomposition on Soil N in Picea mongolica Forest at Different Forest Ages. Forests 2022, 13, 520. [Google Scholar] [CrossRef]
- Xu, L.; Shi, Y.; Fang, H.; Zhou, G.; Xu, X.; Zhou, Y.; Tao, J.; Ji, B.; Xu, J.; Li, C.; et al. Vegetation carbon stocks driven by canopy density and forest age in subtropical forest ecosystems. Sci. Total Environ. 2018, 631-632, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Lucas-Borja, M.E.; de Santiago, J.H.; Yang, Y.; Shen, Y.; Candel-Pérez, D. Nutrient, metal contents and microbiological properties of litter and soil along a tree age gradient in Mediterranean forest ecosystems. Sci. Total Environ. 2019, 650, 749–758. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ran, L.; Fang, N.; Shi, Z. Aggregate stability and associated organic carbon and nitrogen as affected by soil erosion and vegetation rehabilitation on the Loess Plateau. Catena 2018, 167, 257–265. [Google Scholar] [CrossRef]
- Yang, Y.F.; Wei, H.; Lin, L.W.; Deng, Y.S.; Duan, X.Q. Effect of Vegetation Restoration on Soil Humus and Aggregate Stability within the Karst Region of Southwest China. Forests 2024, 15, 292. [Google Scholar] [CrossRef]
- Bai, Y.; Zhou, Y.; He, H. Effects of rehabilitation through afforestation on soil aggregate stability and aggregate-associated carbon after forest fires in subtropical China. Geoderma 2020, 376, 114548. [Google Scholar] [CrossRef]
- Lan, J.C.; Long, Q.X.; Huang, M.Z.; Jiang, Y.X.; Hu, N. Afforestation-induced large macroaggregate formation promotes soil organic carbon accumulation in degraded karst area. For. Ecol. Manag. 2022, 505, 119884. [Google Scholar] [CrossRef]
- Liu, M.; Han, G.L.; Zhang, Q. Effects of agricultural abandonment on soil aggregation, soil organic carbon storage and stabilization: Results from observation in a small karst catchment, Southwest China. Agric. Ecosyst. Environ. 2020, 288, 106719. [Google Scholar] [CrossRef]
- Wang, J.J.; Shu, K.L.; Wang, S.Y.; Zhang, C.; Feng, Y.C.; Gao, M.; Li, Z.H.; Cai, H.G. Soil Enzyme Activities Affect SOC and TN in Aggregate Fractions in Sodic-Alkali Soils, Northeast of China. Agronomy 2022, 12, 2549. [Google Scholar] [CrossRef]
- Wang, S.S.; Wang, Z.Q.; Fan, B.; Mao, X.H.; Luo, H.; Jiang, F.Y.; Liang, C.F.; Chen, J.H.; Qin, H.; Xu, Q.F.; et al. Litter Inputs Control the Pattern of Soil Aggregate-Associated Organic Carbon and Enzyme Activities in Three Typical Subtropical Forests. Forests. 2022, 13, 1210. [Google Scholar] [CrossRef]
- Ma, Y.; Cheng, X.Q.; Kang, F.F.; Han, H.R. Dynamic characteristics of soil aggregate stability and related carbon and nitrogen pools at different developmental stages of plantations in northern China. J. Environ. Manag. 2022, 316, 115283. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Su, X.; Meng, H.; Wang, H.; Yan, X.; Qin, D.; Liu, C.; Men, Y.; Zhang, X.; Song, X.; et al. Long-term cotton stubble return and subsoiling improve soil organic carbon by changing the stability and organic carbon of soil aggregates in coastal saline fields. Soil Tillage Res. 2024, 241, 106127. [Google Scholar] [CrossRef]
- Yongxiang, G.; Haojie, F.; Min, Z.; Yuqing, S.; Jiaqi, W.; Yanli, L.; Chengliang, L. Straw returning combined with controlled-release nitrogen fertilizer affected organic carbon storage and crop yield by changing humic acid composition and aggregate distribution. J. Clean. Prod. 2023, 415, 137783. [Google Scholar]
- Tian, S.Y.; Zhu, B.J.; Yin, R.; Wang, M.W.; Jiang, Y.J.; Zhang, C.Z.; Li, D.M.; Chen, X.Y.; Kardol, P.; Liu, M.Q. Organic fertilization promotes crop productivity through changes in soil aggregation. Soil Biol. Biochem. 2022, 165, 108533. [Google Scholar] [CrossRef]
- Zhao, J.S.; Chen, S.; Hu, R.G.; Li, Y.Y. Aggregate stability and size distribution of red soils under different land uses integrally regulated by soil organic matter, and iron and aluminum oxides. Soil Tillage Res. 2017, 167, 73–79. [Google Scholar] [CrossRef]
- Han, S.; Delgado-Baquerizo, M.; Luo, X.S.; Liu, Y.R.; Van Nostrand, J.D.; Chen, W.L.; Zhou, J.Z.; Huang, Q.Y. Soil aggregate size-dependent relationships between microbial functional diversity and multifunctionality. Soil Biol. Biochem. 2021, 154, 108143. [Google Scholar] [CrossRef]
- Burrell, L.D.; Zehetner, F.; Rampazzo, N.; Wimmer, B.; Soja, G. Long-term effects of biochar on soil physical properties. Geoderma 2016, 282, 96–102. [Google Scholar] [CrossRef]
- Zhang, Q.Q.; Song, Y.F.; Wu, Z.; Yan, X.Y.; Gunina, A.; Kuzyakov, Y.; Xiong, Z.Q. Effects of six-year biochar amendment on soil aggregation, crop growth, and nitrogen and phosphorus use efficiencies in a rice-wheat rotation. J. Clean. Prod. 2020, 242, 118435. [Google Scholar] [CrossRef]
- Sun, F.F.; Lu, S.G. Biochars improve aggregate stability, water retention, and pore- space properties of clayey soil. J. Plant Nutr. Soil Sci. 2014, 177, 26–33. [Google Scholar] [CrossRef]
- Thomaz, E.L.; Araujo-Junior, C.F.; Vendrame, P.R.; de Melo, T.R. Mechanisms of aggregate breakdown in (sub) tropical soils: Effects of the hierarchical resistance. Catena 2022, 216, 106377. [Google Scholar] [CrossRef]
- Tang, X.; Qiu, J.C.; Xu, Y.Q.; Li, J.H.; Chen, J.H.; Li, B.; Lu, Y. Responses of soil aggregate stability to organic C and total N as controlled by land-use type in a region of south China affected by sheet erosion. Catena 2022, 218, 106543. [Google Scholar] [CrossRef]
- Cheng, R.; Kesi, L.; Pengpeng, D.; Xinqing, S.; Dingyuan, Z.; Kaili, W.; Xiqiang, L.; Jiahuan, L.; Kun, W. Soil Nutrients Drive Microbial Changes to Alter Surface Soil Aggregate Stability in Typical Grasslands. J. Soil Sci. Plant Nutr. 2022, 22, 4943–4959. [Google Scholar]
- Zhang, C.; Zhao, X.; Liang, A.J.; Li, Y.Y.; Li, X.Y.; Li, D.P.; Hou, N. Insight into the soil aggregate-mediated restoration mechanism of degraded black soil via biochar addition: Emphasizing the driving role of core microbial communities and nutrient cycling. Environ. Res. 2023, 228, 115895. [Google Scholar] [CrossRef] [PubMed]
- Xuan, L.; Hua, J.F.; Zhang, F.; Wang, Z.Q.; Pei, X.X.; Yang, Y.; Yin, Y.L.; Creech, D.L. Identification and Functional Analysis of ThADH1 and ThADH4 Genes Involved in Tolerance to Waterlogging Stress in Taxodium hybrid ‘Zhongshanshan 406’. Genes 2021, 12, 225. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.J.; Creech, D.L.; Krauss, K.W.; Yunlong, Y.; Kulhavy, D.L. Can We Improve the Salinity Tolerance of Genotypes of Taxodium by Using Varietal and Hybrid Crosses? Hortscience 2010, 45, 1773–1778. [Google Scholar] [CrossRef]
- Shi, Q.; Zhou, Z.D.; Wang, Z.Y.; Lu, Z.G.; Han, J.A.; Xue, J.H.; Creech, D.; Yin, Y.L.; Hua, J.F. Afforestation of Taxodium Hybrid Zhongshanshan Influences Soil Bacterial Community Structure by Altering Soil Properties in the Yangtze River Basin, China. Plants 2022, 11, 3456. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.Y.; Ma, S.L.; Liu, J.; Qin, S.H.; Liu, X.; Li, T.; Liao, Y.; Shi, Y.X.; Zhang, J.C. Organic Materials and AMF Addition Promote Growth of Taxodium ‘zhongshanshan’ by Improving Soil Structure. Forests 2023, 14, 731. [Google Scholar] [CrossRef]
- Tian, Y.; Dou, S.; Zhang, Y.; Wang, C.; Wu, J. Improvement effects of subsurface pipe with different spacing on sodic-alkali soil. Editor. Off. Trans. Chin. Soc. Agric. Eng. 2013, 29, 145–153. [Google Scholar]
- Liu, X.; Cheng, X.F.; Wang, N.; Meng, M.J.; Jia, Z.H.; Wang, J.P.; Ma, S.L.; Tang, Y.Z.; Li, C.; Zhai, L.; et al. Effects of Vegetation Type on Soil Shear Strength in Fengyang Mountain Nature Reserve, China. Forests 2021, 12, 490. [Google Scholar] [CrossRef]
- Cui, H.; Ma, K.; Fan, Y.; Peng, X.; Mao, J.; Zhou, D.; Zhang, Z.; Zhou, J. Stability and heavy metal distribution of soil aggregates affected by application of apatite, lime, and charcoal. Environ. Sci. Pollut. Res. Int. 2016, 23, 10808–10817. [Google Scholar] [CrossRef] [PubMed]
- Setia, R.; Verma, S.L.; Marschner, P. Measuring microbial biomass carbon by direct extraction—Comparison with chloroform fumigation-extraction. Eur. J. Soil Biol. 2012, 53, 103–106. [Google Scholar] [CrossRef]
- Zheng, X.; Liu, Q.; Cao, M.M.; Ji, X.F.; Lu, J.B.; He, L.; Liu, L.J.; Liu, S.L.; Jiang, J. Nitrogen uptake by plants may alleviate N deposition-induced increase in soil N2O emissions in subtropical Chinese fir (Cunninghamia lanceolata) plantations. Plant Soil 2022, 479, 127–142. [Google Scholar] [CrossRef]
- Stemmer, M.; Gerzabek, M.H.; Kandeler, E.J.S.B. Organic matter and enzyme activity in particle-size fractions of soils obtained after low-energy sonication. Soil Biol. Biochem. 1998, 30, 9–17. [Google Scholar] [CrossRef]
- German, D.P.; Weintraub, M.N.; Grandy, A.S.; Lauber, C.L.; Rinkes, Z.L.; Allison, S.D. Optimization of hydrolytic and oxidative enzyme methods for ecosystem studies. Soil Biol. Biochem. 2012, 43, 1387–2011. [Google Scholar] [CrossRef]
- Wang, L.; Jia, Z.Q.; Li, Q.X.; He, L.X.Z.; Tian, J.P.; Ding, W.; Liu, T.; Gao, Y.; Zhang, J.P.; Han, D.; et al. Grazing Impacts on Soil Enzyme Activities Vary with Vegetation Types in the Forest-Steppe Ecotone of Northeastern China. Forests 2023, 14, 2292. [Google Scholar] [CrossRef]
- Li, C.; Jia, Z.H.; Peng, X.N.; Zhai, L.; Zhang, B.; Liu, X.; Zhang, J.C. Functions of mineral-solubilizing microbes and a water retaining agent for the remediation of abandoned mine sites. Sci. Total Environ. 2021, 761, 143215. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Yu, Z.Z.; Lin, J.; Meng, M.J.; Zhao, Y.P.; Jia, Z.H.; Peng, X.N.; Liu, X.; Zhang, J.C. Forest Conversion and Soil Depth Can Modify the Contributions of Organic and Inorganic Colloids to the Stability of Soil Aggregates. Forests 2022, 13, 546. [Google Scholar] [CrossRef]
- Na, L.; Tianyun, S.; Yujie, Z.; Yuchen, C.; Huiying, H.; Qingkai, S.; Xiaohua, L.; Yang, Y.; Xiumei, G.; Zed, R. Effects of planting Melia azedarach L. on soil properties and microbial community in saline-alkali soil. Land Degrad. Dev. 2021, 32, 2951–2961. [Google Scholar]
- Xia, J.; Ren, J.; Zhang, S.; Wang, Y.; Fang, Y. Forest and grass composite patterns improve the soil quality in the coastal saline-alkali land of the Yellow River Delta, China. Geoderma 2019, 349, 25–35. [Google Scholar] [CrossRef]
- Zhu, X.; Shen, Y.; Yuan, X.; Yuan, C.; Jin, L.; Zhao, Z.; Chen, F.; Yang, B.; Jiang, X.; Liu, W. High levels of soil calcium and clay facilitate the recovery and stability of organic carbon: Insights from different land uses in the karst of China. Environ. Sci. Pollut. Res. Int. 2024, 31, 34234–34248. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Chen, X.; Yang, K.; Guo, X.; Liu, G.; Zhuang, G.; Zheng, G.; Fortin, D.; Ma, A. Vegetation restoration in an alpine meadow: Insights from soil microbial communities and resource limitation across soil depth. J. Environ. Manag. 2024, 360, 121129. [Google Scholar] [CrossRef]
- Hu, P.L.; Xiao, J.; Zhang, W.; Xiao, L.M.; Yang, R.; Xiao, D.; Zhao, J.; Wang, K.L. Response of soil microbial communities to natural and managed vegetation restoration in a subtropical karst region. Catena 2020, 195, 104849. [Google Scholar] [CrossRef]
- You, Y.; Li, W.; Chen, Y.; Zhang, Q.; Zhang, K. Soil carbon and nitrogen accumulation during long-term natural vegetation restoration following agricultural abandonment in Qingling Mountains. Ecol. Eng. 2024, 201, 107212. [Google Scholar] [CrossRef]
- Song, W.T.; Hou, Y.K.; Zhu, W.J.; Fan, Y.C.; Xu, H.Y.; Cai, C.Y.; Li, G.H.; Huang, L. Enhancement effects of mangrove restoration on blue carbon storage in Qinzhou Bay. Front. For. Glob. Change 2024, 7, 1328783. [Google Scholar] [CrossRef]
- Van Der Sande, M.T.; Powers, J.S.; Kuyper, T.W.; Norden, N.; Salgado-Negret, B.; Silva de Almeida, J.; Bongers, F.; Delgado, D.; Dent, D.H.; Derroire, G.; et al. Soil resistance and recovery during neotropical forest succession. Philos. Trans. R. Soc. B 2023, 378, 20210074. [Google Scholar] [CrossRef]
- Deng, L.; Liu, G.B.; Shangguan, Z.P. Land-use conversion and changing soil carbon stocks in China’s ‘Grain-for-Green’ Program: A synthesis. Glob. Change Biol. 2014, 20, 3544–3556. [Google Scholar] [CrossRef]
- Karhu, K.; Wall, A.; Vanhala, P.; Liski, J.; Esala, M.; Regina, K. Effects of afforestation and deforestation on boreal soil carbon stocks—Comparison of measured C stocks with Yasso07 model results. Geoderma 2011, 164, 33–45. [Google Scholar] [CrossRef]
- Zhang, K.; Dang, H.; Tan, S.; Cheng, X.; Zhang, Q. Change in soil organic carbon following the ‘Grain-for-Green’ programme in China. Land Degrad. Dev. 2009, 21, 13–23. [Google Scholar] [CrossRef]
- Harper, R.J.; Tibbett, M. The hidden organic carbon in deep mineral soils. Plant Soil 2013, 368, 641–648. [Google Scholar] [CrossRef]
- Fang, X.M.; Chen, F.S.; Wan, S.Z.; Yang, Q.P.; Shi, J.M. Topsoil and Deep Soil Organic Carbon Concentration and Stability Vary with Aggregate Size and Vegetation Type in Subtropical China. PLoS ONE 2015, 10, e0139380. [Google Scholar] [CrossRef]
- Xie, M.; Yuan, J.; Liu, S.; Xu, G.; Lu, Y.; Yan, L.; Li, G. Soil Carbon and Nitrogen Pools and Their Storage Characteristics under Different Vegetation Restoration Types on the Loess Plateau of Longzhong, China. Forests 2024, 15, 173. [Google Scholar] [CrossRef]
- Jiang, J.P.; Xiong, Y.C.; Jiang, H.M.; Ye, D.Y.; Song, Y.J.; Li, F.M. Soil Microbial Activity During Secondary Vegetation Succession in Semiarid Abandoned Lands of Loess Plateau. Pedosphere 2009, 19, 735–747. [Google Scholar] [CrossRef]
- Lucas-Borja, M.E.; Hedo, J.; Cerdá, A.; Candel-Pérez, D.; Viñegla, B. Unravelling the importance of forest age stand and forest structure driving microbiological soil properties, enzymatic activities and soil nutrients content in Mediterranean Spanish black pine (Pinus nigra Ar. ssp. salzmannii) Forest. Sci. Total Environ. 2016, 562, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Kähkönen, M.A.; Hakulinen, R. Hydrolytic enzyme activities, carbon dioxide production and the growth of litter degrading fungi in different soil layers in a coniferous forest in Northern Finland. Eur. J. Soil Biol. 2011, 47, 108–113. [Google Scholar] [CrossRef]
- Tang, L.L.; Wang, S.Q. Dynamics of soil aggregate-related C-N-P stoichiometric characteristics with stand age and soil depth in Chinese fir plantations. Land Degrad. Dev. 2022, 33, 1290–1306. [Google Scholar] [CrossRef]
- Guan, Z.J.; Luo, Q.; Chen, X.; Feng, X.W.; Tang, Z.X.; Wei, W.; Zheng, Y.R. Saline soil enzyme activities of four plant communities in Sangong River basin of Xinjiang, China. J. Arid. Land 2014, 6, 164–173. [Google Scholar] [CrossRef]
- Xiaodong, Y.; Wenjing, Z.; Hui, Z.; Wei, W. Soil Microbial Attributes along a Chronosequence of Scots Pine (Pinus sylvestris var. mongolica) Plantations in Northern China. Pedosphere 2017, 30, 433–442. [Google Scholar]
- Kunito, T.; Akagi, Y.; Park, H.D.; Toda, H. Influences of nitrogen and phosphorus addition on polyphenol oxidase activity in a forested Andisol. Eur. J. For. Res. 2009, 128, 361–366. [Google Scholar] [CrossRef]
- Feng, J.; Xu, X.; Wu, J.J.; Zhang, Q.; Zhang, D.D.; Li, Q.X.; Long, C.Y.; Chen, Q.; Chen, J.W.; Cheng, X.L. Inhibited enzyme activities in soil macroaggregates contribute to enhanced soil carbon sequestration under afforestation in central China. Sci. Total Environ. 2018, 640, 653–661. [Google Scholar] [CrossRef]
- Yong-ling, W.; Bing, W.; Chao, Z.; Wei, D.; Li, P.; University, F. Comprehensive evaluation of soil fertility in different developing stages of Chinese Fir. Plantations 2011, 39, 69–75. [Google Scholar]
- Wang, C.Q.; Xue, L.; Dong, Y.H.; Hou, L.Y.; Wei, Y.H.; Chen, J.Q.; Jiao, R.Z. Contrasting Effects of Chinese Fir Plantations of Different Stand Ages on Soil Enzyme Activities and Microbial Communities. Forests 2019, 10, 11. [Google Scholar] [CrossRef]
- Zhao, F.Z.; Han, X.H.; Yang, G.H.; Feng, Y.Z.; Ren, G.X. Soil structure and carbon distribution in subsoil affected by vegetation restoration Plant Soil Environ. 2014, 64, 21–26. 64.
- Wei, X.R.; Li, X.Z.; Jia, X.X.; Shao, M.G. Accumulation of soil organic carbon in aggregates after afforestation on abandoned farmland. Biol. Fertil. Soils 2013, 49, 637–646. [Google Scholar] [CrossRef]
- Wang, J.Y.; Deng, Y.S.; Li, D.Y.; Liu, Z.F.; Wen, L.L.; Huang, Z.G.; Jiang, D.H.; Lu, Y.P. Soil aggregate stability and its response to overland flow in successive Eucalyptus plantations in subtropical China. Sci. Total Environ. 2022, 807, 151000. [Google Scholar] [CrossRef] [PubMed]
- Le Bissonnais, Y.; Prieto, I.; Roumet, C.; Nespoulous, J.; Metayer, J.; Huon, S.; Villatoro, M.; Stokes, A. Soil aggregate stability in Mediterranean and tropical agro-ecosystems: Effect of plant roots and soil characteristics. Plant Soil 2018, 424, 303–317. [Google Scholar] [CrossRef]
- Laganière, J.; Angers, D.A.; PARÉ, D. Carbon accumulation in agricultural soils after afforestation: A meta-analysis. Glob. Change Biol. 2010, 16, 439–453. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhen, Q.; Ma, W.; Jia, J.; Li, P.; Zhang, X. Dynamic responses of soil aggregate-associated organic carbon and nitrogen to different vegetation restoration patterns in an agro-pastoral ecotone in northern China. Ecol. Eng. 2023, 189, 106895. [Google Scholar] [CrossRef]
- Kurmi, B.; Nath, A.J.; Lal, R.; Das, A.K. Water stable aggregates and the associated active and recalcitrant carbon in soil under rubber plantation. Sci. Total Environ. 2020, 703, 135498. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.Q.; Li, D.H.; Huang, Y.; Deng, Y.S.; Yang, G.R.; Lei, T.W.; Huang, Y.H. Soil matrix infiltration characteristics in differently aged eucalyptus plantations in a southern subtropical area in China. Catena 2022, 217, 106490. [Google Scholar] [CrossRef]
- Yang, M.; Yang, D.; Yu, X. Soil microbial communities and enzyme activities in sea-buckthorn (Hippophae rhamnoides) plantation at different ages. PLoS ONE 2018, 13, e0190959. [Google Scholar] [CrossRef]
- Erdel, E. Effects of Salinity and Alkalinity on Soil Enzyme Activities in Soil Aggregates of Different Sizes. Eurasian Soil Sci. 2022, 55, 759–765. [Google Scholar] [CrossRef]
- Yang, Q.; Zhu, D.Y.; Chen, J. Effects of Planting Patterns on Soil Aggregates and Enzyme Activities in Rocky Desertification Areas of Karst Plateau Mountains. Pol. J. Environ. Stud. 2023, 32, 405–418. [Google Scholar] [CrossRef] [PubMed]
- Haj-Amor, Z.; Araya, T.; Kim, D.G.; Bouri, S.; Lee, J.; Ghilou, W.; Yang, Y.; Kang, H.; Jhariya, M.K.; Banerjee, A.; et al. Soil salinity and its associated effects on soil microorganisms, greenhouse gas emissions, crop yield, biodiversity and desertification: A review. Sci. Total Environ. 2022, 843, 156946. [Google Scholar] [CrossRef] [PubMed]
- Radersma, S.; Grierson, P.F. Phosphorus mobilization in agroforestry: Organic anions, phosphatase activity and phosphorus fractions in the rhizosphere. Plant Soil 2004, 259, 209–219. [Google Scholar] [CrossRef]
- Zhang, D.B.; Yao, Z.Y.; Chen, J.; Yao, P.W.; Zhao, N.; He, W.X.; Li, Y.Y.; Zhang, S.Q.; Zhai, B.N.; Wang, Z.H.; et al. Improving soil aggregation, aggregate-associated C and N, and enzyme activities by green manure crops in the Loess Plateau of China. Eur. J. Soil Sci. 2019, 70, 1267–1279. [Google Scholar]


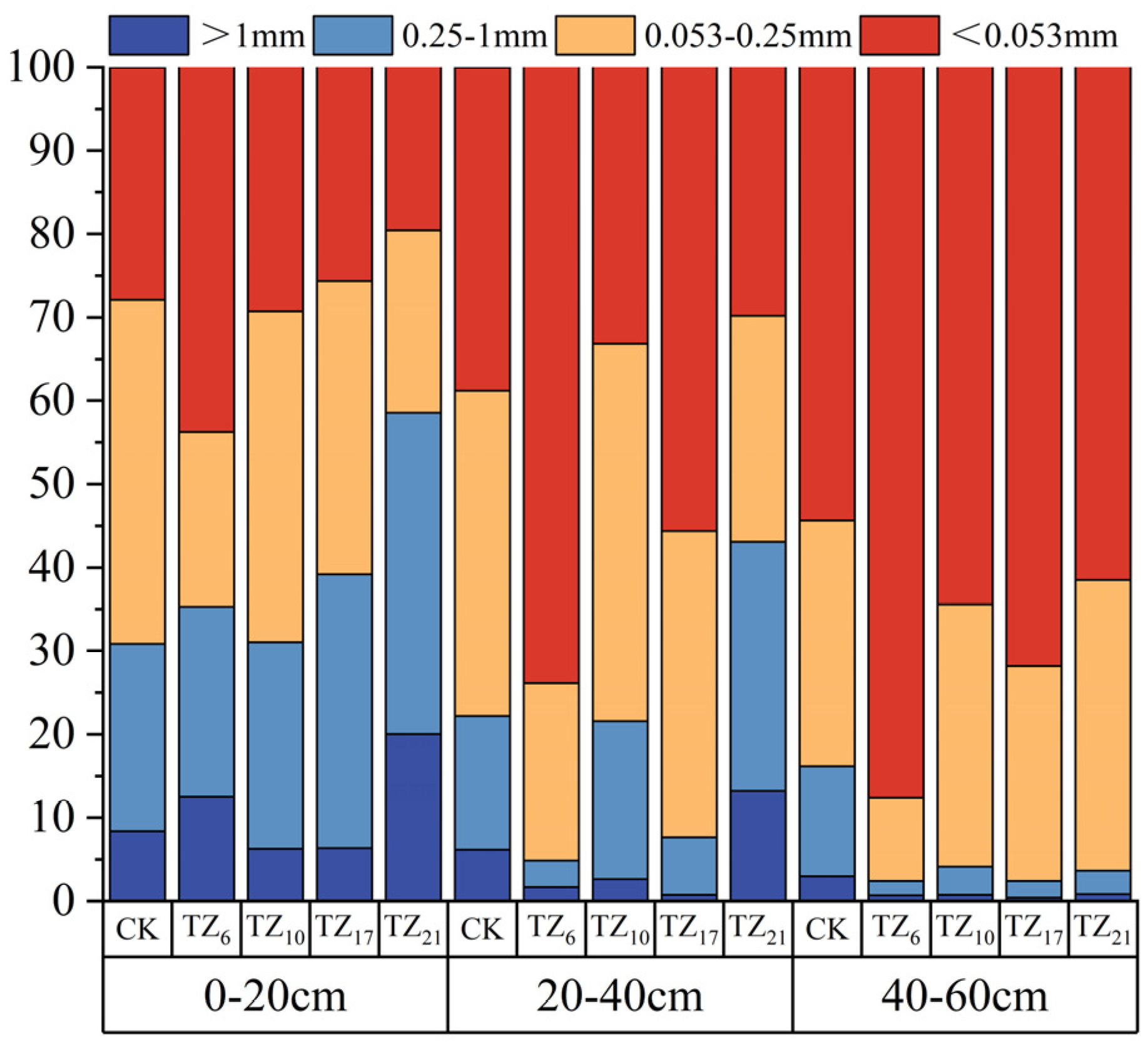



| Soil Layer | Soil Aggregate Fraction | CK | TZ6 | TZ10 | TZ17 | TZ21 |
|---|---|---|---|---|---|---|
| 0–20 cm | >1 mm | 0.084 ± 0.009 Ac | 0.125 ± 0.009 Ab | 0.063 ± 0.005 Ad | 0.063 ± 0.005 Ad | 0.2 ± 0.132 Aa |
| 0.25–1 mm | 0.225 ± 0.021 Ac | 0.228 ± 0.014 Ac | 0.248 ± 0.009 Ac | 0.329 ± 0.006 Ab | 0.385 ± 0.007 Aa | |
| 0.053–0.25 mm | 0.413 ± 0.011 Aa | 0.21 ± 0.005 Ac | 0.397 ± 0.019 Ba | 0.352 ± 0.024 Bb | 0.219 ± 0.006 Cc | |
| <0.053 mm | 0.279 ± 0.008 Cbc | 0.438 ± 0.015 Ca | 0.293 ± 0.025 Bb | 0.256 ± 0.022 Cc | 0.196 ± 0.005 Cd | |
| 20–40 cm | >1 mm | 0.061 ± 0.002 Bb | 0.017 ± 0.004 Bd | 0.026 ± 0.005 Bc | 0.007 ± 0.001 Be | 0.132 ± 0.004 Ba |
| 0.25–1 mm | 0.161 ± 0.015 Bc | 0.032 ± 0.006 Be | 0.19 ± 0.022 Bb | 0.069 ± 0.005 Bd | 0.299 ± 0.013 Ba | |
| 0.053–0.25 mm | 0.39 ± 0.016 Ab | 0.213 ± 0.025 Ad | 0.452 ± 0.009 Aa | 0.367 ± 0.003 Ab | 0.271 ± 0.011 Bc | |
| <0.053 mm | 0.388 ± 0.003 Bc | 0.739 ± 0.033 Ba | 0.332 ± 0.023 Bd | 0.556 ± 0.007 Bb | 0.298 ± 0.018 Bd | |
| 40–60 cm | >1 mm | 0.03 ± 0.003 Ca | 0.007 ± 0.001 Bbc | 0.007 ± 0.003 Cbc | 0.004 ± 0.001 Bc | 0.008 ± 0.002 Cb |
| 0.25–1 mm | 0.132 ± 0.011 Ba | 0.017 ± 0.006 Bc | 0.034 ± 0.006 Cb | 0.02 ± 0.001 Cc | 0.028 ± 0.003 Cbc | |
| 0.053–0.25 mm | 0.295 ± 0.02 Bb | 0.1 ± 0.013 Bd | 0.314 ± 0.01 Cb | 0.258 ± 0.009 Cc | 0.349 ± 0.014 Aa | |
| <0.053 mm | 0.544 ± 0.029 Ad | 0.876 ± 0.018 Aa | 0.644 ± 0.016 Ac | 0.718 ± 0.01 Ab | 0.615 ± 0.015 Ac |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niu, X.; Liu, X.; Li, T.; Lin, J.; Qin, S.; Jing, F.; Zhang, X.; Zhang, J.; Jiang, J. Long-Term Planting of Taxodium Hybrid ‘Zhongshanshan’ Can Effectively Enhance the Soil Aggregate Stability in Saline–Alkali Coastal Areas. Forests 2024, 15, 1376. https://doi.org/10.3390/f15081376
Niu X, Liu X, Li T, Lin J, Qin S, Jing F, Zhang X, Zhang J, Jiang J. Long-Term Planting of Taxodium Hybrid ‘Zhongshanshan’ Can Effectively Enhance the Soil Aggregate Stability in Saline–Alkali Coastal Areas. Forests. 2024; 15(8):1376. https://doi.org/10.3390/f15081376
Chicago/Turabian StyleNiu, Xiaoshu, Xin Liu, Tao Li, Jie Lin, Shenghua Qin, Fulin Jing, Xiang Zhang, Jinchi Zhang, and Jiang Jiang. 2024. "Long-Term Planting of Taxodium Hybrid ‘Zhongshanshan’ Can Effectively Enhance the Soil Aggregate Stability in Saline–Alkali Coastal Areas" Forests 15, no. 8: 1376. https://doi.org/10.3390/f15081376





